Synthesis of Microwave Functionalized, Nanostructured Polylactic Co-Glycolic Acid (nfPLGA) for Incorporation into Hydrophobic Dexamethasone to Enhance Dissolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of nfPLGA
2.3. Synthesis of DXM–nfPLGA
2.4. Characterization of nfPLGA and DXM–nfPLGA Composites
3. Results and Discussion
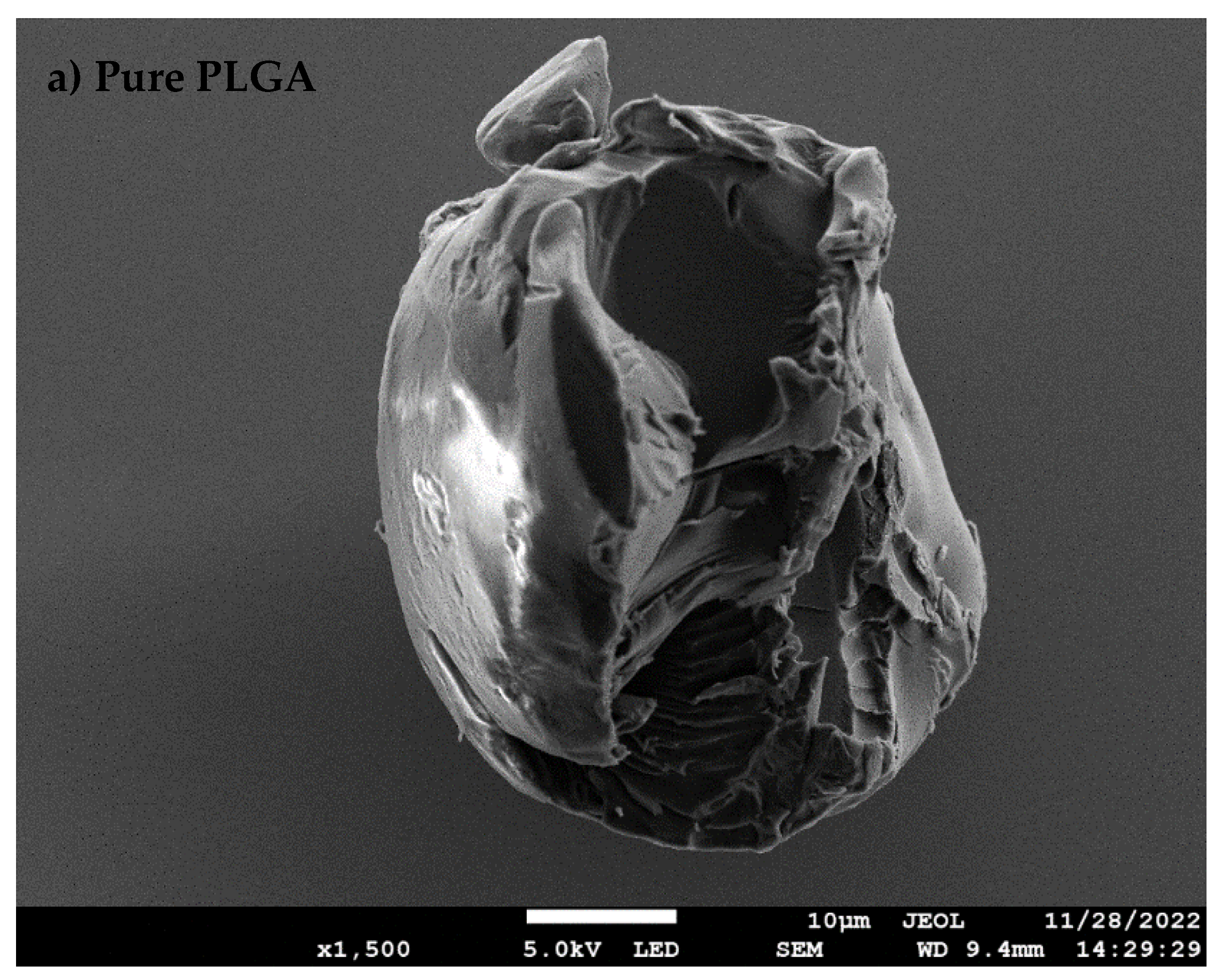
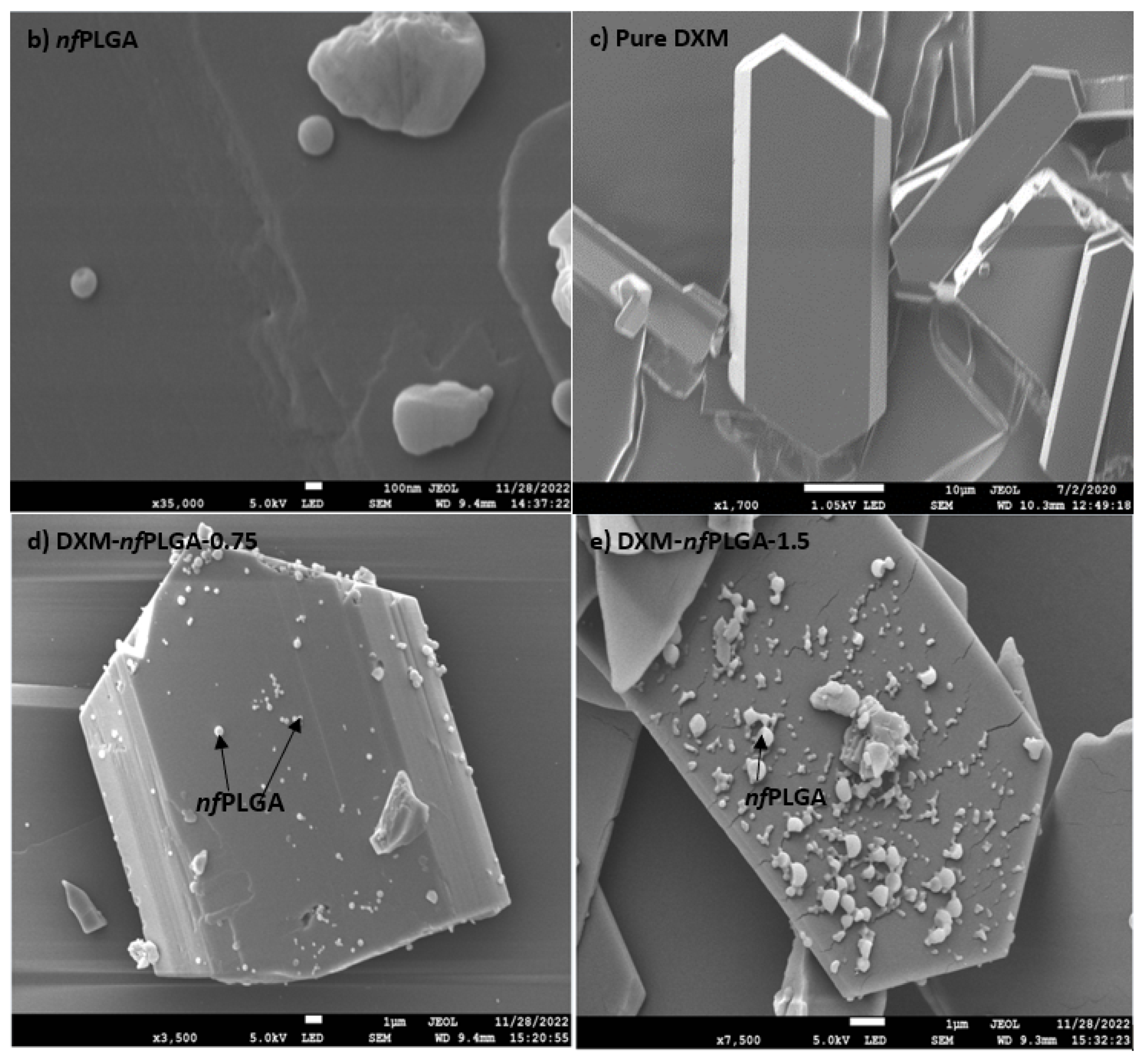
3.1. Synthesis of nfPLGA
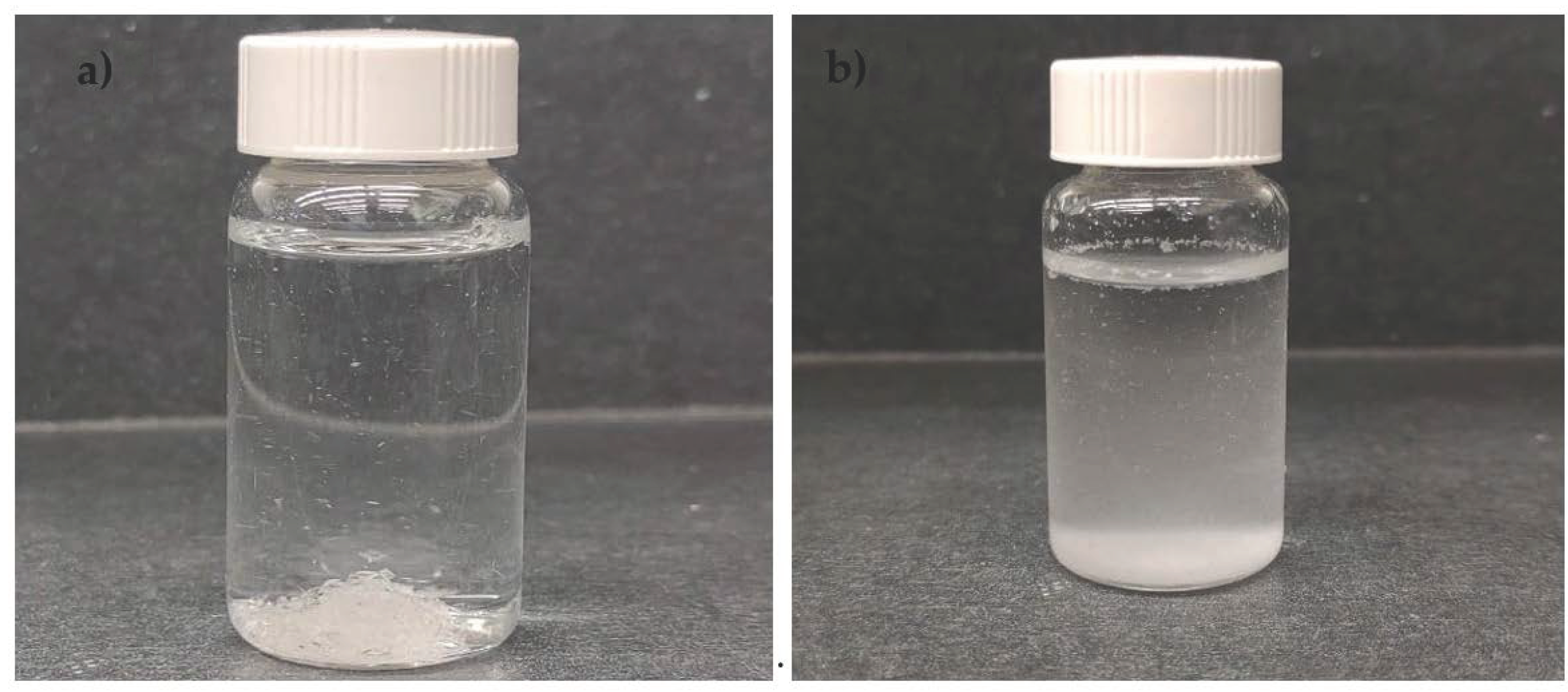
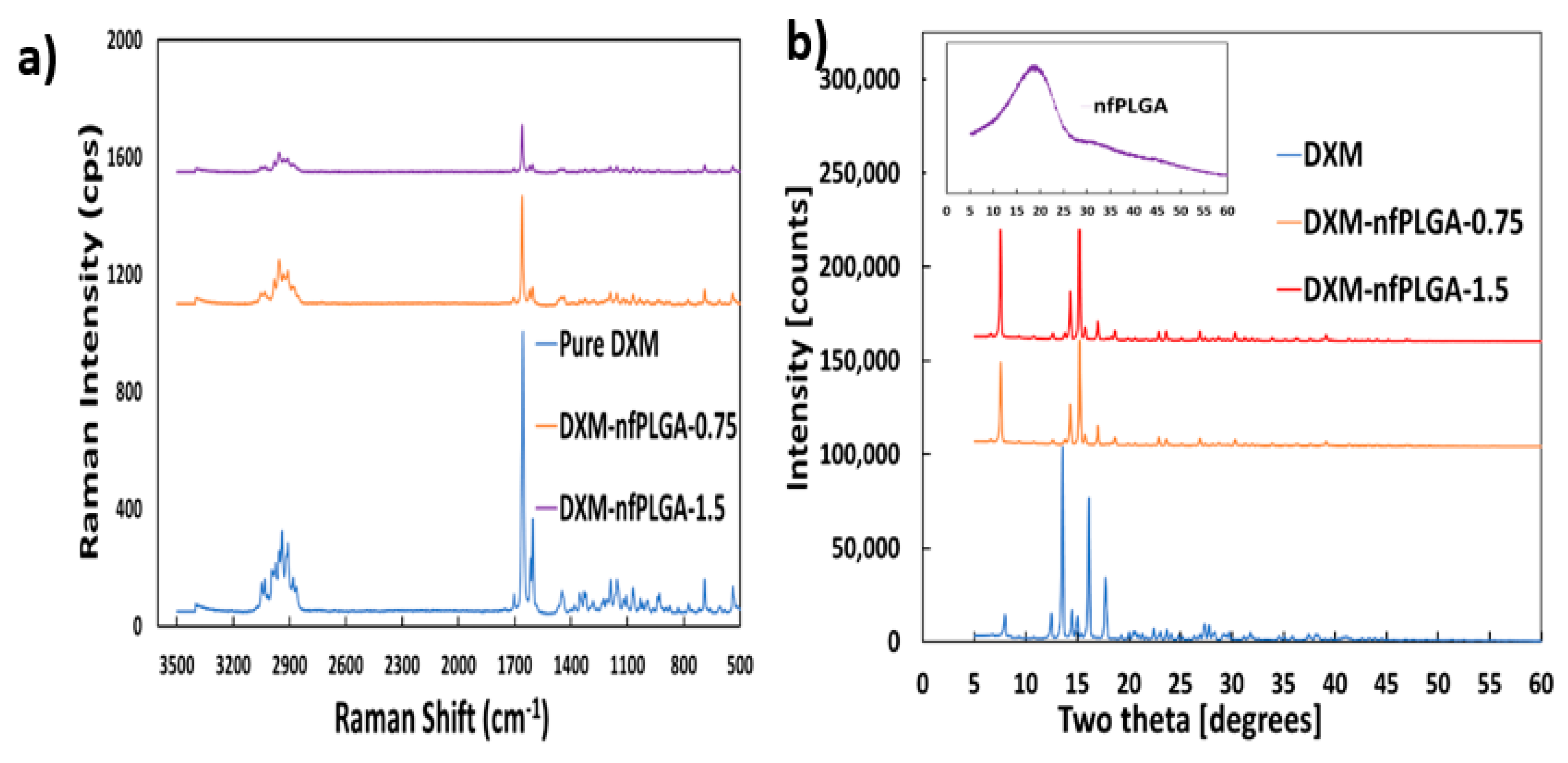
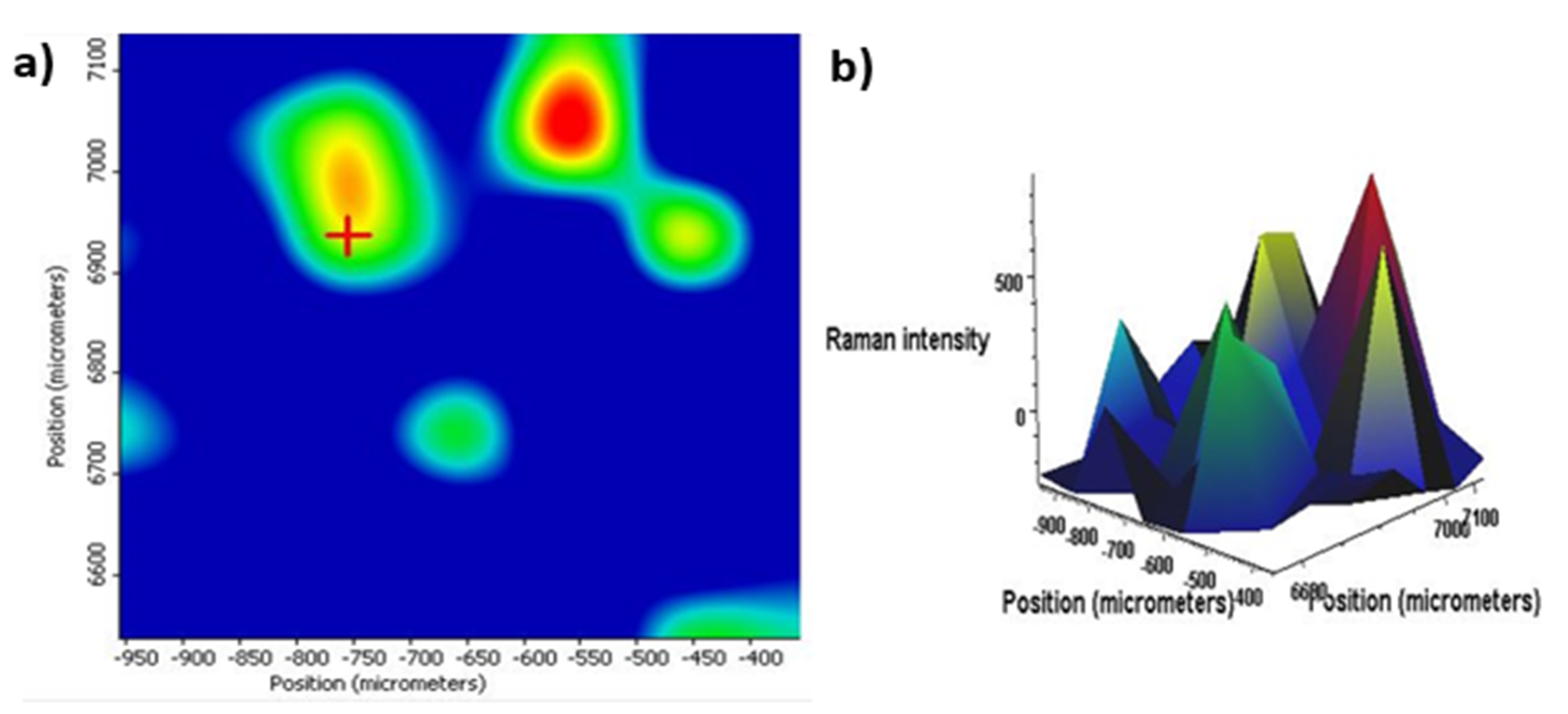
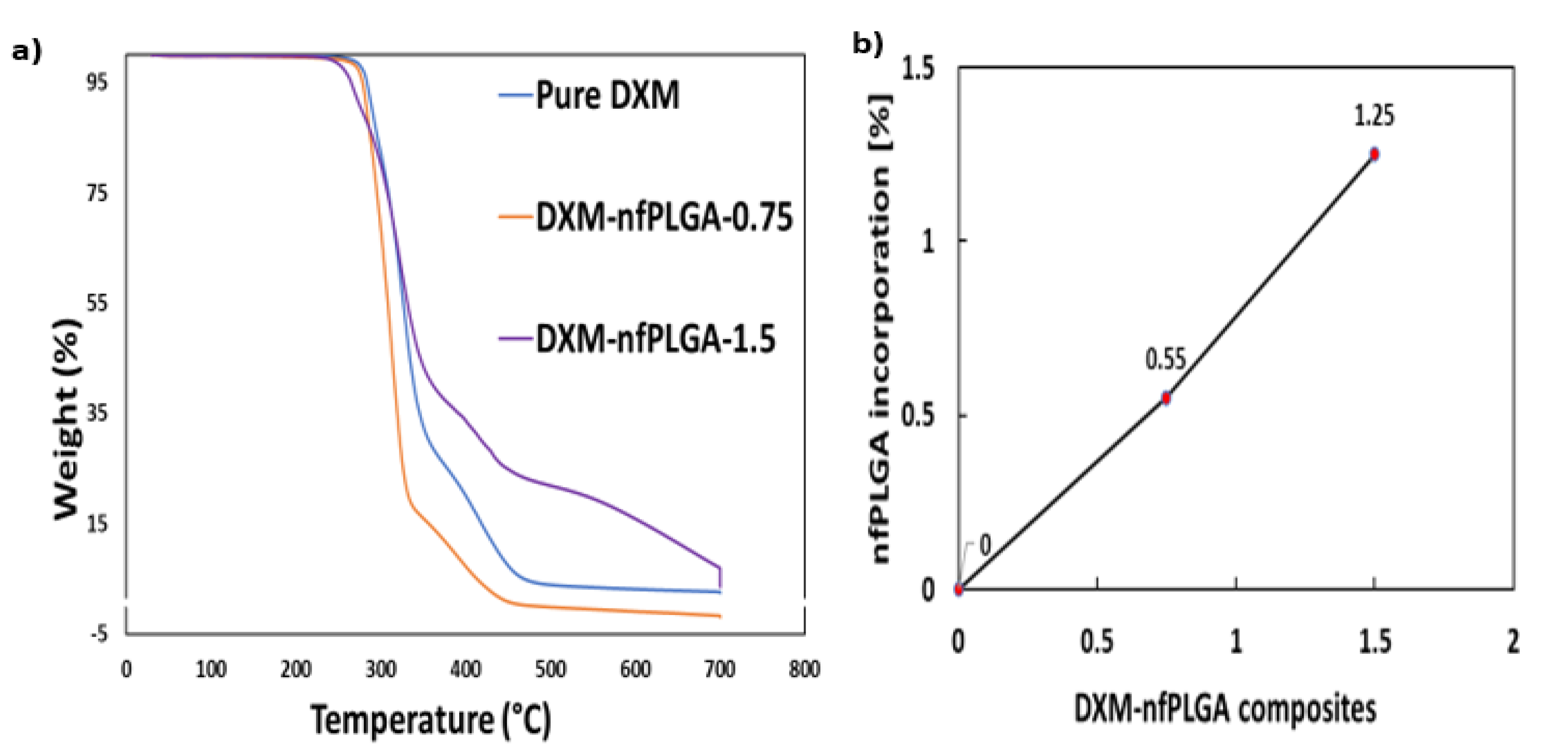
3.2. Characteristics of DXM–nfPLGA Composites
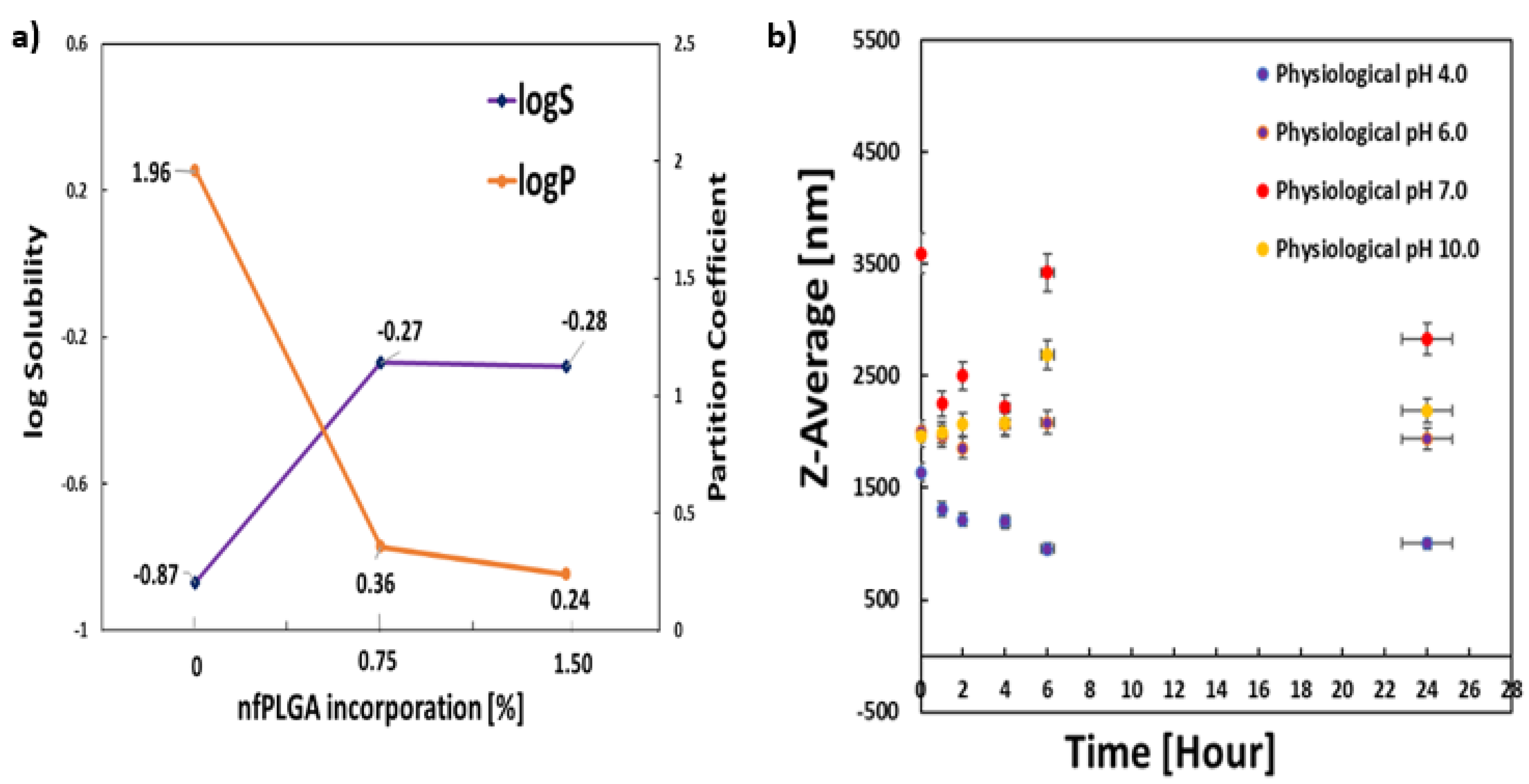
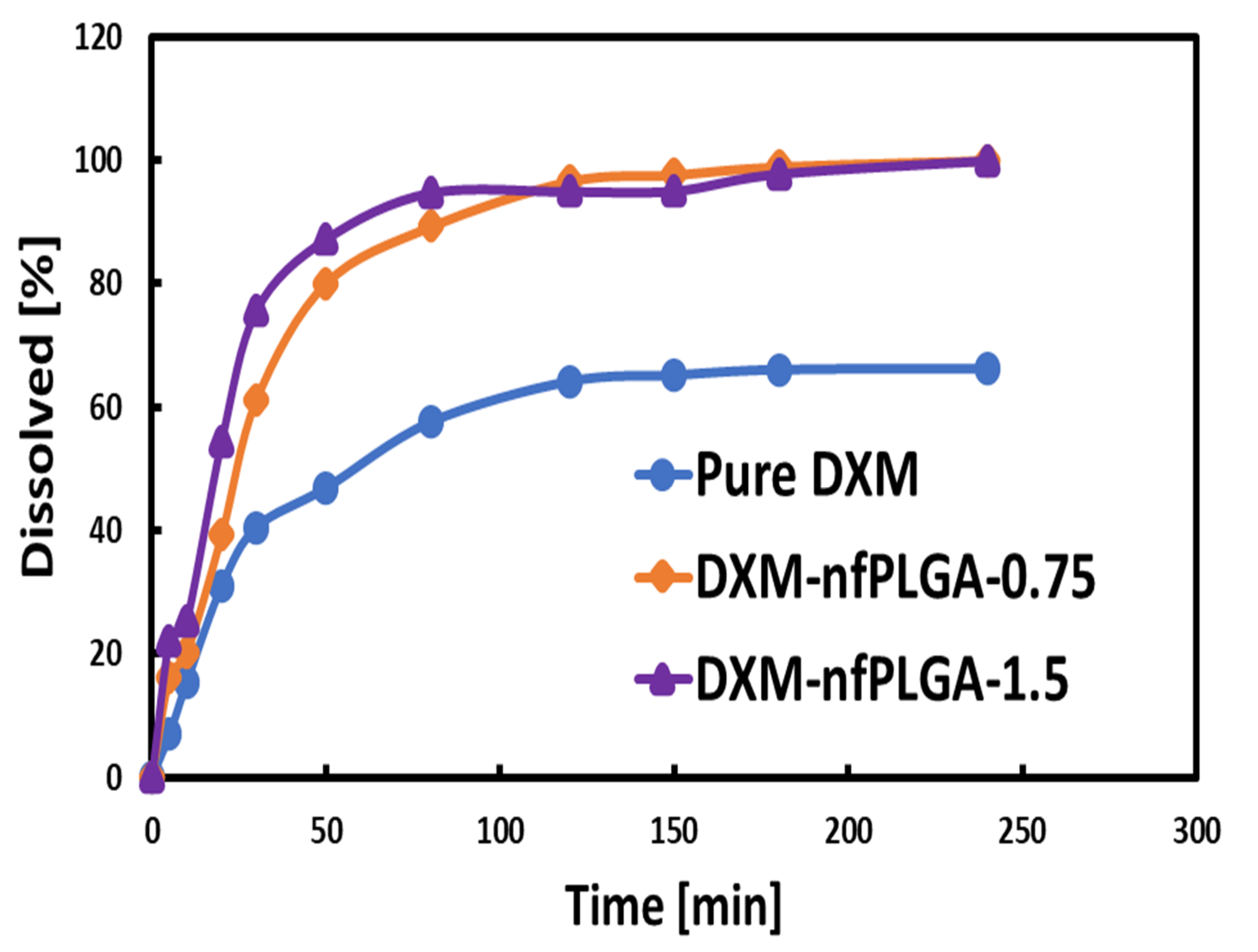
3.3. Dissolution of DXM and DXM–nfPLGA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fink, C.; Sun, D.; Wagner, K.; Schneider, M.; Bauer, H.; Dolgos, H.; Mäder, K.; Peters, S.A. Evaluating the Role of Solubility in Oral Absorption of Poorly Water-Soluble Drugs Using Physiologically-Based Pharmacokinetic Modeling. Clin. Pharmacol. Ther. 2020, 107, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Tsume, Y.; Mudie, D.M.; Langguth, P.; Amidon, G.E.; Amidon, G.L. The Biopharmaceutics Classification System: Subclasses for in vivo predictive dissolution (IPD) methodology and IVIVC. Eur. J. Pharm. Sci. 2014, 57, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Glassman, P.M.; Muzykantov, V.R. Pharmacokinetic and Pharmacodynamic Properties of Drug Delivery Systems. J. Pharmacol. Exp. Ther. 2019, 370, 570–580. [Google Scholar] [CrossRef]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Chandel, A.K.S. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, G. A Critical Appraisal of Solubility Enhancement Techniques of Polyphenols. J. Pharm. 2014, 2014, 180845. [Google Scholar] [CrossRef]
