Wearable Two-Dimensional Nanomaterial-Based Flexible Sensors for Blood Pressure Monitoring: A Review
Abstract
1. Introduction
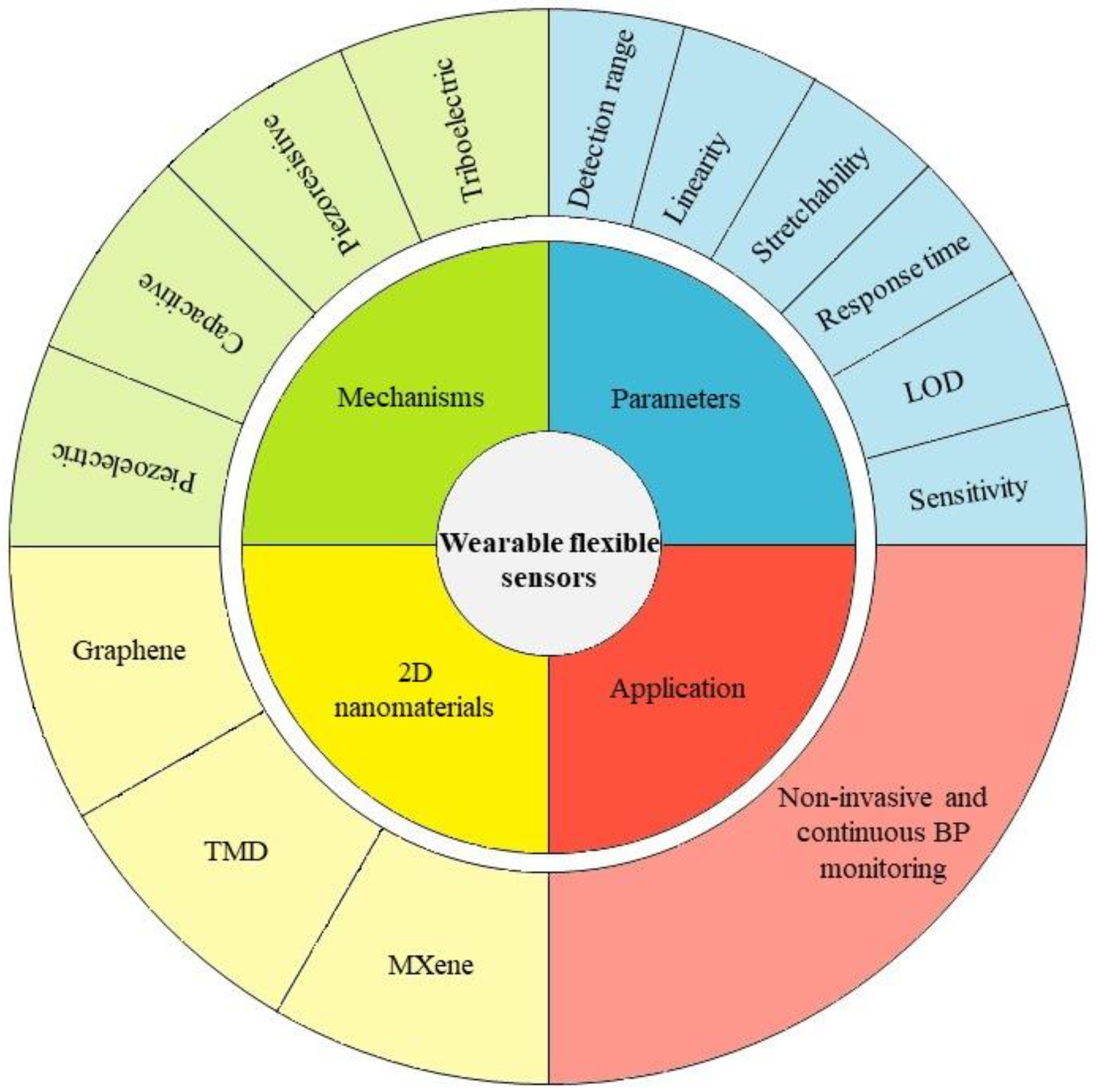
2. Transduction Mechanisms
2.1. Piezoelectric Mechanism
2.2. Capacitive Mechanism
2.3. Piezoresistive Mechanism
2.4. Triboelectric Mechanism
3. Sensing Performance Parameters
4. Two-Dimensional Nanomaterials for Flexible Sensors
4.1. Graphene (Gr)
4.2. Transition Metal Dichalcogenides (TMD)
4.3. Transition Metal Carbides and Nitrides (MXene)
5. Wearable Blood Pressure Monitoring
6. Challenges and Future Outlooks
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, W.; Haick, H. Materials and wearable devices for autonomous monitoring of physiological markers. Adv. Mat. 2018, 30, e1705024. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Z.; Zhang, T. Flexible sensing electronics for wearable/attachable health monitoring. Small 2017, 13, 1602790. [Google Scholar] [CrossRef]
- Guk, K.; Han, G.; Lim, J.; Jeong, K.; Kang, T.; Lim, E.-K.; Jung, J. Evolution of wearable devices with real-time disease monitoring for personalized healthcare. Nanomaterials 2019, 9, 813. [Google Scholar] [CrossRef] [PubMed]
- Tolba, A.; Said, O.; Al-Makhadmeh, Z. MDS: Multi-level decision system for patient behavior analysis based on wearable device information. Comput. Commun. 2019, 147, 180–187. [Google Scholar] [CrossRef]
- Jalal, A.; Batool, M.; Kim, K. Stochastic recognition of physical activity and healthcare using tri-axial inertial wearable sensors. Appl. Sci. 2020, 10, 7122. [Google Scholar] [CrossRef]
- Tang, N.; Zheng, Y.; Cui, D.; Haick, H. Multifunctional dressing for wound diagnosis and rehabilitation. Adv. Healthc. Mater. 2021, 10, 2101292. [Google Scholar] [CrossRef]
- Durán-Vega, L.A.; Santana-Mancilla, P.C.; Buenrostro-Mariscal, R.; Contreras-Castillo, J.; Anido-Rifón, L.E.; García-Ruiz, M.A.; Montesinos-López, O.A.; Estrada-González, F. An IoT system for remote health monitoring in elderly adults through a wearable device and mobile application. Geriatrics 2019, 4, 34. [Google Scholar] [CrossRef]
- Ahmad, W.N.W.; Adib, M.A.H.M.; Ahmad, Z.; Zaihidee, F.M.; Txi, M.R.S. Integration of the Health Monitoring System with IoT Application in Sports Technology: A Review. J. Kejuruter. 2022, 5, 101–109. [Google Scholar] [CrossRef]
- Wen, D.; Zhang, X.; Liu, X.; Lei, J. Evaluating the consistency of current mainstream wearable devices in health monitoring: A comparison under free-living conditions. J. Med. Internet Res. 2017, 19, e68. [Google Scholar] [CrossRef]
- Haghayegh, S.; Khoshnevis, S.; Smolensky, M.H.; Diller, K.R.; Castriotta, R.J. Accuracy of wristband fitbit models in assessing sleep: Systematic review and meta-analysis. J. Med. Internet Res. 2019, 21, e16273. [Google Scholar] [CrossRef]
- Lee, Y.; Chung, J.W.; Lee, G.H.; Kang, H.; Kim, J.-Y.; Bae, C.; Yoo, H.; Jeong, S.; Cho, H.; Kang, S.-G.; et al. Standalone real-time health monitoring patch based on a stretchable organic optoelectronic system. Sci. Adv. 2021, 7, eabg9180. [Google Scholar] [CrossRef] [PubMed]
- Leal, C.; Lopes, P.A.; Serra, A.; Coelho, J.F.J.; de Almeida, A.T.; Tavakoli, M. Untethered disposable health monitoring electronic patches with an integrated Ag2O−Zn battery, a AgInGa current collector, and hydrogel electrodes. ACS Appl. Mater. Interfaces 2020, 12, 3407–3414. [Google Scholar] [CrossRef] [PubMed]
- Nakata, S.; Arie, T.; Akita, S.; Takei, K. Wearable, flexible, and multifunctional healthcare device with an ISFET Chemical sensor for simultaneous sweat pH and skin temperature monitoring. ACS Sens. 2017, 2, 443–448. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, G.; Yuan, X.; Li, C.; Liu, L.; You, H. Substrate-free, ultra-conformable PEDOT: PSS E-tattoo achieved by energy regulation on skin. Biosens. Bioelectron. 2022, 206, 114118. [Google Scholar] [CrossRef] [PubMed]
- Laurila, M.M.; Peltokangas, M.; Montero, K.L.; Verho, J.; Haapala, M.; Oksala, N.; Vehkaoja, A.; Mäntysalo, M. Self-powered, high sensitivity printed e-tattoo sensor for unobtrusive arterial pulse wave monitoring. Nano Energy 2022, 102, 107625. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, Y.; Ameri, S.K.; Jang, H.; Dai, Z.; Huang, Y.A.; Lu, N. Low-cost, μm-thick, tape-free electronic tattoo sensors with minimized motion and sweat artifacts. NPJ Flex. Electron. 2018, 2, 6. [Google Scholar] [CrossRef]
