Exploring the Enhancement of Exchange Bias in Innovative Core/Shell/Shell Structures: Synthesis and Magnetic Properties of Co-Oxide/Co and Co-Oxide/Co/Co-Oxide Inverted Nanostructures
Abstract
1. Introduction
2. Experimental Methods
2.1. Synthesis of Samples
2.2. Structural and Magnetic Characterization Methods
3. Study Results and Discussion
3.1. Structural Charactrization
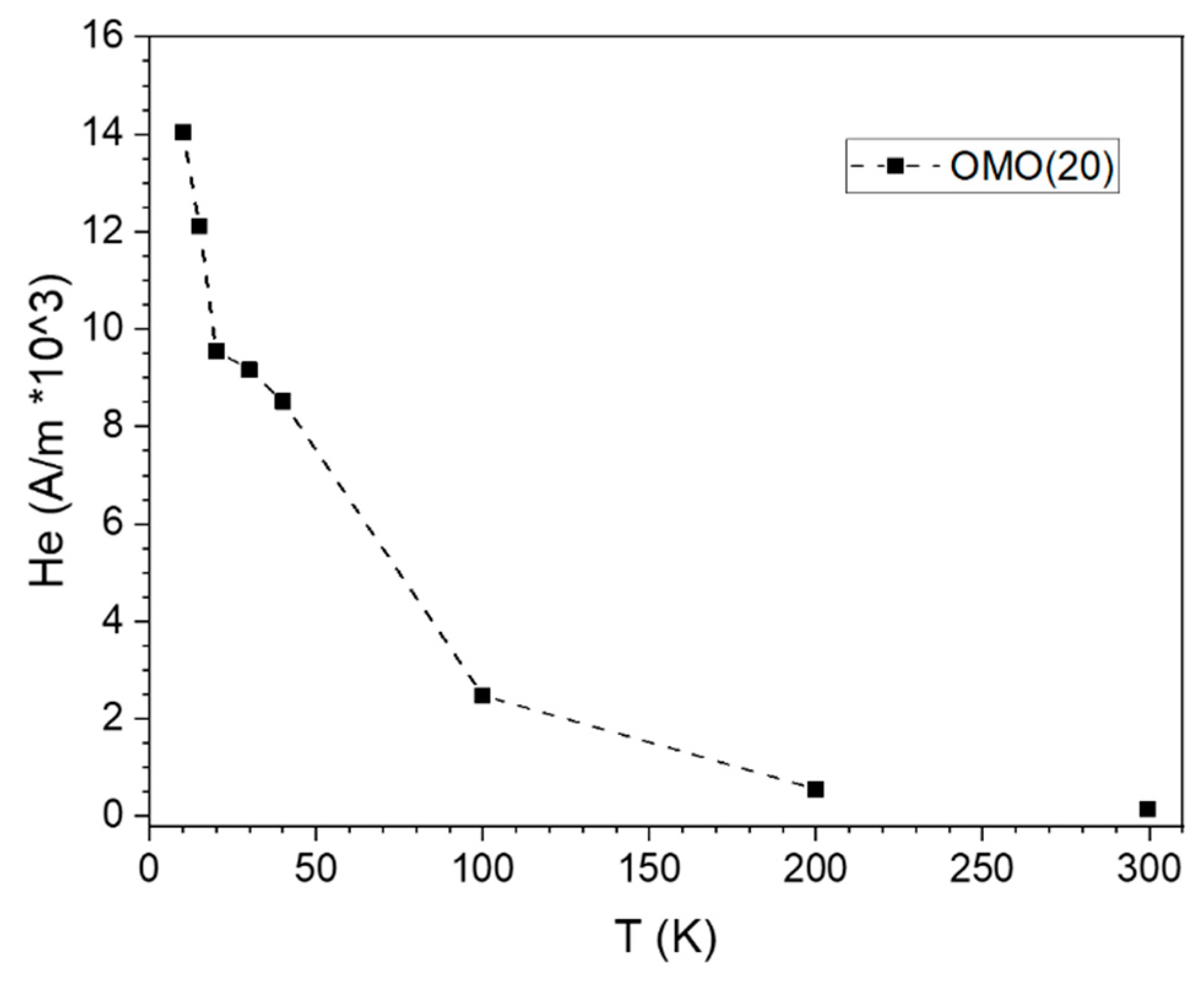
3.2. Magnetic Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nogués, J.; Schuller, I.K. Exchange Bias. J. Magn. Magn. Mater. 1999, 192, 203–232. [Google Scholar] [CrossRef]
- Nogués, J.; Sort, J.; Langlais, V.; Skumryev, V.; Suriñach, S.; Muñoz, J.S.; Baró, M.D. Exchange bias in nanostructures. Phys. Rep. 2005, 422, 65–117. [Google Scholar] [CrossRef]
- López-Ortega, A.; Estrader, M.; Salazar-Alvarez, G.; Roca, A.G.; Nogués, J. Applications of exchange coupled bi-magnetic hard/soft and soft/hard magnetic core/shell nanoparticles. Phys. Rep. 2015, 553, 1–32. [Google Scholar] [CrossRef]
- Sort, J.; Dieny, B.; Nogués, J. Exchange bias in antiferromagnetic-ferromagnetic-antiferromagnetic structures with out-of-plane magnetization. Phys. Rev. B 2005, 72, 1044121–1044126. [Google Scholar] [CrossRef]
- Ghorbani, H.; Eshraghi, M.; Dodaran, A.S.; Kameli, P.; Protasowicki, S.; Vashaee, D. Effect of Yb doping on the structural and magnetic properties of cobalt ferrite nanoparticles. Mater. Res. Bull. 2022, 147, 111642. [Google Scholar] [CrossRef]
- Sharrock, M.P. Recent advances in metal particulate recording media: Toward the ultimate particle. IEEE Trans. Magn. 2000, 36, 2420–2425. [Google Scholar] [CrossRef]
- Falk, R.B.; Hooper, G.D. Elongated Iron-Cobalt: Ferrite, a New, Lightweight, Permanent Magnet Material. J. Appl. Phys. 1961, 32, 190–192. [Google Scholar] [CrossRef]
- Gurney, B.A.; Speriosu, V.S.; Wilhoit, D.R.; Lefakis, H.; Fontana, R.E.; Heim, D.E.; Dovek, M. Can spin valves be reliably deposited for magnetic recording applications? J. Appl. Phys. 1997, 81, 3998–4003. [Google Scholar] [CrossRef]
- Goh, C.K.; Yuan, Z.M.; Liu, B. Magnetization reversal in enclosed composite pattern media structure. J. Appl. Phys. 2009, 105, 83920. [Google Scholar] [CrossRef]
- de Toro, J.A.; Marques, D.P.; Muñiz, P.; Skumryev, V.; Sort, J.; Givord, D.; Nogués, J. High Temperature Magnetic Stabilization of Cobalt Nanoparticles by an Antiferromagnetic Proximity Effect. Phys. Rev. Lett. 2015, 115, 57201. [Google Scholar] [CrossRef]
- Polash, M.M.H.; Vashaee, D. Spin fluctuations yield zT enhancement in ferromagnets. iScience 2021, 24, 103356. [Google Scholar] [CrossRef]