- Bi, X.; Liu, X.; Di, L.; Zu, Q. Improved Oral Bioavailability Using a Solid Self-Microemulsifying Drug Delivery System Containing a Multicomponent Mixture Extracted from Salvia miltiorrhiza. Molecules 2016, 21, 456. [Google Scholar] [CrossRef]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef]
- Albetawi, S.; Abdalhafez, A.; Abu-Zaid, A.; Matrouk, A.; Alhourani, N. Recent solubility and dissolution enhancement techniques for repaglinide a BCS class II drug: A review. Pharmacia 2021, 68, 573–583. [Google Scholar] [CrossRef]
- Alshehri, S.; Imam, S.S.; Hussain, A.; Altamimi, M.A.; Alruwaili, N.K.; Alotaibi, F.; Alanazi, A.; Shakeel, F. Potential of solid dispersions to enhance solubility, bioavailability, and therapeutic efficacy of poorly water-soluble drugs: Newer formulation techniques, current marketed scenario and patents. Drug Deliv. 2020, 27, 1625–1643. [Google Scholar] [CrossRef]
- Islam, M.S.; Renner, F.; Azizighannad, S.; Mitra, S. Direct incorporation of nano graphene oxide (nGO) into hydrophobic drug crystals for enhanced aqueous dissolution. Colloids Surf. B Biointerfaces 2020, 189, 110827. [Google Scholar] [CrossRef]
- Chen, K.; Mitra, S. Incorporation of functionalized carbon nanotubes into hydrophobic drug crystals for enhancing aqueous dissolution. Colloids Surf. B Biointerfaces 2018, 173, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.S.; Matzger, A.J. Effect of Polymer Hydrophobicity on the Stability of Amorphous Solid Dispersions and Supersaturated Solutions of a Hydrophobic Pharmaceutical. Mol. Pharm. 2019, 16, 682–688. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, Y. Biomedical Applications of Supramolecular Systems Based on Host–Guest Interactions. Chem. Rev. 2014, 115, 7794–7839. [Google Scholar] [CrossRef]
