Remarkably Enhanced Lattice Oxygen Participation in Perovskites to Boost Oxygen Evolution Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Syntheses of OER Active Perovskite Materials
2.3. Characterization
2.4. Electrochemical Characterization: OER
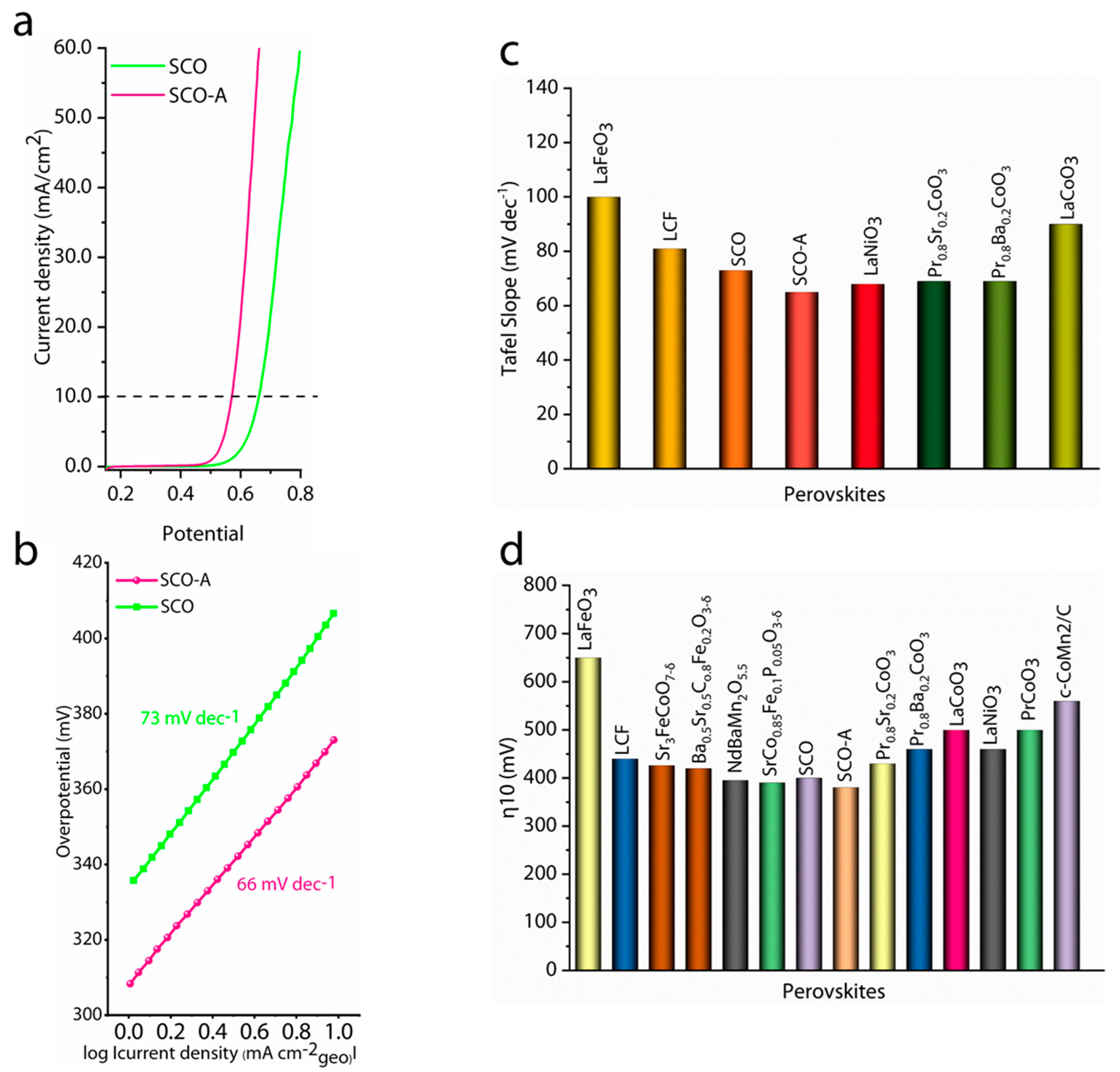
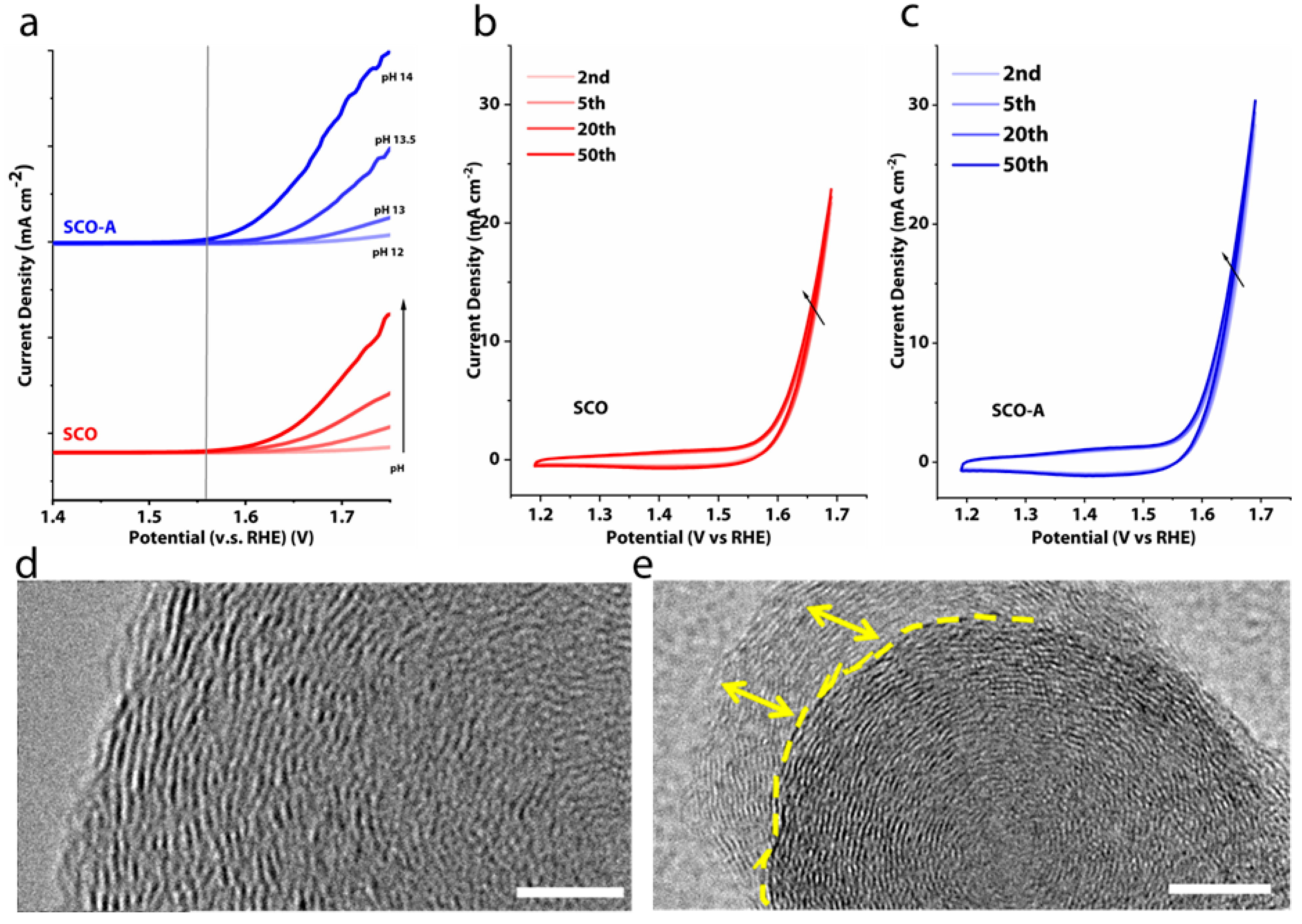
3. Results and Discussions
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surendranath, Y.; Kanan, M.W.; Nocera, D.G. Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH. J. Am. Chem. Soc. 2010, 132, 16501–16509. [Google Scholar] [CrossRef]
- Siegbahn, P.E. An energetic comparison of different models for the oxygen evolving complex of photosystem II. J. Am. Chem. Soc. 2009, 131, 18238–18239. [Google Scholar] [CrossRef]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Singh, A.N.; Kim, M.-H.; Meena, A.; Wi, T.-U.; Lee, H.-W.; Kim, K.S. Na/Al Codoped Layered Cathode with Defects as Bifunctional Electrocatalyst for High-Performance Li-Ion Battery and Oxygen Evolution Reaction. Small 2021, 17, 2005605. [Google Scholar] [CrossRef]
- Sultan, S.; Tiwari, J.N.; Singh, A.N.; Zhumagali, S.; Ha, M.; Myung, C.W.; Thangavel, P.; Kim, K.S. Single Atoms and Clusters Based Nanomaterials for Hydrogen Evolution, Oxygen Evolution Reactions, and Full Water Splitting. Adv. Energy Mater. 2019, 9, 1900624. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Harzandi, A.M.; Ha, M.; Sultan, S.; Myung, C.W.; Park, H.J.; Kim, D.Y.; Thangavel, P.; Singh, A.N.; Sharma, P.; et al. High-Performance Hydrogen Evolution by Ru Single Atoms and Nitrided-Ru Nanoparticles Implanted on N-Doped Graphitic Sheet. Adv. Energy Mater. 2019, 9, 1900931. [Google Scholar] [CrossRef]
- Singh, A.N.; Hajibabaei, A.; Ha, M.; Meena, A.; Kim, H.-S.; Bathula, C.; Nam, K.-W. Reduced Potential Barrier of Sodium-Substituted Disordered Rocksalt Cathode for Oxygen Evolution Electrocatalysts. Nanomaterials 2023, 13, 10. [Google Scholar] [CrossRef]
- Hwang, J.; Rao, R.R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 2017, 358, 751–756. [Google Scholar] [CrossRef]
