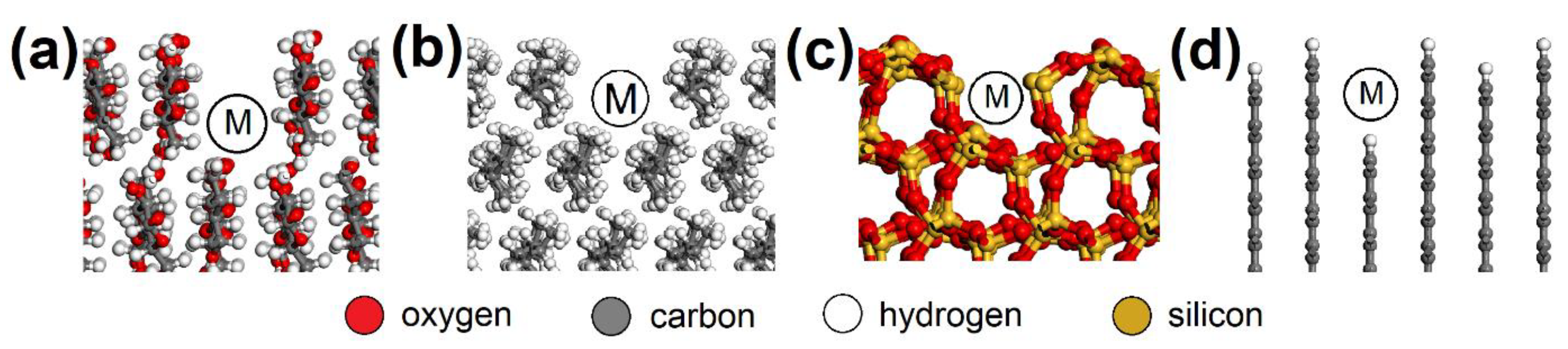Abstract
Dangerous gases arising from combustion processes must be removed from the air simply and cheaply, e.g., by adsorption. This work is focused on competitive adsorption experiments and force field-based molecular modeling of the interactions at the molecular level. Emission gas, containing CO, NO, SO2, and CO2, was adsorbed on activated carbon, clay mineral, silicon dioxide, cellulose, or polypropylene at two different temperatures. At 20 °C, activated carbon had the highest NO and SO2 adsorption capacity (120.83 and 3549.61 μg/g, respectively). At 110 °C, the highest NO and SO2 adsorption capacity (6.20 and 1182.46 μg/g, respectively) was observed for clay. CO was adsorbed very weakly, CO2 not at all. SO2 was adsorbed better than NO, which correlated with modeling results showing positive influence of carboxyl and hydroxyl functional groups on the adsorption. In addition to the wide range of adsorbents, the main novelty of this study is the modeling strategy enabling the simulation of surfaces with pores of controllable sizes and shapes, and the agreement of the results achieved by this strategy with the results obtained by more computationally demanding methods. Moreover, the agreement with experimental data shows the modeling strategy to be a valuable tool for further adsorption studies.
1. Introduction
In recent years, more than ever before, the importance of monitoring the evolution of the amount of pollutants in the environment has increased [1]. People and nature in general need optimal conditions for their efficient functioning, which is related to the reduction of destructive factors and the increase of constructive factors in the environment. Manufacturing industry is accompanied by the disruption of natural resources during the acquisition of input materials, a number of chemical reactions or energy processes during production, and last but not least, the disposal of waste, i.e., unused or unusable products. The main sources of pollution include the manufacturing and processing industry [2] as well as the energy industry [3] and transportation [4], with which the increasing production of specifically air pollutants is connected.
Despite the fact that thermochemical conversion is beginning to be replaced by the use of renewable resources worldwide, the combustion process of various materials (coal, gas, heating oils, wood, biomass, etc.) still contributes significantly to air pollution [5,6]. Even with the effective use of plant waste [7,8], it is still challenging to monitor and reduce the risks related to the production of arising pollutants. While solid pollutants consist primarily of particulate matter of various grain sizes and NH3, gaseous pollutants mainly include CO2, CO, SO2, nitrogen oxides (NOx) [9,10], volatile organic compounds and polycyclic aromatic hydrocarbons [6,10]. Solid particles released during the combustion process can be easily captured by various types of solid or liquid filter systems. However, the removal of emission gases from the air is generally more complex, due to the gaseous state of the pollutants, the required chemical selectivity, etc. The most studied is CO2 removal from the air [11]. Absorption of CO2 in gas-liquid systems with methanol, poly-(ethylene glycol) dimethyl ether [12] or amine solutions [13] as absorbing phases was investigated. However, adsorption methods in gas-solid systems, i.e., weaker physisorption or stronger chemisorption [11,14,15], are used more often. The most effective adsorption materials for CO2 include activated carbons [16,17], zeolites [18], porous oxides (e.g., calcium oxides [19] or silica gel [20]), hydrotalcites [21], amines [22] or metal-organic frameworks [11,23,24].
On the other hand, the adsorption mechanisms and suitable adsorbents of NOx, CO, and SO2 have been considerably less investigated. Research on CO and NO adsorption using Pd catalysts [25], possibly Au alloys [26] or various modifications of activated carbon [27] should be mentioned. In the case of SO2 and NO, adsorption mechanisms on modified activated carbon [27,28] and bimetallic surfaces containing noble and transition metals [29] were investigated. Zeolites are also efficient adsorbents of CO2, SO2, and NO [30].
Research works mostly deal with specific adsorption materials for a specific gas, but materials in various forms sorbing several types of emission gases at the same time by the mechanism of competitive adsorption have not yet been sufficiently investigated. There is also a lack of research on interactions at the molecular level during the competitive adsorption of emission gases. Therefore, in this work, the process of competitive adsorption of emission gases on different materials with different physical and chemical properties was studied both experimentally and via force field-based molecular modeling. Activated carbon is extensively researched and modeled in connection with adsorption [31,32,33,34]. Therefore, it was used for comparing the obtained data and verifying the modeling strategy. Adsorbent materials whose potential in the field of competitive adsorption of emission gases has not yet been established were used, and adsorption capacities were determined. Unlike DTF modeling methods that are usually used, the modeling strategy introduced here enabled the creation of larger models with pores of precisely controllable sizes and shapes. The influence of chemical and structural factors on competitive adsorption was clarified, which can be used further in research on other materials or as a prediction tool of adsorption.
2. Materials and Methods
2.1. Materials
Adsorption experiments were carried out using a special emission gas (EG) having concentrations of emission components similar to the real concentrations produced during the combustion of various alternative fuels, such as biomass, sewage sludge, etc. [35]. The EG consisted of 197.3 vol. ppm NO (199.9 w/w ppm), 197.3 vol. ppm SO2 (426.8 w/w ppm), 395 vol. ppm CO (373.6 w/w ppm), and 9.96 vol. % CO2 (14.80 w/w %) in nitrogen (SIAD Czech s.r.o., Ostrava, Czech Republic). In addition, the nitrogen was also used to purge the adsorption path.
The following materials commonly used in hazardous chemical spill incidents were used as adsorbents in this study: activated carbon (AC; MA C6 D40 CZ, Resorbent, s.r.o., Ostrava, Czech Republic), clay (CL; Bentonite Puranit, LIPERA s.r.o., Velke Bilovice, Czech Republic), and (from REO AMOS s.r.o., Ostrava, Czech Republic) silicon dioxide (SiO2), cellulose (CEL) and polypropylene (PP).
2.2. Characterization of Samples
A photoelectron spectrometer with a hemispherical VG SCIENTA R3000 analyzer (Prevac, Rogów, Poland) was used for XPS analysis of AC. The photoelectron spectra were recorded using a monochromatized aluminum AlKα source (E = 1486.6 eV) and a low-energy electron flood gun (FS40A-PS). The base pressure in the analysis chamber was 5·10−9 mbar. Spectra were recorded with a constant pass energy of 100 eV for the survey and high-resolution spectra. The binding energy scale was calibrated using the Au 4f7/2 line of a cleaned gold sample at 84.0 eV. The composition and chemical surrounding of the sample surface were investigated on the basis of the areas and binding energies of O 1s and C 1s photoelectron peaks. The fitting of high-resolution spectra was performed using CasaXPS software.
The moisture content of the samples was determined by Kern ABT 320-4NM (Kern & Sohn GmbH, Balingen, Germany). Particle density was determined by measuring the volume of the sample in water and the weight with MA 110.R laboratory scale (RADWAG Váhy s.r.o., Šumperk, Czech Republic). Atmospheric pressure and pressure in the adsorption system were measured with GPB 3300 (GHM-Greisinger s.r.o., Regenstauf, Germany) and Kimo MP 210 (Kimo Instruments, Mumbai, India), respectively. The concentration of NO, SO2 and CO was measured by the infrared spectrometer PG-350 (Horiba, Palaiseau, France). Nitrogen physisorption at 77 K was measured using 3Flex apparatus (Micromeritics, Norcross, CA, USA). Prior to physisorption analysis, each material was degassed under pressure of 0.6 bar and temperature of 350 °C. This step was done for all materials to release physisorbed moisture. After such pre-treatment, the nitrogen adsorption–desorption isotherms at 77 K of all materials were measured for the relative pressure range p/p0~1 × 10−7–0.99. Nitrogen adsorption–desorption data were processed using BET theory, the t-plot method and the BJH method applied on the adsorption branch of the nitrogen adsorption–desorption isotherm, by using either the Carbon Black STSA standard isotherm (for carbonaceous materials) or Broekhoff-De Boer standard isotherm (for inorganic materials). The slit or cylindrical-pore geometry of mesopores and macropores characterized by pore width or diameter, respectively, were assumed. From measured data, it was possible to reliably obtain the specific surface area, SBET (m2/g), the mesopore–macropore surface area, Smeso (m2/g), the micropore volume, Vmicro (mmliq3/g) and mesopore–macropore size distribution characterized by the pore width or diameter. The net pore volume, Vnet (mm3liq/g), was evaluated from the adsorbed amount of nitrogen at p/p0~0.99. The micropore size distribution was evaluated from the adsorbed amount of nitrogen at p/p0~1 × 10−7–0.05 using the Horwath–Kawazoe solution and assuming the slit-pore geometry for both carbonaceous and inorganic materials.
X-ray powder diffraction (XRPD) patterns were recorded under CoKα irradiation (λ = 0.1789 nm, U = 35 kV, I = 25 mA) using the Bruker D8 Advance diffractometer (Bruker AXS, Karlsruhe, Germany) equipped with a fast position sensitive detector VÅNTEC 1. Measurements were carried out in the reflection mode, powder samples were pressed in a rotational holder, and a goniometer was used with the Bragg–Brentano geometry in 2θ range from 3 to 80°, step size 0.03°. The phase composition was evaluated using database PDF 2 Release 2020 (International Centre for Diffraction Data).
2.3. Sample Preparation
The powder samples (AC, CL, SiO2) were crushed and sieved into three granulometric fractions—0.16–0.315 mm, 0.315–0.63 mm, 0.63–1.00 mm (Table 1)—to ensure laminar flow in the adsorption chamber (Ad) whose inner tube diameter was 10 mm. CEL and PP were used in their original form, i.e., pellets and nonwoven, respectively. Each type of sample was used in its wet and dried state. The samples were dried in a drying oven Binder FP 400.

Table 1.
Basic information about the adsorbing materials used, such as particle density and material humidity, and its forms and labeling.
Particle density and humidity of the adsorbents were determined, and the samples were labeled according to the granulometric fraction (Table 1).
