A Nanotechnology-Based Approach to Biosensor Application in Current Diabetes Management Practices
Abstract
1. Introduction
2. Different Parameters for the Diagnosis of Diabetes
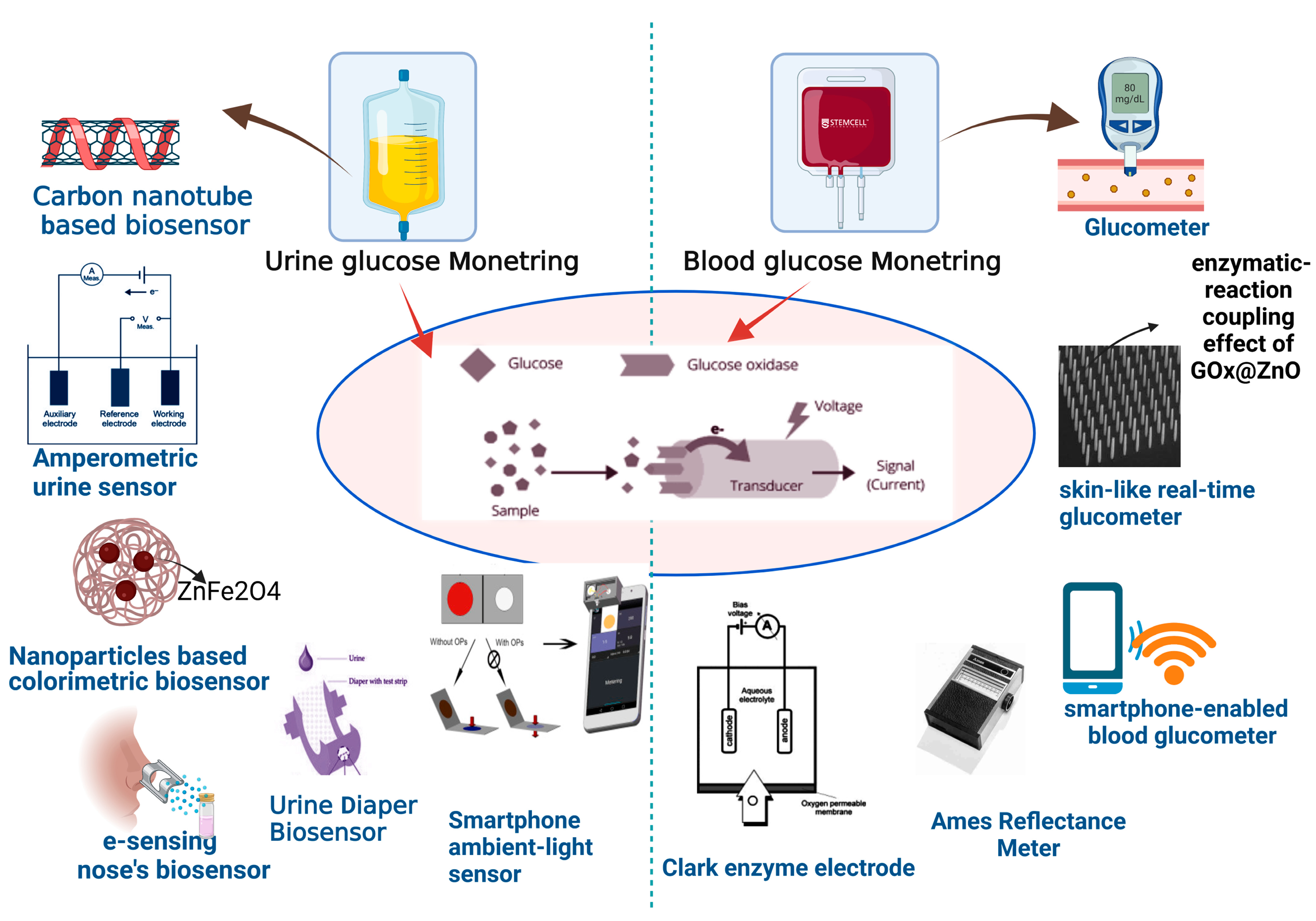
2.1. Urine Glucose Monitoring
2.2. Blood Glucose Monitoring
2.2.1. Glucometer
2.2.2. Colorimetric Strip
2.2.3. Ames Reflectance Meter
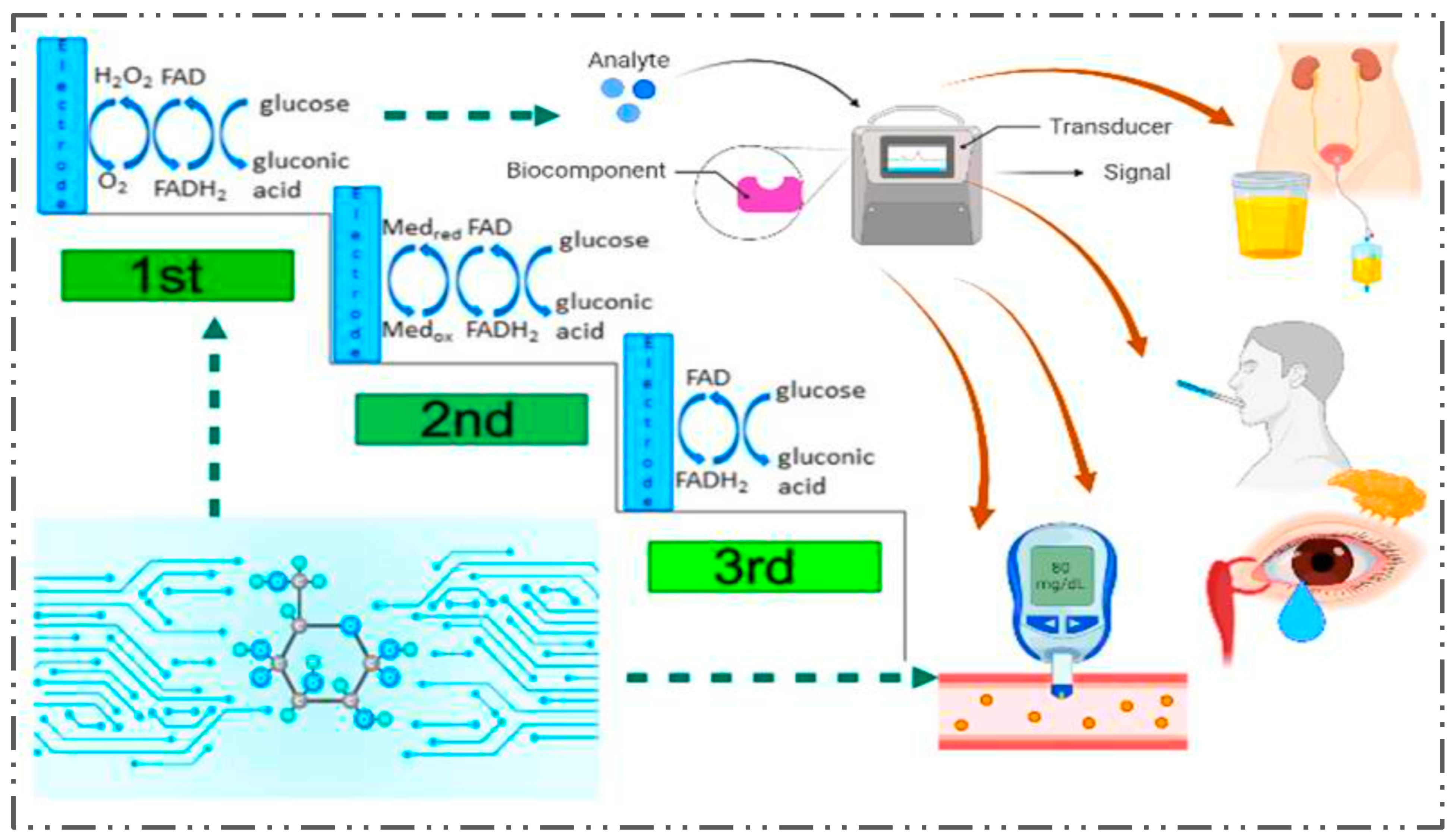
2.3. Glucose Biosensors
2.3.1. The Clark Enzyme Electrode
2.3.2. Yellow Springs Instrument
2.3.3. Mediated Biosensors
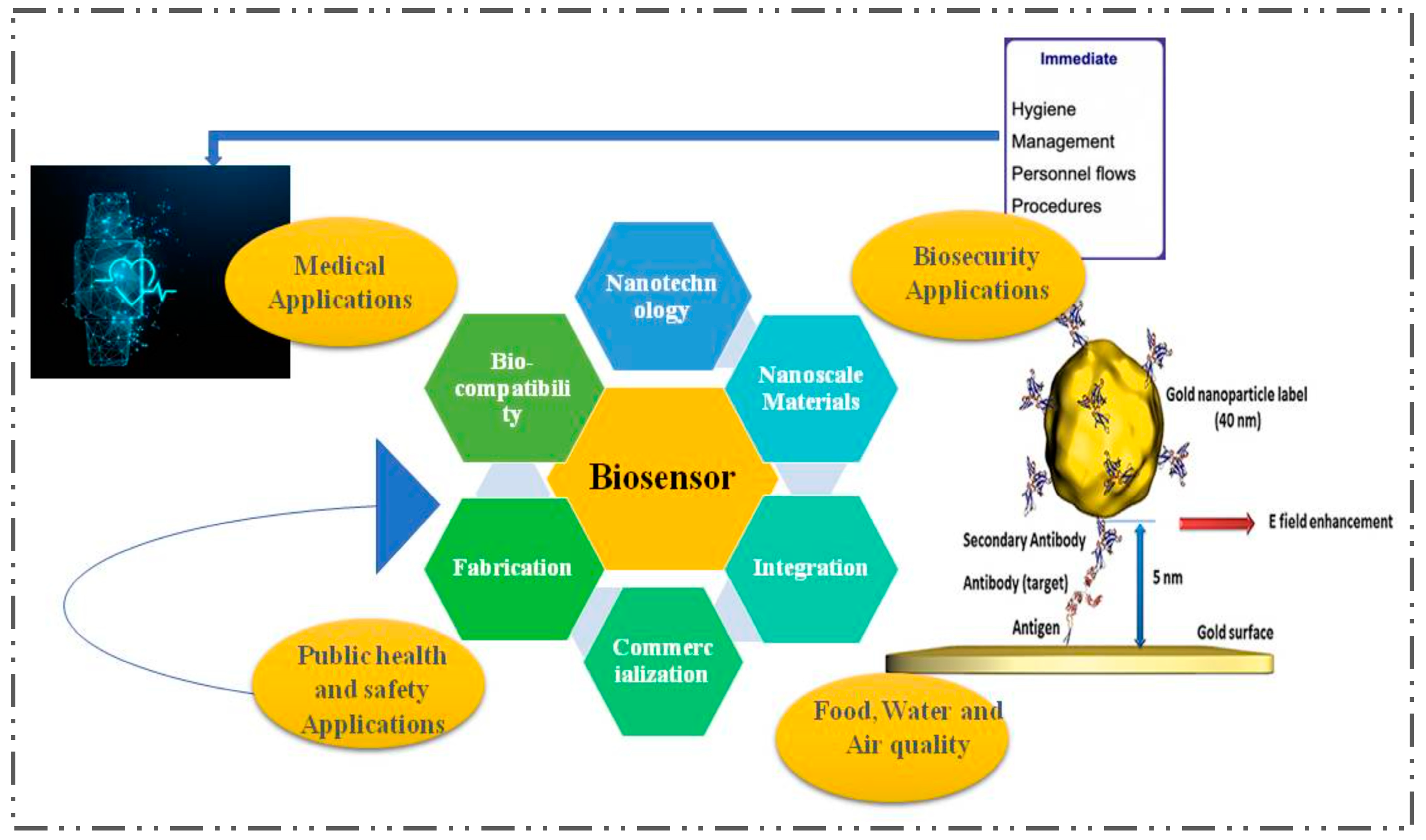
3. Biosensors for Diabetes—A Special Case
3.1. Printed Biosensors and Biosensing Systems
3.2. Implantable Biosensors and Noninvasive Monitoring
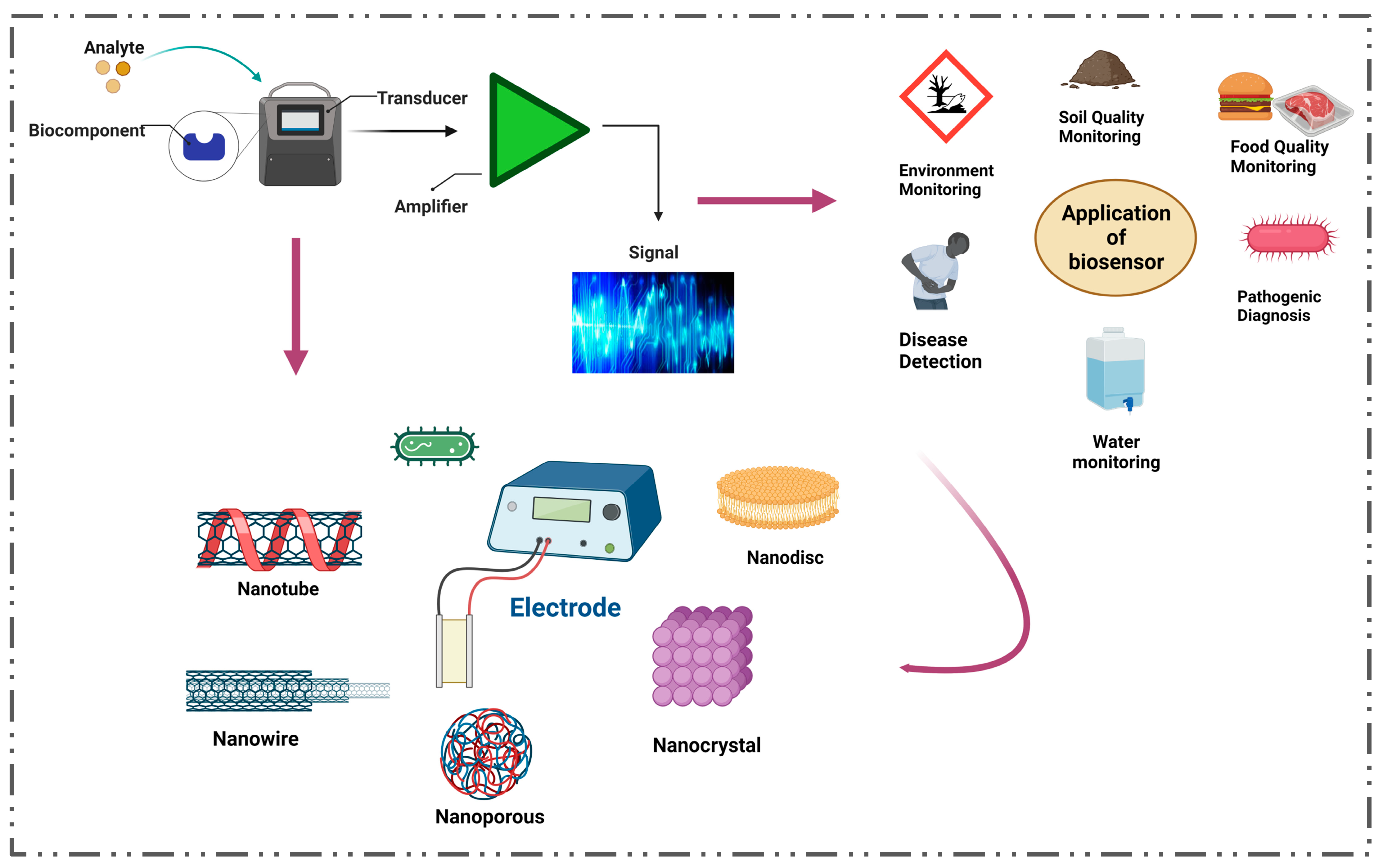
4. The Impact of Nanotechnology in the Development of Biosensors for the Detection of Diabetes
5. Nanomaterials as Potential Additives to Existing Biosensors
6. Hurdles for Biosensors in Clinical Practice
6.1. Hurdles for Composition and Secretion of Epidermal Biofluids Such as Simultaneous Monitoring of Sweat (ISF) Monitoring
6.2. Hurdles in Wearable Tear-Based Biosensors
6.3. Specialty Hurdles in Biosensors
- Optimizing biosensors’ sensitivity, specificity, and accuracy for rapid serological tests (such as for infectious diseases, etc.);
- Improving the production efficiency of disposable, inexpensive, and highly efficient biosensor devices;
- Improving the platforms and performance of wearable and investable biosensors.