- Polash, M.M.H.; Mohaddes, F.; Rasoulianboroujeni, M.; Vashaee, D. Magnon-drag thermopower in antiferromagnets versus ferromagnets. J. Mater. Chem. C Mater. 2020, 8, 4049–4057. [Google Scholar] [CrossRef]
- Polash, M.M.H.; Vashaee, D. Magnon-bipolar carrier drag thermopower in antiferromagnetic/ferromagnetic semiconductors: Theoretical formulation and experimental evidence. Phys. Rev. B 2020, 102, 045202. [Google Scholar] [CrossRef]
- Polash, M.M.H.; Vashaee, D. Anomalous Thermoelectric Transport Properties of Fe-Rich Magnetic FeTe. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2021, 15, 2100231. [Google Scholar] [CrossRef]
- Polash, M.M.H.; Moseley, D.; Zhang, J.; Hermann, R.P.; Vashaee, D. Understanding and design of spin-driven thermoelectrics. Cell Rep. Phys. Sci. 2021, 2, 100614. [Google Scholar] [CrossRef]
- Zheng, Y.; Lu, T.; Polash, M.M.H.; Rasoulianboroujeni, M.; Liu, N.; Manley, M.E.; Deng, Y.; Sun, P.J.; Chen, X.L.; Hermann, R.P.; et al. Paramagnon drag in high thermoelectric figure of merit Li-doped MnTe. Sci. Adv. 2019, 5, eaat9461. [Google Scholar] [CrossRef] [PubMed]
- Polash, M.M.H.; Yalameha, S.; Zhou, H.; Ahadi, K.; Nourbakhsh, Z.; Vashaee, D. Topological quantum matter to topological phase conversion: Fundamentals, materials, physical systems for phase conversions, and device applications. Mater. Sci. Eng. R Rep. 2021, 145, 100620. [Google Scholar] [CrossRef]
- López-Ortega, A.; Lottini, E.; Bertoni, G.; de Julián Fernández, C.; Sangregorio, C. Topotaxial Phase Transformation in Cobalt Doped Iron Oxide Core/Shell Hard Magnetic Nanoparticles. Chem. Mater. 2017, 29, 1279–1289. [Google Scholar] [CrossRef]
- Roca, A.G.; Golosovsky, I.V.; Winkler, E.; López-Ortega, A.; Estrader, M.; Zysler, R.D.; Baró, M.D.; Nogués, J. Unravelling the Elusive Antiferromagnetic Order in Wurtzite and Zinc Blende CoO Polymorph Nanoparticles. Small 2018, 14, 1703963. [Google Scholar] [CrossRef]
- Estrader, M.; López-Ortega, A.; Estradé, S.; Golosovsky, I.V.; Salazar-Alvarez, G.; Vasilakaki, M.; Trohidou, K.N.; Varela, M.; Stanley, D.C.; Sinko, M.; et al. Robust antiferromagnetic coupling in hard-soft bi-magnetic core/shell nanoparticles. Nat. Commun. 2013, 4, 2960. [Google Scholar] [CrossRef]
- Zeng, H.; Li, J.; Wang, Z.L.; Liu, J.P.; Sun, S. Bimagnetic Core/Shell FePt/Fe3O4 Nanoparticles. Nano Lett. 2004, 4, 187–190. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Nayek, C.; Manna, K.; Bhattacharjee, G.; Al-Omari, I.A.; Gismelseed, A. Investigating exchange bias and coercivity in Fe3O4–γ-Fe2O3 core–shell nanoparticles of fixed core diameter and variable shell thicknesses. Nanomaterials 2017, 7, 415. [Google Scholar] [CrossRef] [PubMed]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [PubMed]