2.4. Adsorption System
The adsorption experiments took place in a closed system consisting of PTFE hoses, stainless steel fittings, the stainless steel Ad and analytical instruments (Figure 1). The Ad was a stainless steel tube with an inner diameter of 10 mm. A volume of 3 cm3 was calculated from the volume of the catalyst Vcat according to the formula:
where QV is volumetric gas flow rate per hour (0.5 L/min = 30,000 cm3/h) and GHSV is gas hourly space velocity (10,000 h−1) [36].
Vcat = QV/GHSV
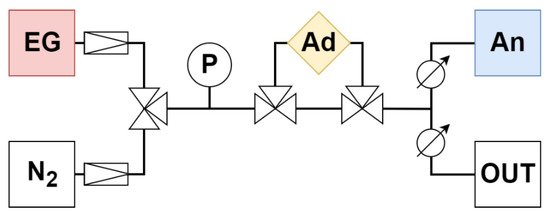
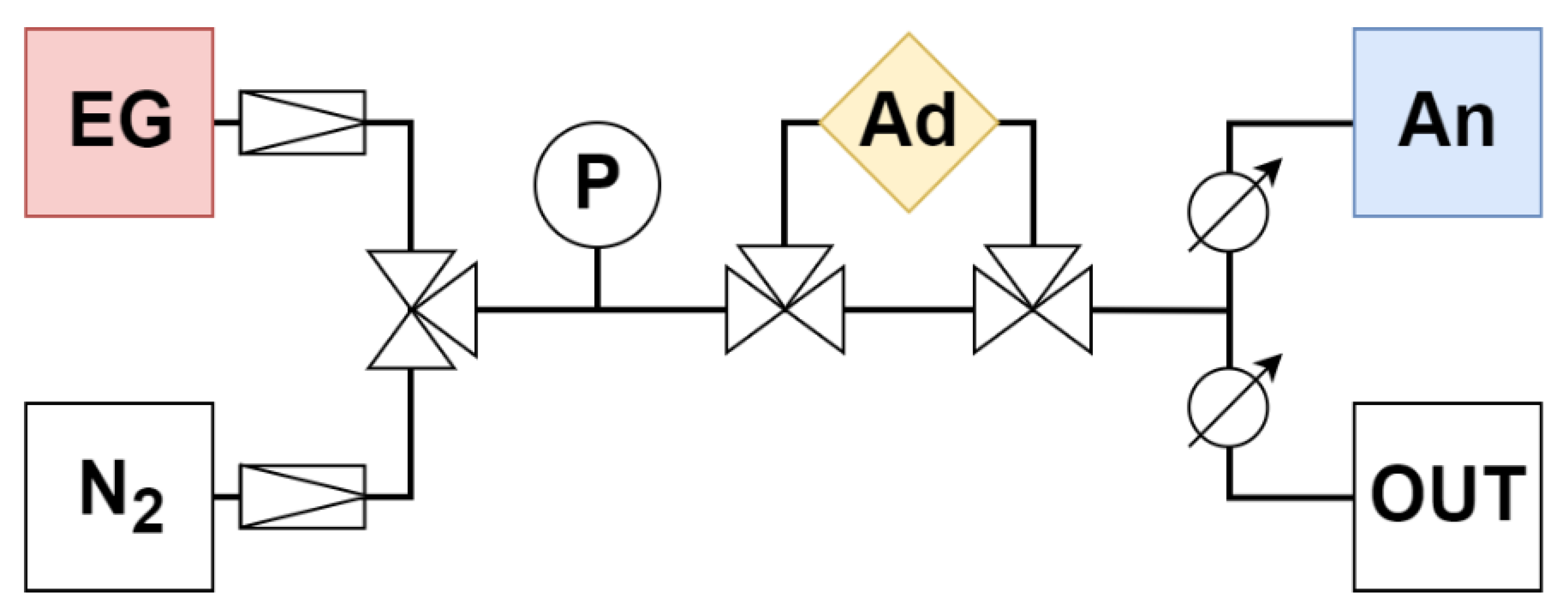
Figure 1.
Diagram of adsorption apparatus with fittings. EG—emission gas, N2—nitrogen, P—pressure gauge, Ad—adsorption chamber, An—analyzer, OUT—overflow branch.
The gas traveled from the gas cylinders (EG—emission gas, N2—nitrogen) through the pressure gauge (P) and the adsorbent (Ad) to the analyzer (An) at a speed of 0.5 L/min (analyzer parameter). The rest was discharged through the overflow branch (OUT) (Figure 1).
First, the entire adsorption apparatus was filled with nitrogen (including the Ad branch). Subsequently, the source gas was switched from N2 to EG through the branch without Ad. The measurement of the concentration of emission components with the analyzer was switched on. As soon as the concentration values stabilized, thereby determining the actual concentrations of gaseous components in EG, the gas path was switched to the Ad branch, where adsorption took place. The measurement was terminated after the initial concentration was again reached (the actual concentration in the EG bottle). The measurement took place at 20 °C (with wet adsorbents) and 110 °C (with dried adsorbents and Ad in the drying oven Binder FP 400 (Binder GMBh, Tuttlingen, Germany). Each sample was measured three times and the results were averaged.
The total amount of each adsorbed gaseous component was obtained by integrating the area between the adsorption curve and the original concentration value of the given emission component, and using the ideal gas equation (where the amount of substance n is expressed as m/M) for expressed mass m of the adsorbed gas component:
where p (Pa) is pressure in the adsorption system, V (dm3) is volume of the adsorbent, M (g·mol−1) is molar mass of the gaseous component, R (J·K−1·mol−1) is the gas constant, and T (K) is temperature in the adsorption system [37].
m = p·V·M/(R·T)
Adsorbed mass m was related to one gram of wet or dried adsorbent expressed in μg/g.
2.5. Atomistic Models and Modeling Strategy
Building of the initial models of EG molecule and adsorbent surface, geometry optimizations, molecular dynamics, and energy calculations were carried out in Biovia Materials Studio 7.0 (MS) modeling environment [38]. Triclinic unit cells of CEL (a = 8.549 Å, b = 9.568 Å, c = 10.000 Å, α = 102.10°, β = 106.54°, γ = 55.46°), and PP (a = 8.348 Å, b = 13.505 Å, c = 10.000 Å, α = 75.68°, β = 39.54°, γ = 61.01°), both containing two polymer chains, were created according to Chen et al. [39] and Verenich et al. [40], respectively (Figure 2).
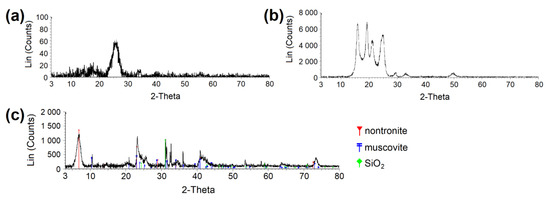
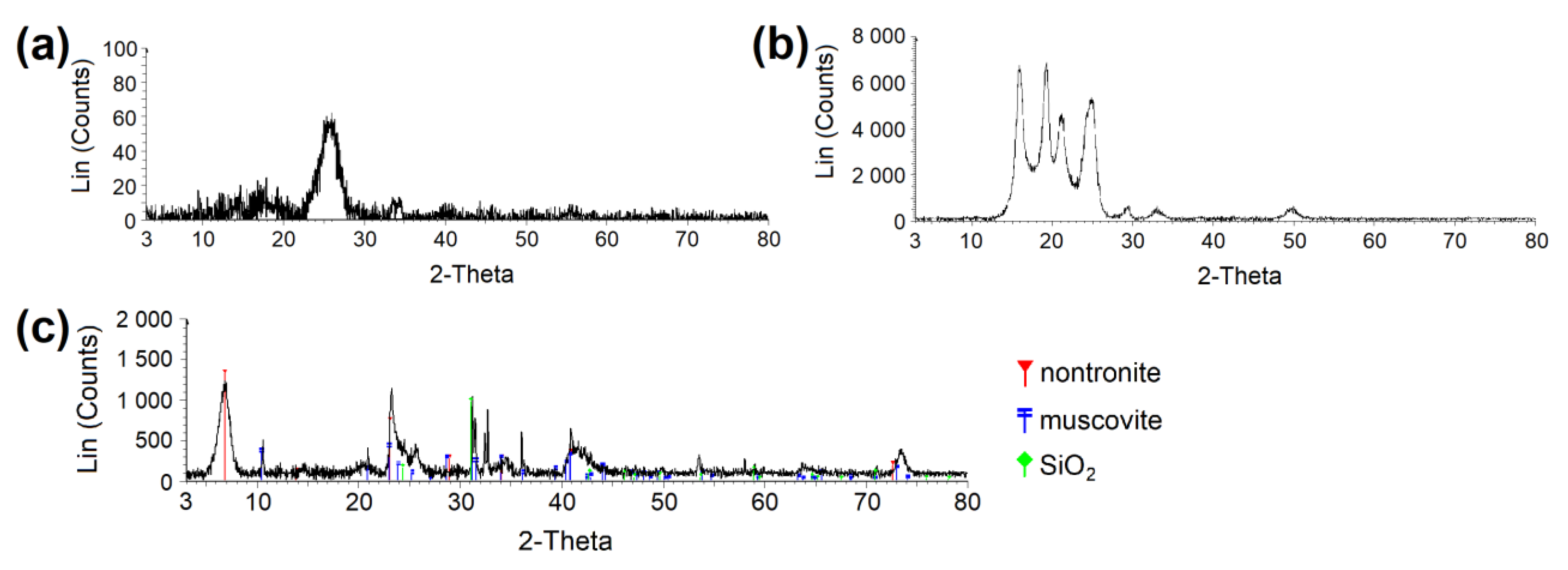
Figure 2.
XRPD diffractogram proving the crystalline phase in (a) CEL and (b) PP. (c) Determination of clay phases in clay material. CEL—cellulose, PP—polypropylene.
AC orthorhombic unit cell (a = b = 2.460 Å, c = 6.670 Å, α = β = 90°, γ = 120°) was prepared according to Wyckoff [41]. SiO2 trigonal (a = b = 4.913 Å, α = β = 90°, γ = 95.18°) and MUS monoclinic unit cells (a = 5.194 Å, b = 8.996 Å, c = 20.096 Å, α = β = 90°, γ = 120°, layer thickness ~6.7 Å, interlayer distance ~3.3 Å) were imported from the MS database. Substitutions and interlayer cations were added to MUS so that MUS and NON unit cells met the chemical formulas and , respectively, according to Weiss et al. [42]. Chemical formulas of prepared MUS and NON models were and , respectively. CEL, PP, SiO2, and AC periodic unit cells were enlarged to ~50 × 50 Å with a surface height and additional vacuum slab height of 50 Å and 350 Å, respectively. The CEL, PP, SiO2, and AC surfaces were further modified to simulate nanopores with dimensions of (width × height) ~30 × 30 Å (Figure 3) and molecular pores with dimensions of 5.1 × 7.1 Å (CEL), 6.5 × 5.9 Å (PP), 5.6 × 5.7 Å (SiO2), and 5.9 × 6.3 Å (AC) (Figure 4).