7. Conclusions
8. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amos, A.F.; McCarty, D.J.; Zimmet, P. The rising global burden of diabetes and its complications: Estimates and projections to the year 2010. Diabet. Med. 1997, 14, S7–S85. [Google Scholar] [CrossRef]
- Rojas, A.; Lindner, C.; Gonzàlez, I.; Morales, M.A. Advanced-glycation end-products axis: A contributor to the risk of severe illness from COVID-19 in diabetes patients. World J. Diabetes 2021, 12, 590. [Google Scholar] [CrossRef]
- Gharravi, A.M.; Jafar, A.; Ebrahimi, M.; Mahmodi, A.; Pourhashemi, E.; Haseli, N.; Talaie, N.; Hajiasgarli, P. Current status of stem cell therapy, scaffolds for the treatment of diabetes mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 1133–1139. [Google Scholar] [CrossRef]
- Wise, J. Type 1 Diabetes Is Still Linked to Lower Life Expectancy; British Medical Journal Publishing Group: London, UK, 2016. [Google Scholar]
- Massaro, J.D.; Polli, C.D.; E Silva, M.C.; Alves, C.C.; Passos, G.A.; Sakamoto-Hojo, E.T.; de Holanda Miranda, W.R.; Cezar, N.J.B.; Rassi, D.M.; Crispim, F. Post-transcriptional markers associated with clinical complications in Type 1 and Type 2 diabetes mellitus. Mol. Cell. Endocrinol. 2019, 490, 1–14. [Google Scholar] [CrossRef]
- Mobasseri, M.; Shirmohammadi, M.; Amiri, T.; Vahed, N.; Fard, H.H.; Ghojazadeh, M. Prevalence and incidence of type 1 diabetes in the world: A systematic review and meta-analysis. Health Promot. Perspect. 2020, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, A.; Salem-Bekhit, M.M.; Siddiqui, H.H.; Dixit, R.K.; Bayomi, M.; Khalid, M.; Shakeel, F. Antidiabetic activity of standardized dried tubers extract of Aconitum napellus in streptozotocin-induced diabetic rats. 3 Biotech 2020, 10, 56. [Google Scholar] [CrossRef]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of type 2 diabetes–global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Middleton, T.L.; Chadban, S.; Molyneaux, L.; D’Souza, M.; Constantino, M.I.; Yue, D.K.; McGill, M.; Wu, T.; Twigg, S.M.; Wong, J. Young adult onset type 2 diabetes versus type 1 diabetes: Progression to and survival on renal replacement therapy. J. Diabetes Its Complicat. 2021, 35, 108023. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, A.; Perry, L.; Gholizadeh, L.; Al-Ganmi, A. Incidence and prevalence rates of diabetes mellitus in Saudi Arabia: An overview. J. Epidemiol. Glob. Health 2017, 7, 211–218. [Google Scholar] [CrossRef]
- Abdulaziz Al Dawish, M.; Alwin Robert, A.; Braham, R.; Abdallah Al Hayek, A.; Al Saeed, A.; Ahmed Ahmed, R.; Sulaiman Al Sabaan, F. Diabetes mellitus in Saudi Arabia: A review of the recent literature. Curr. Diabetes Rev. 2016, 12, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Gasbarrini, A.; Piscaglia, A.C. A natural diet versus modern western diets? A new approach to prevent “Well-Being Syndromes”. Dig. Dis. Sci. 2005, 50, 1–6. [Google Scholar] [CrossRef]
- Sabu, C.; Henna, T.; Raphey, V.; Nivitha, K.; Pramod, K. Advanced biosensors for glucose and insulin. Biosens. Bioelectron. 2019, 141, 111201. [Google Scholar] [CrossRef]
- Pandey, A.; Tripathi, P.; Pandey, R.; Srivatava, R.; Goswami, S. Alternative therapies useful in the management of diabetes: A systematic review. J. Pharm. Bioallied Sci. 2011, 3, 504. [Google Scholar]
- Hadžović, H.; Alić, M.; Dedović, A.; Sušić, A.; Tatlić, B.; Zorlak, Z.; Žigić, N.; Malenica, M.; Bego, T. Use of Biosensors in Diabetes Monitoring: Medical and Economic Aspects. In Proceedings of the CMBEBIH 2019: International Conference on Medical and Biological Engineering, Banja Luka, Bosnia and Herzegovina, 16–18 May 2019; pp. 761–768. [Google Scholar]
- Ding, S.; Schumacher, M. Sensor monitoring of physical activity to improve glucose management in diabetic patients: A review. Sensors 2016, 16, 589. [Google Scholar] [CrossRef]
- Moussy, F. Biosensor/tissue interactions. In Proceedings of the 1st Annual International IEEE-EMBS Special Topic Conference on Microtechnologies in Medicine and Biology. Proceedings (Cat. No. 00EX451), Lyon, France, 12–14 October 2000; pp. 479–482. [Google Scholar]
- Banakar, M.; Hamidi, M.; Khurshid, Z.; Zafar, M.S.; Sapkota, J.; Azizian, R.; Rokaya, D. Electrochemical Biosensors for Pathogen Detection: An Updated Review. Biosensors 2022, 12, 927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, L.; Gao, H.; Yang, W.; Wang, S.; Xing, L.; Xue, X. Self-powered implantable skin-like glucometer for real-time detection of blood glucose level in vivo. Nano-Micro Lett. 2018, 10, 32. [Google Scholar] [CrossRef]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Feng, J.; Zhou, X.; Ren, C.; Li, H.; Chen, X. Colorimetric detection of urine glucose based ZnFe2O4 magnetic nanoparticles. Anal. Chem. 2012, 84, 5753–5758. [Google Scholar] [CrossRef]
- Makaram, P.; Owens, D.; Aceros, J. Trends in nanomaterial-based non-invasive diabetes sensing technologies. Diagnostics 2014, 4, 27–46. [Google Scholar] [CrossRef]
- Miyashita, M.; Ito, N.; Ikeda, S.; Murayama, T.; Oguma, K.; Kimura, J. Development of urine glucose meter based on micro-planer amperometric biosensor and its clinical application for self-monitoring of urine glucose. Biosens. Bioelectron. 2009, 24, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Smith, A.; Gorski, W. Carbon nanotube− chitosan system for electrochemical sensing based on dehydrogenase enzymes. Anal. Chem. 2004, 76, 5045–5050. [Google Scholar] [CrossRef] [PubMed]
- Siyang, S.; Wongchoosuk, C.; Kerdcharoen, T. Diabetes diagnosis by direct measurement from urine odor using electronic nose. In Proceedings of the 5th 2012 Biomedical Engineering International Conference, Macau, China, 29–30 May 2012; pp. 1–4. [Google Scholar]
- Wang, T.-T.; Guo, K.; Hu, X.-M.; Liang, J.; Li, X.-D.; Zhang, Z.-F.; Xie, J. Label-free colorimetric detection of urine glucose based on color fading using smartphone ambient-light sensor. Chemosensors 2020, 8, 10. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Su, H.; Sun, F.; Lu, Z.; Su, A. A wearable self-powered biosensor system integrated with diaper for detecting the urine glucose of diabetic patients. Sens. Actuators B Chem. 2021, 341, 130046. [Google Scholar] [CrossRef]
- Thakur, B.; Amarnath, C.A.; Sawant, S.N. Pectin coated polyaniline nanoparticles for an amperometric glucose biosensor. RSC Adv. 2014, 4, 40917–40923. [Google Scholar] [CrossRef]
- Ji, P.; Murata-Hori, M.; Lodish, H.F. Formation of mammalian erythrocytes: Chromatin condensation and enucleation. Trends Cell Biol. 2011, 21, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Kim, I.; Roh, S.; Jung, H.G.; Lee, S.W.; Kim, H.S.; Yoon, D.S.; Hong, Y.; Lee, G. Selective colorimetric urine glucose detection by paper sensor functionalized with polyaniline nanoparticles and cell membrane. Anal. Chim. Acta 2021, 1158, 338387. [Google Scholar] [CrossRef]
- Niu, Z.; Zhang, W.; Yu, C.; Zhang, J.; Wen, Y. Recent advances in biological sample preparation methods coupled with chromatography, spectrometry and electrochemistry analysis techniques. TrAC Trends Anal. Chem. 2018, 102, 123–146. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Liu, L.; Qiao, H. A review of biosensor technology and algorithms for glucose monitoring. J. Diabetes Its Complicat. 2021, 35, 107929. [Google Scholar] [CrossRef]
- Cui, G.; Kim, S.J.; Choi, S.H.; Nam, H.; Cha, G.S.; Paeng, K.-J. A disposable amperometric sensor screen printed on a nitrocellulose strip: A glucose biosensor employing lead oxide as an interference-removing agent. Anal. Chem. 2000, 72, 1925–1929. [Google Scholar] [CrossRef]
- Vashist, S.K.; Zheng, D.; Al-Rubeaan, K.; Luong, J.H.; Sheu, F.-S. Technology behind commercial devices for blood glucose monitoring in diabetes management: A review. Anal. Chim. Acta 2011, 703, 124–136. [Google Scholar] [CrossRef]
- Forman, D.; Grayson, S.; Slonicki, A. Evaluation of a reagent strip-reflectance meter serum glucose method. Lab. Med. 1972, 3, 26–29. [Google Scholar] [CrossRef]
- Yoo, E.-H.; Lee, S.-Y. Glucose biosensors: An overview of use in clinical practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef] [PubMed]
- Heller, A.; Feldman, B. Electrochemical glucose sensors and their applications in diabetes management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Du, X.; Louie, R.F.; Kost, G.J. Effects of drugs on glucose measurements with handheld glucose meters and a portable glucose analyzer. Am. J. Clin. Pathol. 2000, 113, 75–86. [Google Scholar] [CrossRef] [PubMed]
- ISO 15197:2013; In Vitro Diagnostic Test Systems: Requirements for Blood-Glucose Monitoring Systems for Self-Testing in Managing Diabetes Mellitus. ISO: Geneva, Switzerland, 2003.
