Hybrid Organic–Inorganic Perovskite Superstructures for Ultrapure Green Emissions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
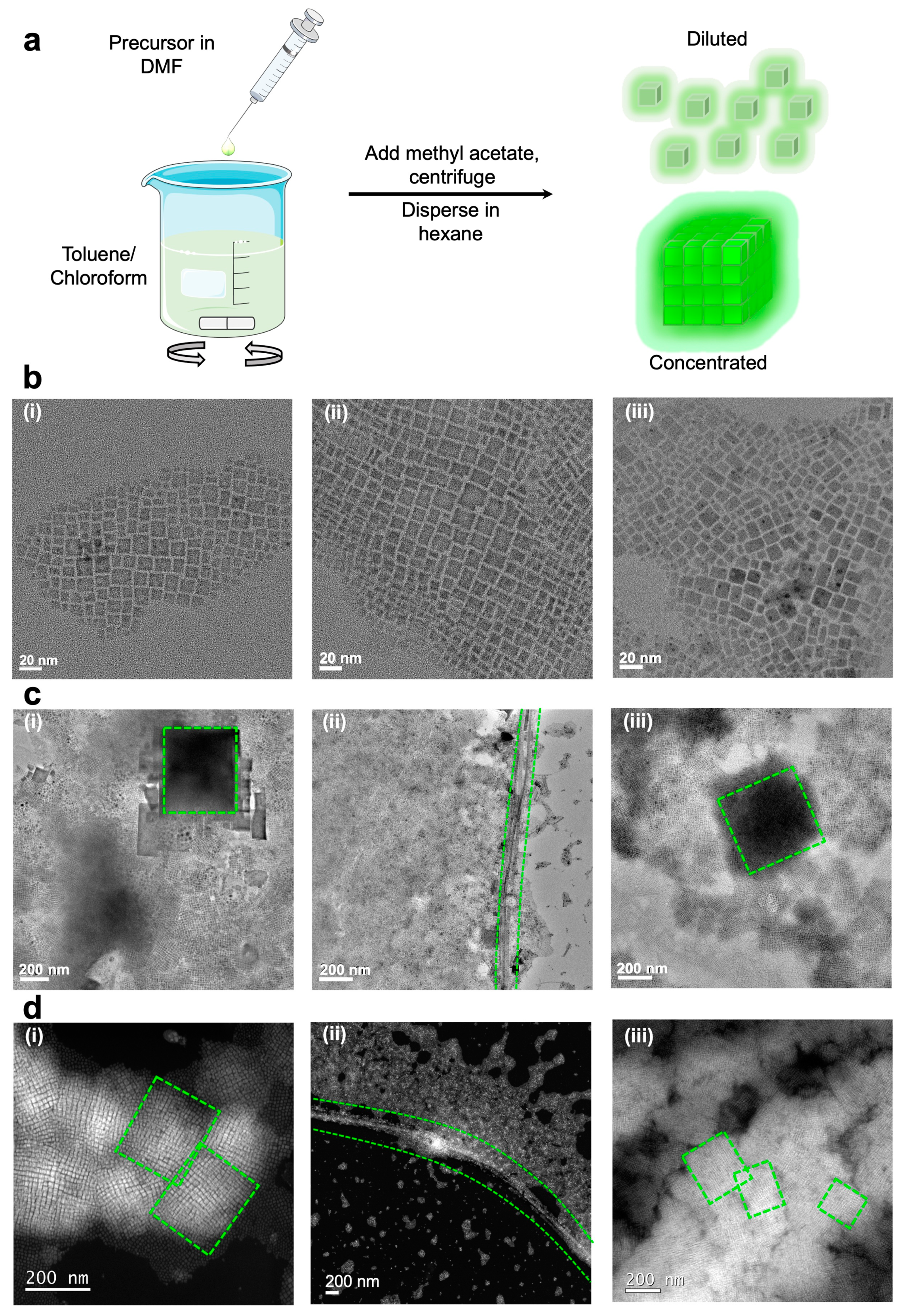
2.2. Synthesis
2.3. Characterization
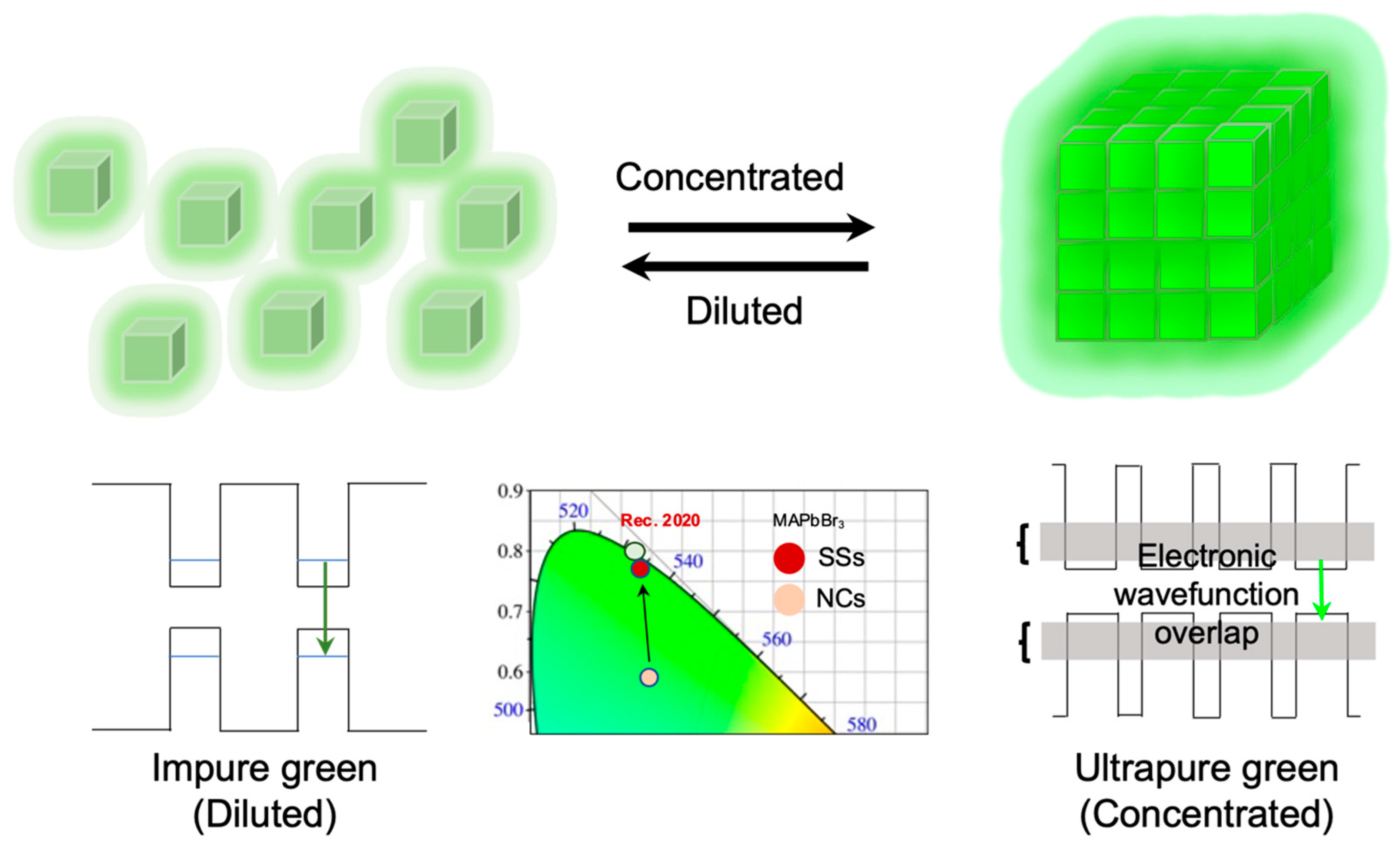
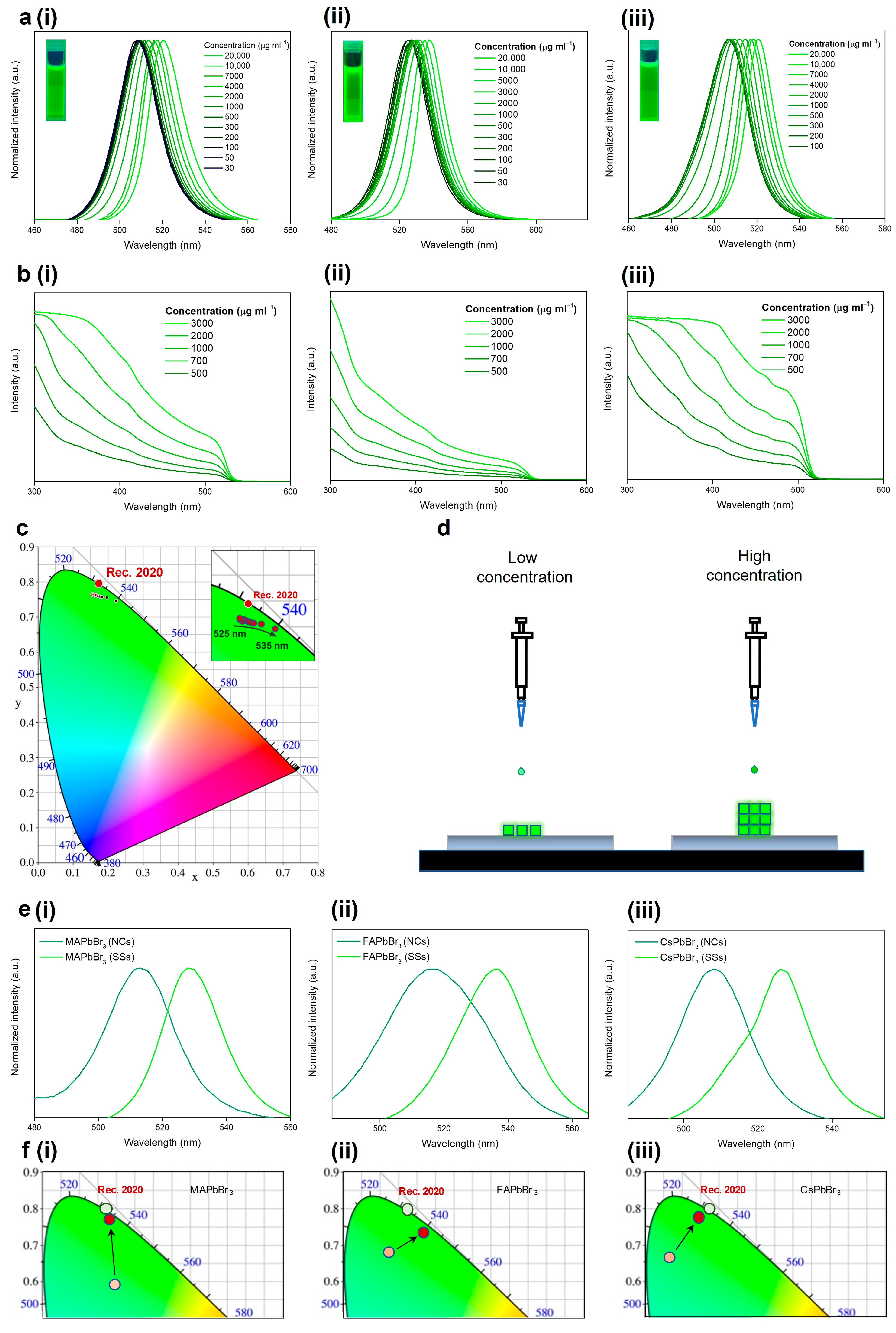
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Quilettes, D.W.; Vorpahl, S.M.; Stranks, S.D.; Nagaoka, H.; Eperon, G.E.; Ziffer, M.E.; Snaith, H.J.; Ginger, D.S. Impact of microstructure on local carrier lifetime in perovskite solar cells. Science 2015, 348, 683–686. [Google Scholar] [CrossRef]
- Yin, W.-J.; Shi, T.; Yan, Y. Unusual defect physics in CH3NH3PbI3 perovskite solar cell absorber. Appl. Phys. Lett. 2014, 104, 063903. [Google Scholar] [CrossRef]
- Tong, Y.; Yao, E.P.; Manzi, A.; Bladt, E.; Wang, K.; Doblinger, M.; Bals, S.; Muller-Buschbaum, P.; Urban, A.S.; Polavarapu, L.; et al. Spontaneous Self-Assembly of Perovskite Nanocrystals into Electronically Coupled Supercrystals: Toward Filling the Green Gap. Adv. Mater. 2018, 30, 1801117. [Google Scholar] [CrossRef]
- Raino, G.; Becker, M.A.; Bodnarchuk, M.I.; Mahrt, R.F.; Kovalenko, M.V.; Stoferle, T. Superfluorescence from lead halide perovskite quantum dot superlattices. Nature 2018, 563, 671–675. [Google Scholar] [CrossRef]
- Sugaya, T.; Amano, T.; Mori, M.; Niki, S. Miniband formation in InGaAs quantum dot superlattice. Appl. Phys. Lett. 2010, 97, 043112. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, J.T.; Liyanage, T.; Sardar, R. Flexible Polymer-Assisted Mesoscale Self-Assembly of Colloidal CsPbBr3 Perovskite Nanocrystals into Higher Order Superstructures with Strong Inter-Nanocrystal Electronic Coupling. J. Am. Chem. Soc. 2019, 141, 1526–1536. [Google Scholar] [CrossRef]
- Zhang, J.; Lutich, A.A.; Susha, A.S.; Döblinger, M.; Mauser, C.; Govorov, A.O.; Rogach, A.L.; Jäckel, F.; Feldmann, J. Thermomechanical control of electronic coupling in quantum dot solids. J. Appl. Phys. 2010, 107, 123516. [Google Scholar] [CrossRef]