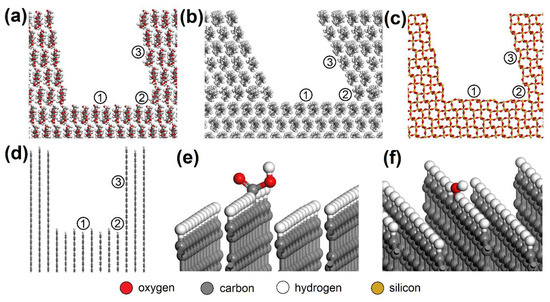
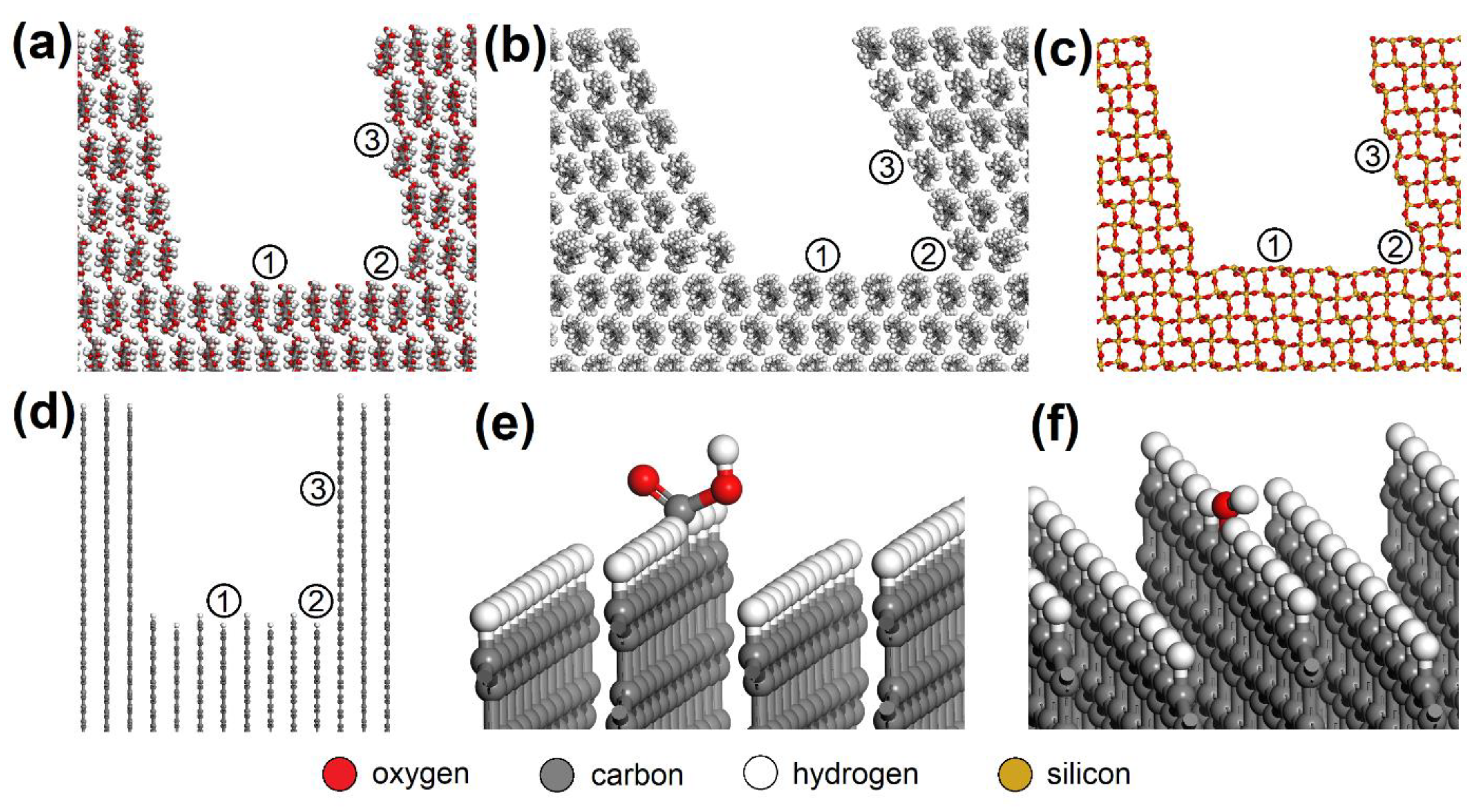
Figure 3.
Nanopores in the (a) CEL, (b) PP, (c) SiO2, and (d) AC surfaces with spots where the NO or SO2 molecule was placed: (1) bottom, (2) corner, (3) side. NO or SO2 molecules were also placed on the AC surfaces with -COOH or -OH groups added, e.g. (e) bottom_COOH and (f) bottom_OH. CEL—cellulose, PP—polypropylene, SiO2—silicon dioxide, AC—activated carbon.
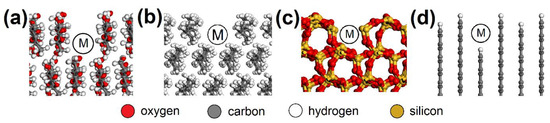
Figure 4.
Molecular pores in the (a) CEL, (b) PP, (c) SiO2, and (d) AC surfaces with spots (M) where the NO or SO2 molecule was placed. CEL—cellulose, PP—polypropylene, SiO2—silicon dioxide, AC—activated carbon.
Due to the XPS analysis of AC adsorbents establishing 3.67 % carboxyl and 4.61 % hydroxyl groups on the surface, models with -COOH (bottom_COOH, corner_COOH, cavity_COOH) and -OH (bottom_OH, corner_OH, cavity_OH) added to the bare AC surfaces (bottom, corner, cavity) were also prepared (Figure 3e,f). The main advantage of using this approach to building AC models based on a graphitic structure is the possibility it offers of creating pores and cavities of controllable sizes [43].
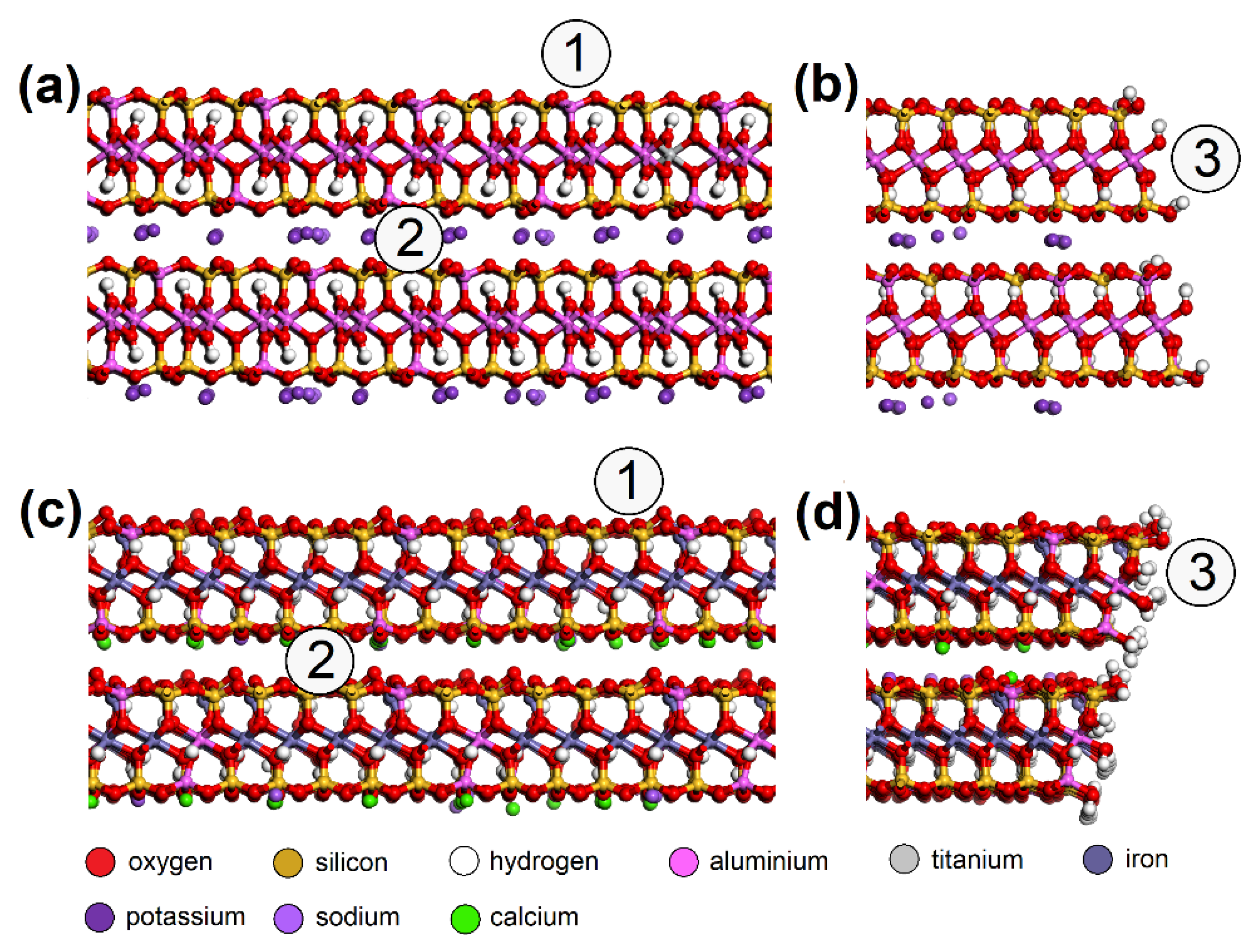
MUS and NON periodic unit cells were enlarged to ~50 × 50 Å with a surface containing two clay layers with two interlayers and a vacuum slab added with a height of 350 Å. NO and SO2 molecules were placed on various spots of the surfaces (Figure 5). Three models were created for each “EG molecule/adsorbent surface spot” combination.
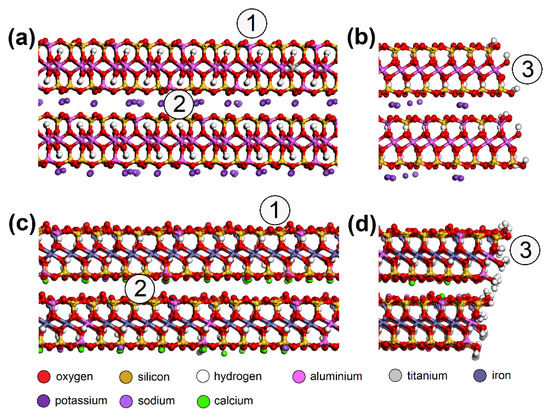
Figure 5.
Molecular models of MUS (a) surface, (b) edge; and NON (c) surface and (d) edge—with spots where the NO or SO2 molecule was placed: (1) surface, (2) interlayer, (3) edge. MUS—muscovite, NON—nontronite.
An MS/Forcite module was used for geometry optimization of each model. The COMPASS force field parameterized atoms and assigned their charges [44], which was verified for CEL [45], PP [46], SiO2 [47], and AC [43]. A universal force field, with the QEq module setting charges, was used for models containing MUS and NON [48].
The Smart algorithm (implemented in MS) with 5·105 steps was used. Convergence thresholds for displacement, force, and energy were 5·10−5 Å, 5·10−3 kcal·mol−1·Å−1, and 1·10−4 kcal·mol−1, respectively. Cell parameters were not optimized. Interaction energy (Eint; kcal/mol) was calculated for each optimized model from potential energies (Ep) using the following equation:
where Ep1 is Ep of a whole model, Ep2 is Ep of a surface, and Ep3 is Ep of a NO/SO2 molecule. The lower the Eint value was, the stronger the interaction between NO/SO2 and the surfaces.