- Doshi, S.M.; Thostenson, E.T. Thin and flexible carbon nanotube-based pressure sensors with ultrawide sensing range. ACS Sens. 2018, 3, 1276–1282. [Google Scholar] [CrossRef]
- Zhong, M.; Zhang, L.; Liu, X.; Zhou, Y.; Zhang, M.; Wang, Y.; Yang, L.; Wei, D. Wide linear range and highly sensitive flexible pressure sensor based on multistage sensing process for health monitoring and human-machine interfaces. J. Chem. Eng. 2021, 412, 128649. [Google Scholar] [CrossRef]
- Yang, D.; Guo, H.; Chen, X.; Wang, L.; Jiang, P.; Zhang, W.; Zhang, L.; Wang, Z.L. A flexible and wide pressure range triboelectric sensor array for real-time pressure detection and distribution mapping. J. Mater. Chem. A 2020, 8, 23827–23833. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Sheng, L.; Liao, T.; Kou, L.; Sun, Z. Single-crystalline ultrathin 2D TiO2 nanosheets: A bridge towards superior photovoltaic devices. Mater. Today Energy 2017, 3, 32–39. [Google Scholar] [CrossRef]
- Zong, B.; Xu, Q.; Li, Q.; Fang, X.; Chen, X.; Liu, C.; Zang, J.; Bo, Z.; Mao, S. Novel insights into the unique intrinsic sensing behaviors of 2D nanomaterials for volatile organic compounds: From graphene to MoS2 and black phosphorous. J. Mater. Chem. A 2021, 9, 14411–14421. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, S.; Wang, J.; Peng, T.; Li, R. Direct Z-Scheme 2D/2D photocatalyst based on ultrathin g-C3N4 and WO3 nanosheets for efficient visible-light-driven H2 generation. ACS Appl. Mater. Interfaces 2019, 11, 27913–27923. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Lei, W.; Sui, X.; Liu, X.; Qi, X.; Tang, K.; Liu, G.; Liu, M. Anchoring black phosphorus quantum dots on molybdenum disulfide nanosheets: A 0D/2D nanohybrid with enhanced visible−and NIR−light photoactivity. Appl. Catal. B Environ. 2018, 238, 444–453. [Google Scholar] [CrossRef]
- Zong, L.; Li, X.; Zhu, L.; You, J.; Li, Z.; Gao, H.; Li, M.; Li, C. Photo-responsive heterojunction nanosheets of reduced graphene oxide for photo-detective flexible energy devices. J. Mater. Chem. A 2019, 7, 7736–7744. [Google Scholar] [CrossRef]
- Guiney, L.M.; Mansukhani, N.D.; Jakus, A.E.; Wallace, S.G.; Shah, R.N.; Hersam, M.C. Three-dimensional printing of cytocompatible, thermally conductive hexagonal boron nitride nanocomposites. Nano Lett. 2018, 18, 3488–3493. [Google Scholar] [CrossRef]
- Hamzah, A.A.; Selvarajan, R.S.; Majlis, B.Y. Graphene for biomedical applications: A review. Sains Malays. 2017, 46, 1125–1139. [Google Scholar] [CrossRef]
- Chen, J.; Ding, P.; Pan, R.; Xuan, W.; Guo, D.; Ye, Z.; Yin, W.; Jin, H.; Wang, X.; Dong, S.; et al. Self-powered transparent glass-based single electrode triboelectric motion tracking sensor array. Nano Energy 2017, 34, 442–448. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.N.; Tao, X.M.; Ding, X. Review of flexible temperature sensing networks for wearable physiological monitoring. Adv. Healthc. Mater. 2017, 6, 1601371. [Google Scholar] [CrossRef]
- Huynh, T.-P.; Haick, H. Autonomous flexible sensors for health monitoring. Adv. Mater. 2018, 30, 1802337. [Google Scholar] [CrossRef]
- Ribas Ripoll, V.; Vellido, A. Blood pressure assessment with differential pulse transit time and deep learning: A proof of concept. Kidney Dis. 2019, 5, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Ellington, F.; Lee, T.-Y.; Vo, K.; Khine, M.; Krishnan, S.K.; Dutt, N.; Cao, H. Continuous non-invasive blood pressure monitoring: A methodological review on measurement techniques. IEEE Access 2020, 8, 212478–212498. [Google Scholar] [CrossRef]
- Quan, X.; Liu, J.; Roxlo, T.; Siddharth, S.; Leong, W.; Muir, A.; Cheong, S.-M.; Rao, A. Advances in non-invasive blood pressure monitoring. Sensors 2021, 21, 4273. [Google Scholar] [CrossRef]
- Stewart, J.; Stewart, P.; Walker, T.; Horner, D.V.; Lucas, B.; White, K.; Muggleton, A.; Morris, M.; Selby, N.M.; Taal, M.W. A Feasibility study of non-invasive continuous estimation of brachial pressure derived from arterial and venous lines during dialysis. IEEE J. Transl. Eng. Health Med. 2020, 9, 2700209. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Nakagomi, A.; Okada, S.; Ohno, Y.; Kobayashi, Y. Invasive validation of a novel brachial cuff-based oscillometric device (SphygmoCorXCEL) for measuring central blood pressure. J. Hypertens. 2017, 35, 69–75. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Pang, Y.; Cui, K. Study on signal processing and uniform deflation of wrist electronic sphygmomanometer. Int. Core J. Eng. 2022, 8, 782–792. [Google Scholar]
