An Insight into Durability, Electrical Properties and Thermal Behavior of Cementitious Materials Engineered with Graphene Oxide: Does the Oxidation Degree Matter?
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Fabrication of GO-Engineered Nanocomposites
2.2. Formulation and Designation of GO-Engineered Nanocomposites
2.3. Electrochemical Characterization of GO-Engineered Nanocomposites
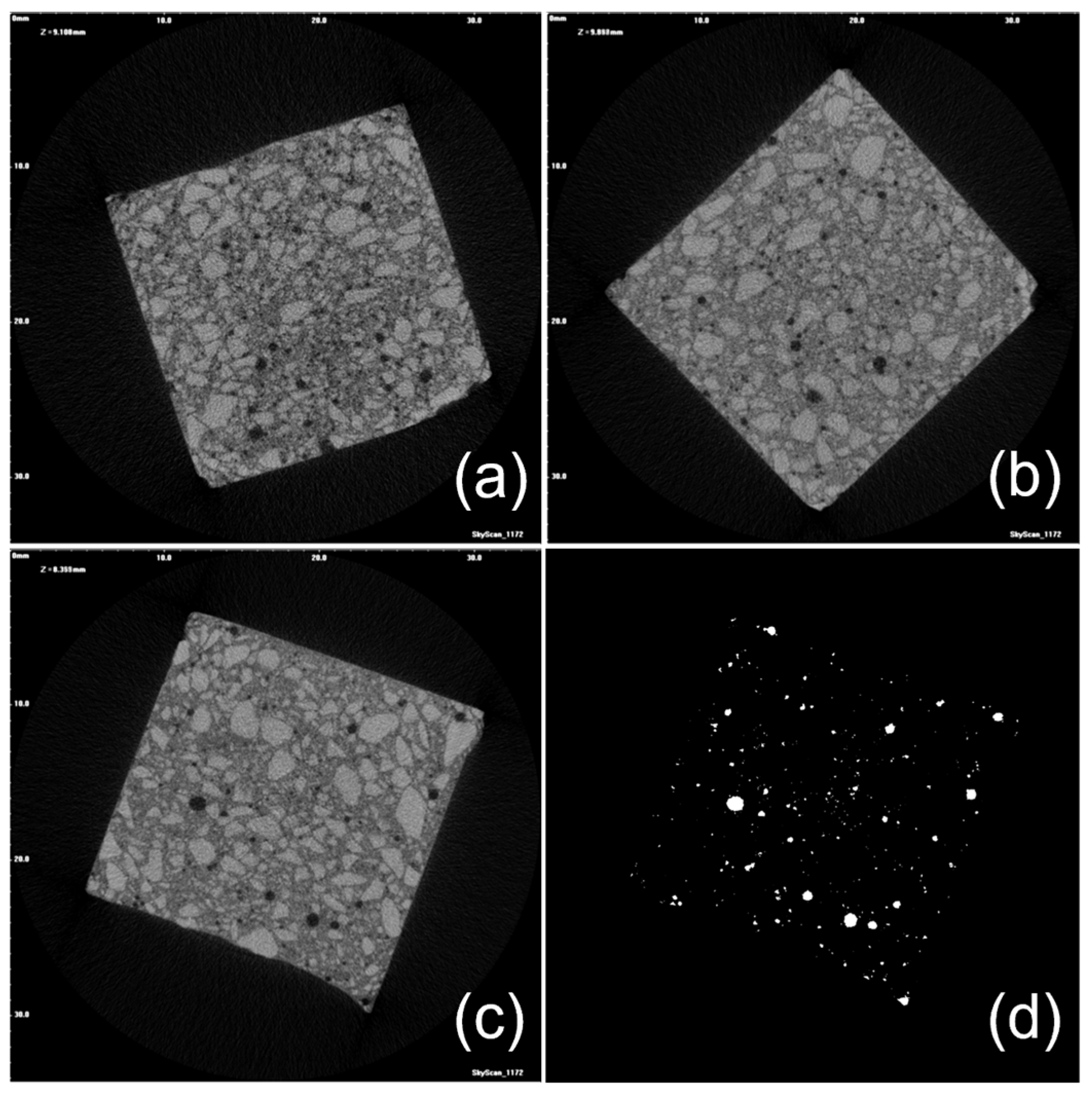
2.4. Micro-Computed Tomography (μ-CT) Analysis of GO-Engineered Nanocomposites
2.5. Permeability Tests of GO-Engineered Nanocomposites
2.6. Thermal Conductivity of GO-Engineered Nanocomposites
3. Results and Discussion
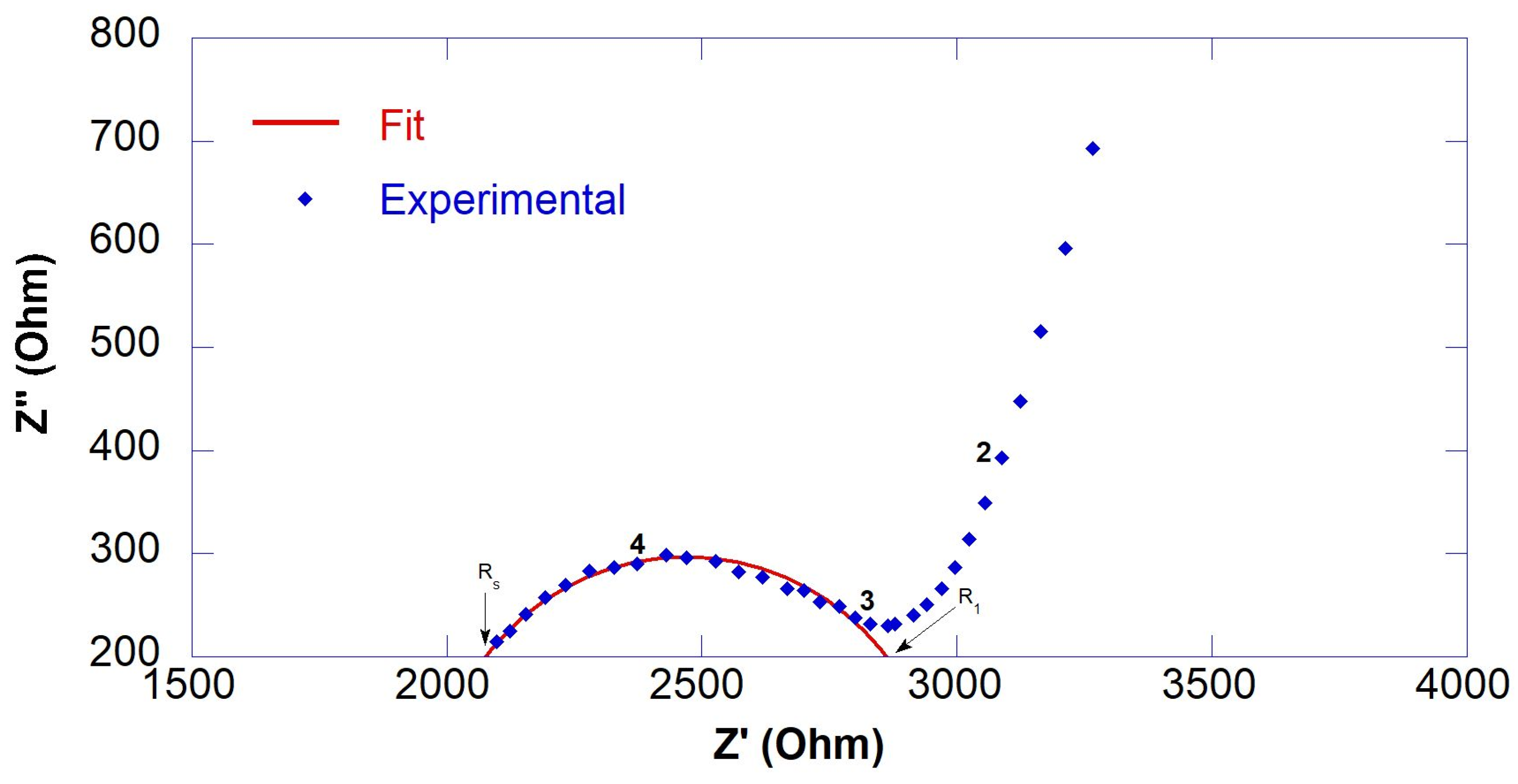
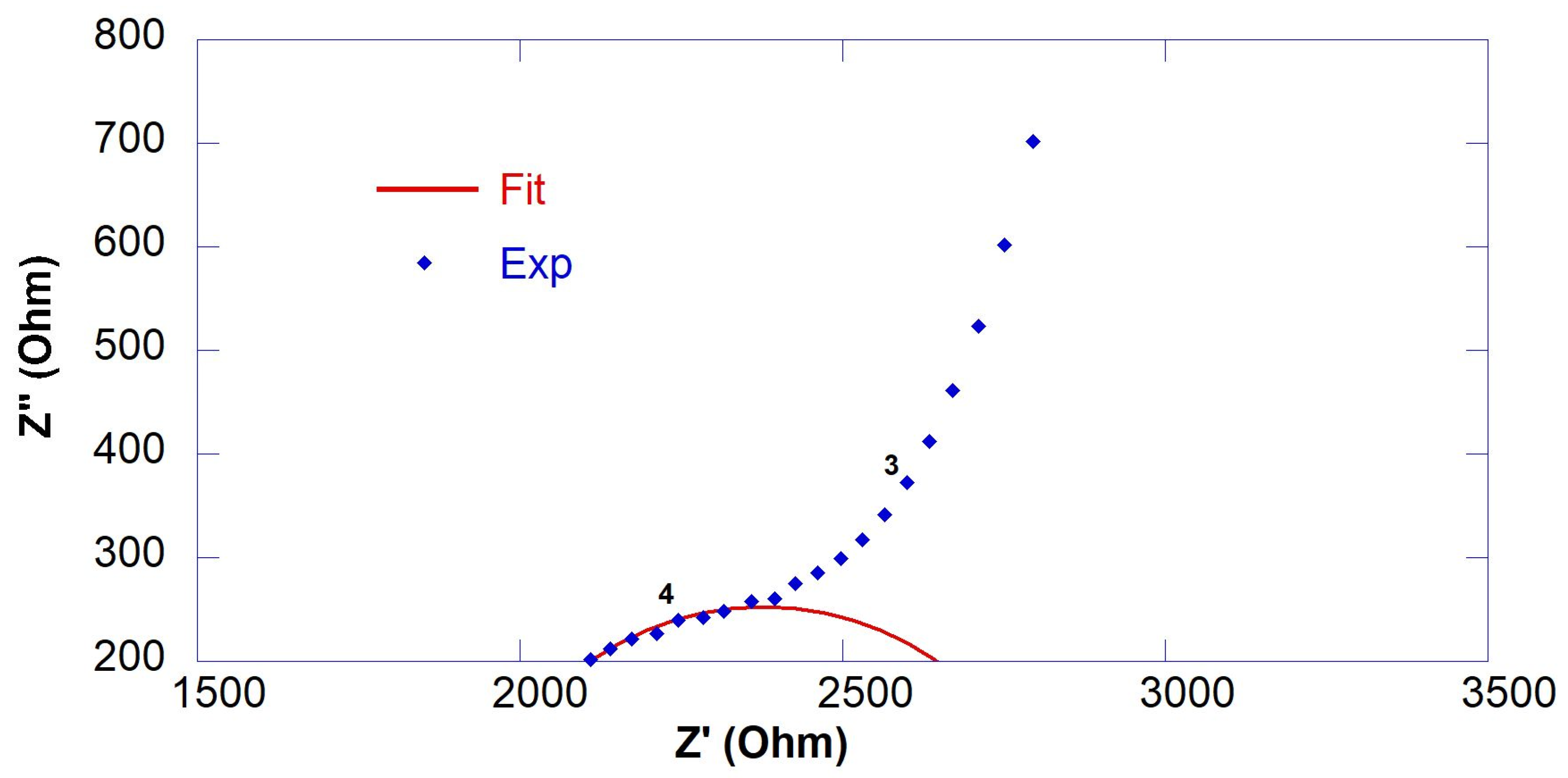
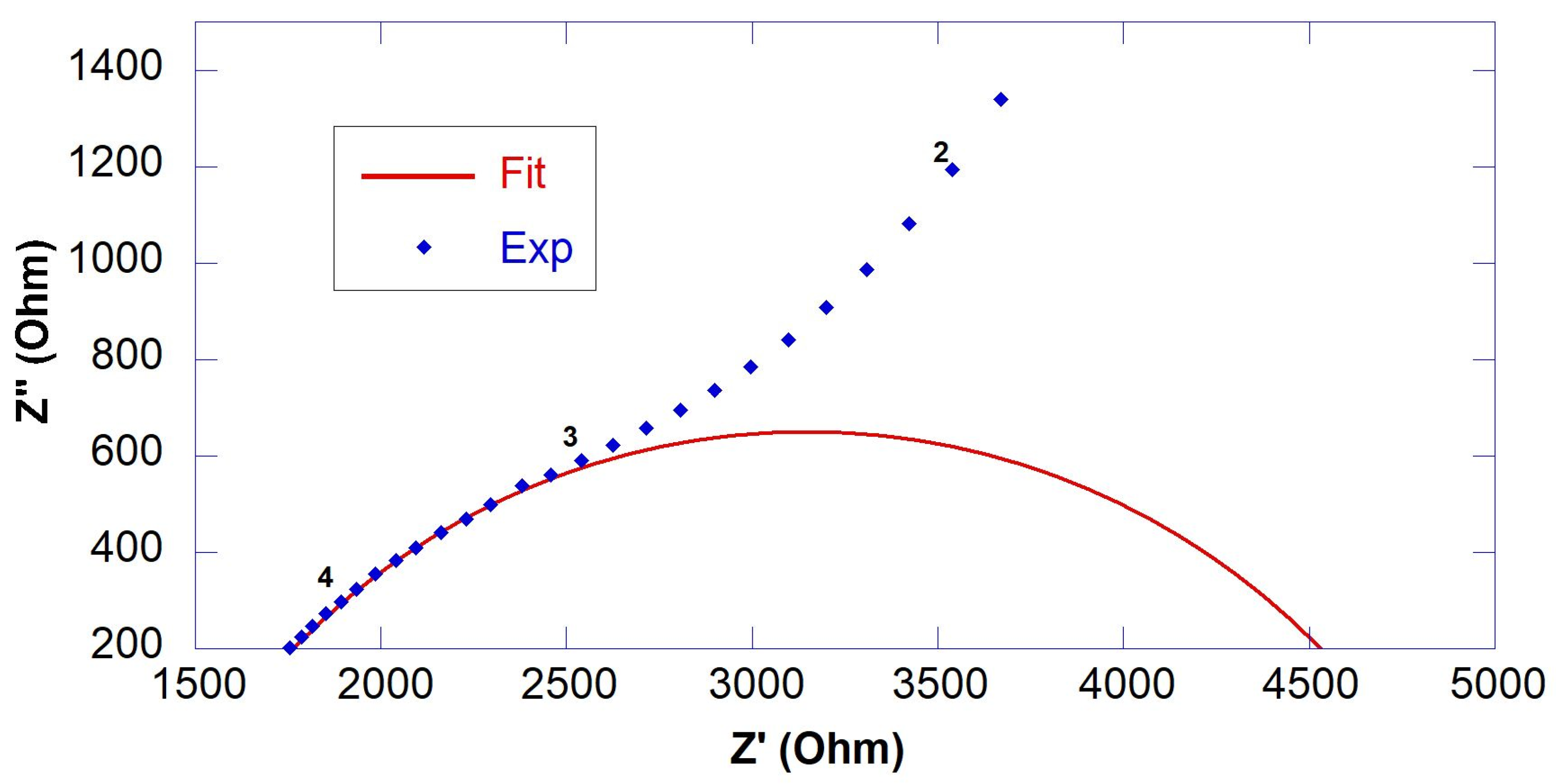
3.1. Resistivity of GO-Engineered Mortars
3.2. Permeability of GO-Engineered Mortars
3.3. Thermal Conductivity of GO-Engineered Mortars
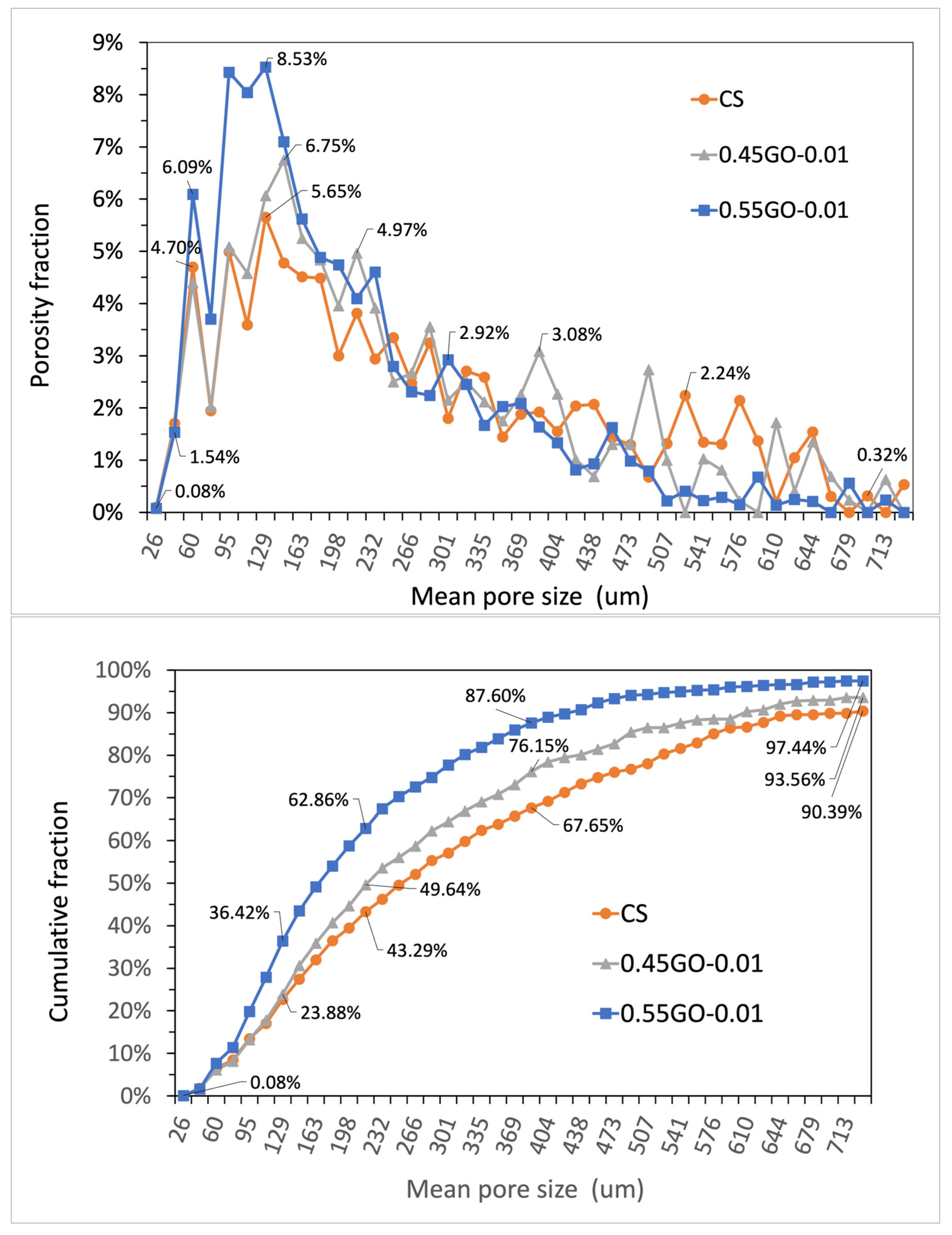
3.4. Micrometric Porosity of GO-Engineered Mortars
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chougan, M.; Ghaffar, S.H.; Nematollahi, B.; Sikora, P.; Dorn, T.; Stephan, D.; Albar, A.; Al-Kheetan, M.J. Effect of natural and calcined halloysite clay minerals as low-cost additives on the performance of 3D-printed alkali-activated materials. Mater. Des. 2022, 223, 111183. [Google Scholar] [CrossRef]