Eint = Ep1 − Ep2 − Ep3
For molecular dynamics, periodic models of AC, MUS, and NON with dimensions of ~17 × 17 Å (α = β = 90°) were prepared. The AC model contained three graphene layers, while the MUS and NON models contained two clay layers with one interlayer. All models had a vacuum slab, 1000 Å high, perpendicular to the surfaces. A total of 28 NO and 28 SO2 molecules were evenly placed on one side of the surfaces. The MD/Forcite module and COMPASS or Universal force field for AC or MUS/NON models, respectively, were used for molecular dynamics with NVT ensemble, Nosé thermostat, random initial velocities, 293 K temperature, and 5 ns total simulation time.
3. Results and Discussion
3.1. Physisorption
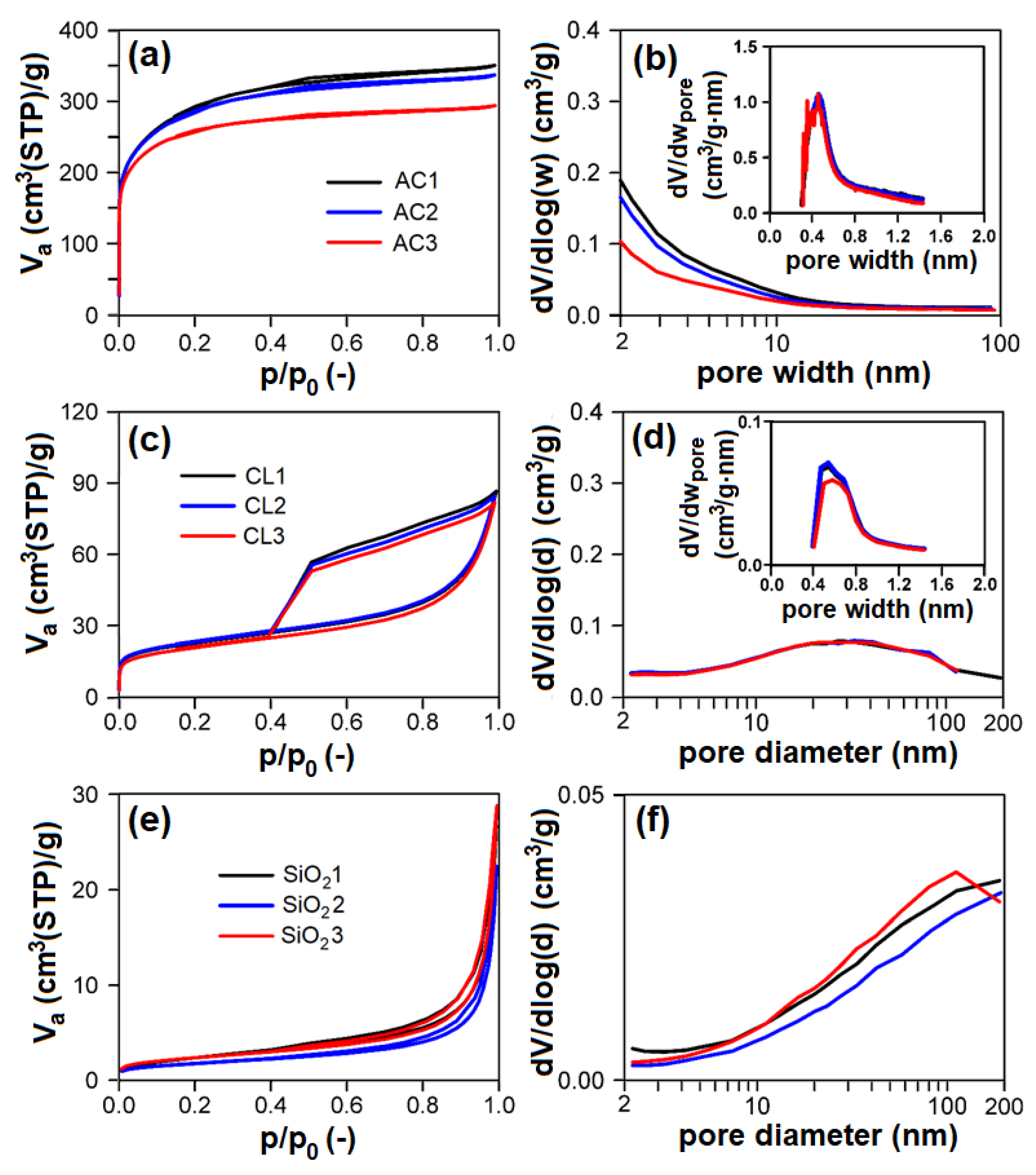
Nitrogen physisorption measurements (Figure 6a,c,e; Table 2) proved that individual groups of adsorbents differ very much concerning their textural properties. According to the surface area and porosity, the adsorbents used can be ordered as follows (from largest to smallest): AC > CL > SiO2 > CEL > PP.
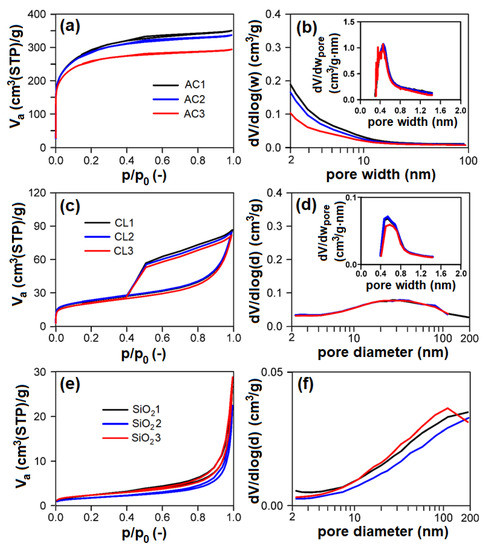
Figure 6.
(left: (a,c,e)) Measured nitrogen adsorption–desorption isotherms at 77 K, (right: (b,d,f)) evaluated mesopore–macropore-size distributions of adsorbents and (inset) evaluated micropore size distributions of adsorbents: (a,b) AC; (c,d) CL; (e,f) SiO2. AC1-3, CL1-3, SiO21-3—activated carbon, clay, and silicon dioxide respectively (1-3—three granulometric fractions; see Table 1), Va—adsorbed volume of nitrogen, STP—standard temperature and pressure, p/p0—relative pressure, dV/dlog(w)—derivation of adsorbed volume of nitrogen divided by derivation of logarithm of pore width, dV/dwpore—derivation of adsorbed volume of nitrogen divided by derivation of pore width, dV/dlog(d)—derivation of adsorbed volume of nitrogen divided by derivation of logarithm of pore diameter.

Table 2.
Determined textural properties of adsorbents.
AC showed the type I adsorption isotherm according to IUPAC classification [49], typical for microporous materials (Figure 6a). Evaluated textural parameters (Table 2) and pore-size distributions (Figure 6b and insets) definitively correspond to this feature. AC showed micropores of 0.48 nm width with a volume of 359–376 mm3liq/g, and mesopore surface area in the range of 114–209 m2/g. With increasing particle-size fraction in the range of 0.16–1 mm, the mesopore surface area and net pore volume of AC logically decreases with no effect on micropore volume.
CL showed the mixed I + IV type of adsorption isotherm (according to IUPAC classification) typical for mesoporous materials with broad mesopores/macropores and some micropores (Figure 6c,d). CL possessed ~14 mm3liq/g of micropores of ~0.6 nm width (Figure 6d inset) and broad mesopores/macropores of 47–50 m2/g surface area (Figure 6d). There are no differences between individual particle-size fractions of CL, the properties of individual particle-size fractions being identical.
Concerning SiO2, these samples show the type III adsorption isotherm (according to IUPAC classification) with narrow steep hysteresis loop (Figure 6e), typical for macroporous/nonporous materials with large macropores, which corresponds to the pore-size distributions shown in Figure 6f, proving the presence of macropores having diameter >100 nm. Textural properties (i.e., pore surface area, net pore volume) of SiO2 are comparable; there are no differences among particle-size fractions. PP and CEL belong among nonporous materials with very low surface area.
3.2. Emission Gas Adsorption
With the exception of PP, all adsorbent materials were able to adsorb at least one of the EG components (Table 3). CO2 was adsorbed on none of the adsorbents. Adsorption of CO on SiO2 was not observed at any temperature. Granulometry had a significant effect on the adsorbed amount of gases—the smaller the particles, the more adsorbed EG. At the adsorption temperature of 20 °C, AC on average adsorbed the most amount of both NO (9× higher than CL, 20× higher than CEL; Table 3) and SO2 (1.5× higher than CL, 3× higher than CEL, and 54× higher than SiO2; Table 3). At the adsorption temperature of 110 °C, all of the adsorbents adsorbed NO in very small amounts in contrast to SO2, which was adsorbed significantly more on average, and most on CL (6× higher than on AC, 7× higher than on CEL and 82× higher than on SiO2; Table 3). CEL was the only one to adsorb CO at 20 °C (33.55 μg/g). on average, CL adsorbed the most amount of CO at 110 °C.

Table 3.
The adsorbents in the wet and dried state and their adsorption capacities for different molecules (NO, SO2, CO) at different adsorbent temperatures (20 and 110 °C).