- Mirdamadi, A.; Etebari, M. Comparison of manual versus automated blood pressure measurement in intensive care unit, coronary care unit, and emergency room. ARYA Atheroscler. 2017, 13, 29–34. [Google Scholar]
- Dal Pont, M.P.; Marques, J.L.B. Reflective photoplethysmography acquisition platform with monitoring modules and noninvasive blood pressure calculation. IEEE Trans. Instrum. Meas. 2020, 69, 5649–5657. [Google Scholar] [CrossRef]
- Ray, D.; Collins, T.; Woolley, S.; Ponnapalli, P. A review of wearable multi-wavelength photoplethysmography. IEEE Rev. Biomed. Eng. 2021, 16, 136–151. [Google Scholar] [CrossRef]
- Chandrasekhar, A.; Yavarimanesh, M.; Natarajan, K.; Hahn, J.-O.; Mukkamala, R. PPG sensor contact pressure should be taken into account for cuff-less blood pressure measurement. IEEE Trans. Biomed. Eng. 2020, 67, 3134–3140. [Google Scholar] [CrossRef]
- Peng, B.; Zhao, F.; Ping, J.; Ying, Y. Recent advances in nanomaterial-enabled wearable sensors: Material synthesis, sensor design, and personal health monitoring. Small 2020, 16, 2002681. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Radhakrishnan, S.; Vaidyanathan, A.; Chakraborty, B.; Rout, C.S. Flexible and wearable electrochemical biosensors based on two-dimensional materials: Recent developments. Anal. Bioanal. Chem. 2021, 413, 727–762. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, A.; Ivanov, M.S.; Noor, M.R.; Silien, C.; Gandhi, A.A.; Soulimane, T.; Kholkin, A.L.; Tofail, S.A.M. Converse piezoelectricity and ferroelectricity in crystals of lysozyme protein revealed by piezoresponse force microscopy. Ferroelectrics 2018, 525, 135–145. [Google Scholar] [CrossRef]
- De Souza Marinho, D.; Pinto, F.R.; Baptista, E.R. Piezoelectric effect as an energy generator: A describal historic of its performance. Int. J. Adv. Eng. Res. Sci. 2019, 6, 228–232. [Google Scholar] [CrossRef]
- Ico, G.; Myung, A.; Kim, B.S.; Myung, N.V.; Nam, J. Transformative piezoelectric enhancement of P(VDF-TrFE) synergistically driven by nanoscale dimensional reduction and thermal treatment. Nanoscale 2018, 10, 2894–2901. [Google Scholar] [CrossRef] [PubMed]
- Zaszczyńska, A.; Gradys, A.; Sajkiewicz, P. Progress in the applications of smart piezoelectric materials for medical devices. Polymers 2020, 12, 2754. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Paria, S.; Karan, S.K.; Si, S.K.; Maitra, A.; Das, A.K.; Halder, L.; Bera, A.; De, A.; Khatua, B.B. Morphological interference of two different cobalt oxides derived from a hydrothermal protocol and a single two-dimensional metal organic framework precursor to stabilize the β-phase of PVDF for flexible piezoelectric nanogenerators. Nanoscale 2019, 11, 22989–22999. [Google Scholar] [CrossRef]
- Tian, G.; Deng, W.; Gao, Y.; Xiong, D.; Yan, C.; He, X.B.; Yang, T.; Jin, L.; Chu, X.; Zhang, H.; et al. Rich lamellar crystal baklava-structured PZT/PVDF piezoelectric sensor toward individual table tennis training. Nano Energy 2019, 59, 574–581. [Google Scholar] [CrossRef]
- Chen, X.-G.; Song, X.-J.; Zhang, Z.-X.; Li, P.-F.; Ge, J.-Z.; Tang, Y.-Y.; Gao, J.-X.; Zhang, W.-Y.; Fu, D.-W.; You, Y.-M.; et al. Two-dimensional layered perovskite ferroelectric with giant piezoelectric voltage coefficient. J. Am. Chem. Soc. 2020, 142, 1077–1082. [Google Scholar] [CrossRef]
- Jin, L.; Ma, S.; Deng, W.; Yan, C.; Yang, T.; Chu, X.; Tian, G.; Xiong, D.; Lu, J.; Yang, W. Polarization-free high-crystallization β-PVDF piezoelectric nanogenerator toward self-powered 3D acceleration sensor. Nano Energy 2018, 50, 632–638. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Sun, Z.; Yu, H. A novel stick–slip piezoelectric actuator based on a triangular compliant driving mechanism. IEEE Trans. Ind. Electron. 2019, 66, 5374–5382. [Google Scholar] [CrossRef]
- Habibi, M.; Hashemabadi, D.; Safarpour, H. Vibration analysis of a high-speed rotating GPLRC nanostructure coupled with a piezoelectric actuator. Eur. Phys. J. Plus 2019, 134, 307. [Google Scholar] [CrossRef]
- Jian, Y.; Huang, D.; Liu, J.; Min, D. High-precision tracking of piezoelectric actuator using iterative learning control and direct inverse compensation of hysteresis. IEEE Trans. Ind. Electron. 2019, 66, 368–377. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Y.; Liu, J.; Xu, D.; Wang, Y. Development of a planar piezoelectric actuator using bending–bending hybrid transducers. IEEE Trans. Ind. Electron. 2019, 66, 6141–6149. [Google Scholar] [CrossRef]