- Tang, Y.; Poonia, D.; van der Laan, M.; Timmerman, D.; Kinge, S.; Siebbeles, L.D.A.; Schall, P. Electronic Coupling of Highly Ordered Perovskite Nanocrystals in Supercrystals. ACS Appl. Energy Mater. 2022, 5, 5415–5422. [Google Scholar] [CrossRef]
- Chan, W.K.; Zhou, D.; Yu, Z.; Tan, T.T.Y. Mechanistic studies of CsPbBr3 superstructure formation. J. Mater. Chem. C 2021, 9, 14699–14708. [Google Scholar] [CrossRef]
- Huang, H.; Feil, M.W.; Fuchs, S.; Debnath, T.; Richter, A.F.; Tong, Y.; Wu, L.; Wang, Y.; Döblinger, M.; Nickel, B. Growth of Perovskite CsPbBr3 Nanocrystals and Their Formed Superstructures Revealed by In Situ Spectroscopy. Chem. Mater. 2020, 32, 8877–8884. [Google Scholar] [CrossRef]
- Mattiotti, F.; Kuno, M.; Borgonovi, F.; Janko, B.; Celardo, G.L. Thermal Decoherence of Superradiance in Lead Halide Perovskite Nanocrystal Superlattices. Nano Lett. 2020, 20, 7382–7388. [Google Scholar] [CrossRef]
- Higgins, K.D.; Benjamin, S.C.; Stace, T.M.; Milburn, G.J.; Lovett, B.W.; Gauger, E.M. Superabsorption of light via quantum engineering. Nat. Commun. 2014, 5, 4705. [Google Scholar] [CrossRef]
- Hu, X.; Damjanovic, A.; Ritz, T.; Schulten, K. Architecture and mechanism of the light-harvesting apparatus of purple bacteria. Proc. Natl. Acad. Sci. USA 1998, 95, 5935–5941. [Google Scholar] [CrossRef]
- Zhou, C.; Zhong, Y.; Dong, H.; Zheng, W.; Tan, J.; Jie, Q.; Pan, A.; Zhang, L.; Xie, W. Cooperative excitonic quantum ensemble in perovskite-assembly superlattice microcavities. Nat. Commun. 2020, 11, 329. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, X.; Mohammed, O.F.; Bakr, O.M. Self-Assembly and Regrowth of Metal Halide Perovskite Nanocrystals for Optoelectronic Applications. Acc. Chem. Res. 2022, 55, 262–274. [Google Scholar] [CrossRef]