- Tonyushkina, K.; Nichols, J.H. Glucose meters: A review of technical challenges to obtaining accurate results. J. Diabetes Sci. Technol. 2009, 3, 971–980. [Google Scholar] [CrossRef]
- Zhu, X.; Sarwar, M.; Yue, Q.; Chen, C.; Li, C.-Z. Biosensing of DNA oxidative damage: A model of using glucose meter for non-glucose biomarker detection. Int. J. Nanomed. 2017, 12, 979. [Google Scholar] [CrossRef]
- Mendosa, D. Meter memories: How Tom, Dick, and Charlie did it. Diabetes Wellness Lett. 2000, 1, 1–10. [Google Scholar]
- Forrest, R.D.; Jackson, C.A.; Yudkin, J.S. Screening for diabetes mellitus in general practice using a reflectance meter system. The Islington Diabetes Survey. Diabetes Res. (Edinb. Scotl.) 1987, 6, 119–122. [Google Scholar]
- Cobelli, C.; Schiavon, M.; Dalla Man, C.; Basu, A.; Basu, R. Interstitial fluid glucose is not just a shifted-in-time but a distorted mirror of blood glucose: Insight from an in silico study. Diabetes Technol. Ther. 2016, 18, 505–511. [Google Scholar] [CrossRef]
- Pu, Z.; Tu, J.; Han, R.; Zhang, X.; Wu, J.; Fang, C.; Wu, H.; Zhang, X.; Yu, H.; Li, D. A flexible enzyme-electrode sensor with cylindrical working electrode modified with a 3D nanostructure for implantable continuous glucose monitoring. Lab A Chip 2018, 18, 3570–3577. [Google Scholar] [CrossRef]
- Peteu, S.F.; Emerson, D.; Worden, R.M. A Clark-type oxidase enzyme-based amperometric microbiosensor for sensing glucose, galactose, or choline. Biosens. Bioelectron. 1996, 11, 1059–1071. [Google Scholar] [CrossRef]
- Han, J.; Nichols, J.H.; Rice, M.; Klonoff, D.C. The end of the road for the YSI 2300 analyzer: Where do we go now? J. Diabetes Sci. Technol. 2020, 14, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.X.; Miyajima, K.; Takahashi, D.; Arakawa, T.; Sano, K.; Sawada, S.-I.; Kudo, H.; Iwasaki, Y.; Akiyoshi, K.; Mochizuki, M. Soft contact lens biosensor for in situ monitoring of tear glucose as non-invasive blood sugar assessment. Talanta 2011, 83, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Pickup, J.C.; Hussain, F.; Evans, N.D.; Sachedina, N. In vivo glucose monitoring: The clinical reality and the promise. Biosens. Bioelectron. 2005, 20, 1897–1902. [Google Scholar] [CrossRef]
- Gouvea, C. Biosensors for health applications. In Biosensors for Health, Environment and Biosecurity; IntechOpen: London, UK, 2011; pp. 71–85. [Google Scholar]
- Cano Perez, J.L.; Gutiérrez-Gutiérrez, J.; Perezcampos Mayoral, C.; Pérez-Campos, E.L.; Pina Canseco, M.d.S.; Tepech Carrillo, L.; Mayoral, L.P.-C.; Vargas Treviño, M.; Apreza, E.L.; Rojas Laguna, R. Fiber optic sensors: A review for glucose measurement. Biosensors 2021, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Purvinis, G.; Cameron, B.D.; Altrogge, D.M. Noninvasive polarimetric-based glucose monitoring: An in vivo study. J. Diabetes Sci. Technol. 2011, 5, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Tripathy, N.; Ahn, M.-S.; Bhat, K.S.; Mahmoudi, T.; Wang, Y.; Yoo, J.-Y.; Kwon, D.-W.; Yang, H.-Y.; Hahn, Y.-B. Highly efficient non-enzymatic glucose sensor based on CuO modified vertically-grown ZnO nanorods on electrode. Sci. Rep. 2017, 7, 5715. [Google Scholar] [CrossRef] [PubMed]
- Chihara, T.; Umezawa, M.; Miyata, K.; Sekiyama, S.; Hosokawa, N.; Okubo, K.; Kamimura, M.; Soga, K. Biological deep temperature imaging with fluorescence lifetime of rare-earth-doped ceramics particles in the second NIR biological window. Sci. Rep. 2019, 9, 12806. [Google Scholar] [CrossRef] [PubMed]
- Oliver, N.; Toumazou, C.; Cass, A.; Johnston, D. Glucose sensors: A review of current and emerging technology. Diabet. Med. 2009, 26, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Gourzi, M.; Rouane, A.; Guelaz, R.; Alavi, M.; McHugh, M.; Nadi, M.; Roth, P. Non-invasive glycaemia blood measurements by electromagnetic sensor: Study in static and dynamic blood circulation. J. Med. Eng. Technol. 2005, 29, 22–26. [Google Scholar] [CrossRef]
- WHO. WHO Global Coordination Mechanism on the Prevention and Control of Non-Communicable Diseases: Progress Report 2014–2016; World Health Organization: Geneva, Switzerland, 2017.
- Abdolrazzaghi, M.; Katchinskiy, N.; Elezzabi, A.Y.; Light, P.E.; Daneshmand, M. Noninvasive glucose sensing in aqueous solutions using an active split-ring resonator. IEEE Sens. J. 2021, 21, 18742–18755. [Google Scholar] [CrossRef]
- Pedraza, E.; Karajić, A.; Raoux, M.; Perrier, R.; Pirog, A.; Lebreton, F.; Arbault, S.; Gaitan, J.; Renaud, S.; Kuhn, A. Guiding pancreatic beta cells to target electrodes in a whole-cell biosensor for diabetes. Lab A Chip 2015, 15, 3880–3890. [Google Scholar] [CrossRef] [PubMed]
- Salek-Maghsoudi, A.; Vakhshiteh, F.; Torabi, R.; Hassani, S.; Ganjali, M.R.; Norouzi, P.; Hosseini, M.; Abdollahi, M. Recent advances in biosensor technology in assessment of early diabetes biomarkers. Biosens. Bioelectron. 2018, 99, 122–135. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Wu, J. Review of non-invasive continuous glucose monitoring based on impedance spectroscopy. Sens. Actuators A Phys. 2020, 311, 112103. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Qiu, Q.; Wen, Q.; Zhang, Q.; Yang, W.; Yuwen, L.; Weng, L.; Wang, L. Efficient biofunctionalization of MoS2 nanosheets with peptides as intracellular fluorescent biosensor for sensitive detection of caspase-3 activity. J. Colloid Interface Sci. 2019, 543, 96–105. [Google Scholar] [CrossRef]
- Wang, G.; Qin, J.; Zhao, Y.; Wei, J. Nanoporous carbon spheres derived from metal-phenolic coordination polymers for supercapacitor and biosensor. J. Colloid Interface Sci. 2019, 544, 241–248. [Google Scholar] [CrossRef]
- Bhat, K.S.; Ahmad, R.; Yoo, J.-Y.; Hahn, Y.-B. Nozzle-jet printed flexible field-effect transistor biosensor for high performance glucose detection. J. Colloid Interface Sci. 2017, 506, 188–196. [Google Scholar] [CrossRef]
- Coyle, V.E.; Kandjani, A.E.; Field, M.R.; Hartley, P.; Chen, M.; Sabri, Y.M.; Bhargava, S.K. Co3O4 needles on Au honeycomb as a non-invasive electrochemical biosensor for glucose in saliva. Biosens. Bioelectron. 2019, 141, 111479. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.W.; Toi, P.T.; Kim, B.Y.; Lee, W.I.; Lee, H.B.; Hanif, A.; Lee, E.H.; Lee, N.-E. Fully stretchable capillary microfluidics-integrated nanoporous gold electrochemical sensor for wearable continuous glucose monitoring. ACS Appl. Mater. Interfaces 2019, 11, 14567–14575. [Google Scholar] [CrossRef]
- Muniswamy, V.; Pattnaik, P.K.; Krishnaswamy, N. Modeling and analysis of SOI gratings-based opto-fluidic biosensor for lab-on-a-chip applications. Photonics 2019, 6, 71. [Google Scholar] [CrossRef]
- Elomaa, J.; Gallegos, L.; Gomez, F.A. Cord-based microfluidic chips as a platform for ELISA and glucose assays. Micromachines 2019, 10, 614. [Google Scholar] [CrossRef]