- Yang, T.; Xie, D.; Li, Z.; Zhu, H. Recent advances in wearable tactile sensors: Materials, sensing mechanisms, and device performance. Mater. Sci. Eng. R Rep. 2022, 115, 1–37. [Google Scholar] [CrossRef]
- Osoinach, B. Proximity Capacitive Sensor Technology for Touch Sensing Applications. Available online: Cache.freescale.com/files/sensors/doc/white_paper/PROXIMITYWP.pdf (accessed on 5 November 2022).
- Berggren, C.; Bjarnason, B.; Johansson, G. Capacitive biosensors. Electroanalysis 2001, 13, 173–180. [Google Scholar] [CrossRef]
- Lin, L.; Chung, C.-K. PDMS Microfabrication and design for microfluidics and sustainable energy application: Review. Micromachines 2021, 12, 1350. [Google Scholar] [CrossRef]
- Tang, C.; Liu, Z.; Li, L. Mechanical sensors for cardiovascular monitoring: From battery-powered to self-powered. Biosensors 2022, 12, 651. [Google Scholar] [CrossRef]
- Chen, W.; Yan, X. Progress in achieving high-performance piezoresistive and capacitive flexible pressure sensors: A review. J. Mater. Sci. Technol. 2020, 43, 175–188. [Google Scholar] [CrossRef]
- Verma, P.; Punetha, D.; Pandey, S.K. Sensitivity optimization of MEMS based piezoresistive pressure sensor for harsh environment. Silicon 2020, 12, 2663–2671. [Google Scholar] [CrossRef]
- Bao, M. Analysis and Design Principles of MEMS Devices; Elsevier Science: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Duan, L.; D’hooge, D.; Cardon, L. Recent progress on flexible and stretchable piezoresistive strain sensors: From design to application. Prog. Mater. Sci. 2020, 114, 100617. [Google Scholar] [CrossRef]
- Wang, Z.L. From contact electrification to triboelectric nanogenerators. Rep. Prog. Phys. 2021, 84, 096502. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Bao, R.; Wang, X.; Peng, Y.; Li, J.; Fu, S.; Pan, C.; Wang, Z.L. Self-powered tactile sensor array systems based on the triboelectric effect. Adv. Funct. Mater. 2018, 29, 1806379. [Google Scholar] [CrossRef]
- Askari, H.; Khajepour, A.; Khamesee, M.B.; Saadatnia, Z.; Wang, Z.L. Piezoelectric and triboelectric nanogenerators: Trends and impacts. Nano Today 2018, 22, 10–13. [Google Scholar] [CrossRef]
- Meng, K.; Chen, J.; Li, X.; Wu, Y.; Fan, W.; Zhou, Z.; He, Q.; Wang, X.; Fan, X.; Zhang, Y.; et al. Flexible weaving constructed self-powered pressure sensor enabling continuous diagnosis of cardiovascular disease and measurement of cuffless blood pressure. Adv. Func. Mater. 2018, 29, 1806388. [Google Scholar] [CrossRef]
- Qiu, A.; Li, P.; Yang, Z.; Yao, Y.; Lee, I.; Ma, J. A path beyond metal and silicon:Polymer/nanomaterial composites for stretchable strain sensors. Adv. Func. Mat. 2019, 29, 1806306. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Hou, X.; Li, G.; Wang, L.; Bai, N.; Cai, M.; Zhao, L.; Wang, Y.; Zhang, J.; et al. Highly stable flexible pressure sensors with a quasi-homogeneous composition and interlinked interfaces. Nat. Commun. 2022, 13, 1317. [Google Scholar] [CrossRef]
- Li, C.; Huang, K.; Yuan, T.; Cong, T.; Fan, Z.; Pan, L. Fabrication and conductive mechanism analysis of stretchable electrodes based on PDMS-Ag nanosheet composite with low resistance, stability, and durability. Nanomaterials 2022, 12, 2628. [Google Scholar] [CrossRef]
- Pan, S.; Liu, Z.; Wang, M.; Jiang, Y.; Luo, Y.; Wan, C.; Qi, D.; Wang, C.; Ge, X.; Chen, X. Mechanocombinatorially screening sensitivity of stretchable strain sensors. Adv. Mater. 2019, 31, 1903130. [Google Scholar] [CrossRef]
- Park, J.; Hwang, J.C.; Kim, G.G.; Park, J.-U. Flexible electronics based on one-dimensional and two-dimensional hybrid nanomaterials. InfoMat 2019, 2, 33–56. [Google Scholar] [CrossRef]
- Naghdi, S.; Rhee, K.Y.; Hui, D.; Park, S.J. A Review of Conductive Metal Nanomaterials as Conductive, Transparent, and Flexible Coatings, Thin Films, and Conductive Fillers: Different Deposition Methods and Applications. Coatings 2018, 8, 278. [Google Scholar] [CrossRef]
- Kamyshny, A.; Magdassi, S. Conductive Nanomaterials for Printed Electronics. Small 2014, 10, 3515–3535. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Chen, Q.; Shen, H.; Li, G. Wearable graphene devices for sensing. J. Electrochem. Soc. 2020, 167, 037541. [Google Scholar] [CrossRef]
- Prakash, J.; Prema, D.; Venkataprasanna, K.S.; Balagangadharan, K.; Selvamurugan, N.; Venkatasubbu, G.D. Nanocomposite chitosan film containing graphene oxide/hydroxyapatite/gold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 154, 62–71. [Google Scholar] [CrossRef]