- Grimaud, A.; May, K.J.; Carlton, C.E.; Lee, Y.-L.; Risch, M.; Hong, W.T.; Zhou, J.; Shao-Horn, Y. Double perovskites as a family of highly active catalysts for oxygen evolution in alkaline solution. Nat. Commun. 2013, 4, 2439. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, X.; Zhong, Y.; Ge, L.; Chen, Y.; Veder, J.-P.M.; Guan, D.; O’Hayre, R.; Li, M.; Wang, G.; et al. Direct evidence of boosted oxygen evolution over perovskite by enhanced lattice oxygen participation. Nat. Commun. 2020, 11, 2002. [Google Scholar] [CrossRef]
- Hu, R.; Zhao, M.; Miao, H.; Liu, F.; Zou, J.; Zhang, C.; Wang, Q.; Tian, Z.; Zhang, Q.; Yuan, J. Rapidly reconstructing the active surface of cobalt-based perovskites for alkaline seawater splitting. Nanoscale 2022, 14, 10118–10124. [Google Scholar] [CrossRef]
- Zou, J.; Qiu, D.; Chen, G.; Miao, H.; Dang, J.; Xia, L.; Wang, Q.; Zhang, H.; Yuan, J. Greatly enhancing the bifunctional oxygen catalytic activities of cobalt-based perovskites by anchoring metal sulfides. J. Alloys Compd. 2023, 933, 167817. [Google Scholar] [CrossRef]
- Yagi, S.; Yamada, I.; Tsukasaki, H.; Seno, A.; Murakami, M.; Fujii, H.; Chen, H.; Umezawa, N.; Abe, H.; Nishiyama, N.; et al. Covalency-reinforced oxygen evolution reaction catalyst. Nat. Commun. 2015, 6, 8249. [Google Scholar] [CrossRef]
- Zhao, F.; Wen, B.; Niu, W.; Chen, Z.; Yan, C.; Selloni, A.; Tully, C.G.; Yang, X.; Koel, B.E. Increasing Iridium Oxide Activity for the Oxygen Evolution Reaction with Hafnium Modification. J. Am. Chem. Soc. 2021, 143, 15616–15623. [Google Scholar] [CrossRef]
- Sabatier, P. Hydrogénations et déshydrogénations par catalyse. Ber. Dtsch. Chem. Ges. 1911, 44, 1984–2001. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Chen, Z.G.; Chen, Y.; Su, C.; Tadé, M.O.; Shao, Z. SrNb0. 1Co0. 7Fe0. 2O3− δ perovskite as a next-generation electrocatalyst for oxygen evolution in alkaline solution. Angew. Chem. 2015, 127, 3969–3973. [Google Scholar] [CrossRef]
- Kim, J.; Yin, X.; Tsao, K.-C.; Fang, S.; Yang, H. Ca2Mn2O5 as Oxygen-Deficient Perovskite Electrocatalyst for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2014, 136, 14646–14649. [Google Scholar] [CrossRef]