- López-Ortega, A.; Roca, A.G.; Torruella, P.; Petrecca, M.; Estradé, S.; Peiró, F.; Puntes, V.; Nogués, J. Galvanic Replacement onto Complex Metal-Oxide Nanoparticles: Impact of Water or Other Oxidizers in the Formation of either Fully Dense Onion-like or Multicomponent Hollow MnOx/FeOx Structures. Chem. Mater. 2016, 28, 8025–8031. [Google Scholar] [CrossRef]
- Ghoshani, M.; Mozaafari, M.; Normile, P.S.; de Toro, J.A.; Al-Nabhani, A. Core size and interface impact on the exchange bias of cobalt/cobalt oxide nanostructures. Magnetochemistry 2021, 7, 40. [Google Scholar] [CrossRef]
- Liu, C.; Cui, J.; He, X.; Shi, H. Large exchange bias with remarkable thermostability in an inverted quasi core/shell CoO/γ-Fe2O3 granular system. J. Nanopart. Res. 2014, 16, 2320–2327. [Google Scholar] [CrossRef]
- Vasilakaki, M.; Trohidou, K.N.; Nogue, J. Enhanced Magnetic Properties in Antiferromagnetic-Core/Ferrimagnetic-Shell Nanoparticles. Sci. Rep. 2015, 5, 96091–96097. [Google Scholar] [CrossRef]
- Nogues, J.; Skumryev, V.; Sort, J.; Stoyanov, S.; Givord, D. Shell-Driven Magnetic Stability in Core-Shell Nanoparticles. Phys. Rev. Lett. 2006, 97, 157203. [Google Scholar] [CrossRef]
- Berkowitz, A.E.; Rodriguez, G.F.; Hong, J.I.; An, K.; Hyeon, T.; Agarwal, N.; Agarwal, N.; Smith, D.J.; Fullerton, E.E. Antiferromagnetic MnO nanoparticles with ferrimagnetic Mn3O4 shells: Doubly inverted core-shell system. Phys. Rev. B Condens. Matter Mater. Phys. 2008, 77, 024403. [Google Scholar] [CrossRef]
- Ghoshani, M.; Sánchez, E.H.; Lee, S.S.; Singh, G.; Yaacoub, N.; Peddis, D.; Mozaffari, M.; Binns, C.; De Toro, J.A.; Normile, P.S. On the detection of surface spin freezing in iron oxide nanoparticles and its long-term evolution under ambient oxidation. Nanotechnology 2021, 32, 065704. [Google Scholar] [CrossRef]
- Zhou, S.M.; Imhoff, D.; Yu-Zhang, K.; Leprince-Wang, Y. Effect of field cooling on magnetic properties of ultrafine CoO/Co particles. Appl. Phys. A Mater. Sci. Process 2005, 81, 115–118. [Google Scholar] [CrossRef]
- Antón, R.L.; González, J.A.; Andrés, J.P.; Canales-Vázquez, J.; De Toro, J.A.; Riveiro, J.M. High-vacuum annealing reduction of Co/CoO nanoparticles. Nanotechnology 2014, 25, 105702. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.M.; Galdino, V.B.; Conceição, O.L.A.; Morales, M.A.; De Araújo, J.H.; MacHado, F.L.A. Critical dimension for magnetic exchange-spring coupled core/shell CoFe2O4/CoFe2 nanoparticles. J. Magn. Magn. Mater. 2013, 326, 81–84. [Google Scholar] [CrossRef]