- García-Segura, T.; Yepes, V.; Alcalá, J. Life cycle greenhouse gas emissions of blended cement concrete including carbonation and durability. Int. J. Life Cycle Assess. 2014, 19, 3–12. [Google Scholar] [CrossRef]
- Hafez, H.; Kurda, R.; Al-Ayish, N.; García-Segura, T.; Cheung, W.M.; Nagaratnam, B. A whole life cycle performance-based ECOnomic and ECOlogical assessment framework (ECO2) for concrete sustainability. J. Clean. Prod. 2021, 292, 126060. [Google Scholar] [CrossRef]
- Said, S.H.; Razak, H.A.; Othman, I. Flexural behavior of engineered cementitious composite (ECC) slabs with polyvinyl alcohol fibers. Constr. Build. Mater. 2015, 75, 176–188. [Google Scholar] [CrossRef]
- Zhang, J.; Gong, C.; Guo, Z.; Zhang, M. Engineered cementitious composite with characteristic of low drying shrinkage. Cem. Concr. Res. 2009, 39, 303–312. [Google Scholar] [CrossRef]
- Mohammed, A.; Sanjayan, J.G.; Duan, W.H.; Nazari, A. Incorporating graphene oxide in cement composites: A study of transport properties. Constr. Build. Mater. 2015, 84, 341–347. [Google Scholar] [CrossRef]