- Yek, S.M.-G.; Azarifar, D.; Nasrollahzadeh, M.; Bagherzadeh, M.; Shokouhimehr, M. Heterogenized Cu(II) complex of 5-aminotetrazole immobilized on graphene oxide nanosheets as an efficient catalyst for treating environmental contaminants. Sep. Purif. Technol. 2020, 247, 116952. [Google Scholar] [CrossRef]
- Devi, S.C.; Khan, R.A. Effect of graphene oxide on mechanical and durability performance of concrete. J. Build. Eng. 2020, 27, 101007. [Google Scholar] [CrossRef]
- Stanford, M.G.; Zhang, C.; Fowlkes, J.D.; Hoffman, A.; Ivanov, I.N.; Rack, P.D.; Tour, J.M. High-resolution laser-induced graphene. Flexible electronics beyond the visible limit. ACS Appl. Mater. Interfaces 2020, 12, 10902–10907. [Google Scholar] [CrossRef]
- Chakraborty, B.; Ray, P.; Garg, N.; Banerjee, S. High capacity reversible hydrogen storage in titanium doped 2D carbon allotrope Ψ-graphene: Density Functional Theory investigations. Int. J. Hydrog. Energy 2021, 46, 4154–4167. [Google Scholar] [CrossRef]
- Kumar, R.; Youssry, S.M.; Soe, H.M.; Abdel-Galeil, M.M.; Kawamura, G.; Matsuda, A. Honeycomb-like open-edged reduced-graphene-oxide-enclosed transition metal oxides (NiO/Co3O4) as improved electrode materials for high-performance supercapacitor. J. Energy Storage 2020, 30, 101539. [Google Scholar] [CrossRef]
- Zulkepli, N.; Yunas, J.; Mohamed, M.A.; Sirat, M.S.; Hamzah, A.A. Atmospheric pressure chemical vapour deposition growth of graphene for the synthesis of SiO2 based graphene ball. Sains Malays. 2022, 51, 1927–1932. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef] [PubMed]
- Olabi, A.G.; Wilberforce, T.; Sayed, E.T.; Elsaid, K.; Rezk, H.; Abdelkareem, M.A. Recent progress of graphene based nanomaterials in bioelectrochemical systems. Sci. Total Environ. 2020, 749, 141225. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Guo, Y.; Ma, W.; Zhou, Z. 2 D materials for electrochemical energy storage: Design, preparation, and application. ChemSusChem 2020, 13, 1155–1171. [Google Scholar] [CrossRef] [PubMed]
- Shazni, M.A.; Lee, M.W.; Lee, H.W. Highly-sensitive graphene-based flexible pressure sensor platform. Sains Malays. 2017, 46, 1155–1161. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, S.; Pu, J.; Xue, Q. Barrier mechanism of multilayers graphene coated copper against atomic oxygen irradiation. Appl. Surf. Sci. 2018, 444, 28–35. [Google Scholar] [CrossRef]
- Govindasamy, M.; Jian, C.-R.; Kuo, C.-F.; Hsieh, A.-H.; Sie, J.-L.; Huang, C.-H. A chemiresistive biosensor for detection of cancer biomarker in biological fluids using CVD-grown bilayer graphene. Microchim. Acta 2022, 189, 374. [Google Scholar] [CrossRef]
- Shen, C.; Li, X.; Li, N.; Xie, K.; Wang, J.-G.; Liu, X.; Wei, B. Graphene-boosted, high-performance aqueous Zn-ion battery. ACS Appl. Mater. Interfaces 2018, 10, 25446–25453. [Google Scholar] [CrossRef]
- Li, G.; Law, W.-C.; Chan, K.C. Floating, highly efficient, and scalable graphene membranes for seawater desalination using solar energy. Green Chem. 2018, 20, 3689–3695. [Google Scholar] [CrossRef]
- Burdanova, M.G.; Kharlamova, M.V.; Kramberger, C.; Nikitin, M.P. Applications of Pristine and Functionalized Carbon Nanotubes, Graphene, and Graphene Nanoribbons in Biomedicine. Nanomaterials 2021, 11, 3020. [Google Scholar] [CrossRef]
- Du, D.; Li, P.; Ouyang, J. Graphene coated nonwoven fabrics as wearable sensors. J. Mater. Chem. C 2016, 4, 3224–3230. [Google Scholar] [CrossRef]
- Ai, Y.; Hsu, T.H.; Wu, D.C.; Lee, L.; Chen, J.H.; Chen, Y.Z.; Wu, S.C.; Wu, C.; Wang, Z.M.; Chueh, Y.L. An ultrasensitive flexible pressure sensor for multimodal wearable electronic skins based on large-scale polystyrene ball@reduced graphene-oxide core–shell nanoparticles. J. Mater. Chem. C 2018, 6, 5514–5520. [Google Scholar] [CrossRef]
- Luo, S.; Zhou, X.; Tang, X.; Li, J.; Wei, D.; Tai, G.; Chen, Z.; Liao, T.; Fu, J.; Wei, D.; et al. Microconformal electrode-dielectric integration with zinc oxide for flexible ultrasensitive robotic tactile sensing. Nano Energy 2021, 80, 105580. [Google Scholar] [CrossRef]
- Wu, Q.; Qiao, Y.; Guo, R.; Naveed, S.; Hirtz, T.; Li, X.; Fu, Y.; Wei, Y.; Deng, G.; Yang, Y.; et al. Triode-mimicking graphene pressure sensor with positive resistance variation for physiology and motion monitoring. ACS Nano 2020, 14, 10104–10114. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Peng, H.; Liu, Z. Synthesis challenges for graphene industry. Nat. Mater. 2019, 18, 520–524. [Google Scholar] [CrossRef]