- Penzo, E.; Loiudice, A.; Barnard, E.S.; Borys, N.J.; Jurow, M.J.; Lorenzon, M.; Rajzbaum, I.; Wong, E.K.; Liu, Y.; Schwartzberg, A.M. Long-range exciton diffusion in two-dimensional assemblies of cesium lead bromide perovskite nanocrystals. ACS Nano 2020, 14, 6999–7007. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, R.; Ou, X.; Fu, K.; Chen, Q.; Ding, Y.; Xu, L.-J.; Liu, L.; Han, Y.; Malko, A.V. Metal halide perovskite nanosheet for X-ray high-resolution scintillation imaging screens. ACS Nano 2019, 13, 2520–2525. [Google Scholar] [CrossRef]
- Kubicki, D.J.; Prochowicz, D.; Hofstetter, A.; Péchy, P.; Zakeeruddin, S.M.; Gratzel, M.; Emsley, L. Cation dynamics in mixed-cation (MA)x (FA)1–x PbI3 hybrid perovskites from solid-state NMR. J. Am. Chem. Soc. 2017, 139, 10055–10061. [Google Scholar] [CrossRef]
- Motta, C.; El-Mellouhi, F.; Kais, S.; Tabet, N.; Alharbi, F.; Sanvito, S. Revealing the role of organic cations in hybrid halide perovskite CH3NH3PbI3. Nat. Commun. 2015, 6, 7026. [Google Scholar] [CrossRef]
- Hao, M.; Bai, Y.; Zeiske, S.; Ren, L.; Liu, J.; Yuan, Y.; Zarrabi, N.; Cheng, N.; Ghasemi, M.; Chen, P.; et al. Ligand-assisted cation-exchange engineering for high-efficiency colloidal Cs1−xFAxPbI3 quantum dot solar cells with reduced phase segregation. Nat. Energy 2020, 5, 79–88. [Google Scholar] [CrossRef]
- Zhu, H.; Miyata, K.; Fu, Y.; Wang, J.; Joshi, P.P.; Niesner, D.; Williams, K.W.; Jin, S.; Zhu, X.Y. Screening in crystalline liquids protects energetic carriers in hybrid perovskites. Science 2016, 353, 1409–1413. [Google Scholar] [CrossRef]
- Biliroglu, M.; Findik, G.; Mendes, J.; Seyitliyev, D.; Lei, L.; Dong, Q.; Mehta, Y.; Temnov, V.V.; So, F.; Gundogdu, K. Room-temperature superfluorescence in hybrid perovskites and its origins. Nat. Photonics 2022, 16, 324–329. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G.; et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef]