- Man, I.C.; Su, H.-Y.; Calle-Vallejo, F.; Hansen, H.A.; Martínez, J.I.; Inoglu, N.G.; Kitchin, J.; Jaramillo, T.F.; Nørskov, J.K.; Rossmeisl, J. Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces. ChemCatChem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, L.; Zhen, D.; Yoo, S.; Ding, Y.; Chen, D.; Chen, Y.; Zhang, Q.; Doyle, B.; Xiong, X.; et al. A tailored double perovskite nanofiber catalyst enables ultrafast oxygen evolution. Nat. Commun. 2017, 8, 14586. [Google Scholar] [CrossRef]
- Ou, G.; Yang, C.; Liang, Y.; Hussain, N.; Ge, B.; Huang, K.; Xu, Y.; Wei, H.; Zhang, R.; Wu, H. Surface Engineering of Perovskite Oxide for Bifunctional Oxygen Electrocatalysis. Small Methods 2019, 3, 1800279. [Google Scholar] [CrossRef]
- Grimaud, A.; Hong, W.T.; Shao-Horn, Y.; Tarascon, J.-M. Anionic redox processes for electrochemical devices. Nat. Mater. 2016, 15, 121–126. [Google Scholar] [CrossRef]
- Yoo, J.S.; Rong, X.; Liu, Y.; Kolpak, A.M. Role of Lattice Oxygen Participation in Understanding Trends in the Oxygen Evolution Reaction on Perovskites. ACS Catal. 2018, 8, 4628–4636. [Google Scholar] [CrossRef]
- Rong, X.; Parolin, J.; Kolpak, A.M. A Fundamental Relationship between Reaction Mechanism and Stability in Metal Oxide Catalysts for Oxygen Evolution. ACS Catal. 2016, 6, 1153–1158. [Google Scholar] [CrossRef]
- Grimaud, A.; Diaz-Morales, O.; Han, B.; Hong, W.T.; Lee, Y.-L.; Giordano, L.; Stoerzinger, K.A.; Koper, M.T.M.; Shao-Horn, Y. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 2017, 9, 457–465. [Google Scholar] [CrossRef]
- Mefford, J.T.; Rong, X.; Abakumov, A.M.; Hardin, W.G.; Dai, S.; Kolpak, A.M.; Johnston, K.P.; Stevenson, K.J. Water electrolysis on La1−xSrxCoO3−δ perovskite electrocatalysts. Nat. Commun. 2016, 7, 11053. [Google Scholar] [CrossRef]
- Yoo, J.S.; Liu, Y.; Rong, X.; Kolpak, A.M. Electronic Origin and Kinetic Feasibility of the Lattice Oxygen Participation During the Oxygen Evolution Reaction on Perovskites. J. Phys. Chem. Lett. 2018, 9, 1473–1479. [Google Scholar] [CrossRef]
- Xu, K.; Song, F.; Gu, J.; Xu, X.; Liu, Z.; Hu, X. Solvent-induced surface hydroxylation of a layered perovskite Sr3FeCoO7−δ for enhanced oxygen evolution catalysis. J. Mater. Chem. A 2018, 6, 14240–14245. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Feng, Q.; Pu, C.; Zhang, L.; Hu, M.; Zhou, X.; Zhong, X.; Yi, W.; Tang, J.; et al. Redox inactive ion meliorated BaCo0.4Fe0.4Zr0.1Y0.1O3−δ perovskite oxides as efficient electrocatalysts for the oxygen evolution reaction. J. Mater. Chem. A 2018, 6, 17288–17296. [Google Scholar] [CrossRef]