- Parlak, O.; İncel, A.; Uzun, L.; Turner, A.P.; Tiwari, A. Structuring Au nanoparticles on two-dimensional MoS2 nanosheets for electrochemical glucose biosensors. Biosens. Bioelectron. 2017, 89, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.; Pemberton, R.M.; Nicholas, P.; Hart, J.P. Fabrication of Miniaturised Screen-printed Glucose Biosensors, Using a Water-based Ink, and the Evaluation of their Electrochemical Behaviour. Electroanalysis 2018, 30, 1616–1620. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Imani, S.; Nunez-Flores, R.; Kumar, R.; Wang, C.; Mohan, A.V.; Wang, J.; Mercier, P.P. Re-usable electrochemical glucose sensors integrated into a smartphone platform. Biosens. Bioelectron. 2018, 101, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Cevik, E.; Cerit, A.; Tombuloglu, H.; Sabit, H.; Yildiz, H.B. Electrochemical glucose biosensors: Whole cell microbial and enzymatic determination based on 10-(4H-Dithieno [3, 2-b: 2′, 3′-d] Pyrrol-4-yl) Decan-1-amine interfaced glassy carbon electrodes. Anal. Lett. 2019, 52, 1138–1152. [Google Scholar] [CrossRef]
- Jiao, K.; Jiang, Y.; Kang, Z.; Peng, R.; Jiao, S.; Hu, Z. Three-dimensional Co3O4@ MWNTs nanocomposite with enhanced electrochemical performance for nonenzymatic glucose biosensors and biofuel cells. R. Soc. Open Sci. 2017, 4, 170991. [Google Scholar] [CrossRef]
- Lu, M.; Zhu, H.; Bazuin, C.G.; Peng, W.; Masson, J.-F. Polymer-templated gold nanoparticles on optical fibers for enhanced-sensitivity localized surface plasmon resonance biosensors. ACS Sens. 2019, 4, 613–622. [Google Scholar] [CrossRef]
- Majdinasab, M.; Mitsubayashi, K.; Marty, J.L. Optical and electrochemical sensors and biosensors for the detection of quinolones. Trends Biotechnol. 2019, 37, 898–915. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Glucose biosensors: 40 years of advances and challenges. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2001, 13, 983–988. [Google Scholar] [CrossRef]
- Murugan, P.; Annamalai, J.; Atchudan, R.; Govindasamy, M.; Nallaswamy, D.; Ganapathy, D.; Reshetilov, A.; Sundramoorthy, A. Electrochemical Sensing of Glucose Using Glucose Oxidase/PEDOT:4-Sulfocalix [4]arene/MXene Composite Modified Electrode. Micromachines 2022, 13, 304–321. [Google Scholar] [CrossRef]
- Pullano, S.A.; Greco, M.; Bianco, M.G.; Foti, D.; Brunetti, A.; Fiorillo, A.S. Glucose biosensors in clinical practice: Principles, limits and perspectives of currently used devices. Theranostics 2022, 12, 493. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Albisser, A.M.; Leibel, B.; Ewart, T.; Davidovac, Z.; Botz, C.; Zingg, W.; Schipper, H.; Gander, R. Clinical control of diabetes by the artificial pancreas. Diabetes 1974, 23, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Shichiri, M.; Yamasaki, Y.; Kawamori, R.; Hakui, N.; Abe, H. Wearable artificial endocrine pancreas with needle-type glucose sensor. Lancet 1982, 320, 1129–1131. [Google Scholar] [CrossRef]
- Matthews, D.; Holman, R.; Bown, E.; Steemson, J.; Watson, A.; Hughes, S.; Scott, D. Pen-sized digital 30-second blood glucose meter. Lancet 1987, 1, 778–779. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Feng, X.-Z.; An, Q.-Q.; Li, S.; Xue, M.; Chen, Z.; Han, G.-C.; Kraatz, H.-B. Enzyme-free glucose sensors with efficient synergistic electro-catalysis based on a ferrocene derivative and two metal nanoparticles. RSC Adv. 2022, 12, 5072–5079. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chen, Y.; Peng, Y. Ginkgo Leaf Inspired Fabrication of Micro/Nanostructures and Demonstration of Flexible Enzyme-Free Glucose Sensors. Sensors 2022, 22, 7507. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Gu, K.; Yao, J.; Shao, Z.; Chen, X. Silk-Based Electrochemical Sensor for the Detection of Glucose in Sweat. Biomacromolecules 2022, 23, 3928–3935. [Google Scholar] [CrossRef]
- Li, G.; Wang, C.; Chen, Y.; Liu, F.; Fan, H.; Yao, B.; Hao, J.; Yu, Y.; Wen, D. Dual Structural Design of Platinum-Nickel Hydrogels for Wearable Glucose Biosensing with Ultrahigh Stability. Small 2023, 2206868. [Google Scholar] [CrossRef]
- Williams, T.J.; Jeevarathinam, A.S.; Jivan, F.; Baldock, V.; Kim, P.; McShane, M.J.; Alge, D.L. Glucose biosensors based on Michael addition crosslinked poly (ethylene glycol) hydrogels with chemo-optical sensing microdomains. J. Mater. Chem. B 2023, 11, 1749–1759. [Google Scholar] [CrossRef]
- Liu, C.-F.; Wang, M.-H.; Jang, L.-S. Microfluidics-based hairpin resonator biosensor for biological cell detection. Sens. Actuators B Chem. 2018, 263, 129–136. [Google Scholar] [CrossRef]
- Matsunaga, M.; Kobayashi, A.; Nakazato, K.; Niitsu, K. Design trade-off between spatial resolution and power consumption in CMOS biosensor circuit based on millimeter-wave LC oscillator array. Jpn. J. Appl. Phys. 2018, 57, 03EC02. [Google Scholar] [CrossRef]
- Nova Max Link™ Blood Glucose Monitor Owner’s Guide. Available online: https://www.northcoastmed.com/pdf/manuals/novamaxlink_userguide.pdf (accessed on 22 February 2023).
- Driscoll, K.A.; Johnson, S.B.; Hogan, J.; Gill, E.; Wright, N.; Deeb, L.C. Insulin bolusing software: The potential to optimize health outcomes in type 1 diabetes mellitus. J. Diabetes Sci. Technol. 2013, 7, 646–652. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Marchetti, A.E.; Apovian, C.; Benchimol, A.K.; Bisschop, P.H.; Bisschop, A.; Hegazi, R.A.; Jenkins, D.; Mendoza, E.; Sanz, M.L.; et al. Diabetes-specific nutrition algorithm: A transcultural program to optimize diabetes and prediabetes care. Curr Diab Rep. 2012, 12, 180–194. [Google Scholar] [CrossRef]
- Yu-Fei, W.; Wei-Ping, J.; Ming-Hsun, W.; Miao-O, C.; Ming-Chang, H.; Chi-Pin, W.; Ming-Shih, L. Accuracy evaluation of 19 blood glucose monitoring systems manufactured in the Asia-Pacific region: A multicenter study. J. Diabetes Sci. Technol. 2017, 11, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Uslan, M.M.; Burton, D.M.; Clements, C.W. Blood glucose meters that are accessible to blind and visually impaired persons. J. Diabetes Sci. Technol. 2008, 2, 284–287. [Google Scholar] [CrossRef]
- Prohaska, E.S.; Herring, C.; Russell, G.B.; Smith, J.D. Accuracy and Precision of the Prodigy AutoCode Blood Glucose Monitor. J. Pharm. Pract. 2012, 25, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Weatherly, J.; Kishnani, S.; Aye, T. Challenges with patient adoption of automated integration of blood glucose meter data in the electronic health record. Diabetes Technol. Ther. 2019, 21, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Tack, C.; Pohlmeier, H.; Behnke, T.; Schmid, V.; Grenningloh, M.; Forst, T.; Pfützner, A. Accuracy evaluation of five blood glucose monitoring systems obtained from the pharmacy: A European multicenter study with 453 subjects. Diabetes Technol. Ther. 2012, 14, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.E.; Kane, M.P.; Bakst, G.; Busch, R.S.; Hamilton, R.A.; Abelseth, J.M. A glucose meter accuracy and precision comparison: The freestyle flash versus the accu-chek advantage, accu-chek compact plus, ascensia contour, and the BD logic. Diabetes Technol. Ther. 2008, 10, 102–110. [Google Scholar] [CrossRef]
- Kwon, M.-J.; Lee, S.-Y. Evaluation of GLUCOCARD X-METER glucose monitoring system. Korean J. Lab. Med. 2008, 28, 8–15. [Google Scholar] [CrossRef]
- Xu, T.; Jin, W.; Wang, Z.; Cheng, H.; Huang, X.; Guo, X.; Ying, Y.; Wu, Y.; Wang, F.; Wen, Y. Electrospun CuO-nanoparticles-modified polycaprolactone@ polypyrrole fibers: An application to sensing glucose in saliva. Nanomaterials 2018, 8, 133. [Google Scholar] [CrossRef]