- Lin, R.; Xiao, K.; Qin, Z.; Han, Q.; Zhang, C.; Wei, M.; Saidaminov, M.I.; Gao, Y.; Xu, J.; Xiao, M.; et al. Monolithic all-perovskite tandem solar cells with 24.8% efficiency exploiting comproportionation to suppress Sn(ii) oxidation in precursor ink. Nat. Energy 2019, 4, 864–873. [Google Scholar] [CrossRef]
- Murugan, P.; Hu, T.; Hu, X.; Chen, Y. Current Development toward Commercialization of Metal-Halide Perovskite Photovoltaics. Adv. Opt. Mater. 2021, 9, 2100390. [Google Scholar] [CrossRef]
- van der Burgt, J.S.; Geuchies, J.J.; van der Meer, B.; Vanrompay, H.; Zanaga, D.; Zhang, Y.; Albrecht, W.; Petukhov, A.V.; Filion, L.; Bals, S.; et al. Cuboidal Supraparticles Self-Assembled from Cubic CsPbBr3 Perovskite Nanocrystals. J. Phys. Chem. C Nanomater. Interfaces 2018, 122, 15706–15712. [Google Scholar] [CrossRef]
- Zu, Y.; Xi, J.; Li, L.; Dai, J.; Wang, S.; Yun, F.; Jiao, B.; Dong, H.; Hou, X.; Wu, Z. High-Brightness and Color-Tunable FAPbBr3 Perovskite Nanocrystals 2.0 Enable Ultrapure Green Luminescence for Achieving Recommendation 2020 Displays. ACS Appl. Mater. Interfaces 2020, 12, 2835–2841. [Google Scholar] [CrossRef]
- Zhu, R.; Luo, Z.; Chen, H.; Dong, Y.; Wu, S.-T. Realizing Rec. 2020 color gamut with quantum dot displays. Optics Express 2015, 23, 23680–23693. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, X.; Zheng, W.; Wan, Q.; Liu, M.; Liao, X.; Hagio, T.; Ichino, R.; Kong, L.; Wang, H.; et al. Band Gap Engineering toward Wavelength Tunable CsPbBr3 Nanocrystals for Achieving Rec. 2020 Displays. Chem. Mater. 2021, 33, 3575–3584. [Google Scholar] [CrossRef]