- Higashi, K.; Ueda, K.; Moribe, K. Intermolecular Interactions between Drugs and Aminoalkyl Methacrylate Copolymer in Solution to Enhance the Concentration of Poorly Water-Soluble Drugs. Chem. Pharm. Bull. 2019, 67, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Renner, F.; Foster, K.; Oderinde, M.S.; Stefanski, K.; Mitra, S. Enhanced aqueous dissolution of hydrophobic apixaban via direct incorporation of hydrophilic nanographene oxide. Colloids Surf. B Biointerfaces 2022, 216, 112512. [Google Scholar] [CrossRef]
- Islam, M.S.; Mitra, S. Development of nano structured graphene oxide incorporated dexamethasone with enhanced dissolution. Colloid Interface Sci. Commun. 2022, 47, 100599. [Google Scholar] [CrossRef]
- Debnath, S.K.; Srivastava, R. Drug Delivery with Carbon-Based Nanomaterials as Versatile Nanocarriers: Progress and Prospects. Front. Nanotechnol. 2021, 3, 644564. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Inoue, Y. Biodegradable polymers. Stud. Phys. Theor. Chem. 1998, 84, 771–817. [Google Scholar] [CrossRef]
- Heller, J. Biodegradable polymers in controlled drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 1984, 1, 39–90. [Google Scholar]
- Dhaliwal, K.; Dosanjh, P. Biodegradable Polymers and their Role in Drug Delivery Systems. Biomed. J. Sci. Tech. Res. 2018, 11, 8315–8320. [Google Scholar] [CrossRef]
- Schindler, A.; Jeffcoat, R.; Kimmel, G.L.; Pitt, C.G.; Wall, M.E.; Zweidinger, R. Biodegradable Polymers for Sustained Drug Delivery. In Contemporary Topics in Polymer Science; Pearce, E.M., Schaefgen, J.R., Eds.; Springer: Boston, MA, USA, 1977. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Bassand, C.; Freitag, J.; Benabed, L.; Verin, J.; Siepmann, F.; Siepmann, J. PLGA implants for controlled drug release: Impact of the diameter. Eur. J. Pharm. Biopharm. 2022, 177, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Oju, J.; Kinam, P. Biodegradable Polymers for Drug Delivery Systems. In Encyclopedia of Surface and Colloid Science; Somasundaran, P., Ed.; Taylor, and Francis: New York, USA, 2009; pp. 1–15. [Google Scholar]