- Soares, J.M.; Conceição, O.L.A.; Machado, F.L.A.; Prakash, A.; Radha, S.; Nigam, A.K. Magnetic couplings in CoFe2O4/FeCo–FeO core–shell nanoparticles. J. Magn. Magn. Mater. 2014, 374, 192–196. [Google Scholar] [CrossRef]
- Soares, J.M.; Galdino, V.B.; Machado, F.L.A. Exchange-bias and exchange-spring coupling in magnetic core—shell nanoparticles. J. Magn. Magn. Mater. 2014, 350, 69–72. [Google Scholar] [CrossRef]
- Kavich, D.W.; Dickerson, J.H. Exchange bias of singly inverted FeO/Fe3O4 core-shell nanocrystals. Phys. Rev. B 2008, 78, 174414-1–174414-6. [Google Scholar] [CrossRef]
- Lak, A.; Niculaes, D.; Anyfantis, G.C.; Bertoni, G.; Barthel, M.; Marras, S.; Cassani, M.; Nitti, S.; Athanassiou, A.; Giannini, C.; et al. Facile transformation of FeO/Fe3O4 core-shell nanocubes to Fe3O4 via magnetic stimulation. Sci. Rep. 2016, 6, 33295. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.L.; Lima, E.; Tobia, D.; Saleta, M.E.; Troiani, H.E.; Agostinelli, E.; Fiorani, D.; Zysler, R.D. Origin of magnetic anisotropy in ZnO/CoFe2O4 and CoO/CoFe2O4 core/shell nanoparticle systems. Appl. Phys. Lett. 2012, 101, 252405. [Google Scholar] [CrossRef]
- Lavorato, G.C.; Lima, E.; Tobia, D.; Fiorani, D.; Troiani, H.E.; Zysler, R.D.; Winkler, E.L. Size effects in bimagnetic CoO/CoFe2O4 core/shell nanoparticles. Nanotechnology 2014, 25, 355704. [Google Scholar] [CrossRef] [PubMed]
- Salazar-alvarez, G.; Sort, J.; Surin, S.; Baro, M.D.; Nogués, J. Synthesis and Size-Dependent Exchange Bias in Inverted Core-Shell MnO|Mn3O4 Nanoparticles. JACS 2007, 129, 9102–9108. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Y.; Du, A. Modeling of exchange bias in the antiferromagnetic (core)/ferromagnetic (shell) nanoparticles with specialized shapes. J. Magn. Magn. Mater. 2011, 323, 2613–2621. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Y.; Du, A.; When, I. Effect of cooling field strength and ferromagnetic shell shape on exchange bias in nanoparticles with inverted ferromagnetic– antiferromagnetic core-shell morphology. Phys. Status Solidi B 2010, 247, 972–978. [Google Scholar] [CrossRef]
- Lopez-ortega, A.; Tobia, D.; Winkler, E.; Golosovsky, I.; Salazar-Alvarez, G.; Estradé, S.; Estrader, M.; Sort, J.; Gonzalez, M.A.; Surinach, S.; et al. Size-Dependent Passivation Shell and Magnetic Properties in Antiferromagnetic/Ferrimagnetic Core/Shell MnO Nanoparticles. J. Am. Chem. Soc. 2010, 132, 9398–9407. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, H.; Chandra, S.; Li, W.; Phan, M.H.; Hadjipanayis, G.C. Synthesis and magnetic properties of core/shell FeO/Fe3O4 nano-octopods. J. Appl. Phys. 2013, 113, 17B508. [Google Scholar] [CrossRef]