- Yu, D.; Cao, F.; Gao, Y.; Xiong, Y.; Zeng, H. Room-temperature ion-exchange-mediated self-assembly toward formamidinium perovskite nanoplates with finely tunable, ultrapure green emissions for achieving rec. 2020 displays. Adv. Funct. Mater. 2018, 28, 1800248. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Xu, X.; Li, J.; Song, J.; Lan, S.; Liu, S.; Cai, B.; Han, B.; Precht, J.T.; et al. Efficient and bright white light-emitting diodes based on single-layer heterophase halide perovskites. Nat. Photonics 2020, 15, 238–244. [Google Scholar] [CrossRef]
- Huang, H.; Susha, A.S.; Kershaw, S.V.; Hung, T.F.; Rogach, A.L. Control of Emission Color of High Quantum Yield CH3NH3PbBr3 Perovskite Quantum Dots by Precipitation Temperature. Adv. Sci. 2015, 2, 1500194. [Google Scholar] [CrossRef]
- Yang, H.S.; Noh, S.H.; Suh, E.H.; Jung, J.; Oh, J.G.; Lee, K.H.; Jang, J. Enhanced Stabilities and Production Yields of MAPbBr3 Quantum Dots and Their Applications as Stretchable and Self-Healable Color Filters. ACS Appl. Mater. Interfaces 2021, 13, 4374–4384. [Google Scholar] [CrossRef]
- Levchuk, I.; Osvet, A.; Tang, X.; Brandl, M.; Perea, J.D.; Hoegl, F.; Matt, G.J.; Hock, R.; Batentschuk, M.; Brabec, C.J. Brightly Luminescent and Color-Tunable Formamidinium Lead Halide Perovskite FAPbX3 (X = Cl, Br, I) Colloidal Nanocrystals. Nano Lett. 2017, 17, 2765–2770. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, W.; Li, L.; Zhang, Z.; Chen, Z.; Lin, Y.; Zhu, J.J. Size-selected and surface-passivated CsPbBr3 perovskite nanocrystals for self-enhanced electrochemiluminescence in aqueous media. Nanoscale 2020, 12, 7321–7329. [Google Scholar] [CrossRef]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef]
- Dai, L.; Deng, Z.; Auras, F.; Goodwin, H.; Zhang, Z.; Walmsley, J.C.; Bristowe, P.D.; Deschler, F.; Greenham, N.C. Slow carrier relaxation in tin-based perovskite nanocrystals. Nat. Photonics 2021, 15, 696–702. [Google Scholar] [CrossRef]
- Brennan, M.C.; Toso, S.; Pavlovetc, I.M.; Zhukovskyi, M.; Marras, S.; Kuno, M.; Manna, L.; Baranov, D. Superlattices are greener on the other side: How light transforms self-assembled mixed halide perovskite nanocrystals. ACS Energy Lett. 2020, 5, 1465–1473. [Google Scholar] [CrossRef]