- Lavanya, K.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Temperature- and pH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater. Sci. Eng. C 2020, 111, 110862. [Google Scholar] [CrossRef] [PubMed]
- Ogaji, I.J.; Nep, E.I.; Audu-Peter, J.D. Advances in Natural Polymers as Pharmaceutical Excipients. Pharm. Anal Acta 2012, 3, 146. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef]
- Wei, B.; Cui, Y.; Ma, S.; Wang, Y.; Guo, X.; Xiao, J.; Li, W.; Pang, A.; Bai, Y. Fluorinated Polymeric Surfactant with a Pluronic-like Structure and Its Application as a Drug Carrier. ACS Appl. Polym. Mater. 2021, 3, 4940–4948. [Google Scholar] [CrossRef]
- Jain, J.P.; Chitkara, D.; Kumar, N. Polyanhydrides as localized drug delivery carrier: An update. Expert Opin. Drug Deliv. 2008, 5, 889–907. [Google Scholar] [CrossRef]
- Kwon, G.S.; Furgeson, D.Y. Biodegradable polymers for drug delivery systems. In 4.3.3 Polyorthoesters, Biomedical Polymers; Poly (ortho Ester)-an overview, ScienceDirect Topics; Woodhead Publishing: Cambridge, UK, 2007. [Google Scholar]
- Moon, S.; Yang, S.-G.; Na, K. An acetylated polysaccharide-PTFE membrane-covered stent for the delivery of gemcitabine for treatment of gastrointestinal cancer and related stenosis. Biomaterials 2011, 32, 3603–3610. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef]
- Koppolu, B.P.; Rahimi, M.; Nattama, S.; Wadajkar, A.; Nguyen, K.T. Development of multiple-layer polymeric particles for targeted and controlled drug delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 355–361. [Google Scholar] [CrossRef]
- Chen, L.; Yan, C.; Zheng, Z. Functional polymer surfaces for controlling cell behaviors. Mater. Today 2017, 21, 38–59. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Alharbi, F.D.; Alhibs, A.S.; Alanazi, N.B.; Alshehri, B.Y.; Saleh, M.A.; Alshehri, F.S.; Algarni, M.A.; Almugaiteeb, T.; Uddin, M.N.; et al. PLGA-Based Nanomedicine: History of Advancement and Development in Clinical Applications of Multiple Diseases. Pharmaceutics 2022, 14, 2728. [Google Scholar] [CrossRef]