- Du, X.; Ai, H.; Chen, M.; Liu, D.; Chen, S.; Wang, X.; Lo, K.H.; Pan, H. PLD-fabricated perovskite oxide nanofilm as efficient electrocatalyst with highly enhanced water oxidation performance. Appl. Catal. B Environ. 2020, 272, 119046. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Petz, A.; Kunsági-Máté, S. Reducing structural defects and improving homogeneity of nitric acid treated multi-walled carbon nanotubes. Carbon 2015, 93, 515–522. [Google Scholar] [CrossRef]
- Li, D.W.; Zou, Q.M.; Huang, X.; Rabiee Golgir, H.; Keramatnejad, K.; Song, J.F.; Xiao, Z.Y.; Fan, L.S.; Hong, X.; Jiang, L.; et al. Controlled defect creation and removal in graphene and MoS2 monolayers. Nanoscale 2017, 9, 8997–9008. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Lyu, K.; Li, L.; Li, J.; Kimberley, L.; Wang, B.; Liu, L.; Cheng, Y.; Frogley, M.D.; Rudić, S.; et al. Integration of mesopores and crystal defects in metal-organic frameworks via templated electrosynthesis. Nat. Commun. 2019, 10, 4466. [Google Scholar] [CrossRef]
- Ding, Y.; Maitra, S.; Wang, C.; Halder, S.; Zheng, R.; Barakat, T.; Roy, S.; Chen, L.-H.; Su, B.-L. Vacancy defect engineering in semiconductors for solar light-driven environmental remediation and sustainable energy production. Interdiscip Mater. 2022, 1, 213–255. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Cui, Z.; Li, Y.; Xin, S.; Zhou, J.; Long, Y.; Jin, C.; Goodenough, J.B. Exceptional oxygen evolution reactivities on CaCoO3 and SrCoO3. Sci. Adv. 2019, 5, eaav6262. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Huang, H.; Lu, Y. Temperature-and magnetic field-dependence of exchange bias in SrCoO2. 29 ceramics. AIP Adv. 2017, 7, 015207. [Google Scholar] [CrossRef]
- Lee, J.; Urban, A.; Li, X.; Su, D.; Hautier, G.; Ceder, G. Unlocking the potential of cation-disordered oxides for rechargeable lithium batteries. Science 2014, 343, 519–522. [Google Scholar] [CrossRef]