- Liao, C.; Mak, C.; Zhang, M.; Chan, H.L.; Yan, F. Flexible organic electrochemical transistors for highly selective enzyme biosensors and used for saliva testing. Adv. Mater. 2015, 27, 676–681. [Google Scholar] [CrossRef]
- Sha, R.; Durai, L.; Badhulika, S. Facile in-situ preparation of few-layered reduced graphene oxide–niobium pentoxide composite for non-enzymatic glucose monitoring. In Proceedings of the 2018 4th IEEE International Conference on Emerging Electronics (ICEE), Bangalore, India, 17–19 December 2018; pp. 1–4. [Google Scholar]
- Xiong, C.; Zhang, T.; Kong, W.; Zhang, Z.; Qu, H.; Chen, W.; Wang, Y.; Luo, L.; Zheng, L. ZIF-67 derived porous Co3O4 hollow nanopolyhedron functionalized solution-gated graphene transistors for simultaneous detection of glucose and uric acid in tears. Biosens. Bioelectron. 2018, 101, 21–28. [Google Scholar] [CrossRef]
- Zou, R.; Shan, S.; Huang, L.; Chen, Z.; Lawson, T.; Lin, M.; Yan, L.; Liu, Y. High-performance intraocular biosensors from chitosan-functionalized nitrogen-containing graphene for the detection of glucose. ACS Biomater. Sci. Eng. 2019, 6, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, L.; Lu, Y.; Sheng, K.; Liu, W.; Chen, C.; Li, Y.; Dong, B.; Song, H. Engineered IrO2@ NiO core–shell nanowires for sensitive non-enzymatic detection of trace glucose in saliva. Anal. Chem. 2016, 88, 12346–12353. [Google Scholar] [CrossRef]
- Gualandi, I.; Tessarolo, M.; Mariani, F.; Arcangeli, D.; Possanzini, L.; Tonelli, D.; Fraboni, B.; Scavetta, E. Layered double hydroxide-modified organic electrochemical transistor for glucose and lactate biosensing. Sensors 2020, 20, 3453. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Poulter, B.; Dudgeon, J.; Li, S.-E.; Ma, X. A highly sensitive nonenzymatic glucose biosensor based on the regulatory effect of glucose on electrochemical behaviors of colloidal silver nanoparticles on MoS2. Sensors 2017, 17, 1807. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Mwafy, E.A.; Hasanin, M.S. One-pot synthesis of nanostructured CdS, CuS, and SnS by pulsed laser ablation in liquid environment and their antimicrobial activity. Opt. Laser Technol. 2020, 121, 105824. [Google Scholar] [CrossRef]
- Nirala, N.R.; Tiwari, M.; Prakash, R. A nanoporous palladium (II) bridged coordination polymer acting as a peroxidase mimic in a method for visual detection of glucose in tear and saliva. Microchim. Acta 2018, 185, 245. [Google Scholar]
- Tarokh, A.; Pebdeni, A.B.; Othman, H.O.; Salehnia, F.; Hosseini, M. Sensitive colorimetric aptasensor based on g-C3N4@Cu2O composites for detection of Salmonella typhimurium in food and water. Microchim. Acta 2021, 188, 87. [Google Scholar] [CrossRef]
- Gharekhani, H.; Olad, A.; Hosseinzadeh, F. Iron/NPK agrochemical formulation from superabsorbent nanocomposite based on maize bran and montmorillonite with functions of water uptake and slow-release fertilizer. New J. Chem. 2018, 42, 13899–13914. [Google Scholar] [CrossRef]
- Chen, L.; Tse, W.H.; Chen, Y.; McDonald, M.W.; Melling, J.; Zhang, J. Nanostructured biosensor for detecting glucose in tear by applying fluorescence resonance energy transfer quenching mechanism. Biosens. Bioelectron. 2017, 91, 393–399. [Google Scholar] [CrossRef]
- Yuan, H.; Ji, W.; Chu, S.; Qian, S.; Wang, F.; Masson, J.-F.; Han, X.; Peng, W. Fiber-optic surface plasmon resonance glucose sensor enhanced with phenylboronic acid modified Au nanoparticles. Biosens. Bioelectron. 2018, 117, 637–643. [Google Scholar] [CrossRef]
- Chen, C.; Dong, Z.-Q.; Shen, J.-H.; Chen, H.-W.; Zhu, Y.-H.; Zhu, Z.-G. 2D photonic crystal hydrogel sensor for tear glucose monitoring. ACS Omega 2018, 3, 3211–3217. [Google Scholar] [CrossRef]
- Zhu, J.; Du, H.-F.; Zhang, Q.; Zhao, J.; Weng, G.-J.; Li, J.-J.; Zhao, J.-W. SERS detection of glucose using graphene-oxide-wrapped gold nanobones with silver coating. J. Mater. Chem. C 2019, 7, 3322–3334. [Google Scholar] [CrossRef]
- Swain, K.; Palai, G.; Prasad, M.; Sahoo, J.; Moharana, J. Realization of accurate urine-glucose sensor using triangular photonic crystal structure. In Proceedings of the 2016 International Conference on Signal Processing, Communication, Power and Embedded System (SCOPES), Paralakhemundi, India, 3–5 October 2016; pp. 1021–1024. [Google Scholar]
- Calvert, P. Inkjet printing for materials and devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Delaney, J.T.; Smith, P.J.; Schubert, U.S. Inkjet printing of proteins. Soft Matter 2009, 5, 4866–4877. [Google Scholar] [CrossRef]
- Yun, Y.H.; Lee, B.K.; Choi, J.S.; Kim, S.; Yoo, B.; Kim, Y.S.; Park, K.; Cho, Y.W. A glucose sensor fabricated by piezoelectric inkjet printing of conducting polymers and bienzymes. Anal. Sci. 2011, 27, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.; Bandodkar, A.J.; Mohan, A.V.; Kumar, R.; Yu, S.; Wang, J.; Mercier, P.P. A wearable chemical–electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 2016, 7, 11650. [Google Scholar] [CrossRef]
- Bihar, E.; Deng, Y.; Miyake, T.; Saadaoui, M.; Malliaras, G.G.; Rolandi, M. A disposable paper breathalyzer with an alcohol sensing organic electrochemical transistor. Sci. Rep. 2016, 6, 27582. [Google Scholar] [CrossRef]
- Yamamoto, S.; Malliaras, G.G. Controlling the neuromorphic behavior of organic electrochemical transistors by blending mixed and ion conductors. ACS Appl. Electron. Mater. 2020, 2, 2224–2228. [Google Scholar] [CrossRef]
- Ferrari, L.M.; Sudha, S.; Tarantino, S.; Esposti, R.; Bolzoni, F.; Cavallari, P.; Cipriani, C.; Mattoli, V.; Greco, F. Ultraconformable temporary tattoo electrodes for electrophysiology. Adv. Sci. 2018, 5, 1700771. [Google Scholar] [CrossRef]
- Bihar, E.; Roberts, T.; Zhang, Y.; Ismailova, E.; Herve, T.; Malliaras, G.G.; De Graaf, J.B.; Inal, S.; Saadaoui, M. Fully printed all-polymer tattoo/textile electronics for electromyography. Flex. Print. Electron. 2018, 3, 034004. [Google Scholar] [CrossRef]
- Bihar, E.; Roberts, T.; Saadaoui, M.; Hervé, T.; De Graaf, J.B.; Malliaras, G.G. Inkjet-printed PEDOT: PSS electrodes on paper for electrocardiography. Adv. Healthc. Mater. 2017, 6, 1601167. [Google Scholar] [CrossRef] [PubMed]
- Karacolak, T.; Hood, A.Z.; Topsakal, E. Design of a dual-band implantable antenna and development of skin mimicking gels for continuous glucose monitoring. IEEE Trans. Microw. Theory Tech. 2008, 56, 1001–1008. [Google Scholar] [CrossRef]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose sensing for diabetes monitoring: Recent developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef]
- Ahmadi, M.M.; Jullien, G.A. A wireless-implantable microsystem for continuous blood glucose monitoring. IEEE Trans. Biomed. Circuits Syst. 2009, 3, 169–180. [Google Scholar] [CrossRef]
- McGreevy, R. Flash Glucose Monitoring Latest Concept in Testing. The Irish Times. 22 October 2013. Available online: https://www.irishtimes.com/life-and-style/health-family/flash-glucose-monitoring-latest-concept-in-testing-1.1568207 (accessed on 15 January 2023).