- Muthu, M.S. Nanoparticles based on PLGA and its co-polymer: An overview. Asian J. Pharm. 2009, 3, 266. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) As biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.-M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 2009, 9, 325–341. [Google Scholar] [CrossRef]
- Zhao, K.; Li, W.; Huang, T.; Luo, X.; Chen, G.; Zhang, Y.; Guo, C.; Dai, C.; Jin, Z.; Zhao, Y.; et al. Preparation and efficacy of Newcastle disease virus DNA vaccine encapsulated in PLGA nanoparticles. PloS ONE 2013, 8, e82648. [Google Scholar] [CrossRef] [PubMed]
- Chereddy, K.K.; Vandermeulen, G.; Préat, V. PLGA based drug delivery systems: Promising carriers for wound healing activity. Wound Repair Regen. 2016, 24, 223–236. [Google Scholar] [CrossRef]
- Rasoulianboroujeni, M.; Fahimipour, F.; Shah, P.; Khoshroo, K.; Tahriri, M.; Eslami, H.; Yadegari, A.; Dashtimoghadam, E.; Tayebi, L. Development of 3D-printed PLGA/TiO2 nanocomposite scaffolds for bone tissue engineering applications. Mater. Sci. Eng. C 2019, 96, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-G.; Gater, D.L.; Kim, Y.-C. Development of transdermal vitamin D3 (VD3) delivery system using combinations of PLGA nanoparticles and microneedles. Drug Deliv. Transl. Res. 2017, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Thiruppathi, R.; Mishra, S.; Ganapathy, M.; Padmanabhan, P.; Gulyás, B. Nanoparticle Functionalization and Its Potentials for Molecular Imaging. Adv. Sci. 2017, 4, 1600279. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Teply, B.A.; Sherifi, I.; Sung, J.; Luther, G.; Gu, F.X.; Levy-Nissenbaum, E.