- Anjum, M.A.R.; Jeong, H.Y.; Lee, M.H.; Shin, H.S.; Lee, J.S. Efficient hydrogen evolution reaction catalysis in alkaline media by all-in-one MoS2 with multifunctional active sites. Adv. Mater. 2018, 30, 1707105. [Google Scholar] [CrossRef]
- Wang, L.; Peng, C.; Lin, H.; Zhao, B. Unraveling the Role of Defects in Electrocatalysts for Water Splitting: Recent Advances and Perspectives. Energy Fuels 2022, 36, 11660–11690. [Google Scholar] [CrossRef]
- Zhu, K.; Wu, T.; Zhu, Y.; Li, X.; Li, M.; Lu, R.; Wang, J.; Zhu, X.; Yang, W. Layered Fe-Substituted LiNiO2 Electrocatalysts for High-Efficiency Oxygen Evolution Reaction. ACS Energy Lett. 2017, 2, 1654–1660. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, K.; Wang, H.; Yao, X. The Role of Defect Sites in Nanomaterials for Electrocatalytic Energy Conversion. Chem 2019, 5, 1371–1397. [Google Scholar] [CrossRef]
- Yang, C.; Grimaud, A. Factors Controlling the Redox Activity of Oxygen in Perovskites: From Theory to Application for Catalytic Reactions. Catalysts 2017, 7, 149. [Google Scholar] [CrossRef]
- May, K.J.; Carlton, C.E.; Stoerzinger, K.A.; Risch, M.; Suntivich, J.; Lee, Y.-L.; Grimaud, A.; Shao-Horn, Y. Influence of Oxygen Evolution during Water Oxidation on the Surface of Perovskite Oxide Catalysts. J. Phys. Chem. Lett. 2012, 3, 3264–3270. [Google Scholar] [CrossRef]
- Jiang, G.; Yu, H.; Yao, D.; Li, Y.; Chi, J.; Zhang, H.; Shao, Z. Boosting the oxygen evolution stability and activity of a heterogeneous IrRu bimetallic coating on a WO3 nano-array electrode for PEM water electrolysis. J. Mater. Chem. A 2022, 10, 11893–11903. [Google Scholar] [CrossRef]
- Hegde, G.S.; Ghosh, A.; Badam, R.; Matsumi, N.; Sundara, R. Role of Defects in Low-Cost Perovskite Catalysts toward ORR and OER in Lithium–Oxygen Batteries. ACS Appl. Energy Mater. 2020, 3, 1338–1348. [Google Scholar] [CrossRef]
- Li, G.; Hou, S.; Gui, L.; Feng, F.; Zhang, D.; He, B.; Zhao, L. Carbon quantum dots decorated Ba0.5Sr0.5Co0.8Fe0.2O3-δ perovskite nanofibers for boosting oxygen evolution reaction. Appl. Catal. B Environ. 2019, 257, 117919. [Google Scholar] [CrossRef]