- Ulian, G.; Moro, D.; Valdrè, G. Electronic and optical properties of graphene/molybdenite bilayer composite. Compos. Struct. 2021, 255, 112978. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotech. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Derakhshi, M.; Daemi, S.; Shahini, P.; Habibzadeh, A.; Mostafavi, E.; Ashkarran, A.A. Two-dimensional nanomaterials beyond graphene for biomedical applications. J. Funct. Biomater. 2022, 13, 27. [Google Scholar] [CrossRef]
- Chand, R.; Ramalingam, S.; Neethirajan, S. A 2D transition-metal dichalcogenide MoS2 based novel nanocomposite and nanocarrier for multiplex miRNA detection. Nanoscale 2018, 10, 8217–8225. [Google Scholar] [CrossRef]
- Andrzejewski, D.; Myja, H.; Heuken, M.; Grundmann, A.; Kalisch, H.; Vescan, A.; Kümmell, T.; Bacher, G. Scalable large-area p–i–n light-emitting diodes based on WS2 monolayers grown via MOCVD. ACS Photonics 2019, 6, 1832–1839. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Krylyuk, S.; Milligan, C.A.; Zhu, Y.; Zemlyanov, D.Y.; Bendersky, L.A.; Burton, B.P.; Davydov, A.V.; Appenzeller, J. Electric-field induced structural transition in vertical MoTe2- and Mo1–xWxTe2-based resistive memories. Nat. Mater. 2018, 18, 55–61. [Google Scholar] [CrossRef]
- Qiu, D.; Chu, Y.; Zeng, H.; Xu, H.; Dan, G. Stretchable MoS2 electromechanical sensors with ultrahigh sensitivity and large detection range for skin-on monitoring. ACS Appl. Mater. Interfaces 2019, 11, 37035–37042. [Google Scholar] [CrossRef]
- Tannarana, M.; Solanki, G.K.; Bhakhar, S.A.; Patel, K.D.; Pathak, V.M.; Pataniya, P.M. 2D-SnSe2 Nanosheets functionalized piezo-resistive flexible sensor for pressure and human breath monitoring. ACS Sustain. Chem. Eng. 2020, 8, 7741–7749. [Google Scholar] [CrossRef]
- Pataniya, P.M.; Bhakhar, S.A.; Tannarana, M.; Zankat, C.; Patel, V.; Solanki, G.K.; Patel, K.D.; Jha, P.K.; Late, D.J.; Sumesh, C.K. Highly sensitive and flexible pressure sensor based on two-dimensional MoSe2 nanosheets for online wrist pulse monitoring. J. Colloid Interface Sci. 2020, 584, 495–504. [Google Scholar] [CrossRef]
- Tedstone, A.T.; Lewis, D.J.; O’Brien, P. Synthesis, properties, and applications of transition metal-doped layered transition metal dichalcogenides. Chem. Mater. 2016, 28, 1965–1974. [Google Scholar] [CrossRef]
- Lan, H.-Y.; Hsieh, Y.-H.; Chiao, Z.-Y.; Jariwala, D.; Shih, M.-H.; Yen, T.-J.; Hess, O.; Lu, Y.-J. Gate-tunable plasmon-enhanced photodetection in a monolayer MoS2 phototransistor with ultrahigh photoresponsivity. Nano Lett. 2021, 21, 3083–3091. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Okubo, M.; Sugahara, A.; Kajiyama, S.; Yamada, A. MXene as a charge storage host. Acc. Chem. Res. 2018, 51, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Deysher, G.; Shuck, C.E.; Hantanasirisakul, K.; Frey, N.C.; Foucher, A.C.; Maleski, K.; Sarycheva, A.; Shenoy, V.B.; Stach, V.B.; Anasori, V.B.; et al. Synthesis of Mo4VAlC4 MAX phase and two-dimensional Mo4VC4 MXene with five atomic layers of transition metals. ACS Nano 2020, 14, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Wang, J.; Shia, Z.; Fan, L. Intercalation and delamination of two-dimensional MXene (Ti3C2Tx) and application in sodium-ion batteries. Mater. Lett. 2018, 219, 45–50. [Google Scholar] [CrossRef]
- Ma, W.; Yang, K.; Wang, H.; Li, H. Poly(vinylidene fluoride-co-hexafluoropropylene)-MXene nanosheet composites for microcapacitors. Appl. Nano Mater. 2020, 3, 7992–8003. [Google Scholar] [CrossRef]
- Ayman, I.; Rasheed, A.; Ajmal, S.; Rehman, A.; Ali, A.; Shakir, I.; Warsi, M.F. CoFe2O4 nanoparticle-decorated 2D MXene: A novel hybrid material for supercapacitor applications. Energy Fuels 2020, 34, 7622–7630. [Google Scholar] [CrossRef]
- Wang, J.; Cai, Z.; Lin, D.; Chen, K.; Zhao, L.; Xie, F.; Su, R.; Xie, W.; Liu, P.; Zhu, R. Plasma oxidized Ti3C2Tx MXene as electron transport layer for efficient perovskite solar cells. Appl. Mater. Interfaces 2021, 13, 32495–32502. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ma, M.; Si, C.; Ji, X.; Wan, P. Flexible MXene-based composites for wearable devices. Adv. Func. Mat. 2021, 31, 2009524. [Google Scholar] [CrossRef]
- Parajuli, D.; Murali, N.; Devendra, K.C.; Karki, B.; Samatha, K.; Kim, A.A.; Park, M.; Pant, B. Advancements in MXene-polymer nanocomposites in energy storage and biomedical applications. Polymers 2022, 14, 3433. [Google Scholar] [CrossRef]