- Lima, E.; Winkler, E.L.; Tobia, D.; Troiani, H.E.; Zysler, R.D.; Agostinelli, E.; Fiorani, D. Bimagnetic CoO core/CoFe2O4 shell nanoparticles: Synthesis and magnetic properties. Chem. Mater. 2012, 24, 512–516. [Google Scholar] [CrossRef]
- Khurshid, H.; Alonso, J.; Nemati, Z.; Phan, M.H.; Mukherjee, P.; Barandiarán, J.M.; Srikanth, H. Anisotropy effects in magnetic hyperthermia: A comparison between spherical and cubic exchange-coupled FeO/Fe3O4 nanoparticles. J. Appl. Phys. 2015, 117, 17A337. [Google Scholar] [CrossRef]
- Krycka, K.L.; Borchers, J.A.; Laver, M.; Salazar-Alvarez, G.; Lopez-Ortega, A.; Estrader, M.; Surinach, S.; Baro, M.D.; Sort, J.; Nogues, J. Correlating material-specific layers and magnetic distributions within onion-like Fe3O4/MnO/ = c-Mn2O3 core/shell nanoparticles. J. Appl. Phys. 2013, 113, 17B531. [Google Scholar] [CrossRef]
- Jin, C.H.; Si, P.Z.; Xiao, X.F.; Feng, H.; Wu, Q.; Ge, H.L.; Zhong, M. Structure and magnetic properties of Cr/Cr2O3/CrO2 microspheres prepared by spark erosion and oxidation under high pressure of oxygen. Mater Lett 2013, 92, 213–215. [Google Scholar] [CrossRef]
- Si, P.Z.; Wang, H.X.; Jiang, W.; Lee, J.G.; Choi, C.J.; Liu, J.J. Synthesis, structure and exchange bias in Cr2O3/CrO2/Cr2O5 particles. Thin Solid Films 2011, 519, 8423–8425. [Google Scholar] [CrossRef]
- Bhowmik, R.N.; Nagarajan, R.; Ranganathan, R. Magnetic enhancement in antiferromagnetic nanoparticle of CoRh2O4. Phys. Rev. B 2004, 69, 054430-1–054430-5. [Google Scholar] [CrossRef]
- Sundaresan, A.; Bhargavi, R.; Rangarajan, N.; Siddesh, U.; Rao, C.N.R. Ferromagnetism as a universal feature of nanoparticles of the otherwise nonmagnetic oxides. Phys. Rev. B 2006, 74, 161306-1–161306-4. [Google Scholar] [CrossRef]
- Dutta, D.P.; Sharma, G.; Manna, P.K.; Tyagi, A.K.; Yusuf, S.M. Room temperature ferromagnetism in CoO nanoparticles obtained from sonochemically synthesized precursors. Nanotechnology 2008, 19, 245609. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Gao, D.; Shi, Z.; Zhang, Z.; Zhang, J.; Xue, D. Room Temperature Ferromagnetism in Vacuum-Annealed CoO Nanospheres. J. Phys. Chem. C 2010, 114, 21989–21993. [Google Scholar] [CrossRef]
- Kisan, B.; Kumar, J.; Saravanan, P.; Perumal, A. Defect induced ferromagnetism in NiO nanocrystals: Insight from experimental and DFT+U study. Physica B 2020, 593, 412319. [Google Scholar] [CrossRef]
- Ghoshani, M.; Mozaffari, M.; Al-nabhani, A. Influence of milling time on the structural and exchange bias of CoO and Co—Co oxide nanocomposite systems. Ceram Int. 2021, 47, 5133–5144. [Google Scholar] [CrossRef]