- Pan, Z.; Wu, C.; Liu, J.; Wang, W.; Liu, J. Study on mechanical properties of cost-effective polyvinyl alcohol engineered cementitious composites (PVA-ECC). Constr. Build. Mater. 2015, 78, 397–404. [Google Scholar] [CrossRef]
- Suo, Y.; Guo, R.; Xia, H.; Yang, Y.; Zhou, B.; Zhao, Z. A review of graphene oxide/cement composites: Performance, functionality, mechanisms, and prospects. J. Build. Eng. 2022, 53, 104502. [Google Scholar] [CrossRef]
- Anwar, A.; Mohammed, B.S.; Wahab, M.A.; Liew, M.S. Enhanced properties of cementitious composite tailored with graphene oxide nanomaterial—A review. Dev. Built Environ. 2020, 1, 100002. [Google Scholar] [CrossRef]
- Jiang, W.; Li, X.; Lv, Y.; Zhou, M.; Liu, Z.; Ren, Z.; Yu, Z. Cement-Based Materials Containing Graphene Oxide and Polyvinyl Alcohol Fiber: Mechanical Properties, Durability, and Microstructure. Nanomaterials 2018, 8, 638. [Google Scholar] [CrossRef]
- Zheng, Q.; Han, B.; Cui, X.; Yu, X.; Ou, J. Graphene-engineered cementitious composites: Small makes a big impact. Nanomater. Nanotechnol. 2017, 7, 1–18. [Google Scholar] [CrossRef]
- Jing, G.; Ye, Z.; Wu, J.; Wang, S.; Cheng, X.; Strokova, V.; Nelyubova, V. Introducing reduced graphene oxide to enhance the thermal properties of cement composites. Cem. Concr. Compos. 2020, 109, 103559. [Google Scholar] [CrossRef]