- Wang, X.; Bannenberg, L. Design and characterization of 2D MXene-based electrode with high-rate capability. MRS Bull. 2021, 46, 755–766. [Google Scholar] [CrossRef]
- Cheng, Y.; Ma, Y.; Li, L.; Zhu, M.; Yue, Y.; Liu, W.; Wang, L.; Jia, S.; Li, C.; Qi, T.; et al. Bioinspired micro-spines for a high-performance spray Ti3C2Tx MXene-based piezoresistive sensor. ACS Nano 2020, 14, 2145–2155. [Google Scholar] [CrossRef]
- Li, L.; Fu, X.; Chen, S.; Uzun, S.; Levitt, A.; Shuck, C.E.; Han, W.; Gogotsi, Y. Hydrophobic and stable MXene-polymer pressure sensors for wearable electronics. ACS Appl. Mater. Interfaces 2020, 12, 15362–15369. [Google Scholar] [CrossRef]
- Su, Y.; Ma, K.; Yuan, F.; Tang, J.; Liu, M.; Zhang, X. High-performance flexible piezoresistive sensor based on Ti3C2Tx MXene with a honeycomb-like structure for human activity monitoring. Micromachines 2022, 13, 821. [Google Scholar] [CrossRef]
- Cheng, Y.; Xie, Y.; Cao, H.; Li, L.; Liu, Z.; Yan, S.; Ma, Y.; Liu, W.; Yue, Y.; Wang, J.; et al. High-strength MXene sheets through interlayer hydrogen bonding for self-healing flexible pressure sensor. J. Chem. Eng. 2023, 453, 139823. [Google Scholar] [CrossRef]
- Wang, L.L.; Wang, D.P.; Wang, K.; Jiang, K.; Shen, G.S. Biocompatible MXene/chitosan-based flexible bimodal devices for real-time pulse and respiratory rate monitoring. ACS Mater. Lett. 2021, 3, 921–929. [Google Scholar] [CrossRef]
- Babar, Z.U.D.; Ventura, B.D.; Velotta, R.; Iannotti, V. Advances and emerging challenges in MXenes and their nanocomposites for biosensing applications. RSC Adv. 2022, 12, 19590–19610. [Google Scholar] [CrossRef] [PubMed]
- Ovbiagele, B.; Diener, H.; Yusuf, S.; Martin, R.H.; Cotton, D.; Vinisko, R.; Donnan, G.A.; Bath, P.M.; PRoFESS Investigators. Level of systolic blood pressure within the normal range and risk of recurrent stroke. JAMA 2011, 306, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Marciniak, M.; Williamson, W.; Huckstep, O.J.; Lapidaire, W.; Mccance, A.; Neubauer, S.; Leeson, P.; Lewandowski, A.J. Association of systolic blood pressure elevation with disproportionate left ventricular remodeling in very preterm-born young adults: The preterm heart and elevated blood pressure. JAMA Cardiol. 2021, 6, 821–829. [Google Scholar] [CrossRef]
- Ismail, S.N.A.; Nayan, N.A.; Jaafar, R.; May, Z. Recent Advances in Non-Invasive Blood Pressure Monitoring and Prediction Using a Machine Learning Approach. Sensors 2022, 22, 6195. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Pang, G.; Pang, Z.; Gu, Y.; Mäntysalo, M.; Yang, H. Non-invasive flexible and stretchable wearable sensors with nano-based enhancement for chronic disease care. IEEE Rev. Biomed. Eng. 2019, 12, 34–71. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.; Yoon, C.; Hyun, E.; Yoon, H.N.; Chung, T.J.; Park, K.S.; Kim, H.C. Ferroelectret film-based patch-type sensor for continuous blood pressure monitoring. Electron. Lett. 2014, 50, 143–144. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Su, Y.; Joe, P.; Yona, R.; Liu, Y.; Kim, Y.S.; Huan, Y.A.; Damadoran, A.R.; Xia, J.; Martin, L.W.; et al. Conformable amplified lead zirconate titanate sensors with enhanced piezoelectric response for cutaneous pressure monitoring. Nat. Commun. 2014, 5, 4496. [Google Scholar] [CrossRef]
- Luo, N.; Dai, W.; Li, C.; Zhou, Z.; Lu, L.; Poon, C.C.Y.; Chen, S.C.; Zhang, Y.; Zhao, N. Flexible piezoresistive sensor patch ena-bling ultralow power cuffless blood pressure measurement. Adv. Funct. Mater. 2016, 26, 1178–1187. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Hu, H.; Zhang, L.; Huang, Z.; Lin, M.; Zhang, Z.; Yin, Z.; Huang, B.; Gong, H.; et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2018, 2, 687–695. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Lin, M.; Yin, L.; De la Paz, E.; Pei, K.; Sonsa-Ard, T.; Silva, A.N.d.L.; Khorshed, A.A.; Zhang, F.; Tostado, N.; et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 2021, 5, 737–748. [Google Scholar] [CrossRef]
- Kireev, D.; Sel, K.; Ibrahim, B.; Kumar, N.; Akbari, A.; Jafari, R.; Akinwande, D. Continuous cuffless monitoring of arterial blood pressure via graphene bioimpedance tattoos. Nat. Nanotechnol. 2022, 17, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Diagnostic and Interventional Cardiology. Available online: https://www.dicardiology.com/content/vivalink-announces-worlds-first-multi-vital-blood-pressure-patch-remote-patient-monitoring (accessed on 1 November 2022).