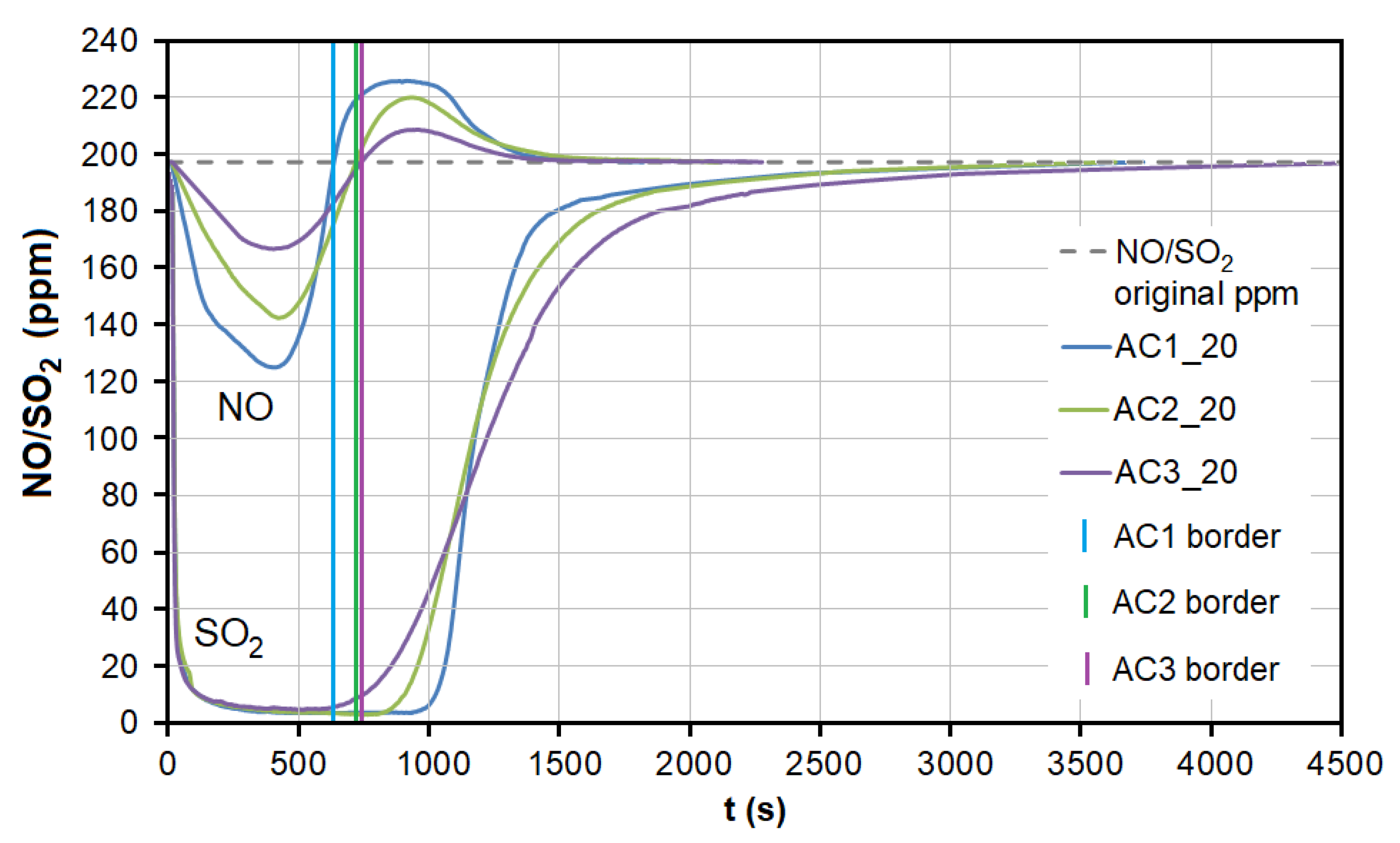
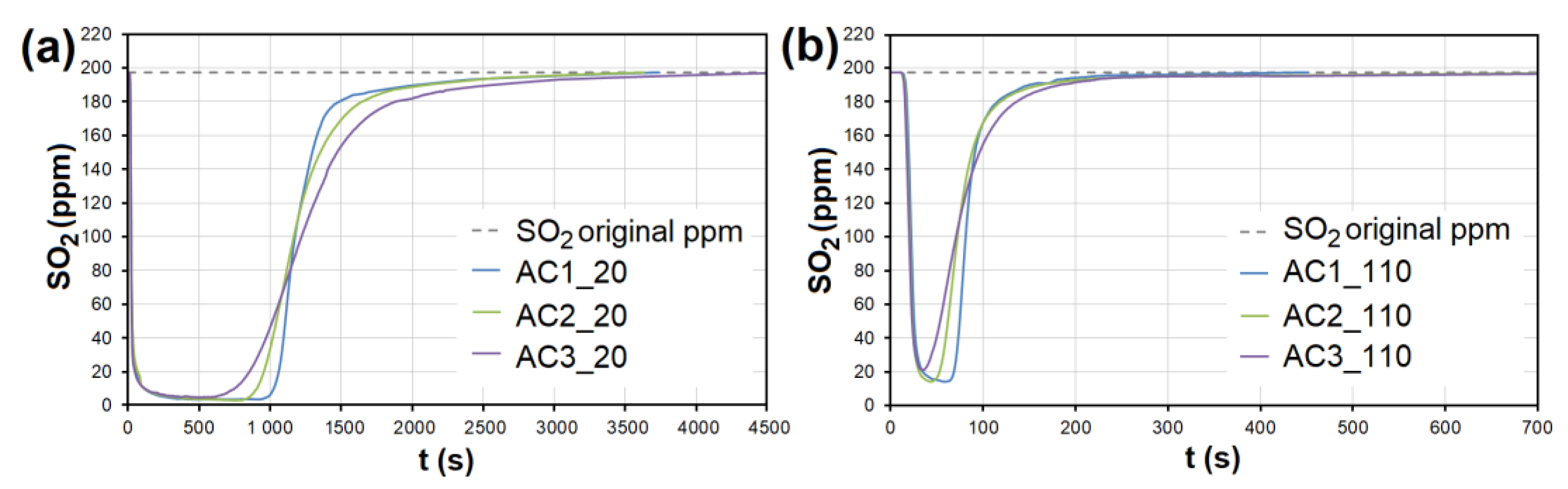
During the adsorption of NO on AC, there was a significant competitive effect between NO and SO2 at both temperatures (Figure 7 and Figure 8). As soon as most of the AC adsorption sites were occupied by SO2 molecules and the adsorbed amount began to decrease (t = 550–950 s at 20 °C, 40–70 s at 110 °C; Figure 7), SO2 began to replace NO molecules, resulting in NO forced desorption. The more porous the AC (smaller granulometric fraction), the earlier the desorption effect occurs (represented by AC1, AC2, and AC3 borderlines in Figure 7).
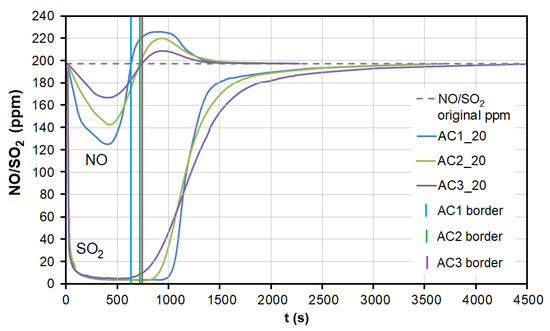
Figure 7.
The dependence between SO2 adsorption and NO desorption at the temperature of 20 °C. AC1, AC2, and AC3 border lines represent the beginning of the NO desorption effect for each adsorbent. AC1-3—activated carbon at different granulometric fractions (see Table 1).
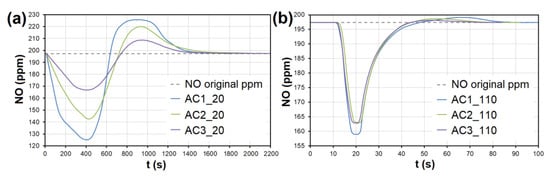
Figure 8.
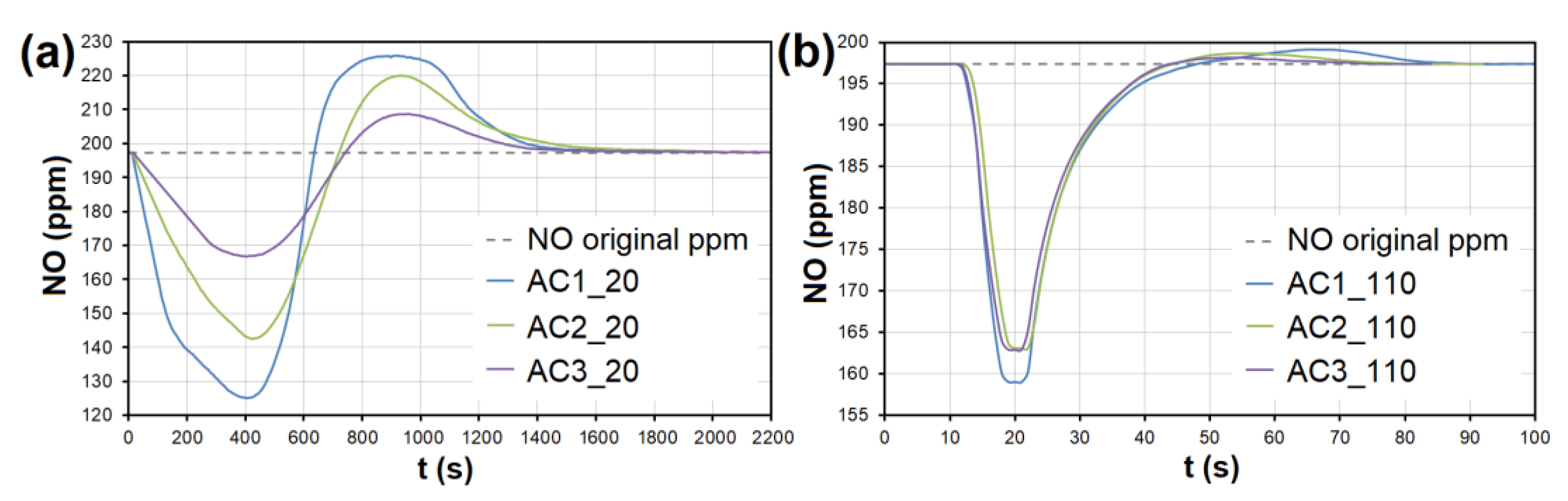
The adsorption curves showing NO adsorption on AC at adsorbent temperatures of (a) 20 °C and (b) 110 °C. The curves also show forced the desorption effect in the ranges of (a) 630–2150 s and (b) 43–93 s. AC1-3—activated carbon at different granulometric fractions (see Table 1).
At the temperature of 20 °C, a total of 214.32/92.66 μg/g of NO was adsorbed/desorbed on AC1, 163.92/60.86 μg/g on AC2, and 106.51/30.03 μg/g on AC3 (Figure 8). This phenomenon was investigated in more detail using molecular dynamics (see Section 3.3). Forced desorption was not observed with any other adsorbent.
In the case of AC, the adsorption maximum of NO was reached gradually (Figure 8), while the SO2 adsorption maximum of SO2 occurred immediately after the EG flow started (Figure 9). The same was observed during SO2 adsorption on CL, SiO2 and CEL (Figure 10 and Figure 11).
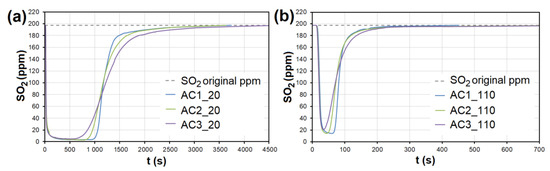
Figure 9.
The adsorption curves showing SO2 adsorption on AC at adsorbent temperatures of (a) 20 °C and (b) 110 °C. AC1-3—activated carbon at different granulometric fractions (see Table 1).
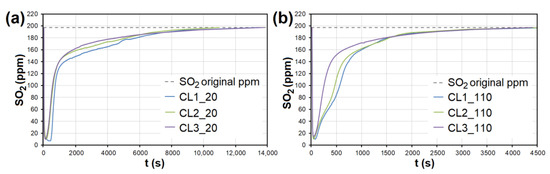
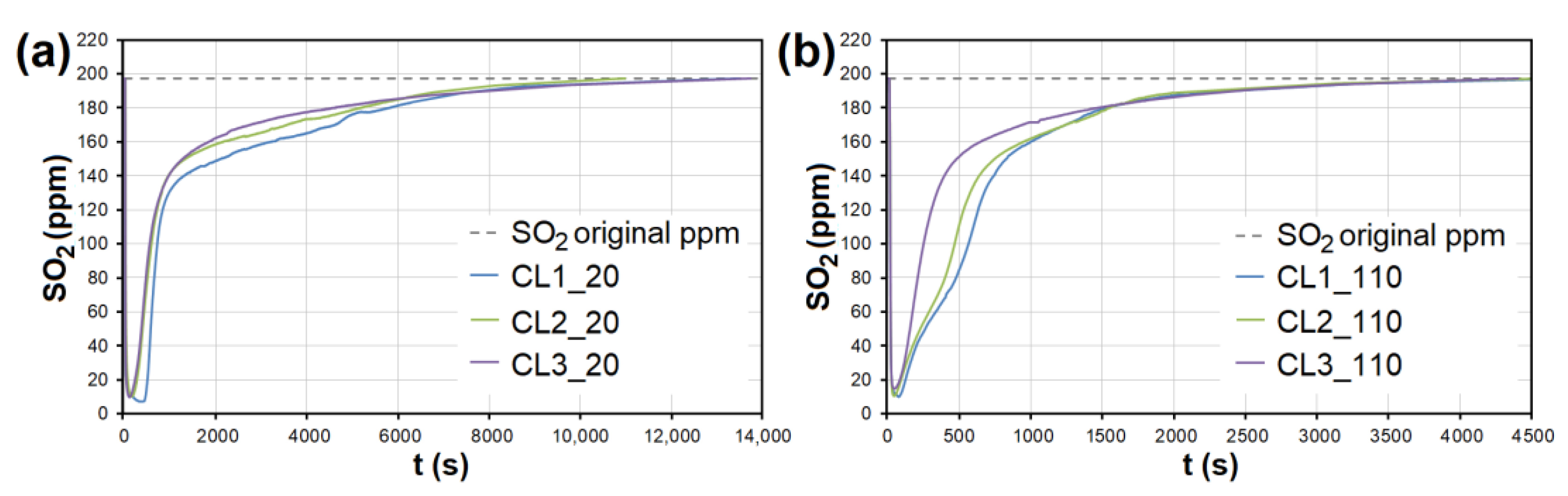
Figure 10.