- Chougan, M.; Lamastra, F.R.; Bolli, E.; Caschera, D.; Kaciulis, S.; Mazzuca, C.; Montesperelli, G.; Ghaffar, S.H.; Al-Kheetan, M.J.; Bianco, A. Extra-low dosage graphene oxide cementitious nanocomposites: A nano- to macroscale approach. Nanomaterials 2021, 11, 3278. [Google Scholar] [CrossRef] [PubMed]
- Chougan, M.; Lamastra, F.R.; Caschera, D.; Kaciulis, S.; Bolli, E.; Mazzuca, C.; Ghaffar, S.H.; Al-Kheetan, M.J.; Montesperelli, G.; Bianco, A. Cementitious nanocomposites engineered with high-oxidized graphene oxide: Spotting the nano to macro correlation. Ceram. Int. 2023, 49, 964–973. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Liu, Y.; Li, W.; Dong, B.; Duan, W.H. Dispersion of graphene oxide agglomerates in cement paste and its effects on electrical resistivity and flexural strength. Cem. Concr. Compos. 2018, 92, 145–154. [Google Scholar] [CrossRef]
- Lu, Z.; Hou, D.; Hanif, A.; Hao, W.; Li, Z.; Sun, G. Comparative evaluation on the dispersion and stability of graphene oxide in water and cement pore solution by incorporating silica fume. Cem. Concr. Compos. 2018, 94, 33–42. [Google Scholar] [CrossRef]