| Type | Advantage | Disadvantage |
|---|---|---|
| Piezoelectric |
|
|
| Capacitive |
|
|
| Piezoresistive |
|
|
| Triboelectric |
|
|
| Materials | Sensing Principles | Sensitivity/ GF | Response Time (ms) | Ref. |
|---|---|---|---|---|
| GNWF | R * | 0.057 kPa−1 | - | [92] |
| rGO/PDMS | R | 50.9 kPa−1 | 50 | [93] |
| GNWs/PDMS/ZnO | C ** | 22.3 kPa−1 | 25 | [94] |
| Gr/Eco-flex | R | 12.3 kPa−1 | - | [95] |
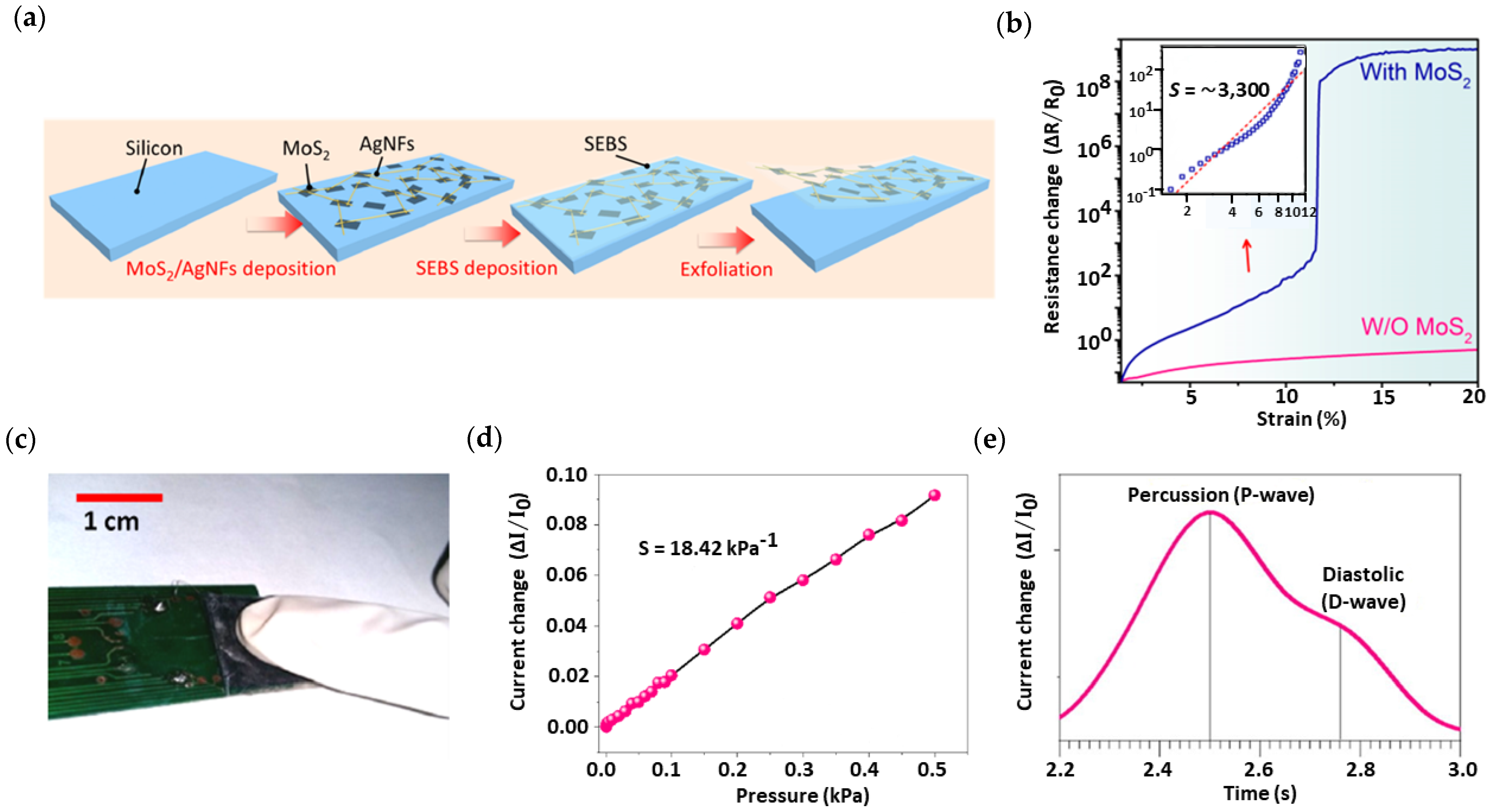
| MoS2/Ag nanofiber | E *** | 3300 | 850 | [103] |
| SnSe2/Paper | R | 1.79 kPa−1 | 100 | [104] |
| MoS2/Cellulose paper | R | 18.42 kPa−1 | 260 | [105] |
| Ti3C2Tx/PDMS | R | 151.4 kPa−1 | <130 | [118] |
| Ti3C2Tx/P(VDF-TrFE) | R | 817.4 kPa−1 | 16 | [119] |
| Ti3C2Tx/PDMS | R | 0.61 kPa−1 | 160 | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, S.N.A.; Nayan, N.A.; Mohammad Haniff, M.A.S.; Jaafar, R.; May, Z. Wearable Two-Dimensional Nanomaterial-Based Flexible Sensors for Blood Pressure Monitoring: A Review. Nanomaterials 2023, 13, 852. https://doi.org/10.3390/nano13050852
Ismail SNA, Nayan NA, Mohammad Haniff MAS, Jaafar R, May Z. Wearable Two-Dimensional Nanomaterial-Based Flexible Sensors for Blood Pressure Monitoring: A Review. Nanomaterials. 2023; 13(5):852. https://doi.org/10.3390/nano13050852
Chicago/Turabian StyleIsmail, Siti Nor Ashikin, Nazrul Anuar Nayan, Muhammad Aniq Shazni Mohammad Haniff, Rosmina Jaafar, and Zazilah May. 2023. "Wearable Two-Dimensional Nanomaterial-Based Flexible Sensors for Blood Pressure Monitoring: A Review" Nanomaterials 13, no. 5: 852. https://doi.org/10.3390/nano13050852
APA StyleIsmail, S. N. A., Nayan, N. A., Mohammad Haniff, M. A. S., Jaafar, R., & May, Z. (2023). Wearable Two-Dimensional Nanomaterial-Based Flexible Sensors for Blood Pressure Monitoring: A Review. Nanomaterials, 13(5), 852. https://doi.org/10.3390/nano13050852