The adsorption curves showing SO2 adsorption on CL at adsorbent temperatures of (a) 20 °C and (b) 110 °C. CL1-3—clay at different granulometric fractions (see Table 1).
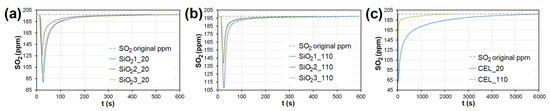
Figure 11.
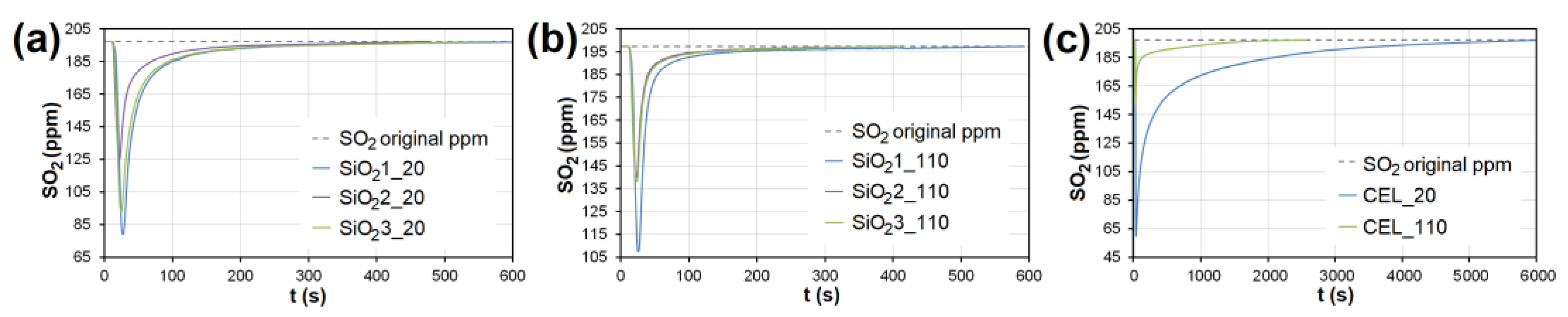
The adsorption curves showing SO2 adsorption on SiO2 at adsorbent temperatures of (a) 20°C and (b) 110 °C. (c) The adsorbing curve showing SO2 adsorption on CEL at adsorbent temperatures of 20°C and 110 °C. SiO21-3—silicon dioxide at different granulometric fractions (see Table 1), CEL—cellulose.
3.3. Molecular Modeling
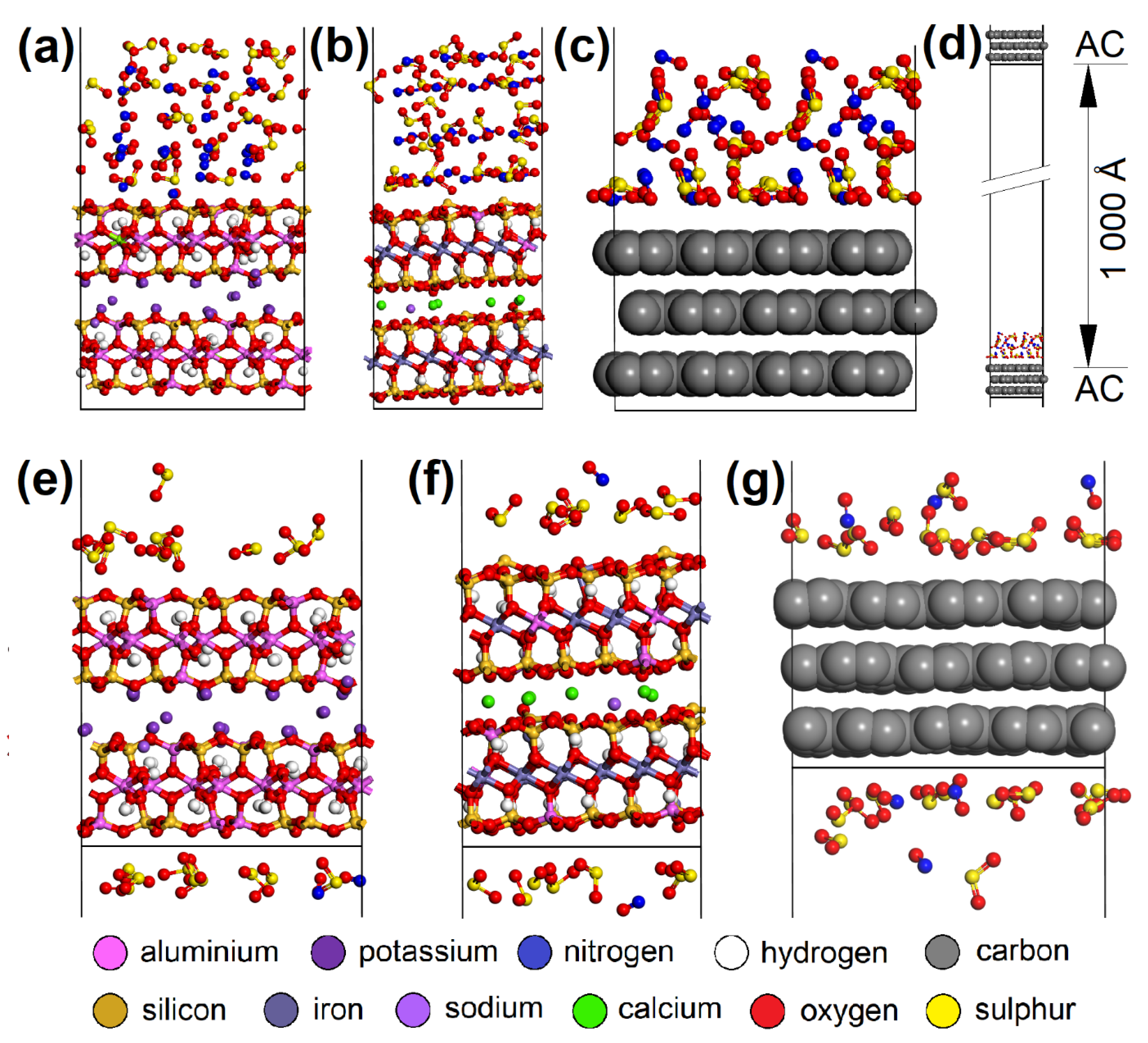
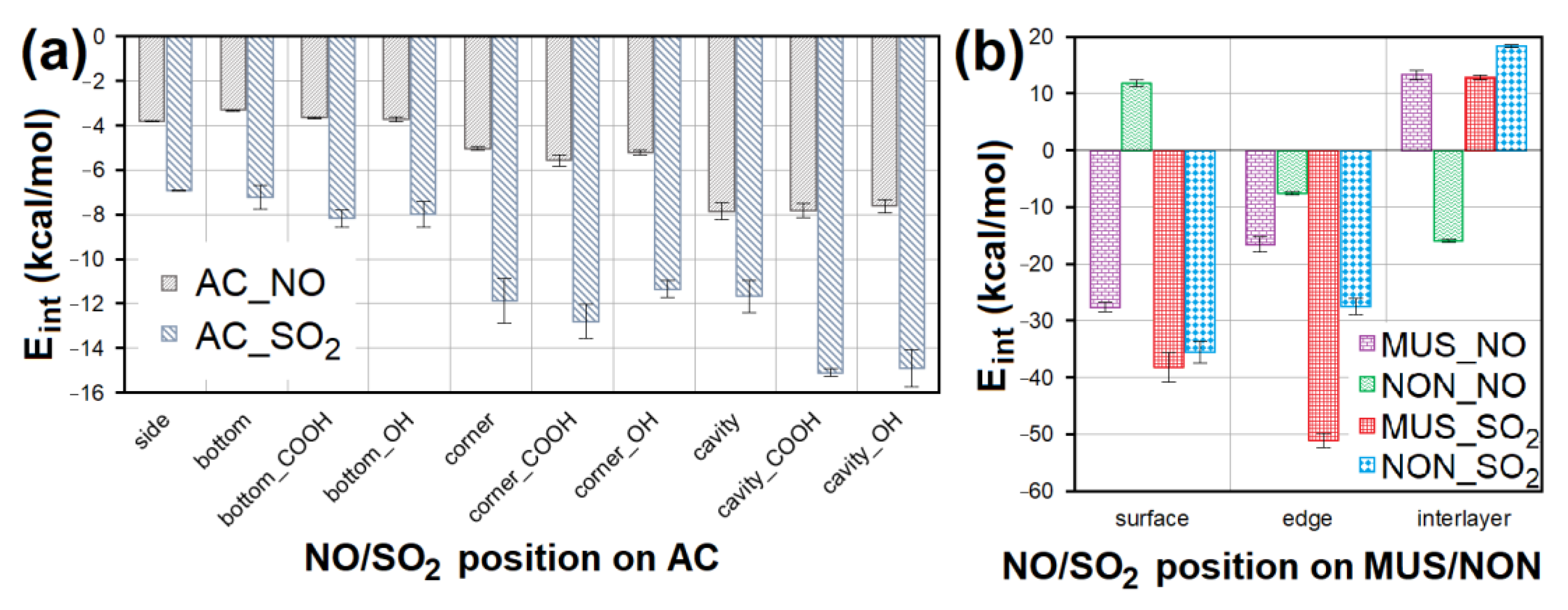
CO2 adsorption was not observed on any of the adsorbents, and CO was only adsorbed in negligible amounts and only at a temperature of 110 °C. Therefore, these two EG components were not molecularly modeled. Molecular dynamics clarified the competitive adsorption process of NO and SO2 on the most adsorbing materials, i.e., AC (having the most specific adsorption process; Figure 12) and CL (MUS and NON). It showed NO forced desorption caused by SO2 adsorption. Consequently, it was found with geometry optimization that SO2 showed a lower Eint with the surfaces than NO (Figure 13a,b), which correlated with the molecular dynamics results. The SO2 molecules gradually occupied the top of the first surface, and the bottom of the second one. According to the notation “EG molecule_EG molecules number on the top of the surface/on the bottom of the surface_in the vacuum slab”, there were SO2_12/13_3 on AC, SO2_10/9_9 on MUS, and SO2_7/7_14 on NON (Figure 12), while most NO molecules were expelled away from the surfaces (NO_3/3_22 for AC, NO_0/2_26 for MUS, and NO_1/1_26 for NON; Figure 12). The molecular dynamics results correspond to the experimentally determined sorption capacities, when higher sorption capacities were measured for SO2 than for NO; and at 20 °C (293 K), AC showed higher sorption capacity than CL (MUS/NON; Table 3).