- Tang, L.; Chen, Z.; Zuo, F.; Hua, B.; Zhou, H.; Li, M.; Li, J.; Sun, Y. Enhancing perovskite electrocatalysis through synergistic functionalization of B-site cation for efficient water splitting. Chem. Eng. J. 2020, 401, 126082. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Chen, D.; Liu, J.; Zhang, Z.; Shao, Z.; Ciucci, F. Water Splitting with an Enhanced Bifunctional Double Perovskite. ACS Catal. 2018, 8, 364–371. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, Y.; Chen, X.; Li, Y.; Su, Z.; Zhao, C. Vertical Growth of Porous Perovskite Nanoarrays on Nickel Foam for Efficient Oxygen Evolution Reaction. ACS Sustain. Chem. Eng. 2020, 8, 4863–4870. [Google Scholar] [CrossRef]
- Hua, B.; Li, M.; Zhang, Y.-Q.; Sun, Y.-F.; Luo, J.-L. All-In-One Perovskite Catalyst: Smart Controls of Architecture and Composition toward Enhanced Oxygen/Hydrogen Evolution Reactions. Adv. Energy Mater. 2017, 7, 1700666. [Google Scholar] [CrossRef]
- Wang, T.; Chen, H.; Yang, Z.; Liang, J.; Dai, S. High-Entropy Perovskite Fluorides: A New Platform for Oxygen Evolution Catalysis. J. Am. Chem. Soc. 2020, 142, 4550–4554. [Google Scholar] [CrossRef]
- Matienzo, D.D.; Kutlusoy, T.; Divanis, S.; Di Bari, C.; Instuli, E. Benchmarking Perovskite Electrocatalysts’ OER Activity as Candidate Materials for Industrial Alkaline Water Electrolysis. Catalysts 2020, 10, 1387. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.N.; Hajibabaei, A.; Diorizky, M.H.; Ba, Q.; Nam, K.-W. Remarkably Enhanced Lattice Oxygen Participation in Perovskites to Boost Oxygen Evolution Reaction. Nanomaterials 2023, 13, 905. https://doi.org/10.3390/nano13050905
Singh AN, Hajibabaei A, Diorizky MH, Ba Q, Nam K-W. Remarkably Enhanced Lattice Oxygen Participation in Perovskites to Boost Oxygen Evolution Reaction. Nanomaterials. 2023; 13(5):905. https://doi.org/10.3390/nano13050905
Chicago/Turabian StyleSingh, Aditya Narayan, Amir Hajibabaei, Muhammad Hanif Diorizky, Qiankai Ba, and Kyung-Wan Nam. 2023. "Remarkably Enhanced Lattice Oxygen Participation in Perovskites to Boost Oxygen Evolution Reaction" Nanomaterials 13, no. 5: 905. https://doi.org/10.3390/nano13050905
APA StyleSingh, A. N., Hajibabaei, A., Diorizky, M. H., Ba, Q., & Nam, K.-W. (2023). Remarkably Enhanced Lattice Oxygen Participation in Perovskites to Boost Oxygen Evolution Reaction. Nanomaterials, 13(5), 905. https://doi.org/10.3390/nano13050905