- Han, S.; Hossain, M.S.; Ha, T.; Yun, K.K. Graphene-oxide-reinforced cement composites mechanical and microstructural characteristics at elevated temperatures. Nanotechnol. Rev. 2022, 11, 3174–3194. [Google Scholar] [CrossRef]
- Liu, B.; Wang, L.; Pan, G.; Li, D. Dispersion of graphene oxide modified polycarboxylate superplasticizer in cement alkali solution for improving cement composites. J. Build. Eng. 2022, 57, 104860. [Google Scholar] [CrossRef]
- Indukuri, C.S.R.; Nerella, R. Enhanced transport properties of graphene oxide based cement composite material. J. Build. Eng. 2021, 37, 102174. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Rafiee, J.; Wang, Z.; Song, H.; Yu, Z.Z.; Koratkar, N. Enhanced Mechanical Properties of Nanocomposites at Low Graphene Content. ACS Nano 2009, 3, 3884–3890. [Google Scholar] [CrossRef]
- Lamastra, F.R.; Chougan, M.; Marotta, E.; Ciattini, S.; Ghaffar, S.H.; Caporali, S.; Vivio, F.; Montesperelli, G.; Ianniruberto, U.; Al-Kheetan, M.J.; et al. Toward a better understanding of multifunctional cement-based materials: The impact of graphite nanoplatelets (GNPs). Ceram. Int. 2021, 47, 20019–20031. [Google Scholar] [CrossRef]