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Formulation of functionalized PLGA–PEG nanoparticles for in vivo targeted drug delivery. Biomaterials 2007, 28, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhou, Q.; Zhu, H.; Zhuang, Y.; Bao, J. Enhanced antitumor efficacy in colon cancer using EGF functionalized PLGA nanoparticles loaded with 5-Fluorouracil and perfluorocarbon. BMC Cancer 2020, 20, 354. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef]
- Liu, H.; Slamovich, E.B.; Webster, T.J. Less harmful acidic degradation of poly(lactic-co-glycolic acid) bone tissue engineering scaffolds through titania nanoparticle addition. Int. J. Nanomed. 2006, 1, 541–545. [Google Scholar] [CrossRef]
- Gauthier, A.; Fisch, A.; Seuwen, K.; Baumgarten, B.; Ruffner, H.; Aebi, A.; Rausch, M.; Kiessling, F.; Bartneck, M.; Weiskirchen, R.; et al. Glucocorticoid-loaded liposomes induce a pro-resolution phenotype in human primary macrophages to support chronic wound healing. Biomaterials 2018, 178, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Usti-anowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Knezevic, N.N.; Anantamongkol, U.; Candido, K.D. Perineural dexamethasone added to local anesthesia for brachial plexus block improves pain but delays block onset and motor blockade recovery. Pain Physician 2015, 18, 1. [Google Scholar] [PubMed]
- Duggan, N.M.; Nagdev, A.; Hayes, B.D.; Shokoohi, H.; Selame, L.A.; Liteplo, A.S.; Goldsmith, A.J. Perineural Dexamethasone as a Peripheral Nerve Block Adjuvant in the Emergency Department: A Case Series. J. Emerg. Med. 2021, 61, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jiang, Y.; Zhou, J.; Gong, H.; Mohapatra, A.; Heo, J.; Gao, W.; Fang, R.H.; Zhang, L. Genetically engineered cell membrane–coated nanoparticles for targeted delivery of dexamethasone to inflamed lungs. Sci. Adv. 2021, 7, eabf7820. [Google Scholar] [CrossRef]