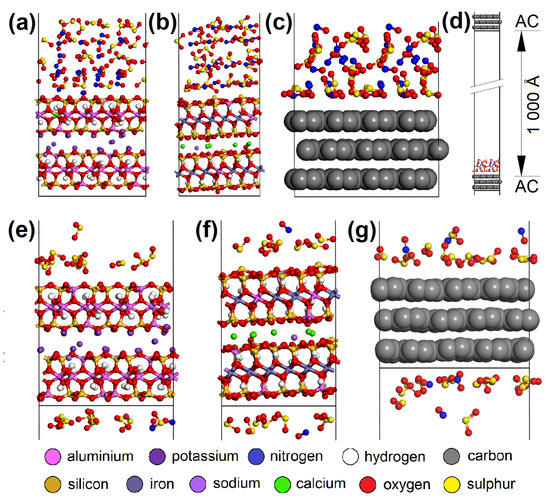
Figure 12.
The initial molecular models containing 28 SO2 and 28 NO molecules on the (a) MUS, (b) NON, and (c) AC surfaces. (d) The initial AC cell with EG molecules and the vacuum slab. The models after molecular dynamics showing the preference of SO2 over NO molecules during the adsorption process on the (e) MUS, (f) NON, and (g) AC surfaces. MUS—muscovite, NON—nontronite, AC—activated carbon; EG—emission gas.
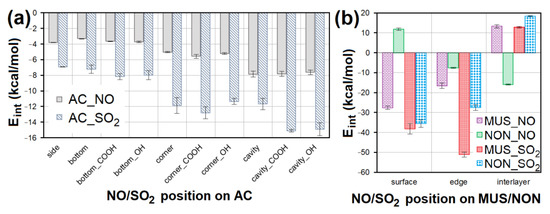
Figure 13.
The Eint comparison of models containing NO/SO2 at different sites of (a) AC surface without or with -COOH/-OH groups and (b) MUS/NON surfaces. AC—activated carbon, MUS—muscovite, NON—nontronite, Eint—interaction energy.
Due to the established correlation of molecular dynamics and geometry optimization results, the other models were only geometry optimized with one EG molecule on the surfaces in order to determine Eint and observe molecular interactions in more detail.
Geometry optimization showed the suitability of the chemical composition of all sorbent materials for NO and SO2 adsorption. An attractive interaction with NO or SO2 was observed for all models containing AC. Average Eint of models containing NO was 2× higher than models containing SO2 on AC (Figure 13a). NO interacted most strongly with the cavities, cavity_COOH (Eint = −8.312 kcal/mol) and cavity_OH. The second strongest NO interactions were observed with the AC corner surfaces (Eint = −5.962 kcal/mol). In the case of SO2, the strongest interactions were observed for cavity_COOH (Eint = −15.360 kcal/mol) and cavity_OH. Corner_COOH (Eint = −13.877 kcal/mol) showed the second strongest interactions with SO2. These findings prove that the interaction of NO/SO2 with AC is stronger, the more numerous surface atoms participating in the non-bonding interaction. The presence of the functional groups (mainly -COOH) is another important factor supporting the strength of AC interaction with NO/SO2 via hydrogen bonds.
NO showed the strongest interaction with the MUS surface (Eint = −28.785 kcal/mol) and NON interlayer (Eint = −16.177 kcal/mol). The lowest Eint showed the model containing SO2 at the MUS edge (Eint = −52.357 kcal/mol); and the second strongest SO2 interaction was with MUS and NON edge (Figure 13b). The MUS and NON interlayers in most cases showed a positive Eint (repulsive force) with NO and SO2, due to a large number of cations in the interlayer of clay minerals causing a strong interaction between clay layers, and making it impossible for NO or SO2 to penetrate. At the same time, these cations occupied all chemically suitable sites for interaction with NO and SO2. The average Eint of models containing SO2 on MUS and NON is significantly lower than in the case of models with NO.
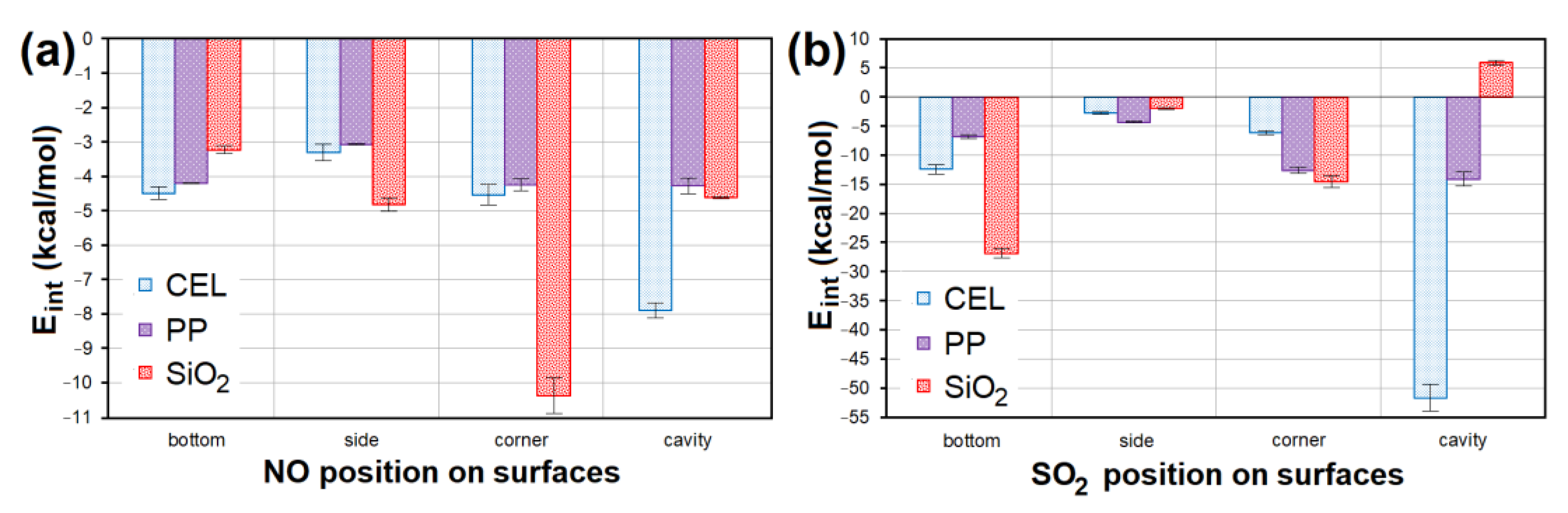
All models containing NO placed at all sites of the CEL, PP and SiO2 surfaces showed a weak negative interaction energy. The strongest interaction (Eint = −10.936 kcal/mol; Figure 14a) was achieved in the case of NO lying in the corner of SiO2. The second lowest Eint (−8.137 kcal/mol; Figure 14a) was achieved for NO placed in the CEL cavity. SO2 interacted with CEL, PP and SiO2 surfaces several times stronger. The model with the strongest interaction of SO2 in the CEL cavity showed 5× lower Eint (−55.491 kcal/mol; Figure 14b) than the model with the strongest interaction with NO. The second strongest interaction (Eint = −27.872 kcal/mol; Figure 14b) was shown by SO2 on the SiO2 bottom. The average Eint of models containing NO/SO2 on PP was the highest.
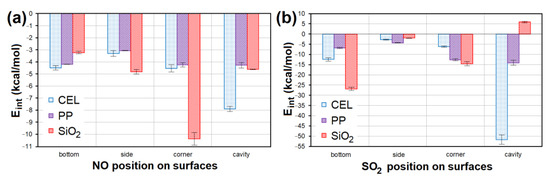
Figure 14.
The Eint comparison of models containing (a) NO and (b) SO2 at different sites of CEL, PP and SiO2 surfaces. CEL—cellulose, PP—polypropylene, SiO2—silicon dioxide, Eint—interaction energy.
3.4. Adsorption Factors
The importance of chemical (Eint) and textural (SBET, Smeso, Vmicro, Vnet) properties on the adsorption capacity of adsorbents was determined (Table 4). AC adsorption capacity was high for both NO and SO2, which was mainly correlated with the large surface area in spite of high Eint values (i.e., weak interactions). The high CL adsorption capacity was due to a combination of both chemical and textural properties. A dominant factor of low SiO2 and PP adsorption capacity was primarily textural properties (despite strong interactions). The high CEL adsorption capacity was mainly provided by the presence of functional groups strongly interacting with SO2 molecules.

Table 4.
Determining the importance of Eint or textural properties (SBET, Smeso, Vmicro, Vnet) on adsorption capacity of the adsorbents having 0.16–0.315 mm granulometry.
Furthermore, agreement between the experiment and molecular modeling was generally more common for lower sorption capacities for NO (corresponding to higher Eint values, i.e., weaker interaction) compared to higher SO2 capacities (corresponding to lower Eint values, i.e., stronger interaction).
In general, a significantly lower Eint can be observed for models containing a molecule in a molecular pore (mainly CEL), which appears to be an ideal combination of chemical (more non-bonding interactions) and structural/steric (molecule size correlates with pore size) factors. The agreement of the force field-based molecular modeling results and experimental data demonstrate the modeling strategy used to be a suitable tool for analysis and prediction of interactions during the sorption process at the molecular level.
However, other modeling studies performed using more computationally demanding DFT methods have led to similar results for interactions between AC and NO/SO2 [32,34]. Wang et al. [34] reported the same behavior of NO on AC—the strongest interactions were observed on AC basal plane (compare with “side” in Figure 13a), weaker interactions with -OH group (compare with “bottom_OH” in Figure 13a), and the weakest with -H (compare with “bottom” in Figure 13a). Zhao et al. [32] found the same Eint trend for SO2 on AC, where the molecule interacted most strongly with -COOH groups (compare with “bottom_COOH” in Figure 13a), more weakly with -OH (compare with “bottom_OH” in Figure 13a), and the weakest with -H (compare with “bottom” in Figure 13a).
The comparison demonstrates the usefulness of computationally less demanding force field-based molecular modeling in adsorption research. It also reveals that there is no need to take into account the partial amorphousness of AC, e.g., by a large amount of polyaromatic hydrocarbons [32]. Approximation of AC using graphite, which additionally enables the preparation of pores of the required size, is sufficient for the given purpose.
4. Conclusions
In this work, the competitive adsorption of emission gas, containing CO, NO, SO2 and CO2, on adsorption materials with different chemical compositions was studied.
CO2 was not adsorbed on any material. CO was adsorbed in small amounts and mainly at a temperature of 110 °C on AC, CL and CEL. At 20 °C, the AC showed the highest NO and SO2 sorption capacity. At 110 °C, the highest NO and SO2 sorption capacity was found for the CL.
The strongest interactions were exhibited between NO/SO2 and corner or cavity surface types due to the larger number of interacting atoms. Carboxyl and hydroxyl functional groups strengthened the interaction of AC mainly with SO2, where hydrogen bonds were formed. NO and SO2 showed attractive interactions with MUS and NON mainly on the surface and edge. Strong interactions between clay layers and interlayer cations prevented the penetration of NO and SO2 into the interlayer space.
The dominant factor determining the sorption capacity of AC, SiO2 and PP was textural (surface area, porosity)—while in the case of CEL, it was the chemical factors (chemical composition, functional groups). Both textural and chemical factors played an important role in adsorption on CL.
The effects of competitive adsorption of emission components on sorption capacity were determined and further clarified using force field-based molecular modeling. The modeling strategy made it possible to simulate surfaces with different and controllable structural character. Geometry optimization and molecular dynamics results agreed well with experimental data allowing the modeling strategy to be further used in studies of interactions during adsorption, or for a prediction of adsorption phenomena.