- Cabeza, M.; Keddam, M.; Nóvoa, X.R.; Sánchez, I.; Takenouti, H. Impedance spectroscopy to characterize the pore structure during the hardening process of Portland cement paste. Electrochim. Acta 2006, 51, 1831–1841. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Mansfeld, F. Concerning the Conversion of the Constant Phase Element Parameter Y0 into a Capacitance. Corrosion 2001, 57, 747–748. [Google Scholar] [CrossRef]
- Lopez-Heredia, M.A.; Sariibrahimoglu, K.; Yang, W.; Bohner, M.; Yamashita, D.; Kunstar, A.; van Apeldoorn, A.A.; Bronkhorst, E.M.; Félix Lanao, R.P.; Sander, C.G.; et al. Influence of the pore generator on the evolution of the mechanical properties and the porosity and interconnectivity of a calcium phosphate cement. Acta Biomater. 2012, 8, 404–414. [Google Scholar] [CrossRef]
- Chougan, M.; Marotta, E.; Lamastra, F.R.; Vivio, F.; Montesperelli, G.; Ianniruberto, U.; Ghaffar, S.H.; Al-Kheetan, M.J.; Bianco, A. High performance cementitious nanocomposites: The effectiveness of nano-Graphite (nG). Constr. Build. Mater. 2020, 259, 119687. [Google Scholar] [CrossRef]
- ASTM C 642-06; Standard Test Method for Density, Absorption, and Voids in Hardened Concrete. Annual Book of ASTM Standards. American Society for Testing of Materials: Philadelphia, PA, USA, 2013; Volume 04.02.