- Bautin, V.A.; Seferyan, A.G.; Nesmeyanov, M.S.; Usov, N.A. Magnetic properties of polycrystalline cobalt nanoparticles. AIP Adv. 2017, 7, 045103. [Google Scholar] [CrossRef]
- Srikala, D.; Singh, V.N.; Banerjee, A.; Mehta, B.R.; Patnaik, S. Synthesis and Characterization of Ferromagnetic Cobalt Nanospheres, Nanodiscs and Nanocubes. J. Nanosci. Nanotechnol. 2009, 9, 5627–5632. [Google Scholar] [CrossRef]
- Antón, R.L.; González, J.A.; Andrés, J.P.; Normile, P.S.; Canales-Vázquez, J.; Muñiz, P.; Riveiro, J.M.; De Toro, J.A. Exchange bias optimization by controlled oxidation of cobalt nanoparticle films prepared by sputter gas aggregation. Nanomaterials 2017, 7, 61. [Google Scholar] [CrossRef]
- González, J.A.; Andres, J.P.; De Toro, J.A.; Muniz, P.; Munoz, T.; Crisan, O.; Binns, C.; Riveiro, J.M. Co-CoO nanoparticles prepared by reactive gas-phase aggregation. J. Nanopart. Res. 2009, 11, 2105–2111. [Google Scholar] [CrossRef]
- Kovylina, M.; Muro, M.G.D.; Konstantinović, Z.; Varela, M.; Iglesias, O.; Labarta, A.; Batlle, X. Controlling exchange bias in Co-CoOx nanoparticles by oxygen content. Nanotechnology 2009, 20, 175702. [Google Scholar] [CrossRef]
- Mumtaz, A.; Maaz, K.; Janjua, B.; Hasanain, S.K.; Bertino, M.F. Exchange bias and vertical shift in CoFe2O4 nanoparticles. J. Magn. Magn. Mater. 2007, 313, 266–272. [Google Scholar] [CrossRef]
- Feygenson, M.; Yiu, Y.; Kou, A.; Kim, K.S.; Aronson, M.C. Controlling the exchange bias field in Co core/CoO shell nanoparticles. Phys. Rev. B Condens. Matter Mater. Phys. 2010, 81, 195445. [Google Scholar] [CrossRef]
- Li, Q.; Kartikowati, C.W.; Horie, S.; Ogi, T.; Iwaki, T.; Okuyama, K. Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci. Rep. 2017, 7, 9894. [Google Scholar] [CrossRef]
- Dobrynin, A.N.; Levlev, D.N.; Temst, K.; Lievens, P.; Margueritat, J. Critical size for exchange bias in ferromagnetic-antiferromagnetic particles. Appl. Phys. Lett. 2005, 87, 012501. [Google Scholar] [CrossRef]
- Morrish, A.H. The Physical Principles of Magnetism; IEEE Press: New York, NY, USA, 2001. [Google Scholar]
- Aslibeiki, B.; Kameli, P.; Salamati, H. The effect of dipole-dipole interactions on coercivity, anisotropy constant, and blocking temperature of MnFe2O4 nanoparticles. J. Appl. Phys. 2016, 119, 063901. [Google Scholar] [CrossRef]
- Gangopadhyay, S.; Hadjipanayis, G.C.; Sorensen, C.M.; Klabunde, K.J. Effect of particle size and surface chemistry on the interactions among fine metallic particles. IEEE Trans. Magn. 1993, 29, 2619–2621. [Google Scholar] [CrossRef]