Author Contributions
Conceptualization, A.V.; methodology, A.V. and J.T.; software, A.V. and J.T.; validation, A.V. and T.N.; formal analysis, A.V., L.M. and K.M.K.; investigation, A.V. and T.N.; resources, J.K. and V.P.; data curation, A.V.; writing—original draft preparation, A.V.; writing—review and editing, J.T.; visualization, A.V.; supervision, J.T.; project administration, A.V.; funding acquisition, A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Doctoral grant competition VSB—Technical University of Ostrava, reg. no. CZ.02.2.69/0.0/0.0/19_073/0016945 within the Operational Programme Research, Development and Education, under project DGS/TEAM/2020-005 “Low-potencial waste heat recovery”. Large Research Infrastructure ENREGAT supported by the Ministry of Education, Youth and Sports of the Czech Republic (project No. LM2018098) also contributed to the realization of this work.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brusseau, M.L.; Pepper, I.L.; Gerba, C.P. Environmental and Pollution Science, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Henzel, C.K. Investigating Manufacturing Pollution, 1st ed.; Child’s World: Parker, CO, USA, 2022. [Google Scholar]
- Tillman, D.A. Coal-Fired Electricity and Emissions Control: Efficiency and Effectiveness, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2018. [Google Scholar]
- Matz, C.J.; Egyed, M.; Hocking, R.; Seenundun, S.; Charman, N.; Edmonds, N. Human health effects of traffic-related air pollution (TRAP): A scoping review protocol. Syst. Rev. 2019, 8, 223. [Google Scholar] [CrossRef]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Werther, J.; Saenger, M.; Hartge, E.U.; Ogada, T.; Siagi, Z. Combustion of agricultural residues. Prog. Energy Combust. Sci. 2000, 26, 1–27. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, Z.; Watanabe, M. Production of solid fuels by hydrothermal treatment of wastes of biomass, plastic, and biomass/plastic mixtures: A review. J. Bioresour. Bioprod. 2022, 7, 221–244. [Google Scholar] [CrossRef]
- Bai, J.; Yu, C.; Li, L.; Wu, P.; Luo, Z.; Ni, M. Experimental study on the NO and N2O formation characteristics during biomass combustion. Energy Fuels 2013, 27, 515–522. [Google Scholar] [CrossRef]
- Jones, J.M.; Lea-Langton, A.R.; Ma, L.; Pourkashanian, M.; Williams, A. The Combustion of Solid Biomass. In Pollutants Generated by the Combustion of Solid Biomass Fuels, 1st ed.; Springer: London, UK, 2014; pp. 25–43. [Google Scholar]
- Yu, C.H.; Huang, C.H.; Tan, C.S. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Sircar, S. Basic research needs for design of adsorptive gas separation processes. Ind. Eng. Chem. Res. 2006, 45, 5435–5448. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, Q.; Zhou, Q.; Yang, J.; Ding, J.; Wen, J. Absorption of carbon dioxide from flue gas using blended amine solutions. Chem. Eng. Technol. 2014, 37, 635–642. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.; Sarkar, P.; Gupta, R. Post-combustion CO2 capture using solid sorbents: A review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Liu, C.; Zhou, L. Comparative studies of CO2 and CH4 sorption on activated carbon in presence of water. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 322, 14–18. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, K.; Clennell, M.B.; Dewhurst, D.N.; Pan, Z.; Pervukhina, M.; Han, T. Molecular simulation studies of hydrocarbon and carbon dioxide adsorption on coal. Pet. Sci. 2015, 12, 692–704. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Oliveira, R.S.; Vieira, R.S.; Cavalcante, C.L.; Azevedo, D. Adsorption of CO2 on nitrogen-enriched activated carbon and zeolite 13X. Adsorption 2011, 17, 235–246. [Google Scholar] [CrossRef]
- Li, Z.S.; Cai, N.S.; Huang, Y.Y.; Han, H.J. Synthesis, experimental studies, and analysis of a new calcium-based carbon dioxide absorbent. Energy Fuels 2005, 19, 1447–1452. [Google Scholar] [CrossRef]
- Franchi, R.S.; Harlick, P.J.; Sayari, A. Applications of pore-expanded mesoporous silica. 2. Development of a high-capacity, water-tolerant adsorbent for CO2. Ind. Eng. Chem. Res. 2005, 44, 8007–8013. [Google Scholar] [CrossRef]
- Ding, Y.; Alpay, E. Equilibria and kinetics of CO2 adsorption on hydrotalcite adsorbent. Chem. Eng. Sci. 2000, 55, 3461–3474. [Google Scholar] [CrossRef]
- Srivatsa, S.C.; Bhattacharya, S. Amine-based CO2 capture sorbents: A potential CO2 hydrogenation catalyst. J. CO2 Util. 2018, 26, 397–407. [Google Scholar] [CrossRef]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Wang, H.; Bai, J.Q.; Yin, Y.; Wang, S.F. Experimental and numerical study of SO2 removal from a CO2/SO2 gas mixture in a Cu-BTC metal organic framework. J. Mol. Graph. Model. 2020, 96, 107533. [Google Scholar] [CrossRef]
- Ozensoy, E.; Goodman, D.W. Vibrational spectroscopic studies on CO adsorption, NO adsorption CO+ NO reaction on Pd model catalysts. Phys. Chem. Chem. Phys. 2004, 6, 3765–3778. [Google Scholar] [CrossRef]
- Joshi, A.M.; Tucker, M.H.; Delgass, W.N.; Thomson, K.T. CO adsorption on pure and binary-alloy gold clusters: A quantum chemical study. J. Chem. Phys. 2006, 125, 194707. [Google Scholar] [CrossRef] [PubMed]
- Abdulrasheed, A.A.; Jalil, A.A.; Triwahyono, S.; Zaini, M.A.A.; Gambo, Y.; Ibrahim, M. Surface modification of activated carbon for adsorption of SO2 and NOX: A review of existing and emerging technologies. Renew. Sustain. Energ. Rev. 2018, 94, 1067–1085. [Google Scholar] [CrossRef]
- Niu, J.; Miao, J.; Zhang, H.; Guo, Y.; Li, L.; Cheng, F. Focusing on the impact of inherent minerals in coal on activated carbon production and its performance: The role of trace sodium on SO2 and/or NO removal. Energy 2023, 263, 125638. [Google Scholar] [CrossRef]
- Rodriguez, J.A. The chemical properties of bimetallic surfaces: Importance of ensemble and electronic effects in the adsorption of sulfur and SO2. Prog. Surf. Sci. 2006, 81, 141–189. [Google Scholar] [CrossRef]
- Yi, H.; Deng, H.; Tang, X.; Yu, Q.; Zhou, X.; Liu, H. Adsorption equilibrium and kinetics for SO2, NO, CO2 on zeolites FAU and LTA. J. Hazard. Mater. 2012, 203, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xuan, C.; Zhang, X.; Sun, R.; Cheng, X.; Wang, Z.; Ma, C. NOx Adsorption Mechanism of Coal-Based Activated Carbon Modified with Trace Potassium: In Situ DRIFTS and DFT Study. Energy Fuels 2022, 36, 7633–7650. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, G.; Wei, G.; Gao, J.; Lu, H. Analysis of SO2 Physisorption by Edge-Functionalized Nanoporous Carbons Using Grand Canonical Monte Carlo Methods and Density Functional Theory: Implications for SO2 Removal. ACS Omega 2021, 6, 33735–33746. [Google Scholar] [CrossRef]
- Zeng, W.; Tan, S.J.; Liu, M.; Zhang, D.; Liu, L.; Do, D.D. New Insights into the Capture of Low-level Gaseous Pollutants in Indoor Environment by Carbonaceous Materials: Effects of Functional Groups, Pore Size, and Presence of Moist. Sep. Purif. Technol. 2022, 298, 121652. [Google Scholar] [CrossRef]
- Wang, J.; Yang, M.; Deng, D.; Qiu, S. The adsorption of NO, NH3, N2 on carbon surface: A density functional theory study. J. Mol. Model. 2017, 23, 262. [Google Scholar] [CrossRef]
- Najser, T.; Gaze, B.; Knutel, B.; Verner, A.; Najser, J.; Mikeska, M.; Chojnacki, J.; Němček, O. Analysis of the Effect of Catalytic Additives in the Agricultural Waste Combustion Process. Materials 2022, 15, 3526. [Google Scholar] [CrossRef]
- Towler, G.; Sinnott, R. Process Flowsheet Development. In Chemical Engineering Design: Principles, Practice and Economic of Plant and Process Design, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 33–102. [Google Scholar]
- Das, T.K. Industrial Pollution Sources, Its Characterization, Estimation, and Treatment. In Industrial Environmental Management: Engineering, Science, and Policy, 1st ed.; Wiley: Hoboken, NJ, USA, 2020; pp. 71–113. [Google Scholar]
- BIOVIA. Materials Studio 7.0; Dassault Systèmes: San Diego, CA, USA, 2013. [Google Scholar]
- Chen, P.; Nishiyama, Y.; Putaux, J.L.; Mazeau, K. Diversity of potential hydrogen bonds in cellulose I revealed by molecular dynamics simulation. Cellulose 2014, 21, 897–908. [Google Scholar] [CrossRef]
- Verenich, S.; Paul, S.; Pourdeyhimi, B. Surface and bulk properties of glycidyl methacrylate modified polypropylene: Experimental and molecular modeling studies. J. Appl. Polym. Sci. 2008, 108, 2983–2987. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. Crystal Structures, 2nd ed.; Wiley: New York, NY, USA, 1963. [Google Scholar]
- Weiss, Z.; Kužvart, M. Clay Minerals: Their Nanostructure and Utilization, 1st ed.; Karolinum: Prague, Czech Republic, 2005. [Google Scholar]
- Bednárek, J.; Matějová, L.; Jankovská, Z.; Vaštyl, M.; Sokolová, B.; Peikertová, P.; Šiler, P.; Verner, A.; Tokarský, J.; Koutník, I.; et al. The Influence of Structural Properties on the Adsorption Capacities of Microwave-assisted Biochars for Metazachlor Removal from Aqueous Solutions. J. Environ. Chem. Eng. 2022, 10, 108003. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab initio force-field optimized for condensed-phase applications—Overview with details on alkane and benzene compounds. J. Phys. Chem. 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Bazooyar, F.; Momany, F.A.; Bolton, K. Validating empirical force fields for molecular-level simulation of cellulose dissolution. Comput. Theor. Chem. 2012, 984, 119–127. [Google Scholar] [CrossRef]
- Deckers, F.; Rasim, K.; Schröder, C. Molecular dynamics simulation of polypropylene: Diffusion and sorption of H2O, H2O2, H2, O2 and determination of the glass transition temperature. J. Polym. Res. 2022, 29, 463. [Google Scholar] [CrossRef]
- Vijayakumar, S.D.; Ridzuan, N. Molecular interaction study on Gemini surfactant and nanoparticles in wax inhibition of Malaysian crude oil. Asia-Pac. J. Chem. Eng. 2021, 16, e2700. [Google Scholar] [CrossRef]
- Rai, B.; Sathish, P.; Tanwar, J.; Moon, K.S.; Fuerstenau, D.W. A molecular dynamics study of the interaction of oleate and dodecylammonium chloride surfactants with complex aluminosilicate minerals. J. Colloid Interface Sci. 2011, 362, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).