- Chiang, C.T.; Yang, C.C. Relation between the diffusion characteristic of concrete from salt ponding test and accelerated chloride migration test. Mater. Chem. Phys. 2007, 106, 240–246. [Google Scholar] [CrossRef]
- Rahman, M.M.; Chamberlain, D.A.; Balakhrishna, M.N.; Kipling, J. Performance of pore-lining impregnants in concrete protection by unidirectional salt-ponding test, (n.d.). Transp. Res. Rec. 2013, 2342, 17–25. [Google Scholar] [CrossRef]
- Wang, R.; He, F.; Shi, C.; Zhang, D.; Chen, C.; Dai, L. AC impedance spectroscopy of cement-based materials: Measurement and interpretation. Cem. Concr. Compos. 2022, 131, 104591. [Google Scholar] [CrossRef]
- Song, G. Equivalent circuit model for AC electrochemical impedance spectroscopy of concrete. Cem. Concr. Res. 2000, 30, 1723–1730. [Google Scholar] [CrossRef]
- Hu, X.; Shi, C.; Liu, X.; Zhang, J.; de Schutter, G. A review on microstructural characterization of cement-based materials by AC impedance spectroscopy. Cem. Concr. Compos. 2019, 100, 1–14. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Materazzi, A.L.; Ubertini, F. Nanotechnology in Cement-Based Construction; CRC Press, Jenny Standford Publishing Pte. Ltd.: Singapore, 2020. [Google Scholar]
- Garboczi, E.J.; Snyder, K.A.; Douglas, J.F. Geometrical percolation threshold of overlapping ellipsoids. Phys. Rev. E 1995, 52, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, G.; Ulm, F.-J. The effect of two types of C-S-H on the elasticity of cement-based materials: Results from nanoindentation and micromechanical modeling. Cem. Concr. Res. 2004, 34, 67–80. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Yao, H.; Farhan, S.; Zheng, S.; Du, C. Study on the three dimensional mechanism of graphene oxide nanosheets modified cement. Constr. Build. Mater. 2016, 126, 730–739. [Google Scholar] [CrossRef]
- Dimov, D.; Amit, I.; Gorrie, O.; Barnes, M.D.; Townsend, N.J.; Neves, A.I.S.; Withers, F.; Russo, S.; Craciun, M.F. Ultrahigh Performance Nanoengineered Graphene–Concrete Composites for Multifunctional Applications. Adv. Funct. Mater. 2018, 28, 1705183. [Google Scholar] [CrossRef]
- Mohammed, B.S.; Baharun, M.H.; Nuruddin, M.F.; Erikole, O.P.D.; Murshed, N.A. Mechanical properties of engineered cementitious composites mixture. Appl. Mech. Mater. 2014, 567, 428–433. [Google Scholar] [CrossRef]
- Du, H.; Gao, H.J.; Pang, S.D. Improvement in concrete resistance against water and chloride ingress by adding graphene nanoplatelet. Cem. Concr. Res. 2016, 83, 114–123. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ansari, R.; Rouhi, S. Investigating the thermal conductivity of concrete/graphene nanocomposite by a multi-scale modeling approach. Int. J. Mod. Phys. B 2018, 32, 1850171. [Google Scholar] [CrossRef]
- Ghosh, S.; Bao, W.; Nika, D.L.; Subrina, S.; Pokatilov, E.P.; Lau, C.N.; Balandin, A.A. Dimensional crossover of thermal transport in few-layer graphene. Nat. Mater. 2010, 9, 555–558. [Google Scholar] [CrossRef]
- Chen, J.; Li, L. Effect of oxidation degree on the thermal properties of graphene oxide. J. Mater. Res. Technol. 2020, 9, 13740–13748. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, P.; Zhang, Z.; Pan, Y. Influencing factors of parameters of air-void structure in fresh cement mortars. Int. J. Pavement Res. Technol. 2020, 13, 417–422. [Google Scholar] [CrossRef]






| Nanofiller | Oxidation Degree (O/C) | Designation | Width (W) | Length (L) | Thickness (t) | Aspect Ratio (Average Lateral Size/t) | Interlayer Spacing (t′) |
|---|---|---|---|---|---|---|---|
| GO (Aldrich) | 0.45 | 0.45GO | 600 nm | 800 nm | 2 nm | 350 | 0.7835 nm |