- Sánchez, E.H.; Vasilakaki, M.; Lee, S.S.; Normile, P.S.; Muscas, G.; Murgia, M.; Andersson, M.S.; Singh, G.; Mathieu, R.; Nordblad, P. Simultaneous Individual and Dipolar Collective Properties in Binary Assemblies of Magnetic Nanoparticles. Chem. Mater. 2020, 32, 969–981. [Google Scholar] [CrossRef]
- Jónsson, P.E. Effects of interparticle interaction in ferromagnetic nanoparticle systems. J. Nanosci. Nanotechnol. 2010, 10, 6067–6071. [Google Scholar] [CrossRef] [PubMed]
- González, J.A.; Andrés, J.P.; López Antón, R.; De Toro, J.A.; Normile, P.S.; Muniz, P.; Riveiro, J.M.; Nogués, J. Maximizing Exchange Bias in Co/CoO Core/Shell Nanoparticles by Lattice Matching between the Shell and the Embedding Matrix. Chem. Mater. 2017, 29, 5200–5206. [Google Scholar] [CrossRef]
- Eftaxias, E.; Trohidou, K.N. Numerical study of the exchange bias effects in magnetic nanoparticles with core/shell morphology. Phys. Rev. B 2005, 71, 134406. [Google Scholar] [CrossRef]
- Iglesias, Ò.; Labarta, A.; Batlle, X. Exchange Bias Phenomenology and Models of Core/Shell Nanoparticles. J. Nanosci. Nanotechnol. 2008, 8, 2761–2780. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Patra, M.; Majumdar, S. Exchange bias effect in alloys and compounds. J. Phys. Condens. Matter 2011, 23, 073201. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Y.; Qi, Y.; Duy, A. Effect of antiferromagnetic anisotropy on exchange bias in a single composite nanoparticle with unconventional antiferromagnetic-ferromagnetic core-shell morphology. e-J. Surf. Sci. Nanotechnol. 2011, 9, 67–71. [Google Scholar] [CrossRef]
- Nogués, J.; Leighton, C.; Schuller, I.K. Correlation between antiferromagnetic interface coupling and positive exchange bias. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 61, 1315–1317. [Google Scholar] [CrossRef]
- Miltényi, P.; Gierlings, M.; Keller, J.; Beschoten, B.; Güntherodt, G.; Nowak, U.; Usadel, K.D. Diluted antiferromagnets in exchange bias: Proof of the domain state model. Phys. Rev. Lett. 2000, 84, 4224–4227. [Google Scholar] [CrossRef]
- Hoffmann, A.; Seo, J.W.; Fitzsimmons, M.R.; Siegwart, H.; Fompeyrine, J.; Locquet, J.P.; Dura, J.A.; Majkrzak, C.F. Induced magnetic moments at a ferromagnet-antiferromagnet interface. Phys. Rev. B Condens. Matter Mater. Phys. 2002, 66, 220406. [Google Scholar] [CrossRef]
- Roy, S.; Fitzsimmons, M.R.; Park, S.; Dorn, M.; Petracic, O.; Roshchin, I.V.; Li, Z.P.; Batlle, X.; Morales, R.; Misra, A.; et al. Depth profile of uncompensated spins in an exchange bias system. Phys. Rev. Lett. 2005, 95, 047201. [Google Scholar] [CrossRef]