- Anthony, P.; Turner, A. Biosensors: Sense and sensibility. Chem. Soc. Rev 2013, 42, 3184–3196. [Google Scholar]
- Worsley, G.J.; Tourniaire, G.A.; Medlock, K.E.; Sartain, F.K.; Harmer, H.E.; Thatcher, M.; Horgan, A.M.; Pritchard, J. Continuous blood glucose monitoring with a thin-film optical sensor. Clin. Chem. 2007, 53, 1820–1826. [Google Scholar] [CrossRef]
- Hanashi, T.; Yamazaki, T.; Tsugawa, W.; Ikebukuro, K.; Sode, K. BioRadioTransmitter: A Self-Powered Wireless Glucose-Sensing System; SAGE Publications: New York, NY, USA, 2011. [Google Scholar]
- Choi, H.; Naylon, J.; Luzio, S.; Beutler, J.; Birchall, J.; Martin, C.; Porch, A. Design and in vitro interference test of microwave noninvasive blood glucose monitoring sensor. IEEE Trans. Microw. Theory Tech. 2015, 63, 3016–3025. [Google Scholar] [CrossRef] [PubMed]
- Villena Gonzales, W.; Mobashsher, A.T.; Abbosh, A. The progress of glucose monitoring—A review of invasive to minimally and non-invasive techniques, devices and sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Marco, M.-P.; De Alda, M.L.; Barceló, D. Biosensors for environmental applications: Future development trends. Pure Appl. Chem. 2004, 76, 723–752. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Lopez de Alda, M.J.; Barceló, D. Biosensors as useful tools for environmental analysis and monitoring. Anal. Bioanal. Chem. 2006, 386, 1025–1041. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Maity, S.; Mastrangelo, C.H. Nanostructures for biosensing, with a brief overview on cancer detection, IoT, and the role of machine learning in smart biosensors. Sensors 2021, 21, 1253. [Google Scholar] [CrossRef]
- Banerjee, A.; Maity, S.; Mastrangelo, C.H. Nanotechnology for biosensors: A Review. arXiv 2021, arXiv:2101.02430. [Google Scholar]
- Khan, M.E.; Mohammad, A.; Yoon, T. State-of-the-art developments in carbon quantum dots (CQDs): Photo-catalysis, bio-imaging, and bio-sensing applications. Chemosphere 2022, 302, 134815. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.E.; Mohammad, A.; Ali, W.; Khan, A.U.; Hazmi, W.; Zakri, W.; Yoon, T. Excellent visible-light photocatalytic activity towards the degradation of tetracycline antibiotic and electrochemical sensing of hydrazine by SnO2–CdS nanostructures. J. Clean. Prod. 2022, 349, 131249. [Google Scholar] [CrossRef]
- Pandit, S.; Dasgupta, D.; Dewan, N.; Prince, A. Nanotechnology based biosensors and its application. Pharma Innov. 2016, 5, 18. [Google Scholar]
- Mohammad, A.; Khan, M.E.; Cho, M.H. Sulfur-doped-graphitic-carbon nitride (Sg-C3N4) for low cost electrochemical sensing of hydrazine. J. Alloy. Compd. 2020, 816, 152522. [Google Scholar] [CrossRef]
- Altintas, Z. Biosensors and Nanotechnology: Applications in Health Care Diagnostics; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Cash, K.J.; Clark, H.A. Nanosensors and nanomaterials for monitoring glucose in diabetes. Trends Mol. Med. 2010, 16, 584–593. [Google Scholar] [CrossRef]
- Chang, L.; Hu, J.; Chen, F.; Chen, Z.; Shi, J.; Yang, Z.; Li, Y.; Lee, L.J. Nanoscale bio-platforms for living cell interrogation: Current status and future perspectives. Nanoscale 2016, 8, 3181–3206. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Q.; Cui, D. Recent advances in nanotechnology applied to biosensors. Sensors 2009, 9, 1033–1053. [Google Scholar] [CrossRef]
- Arora, N. Recent advances in biosensors technology: A review. Octa J. Biosci. 2013, 1, 147–150. [Google Scholar]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Maity, P.P.; Ganguly, S.; Ghosh, S.; Baral, J.; Bose, M.; Choudhary, S.; Gangopadhyay, S.; Dhara, S.; Das, A.K. Biocompatible carbon dots derived from κ-carrageenan and phenyl boronic acid for dual modality sensing platform of sugar and its anti-diabetic drug release behavior. Int. J. Biol. Macromol. 2019, 132, 316–329. [Google Scholar] [CrossRef]
- Yao, J.; Yang, M.; Duan, Y. Chemistry, biology, and medicine of fluorescent nanomaterials and related systems: New insights into biosensing, bioimaging, genomics, diagnostics, and therapy. Chem. Rev. 2014, 114, 6130–6178. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Ganguly, S.; Bose, M.; Mondal, S.; Choudhary, S.; Gangopadhyay, S.; Das, A.K.; Banerjee, S.; Das, N.C. Zinc and nitrogen ornamented bluish white luminescent carbon dots for engrossing bacteriostatic activity and Fenton based bio-sensor. Mater. Sci. Eng. C 2018, 88, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Sanchez-Gaytan, B.L.; Qian, Z.; Park, S.J. Noble metal nanoparticles in DNA detection and delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2012, 4, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Hang, T.; Ling, H.; Hu, A.; Li, M. High-performance Si-based 3D Cu nanostructured electrode assembly for rechargeable lithium batteries. J. Mater. Chem. A 2015, 3, 11912–11919. [Google Scholar] [CrossRef]
- Weber, J.; Jeedigunta, S.; Kumar, A. Fabrication and characterization of ZnO nanowire arrays with an investigation into electrochemical sensing capabilities. J. Nanomater. 2008, 2008, 638523. [Google Scholar] [CrossRef]
- Ali, S.M.U.; Nur, O.; Willander, M.; Danielsson, B. A fast and sensitive potentiometric glucose microsensor based on glucose oxidase coated ZnO nanowires grown on a thin silver wire. Sens. Actuators B Chem. 2010, 145, 869–874. [Google Scholar]
- Kong, T.; Chen, Y.; Ye, Y.; Zhang, K.; Wang, Z.; Wang, X. An amperometric glucose biosensor based on the immobilization of glucose oxidase on the ZnO nanotubes. Sens. Actuators B Chem. 2009, 138, 344–350. [Google Scholar] [CrossRef]
- Chi, B.-Z.; Zeng, Q.; Jiang, J.-H.; Shen, G.-L.; Yu, R.-Q. Synthesis of ruthenium purple nanowire array for construction of sensitive and selective biosensors for glucose detection. Sens. Actuators B Chem. 2009, 140, 591–596. [Google Scholar] [CrossRef]
- Radhakumary, C.; Sreenivasan, K. Naked eye detection of glucose in urine using glucose oxidase immobilized gold nanoparticles. Anal. Chem. 2011, 83, 2829–2833. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Zhao, F.; Mei, D.; Mo, Z.; Zeng, B. Nonenzymatic glucose sensor based on ultrasonic-electrodeposition of bimetallic PtM (M = Ru, Pd and Au) nanoparticles on carbon nanotubes–ionic liquid composite film. Biosens. Bioelectron. 2009, 24, 3481–3486. [Google Scholar] [CrossRef]
- Chen, X.-M.; Lin, Z.-J.; Chen, D.-J.; Jia, T.-T.; Cai, Z.-M.; Wang, X.-R.; Chen, X.; Chen, G.-N.; Oyama, M. Nonenzymatic amperometric sensing of glucose by using palladium nanoparticles supported on functional carbon nanotubes. Biosens. Bioelectron. 2010, 25, 1803–1808. [Google Scholar] [CrossRef]
- Zhiguo, G.; Shuping, Y.; Zaijun, L.; Xiulan, S.; Guangli, W.; Yinjun, F.; Junkang, L. An ultrasensitive electrochemical biosensor for glucose using CdTe-CdS core–shell quantum dot as ultrafast electron transfer relay between graphene-gold nanocomposite and gold nanoparticle. Electrochim. Acta 2011, 56, 9162–9167. [Google Scholar] [CrossRef]
- Lerner, M.B.; Kybert, N.; Mendoza, R.; Villechenon, R.; Bonilla Lopez, M.A.; Charlie Johnson, A. Scalable, non-invasive glucose sensor based on boronic acid functionalized carbon nanotube transistors. Appl. Phys. Lett. 2013, 102, 183113. [Google Scholar] [CrossRef]
- Liao, C.; Zhang, M.; Niu, L.; Zheng, Z.; Yan, F. Highly selective and sensitive glucose sensors based on organic electrochemical transistors with graphene-modified gate electrodes. J. Mater. Chem. B 2013, 1, 3820–3829. [Google Scholar] [CrossRef]
- Hudspeth, M.A.; Kaya, T. Collagen as a humidity sensing dielectric material. MRS Online Proc. Libr. 2012, 1427, 74–79. [Google Scholar] [CrossRef]
- Yao, H.; Shum, A.J.; Cowan, M.; Lähdesmäki, I.; Parviz, B.A. A contact lens with embedded sensor for monitoring tear glucose level. Biosens. Bioelectron. 2011, 26, 3290–3296. [Google Scholar] [CrossRef]
- Claussen, J.C.; Kumar, A.; Jaroch, D.B.; Khawaja, M.H.; Hibbard, A.B.; Porterfield, D.M.; Fisher, T.S. Nanostructuring platinum nanoparticles on multilayered graphene petal nanosheets for electrochemical biosensing. Adv. Funct. Mater. 2012, 22, 3399–3405. [Google Scholar] [CrossRef]
- Manju, S.; Sreenivasan, K. Detection of glucose in synthetic tear fluid using dually functionalized gold nanoparticles. Talanta 2011, 85, 2643–2649. [Google Scholar] [CrossRef]