| GONan (Nanesa) | 0.55 | 0.55GO | 600 nm | 800 nm | 2 nm | 350 | 0.7795 nm |
| Sample | Graphene Oxide (GO) | Weight Percentage (by Weight of Cement) | Weight Fraction Percentage (Wp) 10−3 | Volume Fraction Percentage @28 Days (ϕv) 10−3 | a Density @28 Days g/cm3 |
|---|---|---|---|---|---|
| Control Sample (CS) | - | - | - | - | 1.945 ± 0.063 |
| 0.45GO-0.01 | nanopowder | 0.01% | 2.29% | 4.56% b | 2.210 ± 0.038 |
| (Aldrich) | 5.40% c | ||||
| 0.55GO-0.01 | suspension | 0.01% | 2.29% | 4.54% b | 2.178 ± 0.043 |
| (Nanesa) | 5.37% c | ||||
| 0.55GO-0.1 | suspension | 0.1% | 22.90% | 45.40% b | 2.176 ± 0.029 |
| (Nanesa) | 53.7% c | ||||
| 0.55GO-0.2 | suspension | 0.2% | 45.74% | 90.68% b | 2.155 ± 0.310 |
| (Nanesa) | 107.5% c |
| Sample | RS (Ohm) | R1 (Ohm) | Rccp (Ohm) | ρ (Ohm∙m) |
|---|---|---|---|---|
| CS@7days CS@14days CS@28days | - - 1836 ± 47 | - - 2874 ± 200 | * 1485 * 2104 4710 | 14.9 21.0 47.1 |
| 0.45GO-0.01@7days 0.45GO-0.01@14days 0.45GO-0.01@28days | - 1632 ± 54 1680 ± 16 | - 993 ± 178 1846 ± 104 | * 1624 2625 3526 | 16.2 26.2 35.3 |
| 0.55GO-0.01@7days 0.55GO-0.01@14days 0.55GO-0.01@28days | - - 1811 ± 65 | - - 1651 ± 113 | * 1541 * 3760 3462 | 15.4 37.6 34.6 |
| 0.55GO-0.1@7days 0.55GO-0.1@14days 0.55GO-0.1@28days | - 1647 ± 44 1743 ± 11 | - 2366 ± 657 1691 ± 142 | * 1657 4013 3434 | 16.6 40.1 34.3 |
| 0.55GO-0.2@7days 0.55GO-0.2@14days 0.55GO-0.2@28days | 1275 ± 62 1584 ± 28 1545 ± 27 | 1210 ± 94 3734 ± 258 3122 ± 225 | 2485 5318 4667 | 24.8 53.2 46.7 |
| Sample Density (g/cm3) a | Volume of Interest (VOI) (mm3) | Porosity (%) | Total Volume of Micrometric Pores (mm3) | Number of Micrometric Pores * Pore Density (pores/mm3) | Mean micrometric Pore Size (Numeral) * St. Dev. (μm) | Mean Micrometric Pore Size (Volumetric) * St. Dev. (μm) |
|---|---|---|---|---|---|---|
| CS (1.945 ± 0.063) | 6482 | 1.97 | 127 | 59265 * 9.1 | 57.3 * 45.9 | 337.5 * 249.2 |
| 0.45GO-0.01 (2.210 ± 0.038) | 6864 | 2.11 | 145 | 65835 * 9.6 | 56.5 * 42.7 | 300.9 * 237.9 |
| 0.55GO-0.01 (2.178 ± 0.043) | 6553 | 3.20 | 209 | 95332 * 14.5 | 60.2 * 38.7 | 233.8 * 252.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamastra, F.R.; Montesperelli, G.; Galvanetto, E.; Chougan, M.; Ghaffar, S.H.; Al-Kheetan, M.J.; Bianco, A. An Insight into Durability, Electrical Properties and Thermal Behavior of Cementitious Materials Engineered with Graphene Oxide: Does the Oxidation Degree Matter? Nanomaterials 2023, 13, 726. https://doi.org/10.3390/nano13040726
Lamastra FR, Montesperelli G, Galvanetto E, Chougan M, Ghaffar SH, Al-Kheetan MJ, Bianco A. An Insight into Durability, Electrical Properties and Thermal Behavior of Cementitious Materials Engineered with Graphene Oxide: Does the Oxidation Degree Matter? Nanomaterials. 2023; 13(4):726. https://doi.org/10.3390/nano13040726
Chicago/Turabian StyleLamastra, Francesca Romana, Giampiero Montesperelli, Emanuele Galvanetto, Mehdi Chougan, Seyed Hamidreza Ghaffar, Mazen J. Al-Kheetan, and Alessandra Bianco. 2023. "An Insight into Durability, Electrical Properties and Thermal Behavior of Cementitious Materials Engineered with Graphene Oxide: Does the Oxidation Degree Matter?" Nanomaterials 13, no. 4: 726. https://doi.org/10.3390/nano13040726
APA StyleLamastra, F. R., Montesperelli, G., Galvanetto, E., Chougan, M., Ghaffar, S. H., Al-Kheetan, M. J., & Bianco, A. (2023). An Insight into Durability, Electrical Properties and Thermal Behavior of Cementitious Materials Engineered with Graphene Oxide: Does the Oxidation Degree Matter? Nanomaterials, 13(4), 726. https://doi.org/10.3390/nano13040726