- Toso, S.; Baranov, D.; Giannini, C.; Marras, S.; Manna, L. Wide-Angle X-ray Diffraction Evidence of Structural Coherence in CsPbBr3 Nanocrystal Superlattices. ACS Mater. Lett. 2019, 1, 272–276. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Wang, P.; Bai, X. Concentration- and temperature-dependent photoluminescence of CsPbBr3 perovskite quantum dots. Optik 2017, 139, 56–60. [Google Scholar] [CrossRef]
- Gan, Z.; Wen, X.; Chen, W.; Zhou, C.; Yang, S.; Cao, G.; Ghiggino, K.P.; Zhang, H.; Jia, B. The Dominant Energy Transport Pathway in Halide Perovskites: Photon Recycling or Carrier Diffusion? Adv. Energy Mater. 2019, 9, 1900185. [Google Scholar] [CrossRef]
- Pazos-Outon, L.M.; Szumilo, M.; Lamboll, R.; Richter, J.M.; Crespo-Quesada, M.; Abdi-Jalebi, M.; Beeson, H.J.; Vrucinic, M.; Alsari, M.; Snaith, H.J.; et al. Photon recycling in lead iodide perovskite solar cells. Science 2016, 351, 1430–1433. [Google Scholar] [CrossRef]
- Cherniukh, I.; Raino, G.; Stoferle, T.; Burian, M.; Travesset, A.; Naumenko, D.; Amenitsch, H.; Erni, R.; Mahrt, R.F.; Bodnarchuk, M.I.; et al. Perovskite-type superlattices from lead halide perovskite nanocubes. Nature 2021, 593, 535–542. [Google Scholar] [CrossRef]
- Chen, Y.; Song, J.; He, G.S.; Hu, W.; Yu, Z.; Zhou, T.; Qu, J.; Prasad, P.N. Polymer-assisted room-temperature synthesis of highly luminescent perovskite nanocrystals with superior water resistance for WLED. Mater. Lett. 2018, 232, 138–141. [Google Scholar] [CrossRef]
- Yang, C.; Zhuang, B.; Lin, J.; Wang, S.; Liu, M.; Jiang, N.; Chen, D. Ultrastable glass-protected all-inorganic perovskite quantum dots with finely tunable green emissions for approaching Rec. 2020 backlit display. Chem. Eng. J. 2020, 398, 125616. [Google Scholar] [CrossRef]