- Malozemoff, A.P. heisenberg-to-Ising crossover in a random-field model with uniaxial anisotropy. Phys. Rev. B 1988, 37, 7673. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Marrows, C.H.; Hickey, B.J. Antiferromagnetic layer thickness dependence of the IrMn_Co exchange-bias system. Phys. Rev. B 2003, 68, 214420. [Google Scholar] [CrossRef]
- Baltz, V.; Sort, J.; Rodmacq, B.; Dieny, B.; Landis, S. Thermal activation effects on the exchange bias in ferromagnetic- antiferromagnetic nanostructures. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 72, 104419. [Google Scholar] [CrossRef]
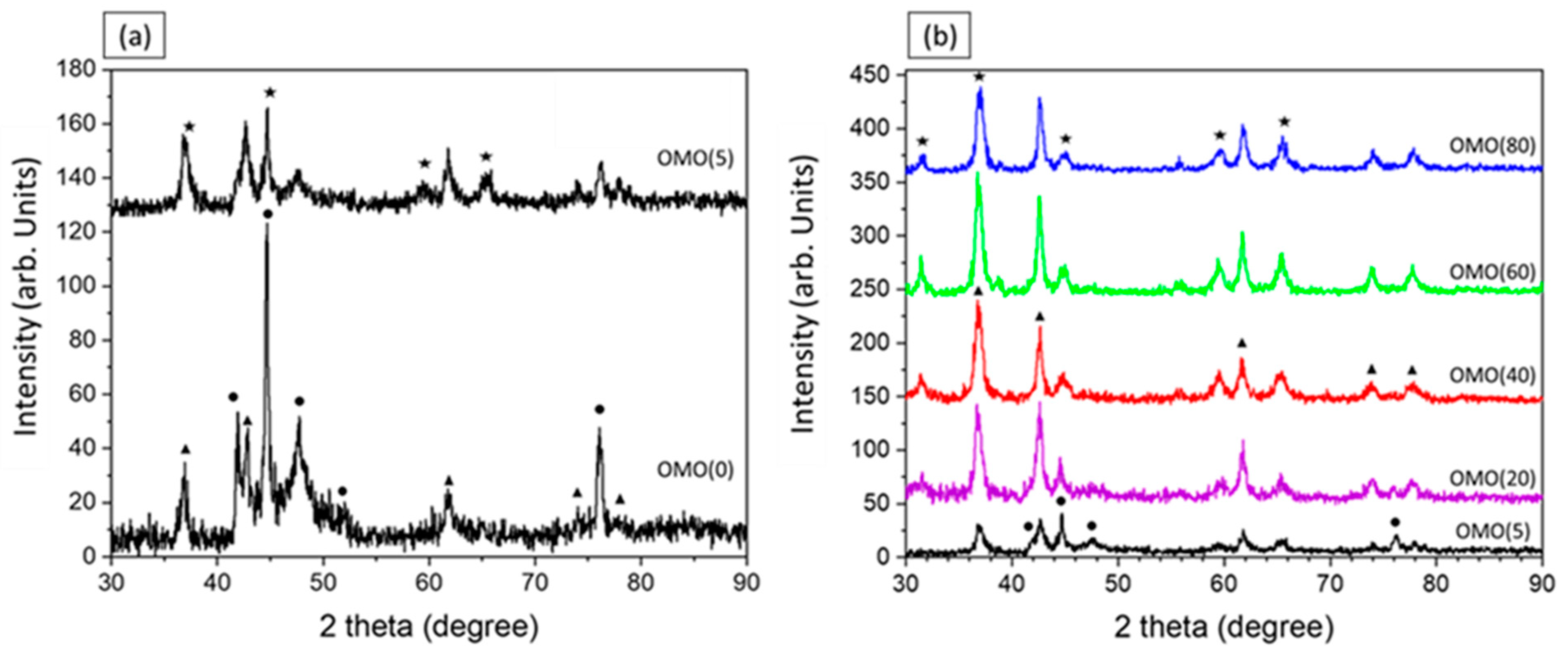
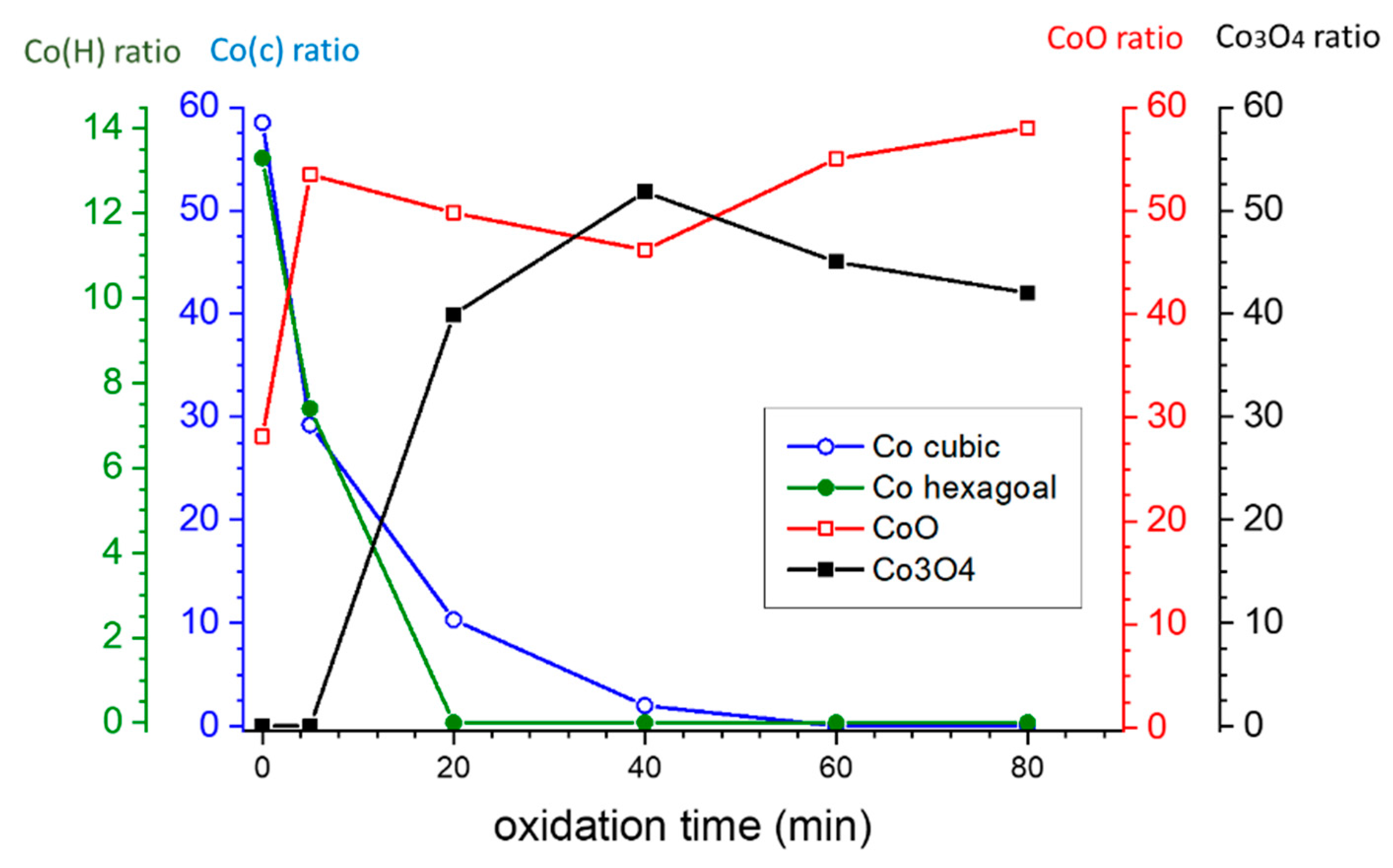

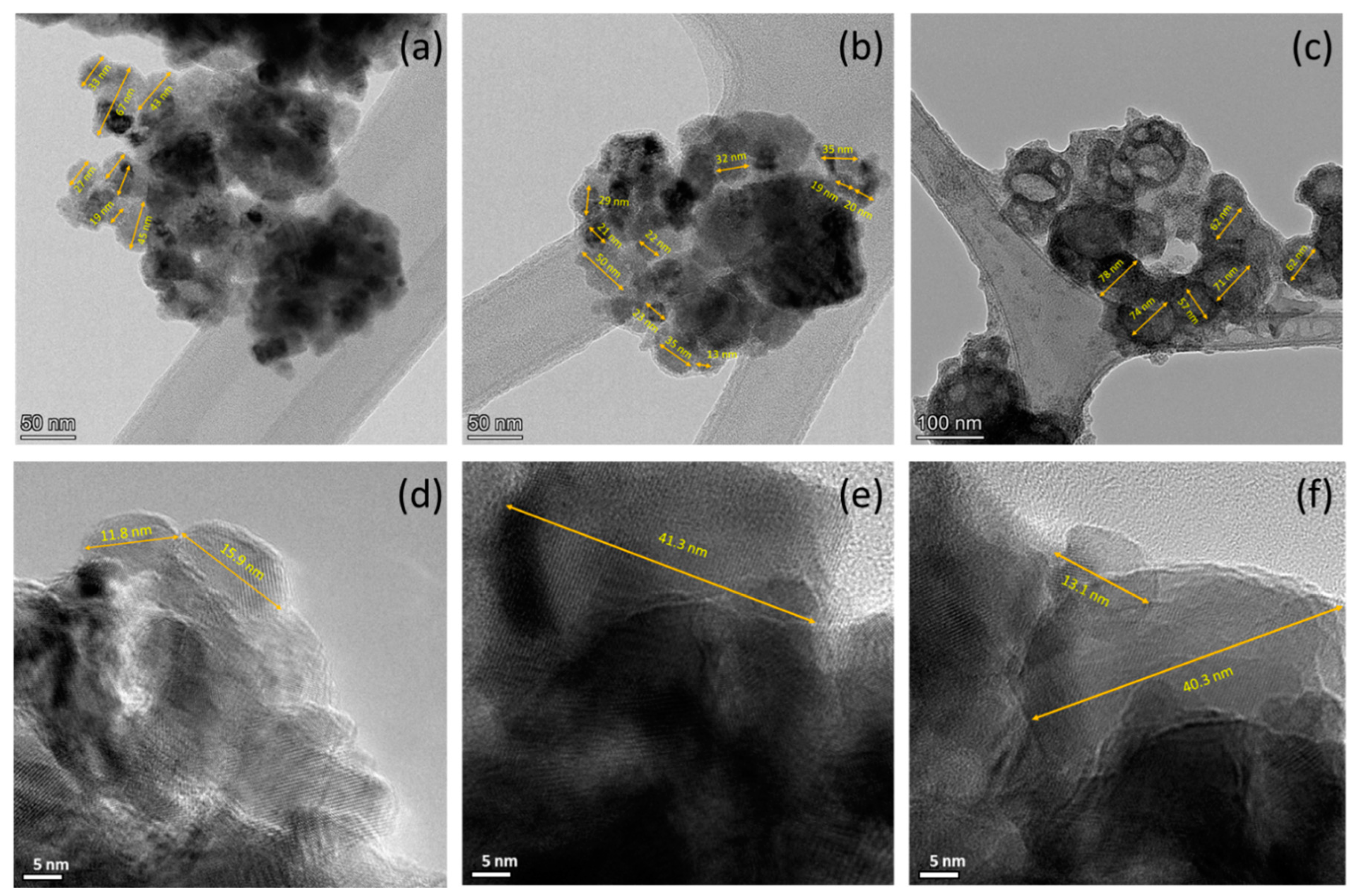
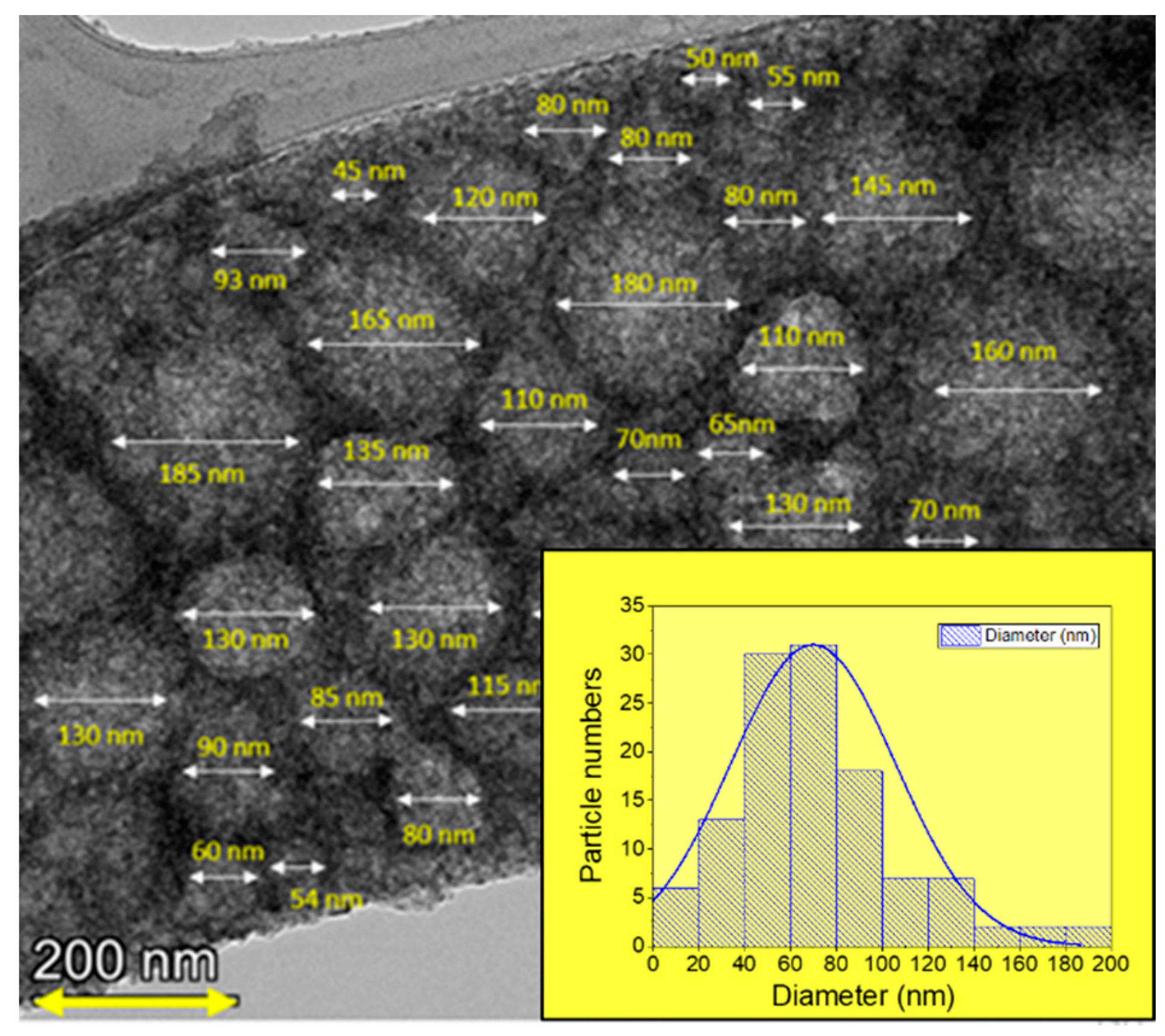
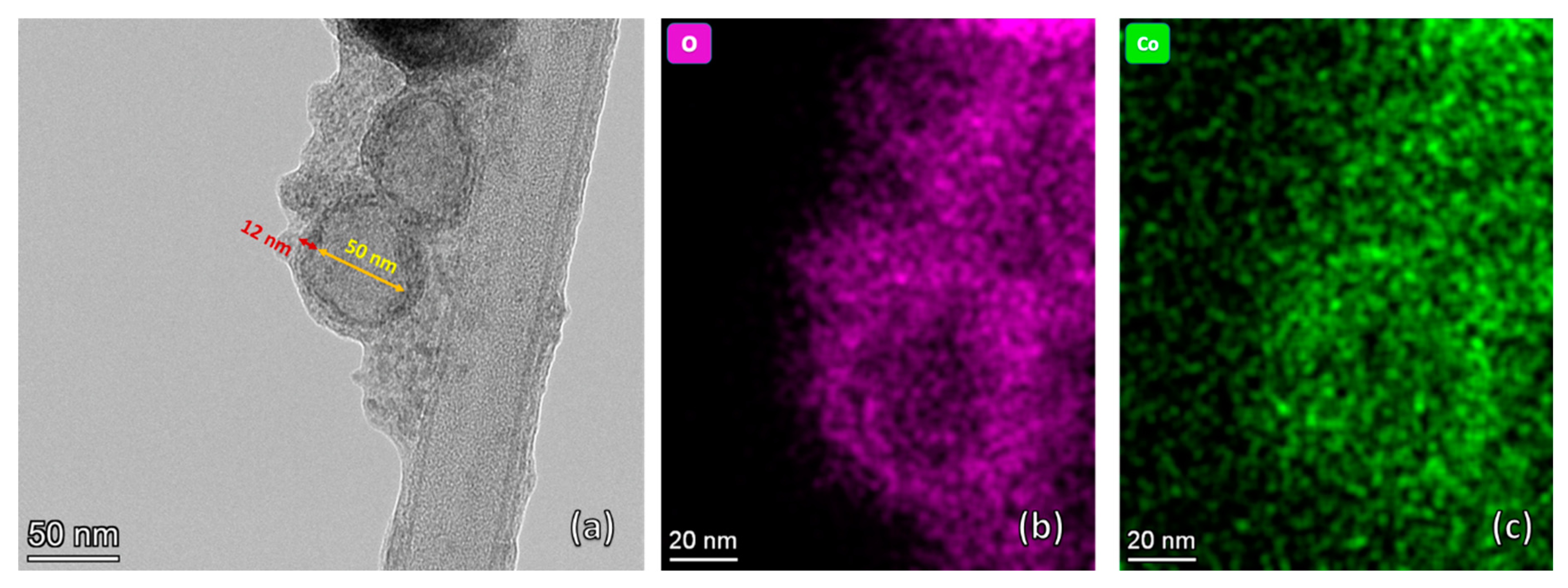

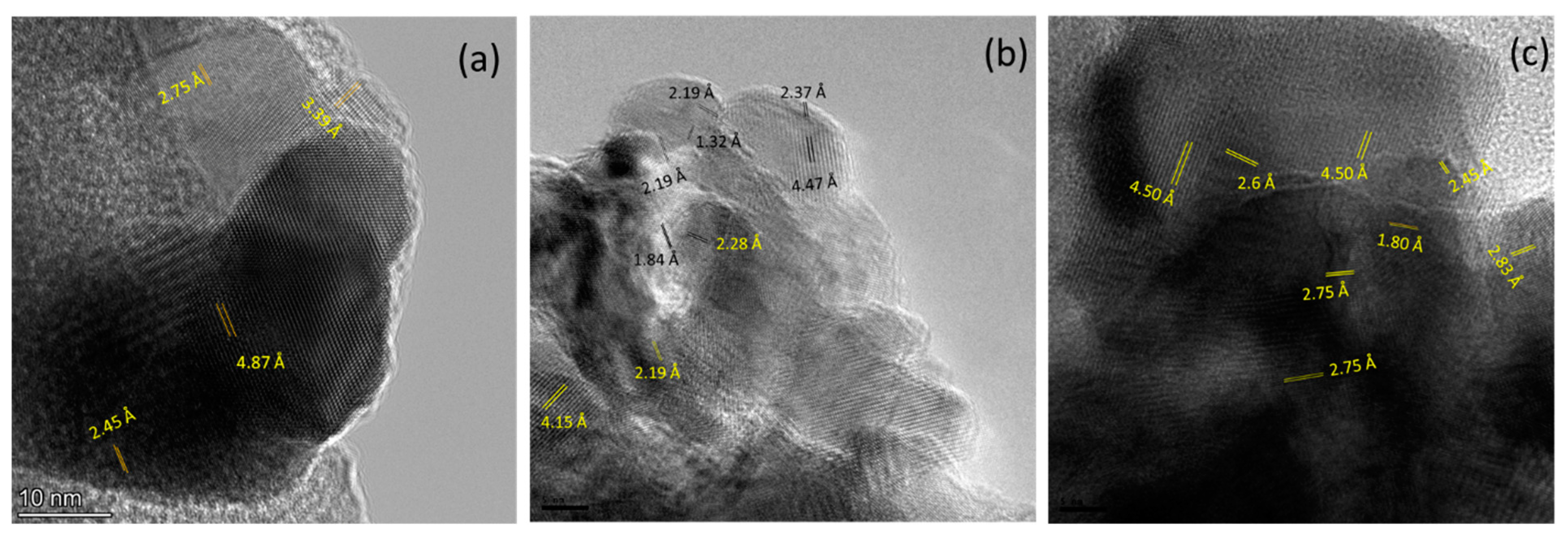
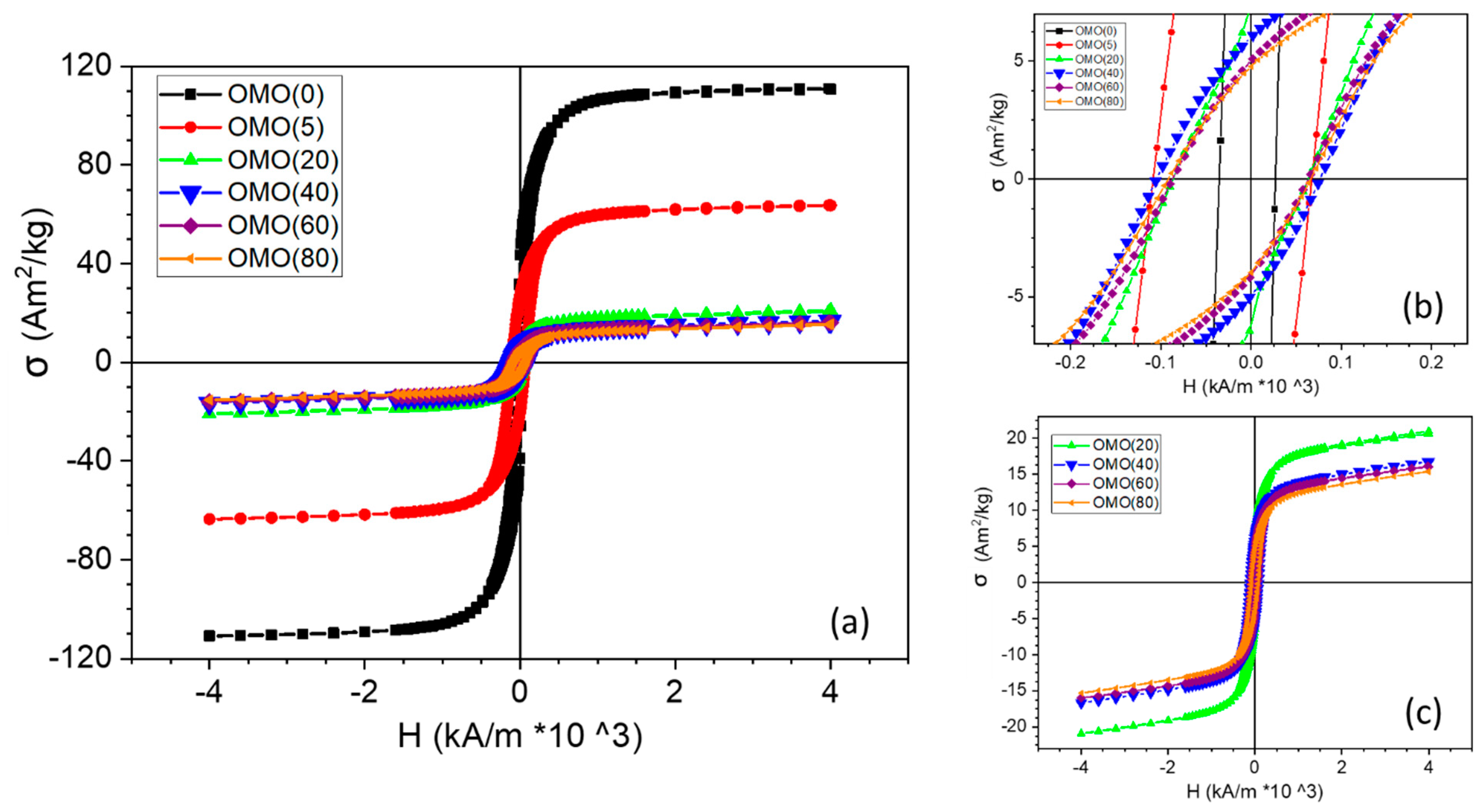
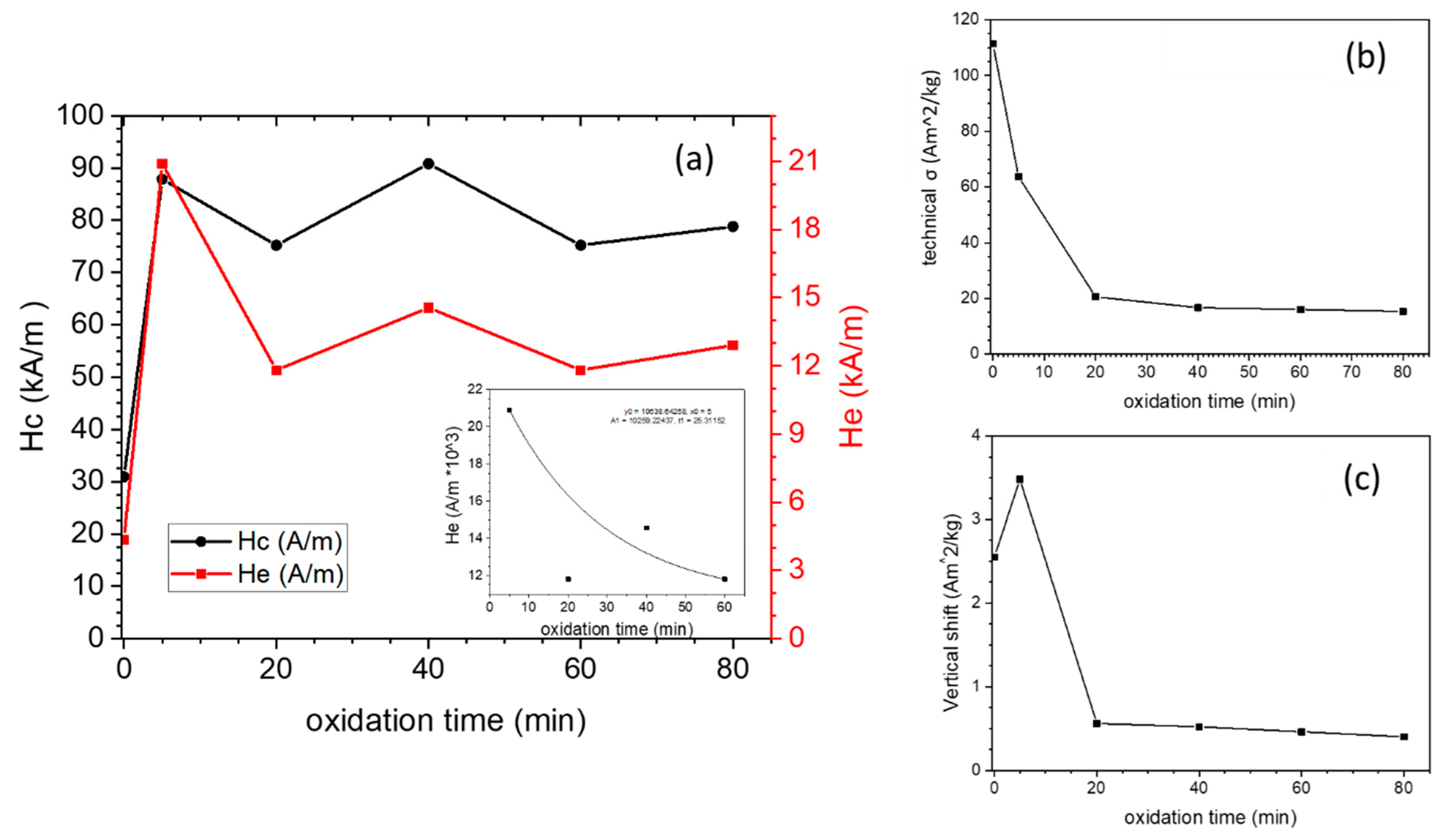
| Co % (σ/σbulk) | Co-Oxide % (1-σ/σbulk) | Co % (XRD) | Co-Oxide % (XRD) | |
|---|---|---|---|---|
| OMO(0) | 69.6 | 30.4 | 71.8 | 28.2 |
| OMO(5) | 39.7 | 60.3 | 36.6 | 63.4 |
| OMO(20) | 12.9 | 87.1 | 10.3 | 89.7 |
| OMO(40) | 10.4 | 89.6 | 2 | 98 |
| OMO(60) | 10.0 | 90 | 0 | 100 |
| OMO(80) | 9.6 | 90.4 | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghoshani, M.; Mozaffari, M.; Acet, M.; Hosseini, M.; Vashaee, D. Exploring the Enhancement of Exchange Bias in Innovative Core/Shell/Shell Structures: Synthesis and Magnetic Properties of Co-Oxide/Co and Co-Oxide/Co/Co-Oxide Inverted Nanostructures. Nanomaterials 2023, 13, 880. https://doi.org/10.3390/nano13050880
Ghoshani M, Mozaffari M, Acet M, Hosseini M, Vashaee D. Exploring the Enhancement of Exchange Bias in Innovative Core/Shell/Shell Structures: Synthesis and Magnetic Properties of Co-Oxide/Co and Co-Oxide/Co/Co-Oxide Inverted Nanostructures. Nanomaterials. 2023; 13(5):880. https://doi.org/10.3390/nano13050880
Chicago/Turabian StyleGhoshani, Maral, Morteza Mozaffari, Mehmet Acet, Mahshid Hosseini, and Daryoosh Vashaee. 2023. "Exploring the Enhancement of Exchange Bias in Innovative Core/Shell/Shell Structures: Synthesis and Magnetic Properties of Co-Oxide/Co and Co-Oxide/Co/Co-Oxide Inverted Nanostructures" Nanomaterials 13, no. 5: 880. https://doi.org/10.3390/nano13050880
APA StyleGhoshani, M., Mozaffari, M., Acet, M., Hosseini, M., & Vashaee, D. (2023). Exploring the Enhancement of Exchange Bias in Innovative Core/Shell/Shell Structures: Synthesis and Magnetic Properties of Co-Oxide/Co and Co-Oxide/Co/Co-Oxide Inverted Nanostructures. Nanomaterials, 13(5), 880. https://doi.org/10.3390/nano13050880