- Bartneck, M.; Peters, F.M.; Warzecha, K.T.; Bienert, M.; van Bloois, L.; Trautwein, C.; Lammers, T.; Tacke, F. Liposomal encapsulation of dexamethasone modulates cytotoxicity, inflammatory cytokine response, and migratory properties of primary human macrophages. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H. Coronavirus breakthrough: Dexamethasone is first drug shown to save lives. Nature 2020, 582, 469. [Google Scholar] [CrossRef] [PubMed]
- Chaib, F. World Health Organization (WHO) wel-Comes-Preliminary-Results-about-Dexamethasone-Use-in-Treating-Critically-Ill-COVID-19-Patients. 2020. Available online: https://www.who.int/news/item/16-06-2020 (accessed on 16 June 2020).
- Matthay, M.A.; Thompson, B.T. Dexamethasone in hospitalised patients with COVID-19: Addressing uncertainties. Lancet Respir. Med. 2020, 8, 1170–1172. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Sofias, A.M.; Van Der Meel, R.; Schiffelers, R.; Storm, G.; Tacke, F.; Koschmieder, S.; Brümmendorf, T.H.; Kiessling, F.; Metselaar, J.M. Dexamethasone nanomedicines for COVID-19. Nat. Nanotechnol. 2020, 15, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.T. Dexamethasone: First Drug Found to Reduce Mortality in People with COVID-19. N. Engl. J. Med. Wat. 2020. Corpus ID: 225775049. [Google Scholar] [CrossRef]
- Thakkar, M.; Islam, M.S.; Railkar, A.; Mitra, S. Antisolvent precipitative immobilization of micro and nanostructured griseofulvin on laboratory cultured diatom frustules for enhanced aqueous dissolution. Colloids Surf. B Biointerfaces 2020, 196, 11308. [Google Scholar] [CrossRef]
- Williams, D.L.; Kuhn, A.T.; Amann, M.A.; Hausinger, M.B.; Konarik, M.M.; Nesselrode, E.I. Computerized Measurement of Contact Angles. Galvanotechnik 2010, 101, 2502–2512. [Google Scholar]
- Pradhan, S.; Kumar, P.; Mehrotra, I. Characterization of Aqueous Organics by Specific Ultraviolet Absorbance and Octanol Water Partition Coefficient. J. Environ. Eng. 2014, 140, 06013001. [Google Scholar] [CrossRef]