- Vázquez, R.J.; Kim, H.; Kobilka, B.M.; Hale, B.J.; Jeffries-El, M.; Zimmerman, P.; Goodson, T. Evaluating the Effect of Heteroatoms on the Photophysical Properties of Donor–Acceptor Conjugated Polymers Based on 2,6-Di(thiophen-2-yl)benzo[1,2-b:4,5-b′]difuran: Two-Photon Cross-Section and Ultrafast Time-Resolved Spectroscopy. J. Phys. Chem. C 2017, 121, 14382–14392. [Google Scholar] [CrossRef]
- Vázquez, R.J.; Kim, H.; Zimmerman, P.M.; Goodson, T. Using ultra-fast spectroscopy to probe the excited state dynamics of a reported highly efficient thermally activated delayed fluorescence chromophore. J. Mater. Chem. C 2019, 7, 4210–4221. [Google Scholar] [CrossRef]
- Perumal, A.; Shendre, S.; Li, M.; Tay, Y.K.E.; Sharma, V.K.; Chen, S.; Wei, Z.; Liu, Q.; Gao, Y.; Buenconsejo, P.J.S. High brightness formamidinium lead bromide perovskite nanocrystal light emitting devices. Sci. Rep. 2016, 6, 36733. [Google Scholar] [CrossRef]
- Chernikov, A.; Berkelbach, T.C.; Hill, H.M.; Rigosi, A.; Li, Y.; Aslan, B.; Reichman, D.R.; Hybertsen, M.S.; Heinz, T.F. Exciton binding energy and nonhydrogenic Rydberg series in monolayer WS2. Phys. Rev. Lett. 2014, 113, 076802. [Google Scholar] [CrossRef]
- Kim, Y.; Liu, H.; Liu, Y.; Jin, B.; Zhang, H.; Tian, W.; Im, C. Long-lasting photoluminescence quantum yield of cesium lead halide perovskite-type quantum dots. Front. Chem. Sci. Eng. 2021, 15, 187–197. [Google Scholar] [CrossRef]
- Mahon, N.S.; Korolik, O.V.; Khenkin, M.V.; Arnaoutakis, G.E.; Galagan, Y.; Soriūtė, V.; Litvinas, D.; Ščajev, P.; Katz, E.A.; Mazanik, A.V. Photoluminescence kinetics for monitoring photoinduced processes in perovskite solar cells. Solar Energy 2020, 195, 114–120. [Google Scholar] [CrossRef]
- Frost, J.M.; Butler, K.T.; Brivio, F.; Hendon, C.H.; van Schilfgaarde, M.; Walsh, A. Atomistic Origins of High-Performance in Hybrid Halide Perovskite Solar Cells. Nano Lett. 2014, 14, 2584–2590. [Google Scholar] [CrossRef]