- Yu, J.-B.; Byun, H.-G.; So, M.-S.; Huh, J.-S. Analysis of diabetic patient’s breath with conducting polymer sensor array. Sens. Actuators B Chem. 2005, 108, 305–308. [Google Scholar] [CrossRef]
- Ping, W.; Yi, T.; Haibao, X.; Farong, S. A novel method for diabetes diagnosis based on electronic nose. Biosens. Bioelectron. 1997, 12, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Kao, K.-W.; Hsu, M.-C.; Chang, Y.-H.; Gwo, S.; Yeh, J.A. A sub-ppm acetone gas sensor for diabetes detection using 10 nm thick ultrathin InN FETs. Sensors 2012, 12, 7157–7168. [Google Scholar] [CrossRef]
- Ding, M.; Sorescu, D.C.; Star, A. Photoinduced charge transfer and acetone sensitivity of single-walled carbon nanotube–titanium dioxide hybrids. J. Am. Chem. Soc. 2013, 135, 9015–9022. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, L.S. Biosensors: Blockbuster or bomb? Electrochemical biosensors for diabetes monitoring. Electrochem. Soc. Interface 1998, 7, 26. [Google Scholar] [CrossRef]
- Abdullah, B.S. Study of Blood Glucose Measurement Based on Blood Resistivity. Master’s Thesis, Sudan University of Science and Technology, Khartoum, Sudan, June 2014. [Google Scholar]
- Machein, M.R.; Kullmer, J.; Fiebich, B.L.; Plate, K.H.; Warnke, P.C. Vascular endothelial growth factor expression, vascular volume, and, capillary permeability in human brain tumors. Neurosurgery 1999, 44, 732–740; discussion 740. [Google Scholar] [CrossRef]
- Calbimonte, J.-P.; Aidonopoulos, O.; Dubosson, F.; Pocklington, B.; Kebets, I.; Legris, P.-M.; Schumacher, M. Decentralized semantic provision of personal health streams. J. Web Semant. 2023, 76, 100774. [Google Scholar] [CrossRef]
| Year | Events | References |
|---|---|---|
| 1962 | The Children’s Hospital of Cincinnati’s Clark and Lyons provided the first description of a biosensor. | [76,77] |
| 1967 | Updike and Hicks developed the first functional enzyme electrode. | [36] |
| 1973 | Electrode for measuring glucose based on hydrogen peroxide detection. | [78] |
| 1975 | The first commercial biosensor reintroduced after a hiatus, i.e., Yellow Springs Instrument Company analyzer (Model 23A YSI analyzer). | [36,79] |
| 1976 | The world’s first fully functional artificial pancreas for use in the hospital (Miles). | [36,80] |
| 1982 | Shichiri made the first needle-shaped enzyme electrode for implantation under the skin. | [81] |
| 1984 | Cass made the first glucose biosensor based on ferrocene and amperometry. | [36,82] |
| 1987 | The MediSense ExacTech blood glucose biosensor is now on the market. | [36] |
| 1999 | Commercialization of an in vivo glucose sensor (MiniMed). | [83,84] |
| 2000 | The first noninvasive glucose meter that can be worn (GlucoWatch). | [36] |
| 2010 | Subcutaneous self-powered sensor, Paper-based sensor. | [36] |
| 2015 | Non-enzymatic sensors; Extensive use of noninvasive biofluids; on-body patch sensor. | [36] |
| 2022 | Enzyme-free glucose sensors utilizing two metal nanoparticles and a ferrocene derivative for effective synergistic electrocatalysis; a flexible enzyme-free glucose sensor fabricated from leaf-inspired micro/nanostructures; silk-based electrochemical sensor. | [83,84,85] |
| 2023 | Continuous glucose and lactate monitoring with NIR Luminescent Oxygen Nanoparticles; wearable glucose biosensing with platinum and nickel hydrogels; carbon nanotube for electrochemical glucose sensors; glucose biosensors with chemo-optical sensing microdomains; wearable noninvasive glucose sensor. | [85,86,87] |
| S. No. | Assay Method | Minimal Sample Volume (µL) | TEST Time (second) | Assay Range (mg/dL) | Hematocrit Range (%) | Memory | Manufacturer | Brand | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | GOD | 0.3 | 5 | 20–600 | 25–60 | 400 | Nova Biomedical | Nova Max | [90] |
| 1.0 | 5 | 20–600 | 30–55 | 500 | LifeScan | OneTouch UltraLink | [91] | ||
| 0.5 | 4 | 20–600 | 20–60 | 300 | AgaMatrix | WaveSense KeyNote | [92] | ||
| 1.4 | 8 | 20–600 | 30–55 | 300 | Bionime | Rightest GM300 | [93] | ||
| 0.7 | 7 | 20–600 | 20–60 | 450 | Diabetes Supply of Suncoast | Advocate Redi-Code | [94] | ||
| 0.6 | 6 | 20–600 | 20–60 | 450 | Diagnostic Devices | Prodigy Autocode | [95] | ||
| 2 | GDH-PQQ | 0.6 | 5 | 10–600 | 20–70 | 500 | Roche | Accu-Chek Aviva | [96] |
| 0.3 | 5 | 20–500 | 15–65 | 400 | Abbott | FreeStyle Freedom Lite | [97] | ||
| 3 | GDH-FAD | 0.6 | 5 | 10–600 | 0–70 | 480 | Bayer | Ascensia Contour | [98] |
| 4 | GDH | 0.3 | 5 | 10–600 | 30–52 | 360 | Arkray | Glucocard X-meter | [99] |
| S. No. | Assay Method | Sample | Limit of Detection Range | Limit of Detection | Noninvasive Biosensor | References |
|---|---|---|---|---|---|---|
| 1 | Electrochemistry | Saliva | 2 µM–9 mM | 0.8 µM | CuO/PCL@PPy/ITO | [100] |
| Saliva | 20–100 µM | 20 µM | Co3O4 needles on Au honeycomb | [65] | ||
| Saliva | 0.1 µM–1 mM | 0.01 µM | Flexible OECTs- GOx-GO/PANI/Nafion-graphene/Pt | [101] | ||
| Tears, Urine, Saliva | 1–10 mM | 1 mM | rGO-modified Nb2O5 | [102] | ||
| Tears | 0–100 nM | 100 nM | GOx-CHIT/Co3O4 /Au | [103] | ||
| Tears | 0–12 mM | 9.5 µM | Chitosan-functionalized NG | [104] | ||
| Saliva | 0.5 µM–2.5 mM | 0.31 µM | IrO2@ NiO nanowires | [105] | ||
| Saliva | 0.1–8.0 mM | 0.02 mM | PEDOT: PSS with Ni/Al LDH | [106] | ||
| Saliva, Sweat | 0.1–1000 µM | 0.03 µM | AgNPs/MoS2 | [107] | ||
| Saliva | 0.05–2 mmol/L | 0.1 mmol/L | Hb-deposited LPG | [108] | ||
| Tears, Urine, Saliva | 1–10 mM | 1 mM | rGO-modified Nb2O5 | [102] | ||
| 2 | Colorimetry | Tears Saliva | 0–47 nM | 61 nM 91 nM | Nanoporous palladium (II) bridged coordination polymer | [109] |
| Tears, Saliva | 0.1–50 mM | 1 pM | Pt/Ni@NGT paper-based device | [110] | ||
| 3 | Chemiluminescence | Tears, Saliva | 3.0 × 10−9–4.0 × 10−5 mol L−1 | 6.4 × 10−10 mol L−1 | PG/Co (OH)2 | [111] |
| 4 | Fluorescence resonance energy transfer | Tears | 0.03–3 mmol/L | 0.03 mmol/L | CdSe/ZnS donor, malachite green dextran acceptor on ZnO nanorods–silicon hydrogel lens | [112] |
| 5 | SPR | Urine | 8 × 10−8–5 × 10−2 M | 0.8 µM | PMBA@Au/optical fiber with AET/AuNPs | [113] |
| 6 | Diffraction spectroscopy | Tears, Blood | 0–20 mM | 20 mM | PS-MCC | [114] |
| 7 | Raman spectroscopy/SPR | Urine | 0.01–10−4 µM | 80 nM | GO-decorated AuNBs@Ag | [115] |
| 8 | Photonic band gap | Urine | 0 gm/dL to 10 gm/dL | - | Two-dimensional triangular photonic crystal structure | [116] |
| S. No. | Nanostructured Materials | Medium | Biomarkers | Concentration | References |
|---|---|---|---|---|---|
| 1 | ZnO Metal nanoparticles Metal Oxides Carbon nanotubes | Blood | Glucose | 2–30 mM | [155,156,157] |
| 2 | Metal nanoparticles Platinum nanoparticles Carbon nanotubes | Urine | Glucose | 2.78–5.5 mM | [158,159,160] |
| 3 | Polymer nanostructure Quantum dots Graphene Carbon nanotubes | Saliva | Glucose | 0.008–0.21 mM | [160,161,162,163] |
| 4 | Polymer nanostructure Carbon nanotubes | Sweat | Glucose | 0.277–1.11 mM | [164] |
| 5 | Polymer nanostructure Graphene sheet Metal/Metal oxides nanostructures | Tears | Glucose | 0.1–0.6 | [165,166,167] |
| 6 | Polymer nanostructures Metal oxides nanostructures Carbon nanotubes | Breath | Acetone | 21–0.5 ppm | [168,169,170,171] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoaib, A.; Darraj, A.; Khan, M.E.; Azmi, L.; Alalwan, A.; Alamri, O.; Tabish, M.; Khan, A.U. A Nanotechnology-Based Approach to Biosensor Application in Current Diabetes Management Practices. Nanomaterials 2023, 13, 867. https://doi.org/10.3390/nano13050867
Shoaib A, Darraj A, Khan ME, Azmi L, Alalwan A, Alamri O, Tabish M, Khan AU. A Nanotechnology-Based Approach to Biosensor Application in Current Diabetes Management Practices. Nanomaterials. 2023; 13(5):867. https://doi.org/10.3390/nano13050867
Chicago/Turabian StyleShoaib, Ambreen, Ali Darraj, Mohammad Ehtisham Khan, Lubna Azmi, Abdulaziz Alalwan, Osamah Alamri, Mohammad Tabish, and Anwar Ulla Khan. 2023. "A Nanotechnology-Based Approach to Biosensor Application in Current Diabetes Management Practices" Nanomaterials 13, no. 5: 867. https://doi.org/10.3390/nano13050867
APA StyleShoaib, A., Darraj, A., Khan, M. E., Azmi, L., Alalwan, A., Alamri, O., Tabish, M., & Khan, A. U. (2023). A Nanotechnology-Based Approach to Biosensor Application in Current Diabetes Management Practices. Nanomaterials, 13(5), 867. https://doi.org/10.3390/nano13050867