| Properties | Original PLGA | nfPLGA |
|---|---|---|
| Carbon (wt. %) | 75.24 | 46.93 |
| Oxygen (wt. %) | 24.76 | 53.07 |
| Mw | 47.9 | 38.3 |
| Mn | 34.1 | 18.4 |
| Particle size (nm) | large | ~161.0 nm |
| Zeta potential [mV] | −13.1 | −31.7 |
| Tm [°C] | 338.03 | 331.78 |
| Tg [℃] | 49.01 | 46.14 |
| Dispersibility [mg/mL] | Non-disperse | 4 |
| Contact angle [°] | 82 | 36 |
| Formulations | (T50) | (T80) | DR | MP | Z.P |
|---|---|---|---|---|---|
| (min) | (min) | (µg/min) | (°C) | [mV] | |
| Pure DXM | 57 | n.d. | 289.7 | 261.74 | −17.2 |
| DXM–nfPLGA-0.75 | 24 | 50 | 422.4 | 260.22 | −34.8 |
| DXM–nfPLGA-1.5 | 18 | 35 | 513.02 | 259.38 | −44.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.S.; Mitra, S. Synthesis of Microwave Functionalized, Nanostructured Polylactic Co-Glycolic Acid (nfPLGA) for Incorporation into Hydrophobic Dexamethasone to Enhance Dissolution. Nanomaterials 2023, 13, 943. https://doi.org/10.3390/nano13050943
Islam MS, Mitra S. Synthesis of Microwave Functionalized, Nanostructured Polylactic Co-Glycolic Acid (nfPLGA) for Incorporation into Hydrophobic Dexamethasone to Enhance Dissolution. Nanomaterials. 2023; 13(5):943. https://doi.org/10.3390/nano13050943
Chicago/Turabian StyleIslam, Mohammad Saiful, and Somenath Mitra. 2023. "Synthesis of Microwave Functionalized, Nanostructured Polylactic Co-Glycolic Acid (nfPLGA) for Incorporation into Hydrophobic Dexamethasone to Enhance Dissolution" Nanomaterials 13, no. 5: 943. https://doi.org/10.3390/nano13050943
APA StyleIslam, M. S., & Mitra, S. (2023). Synthesis of Microwave Functionalized, Nanostructured Polylactic Co-Glycolic Acid (nfPLGA) for Incorporation into Hydrophobic Dexamethasone to Enhance Dissolution. Nanomaterials, 13(5), 943. https://doi.org/10.3390/nano13050943