- Ham, S.; Chung, H.; Kim, T.-W.; Kim, J.; Kim, D. Composition-dependent emission linewidth broadening in lead bromide perovskite (APbBr3, A = Cs and CH3 NH3) nanoparticles. Nanoscale 2018, 10, 2207–2212. [Google Scholar] [CrossRef]
- Zhou, D.; Tao, L.; Yu, Z.; Jiao, J.; Xu, W. Efficient chromium ion passivated CsPbCl3:Mn perovskite quantum dots for photon energy conversion in perovskite solar cells. J. Mater. Chem. C 2020, 8, 12323–12329. [Google Scholar] [CrossRef]

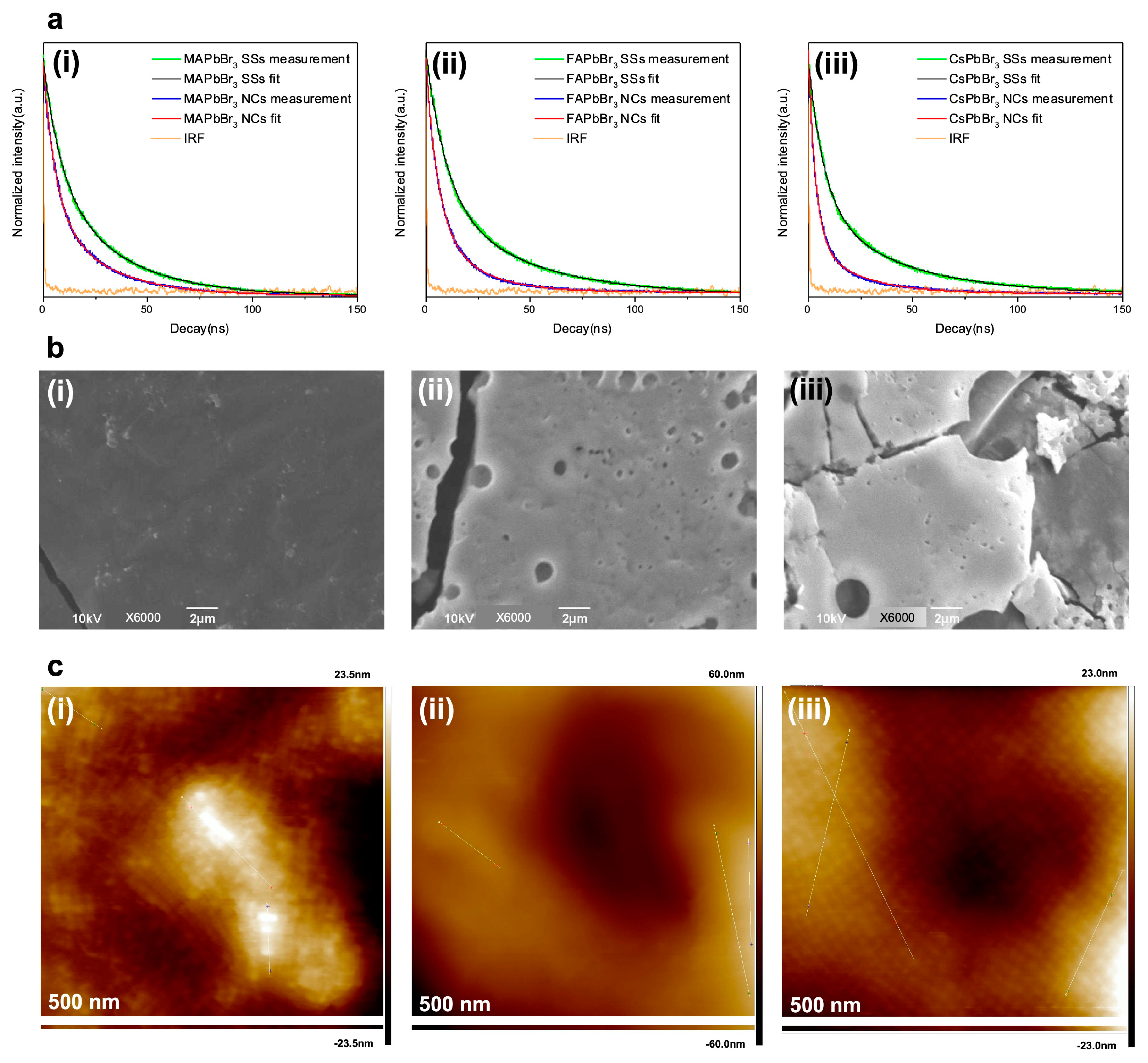
| Sample | SSs τaverage (ns) | NCs τaverage (ns) | Enhancement Factor |
|---|---|---|---|
| MAPbBr3 | 27.3 | 20.8 | 1.3 |
| FAPbBr3 | 34.4 | 14.9 | 2.3 |
| CsPbBr3 | 31.7 | 15.1 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, W.K.; Chen, J.; Zhou, D.; Ye, J.; Vázquez, R.J.; Zhou, C.; Bazan, G.C.; Rao, A.; Yu, Z.; Tan, T.T.Y. Hybrid Organic–Inorganic Perovskite Superstructures for Ultrapure Green Emissions. Nanomaterials 2023, 13, 815. https://doi.org/10.3390/nano13050815
Chan WK, Chen J, Zhou D, Ye J, Vázquez RJ, Zhou C, Bazan GC, Rao A, Yu Z, Tan TTY. Hybrid Organic–Inorganic Perovskite Superstructures for Ultrapure Green Emissions. Nanomaterials. 2023; 13(5):815. https://doi.org/10.3390/nano13050815
Chicago/Turabian StyleChan, Wen Kiat, Jiawei Chen, Donglei Zhou, Junzhi Ye, Ricardo Javier Vázquez, Cheng Zhou, Guillermo Carlos Bazan, Akshay Rao, Zhongzheng Yu, and Timothy Thatt Yang Tan. 2023. "Hybrid Organic–Inorganic Perovskite Superstructures for Ultrapure Green Emissions" Nanomaterials 13, no. 5: 815. https://doi.org/10.3390/nano13050815
APA StyleChan, W. K., Chen, J., Zhou, D., Ye, J., Vázquez, R. J., Zhou, C., Bazan, G. C., Rao, A., Yu, Z., & Tan, T. T. Y. (2023). Hybrid Organic–Inorganic Perovskite Superstructures for Ultrapure Green Emissions. Nanomaterials, 13(5), 815. https://doi.org/10.3390/nano13050815







