MOF-Derived CoNi Nanoalloy Particles Encapsulated in Nitrogen-Doped Carbon as Superdurable Bifunctional Oxygen Electrocatalyst
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
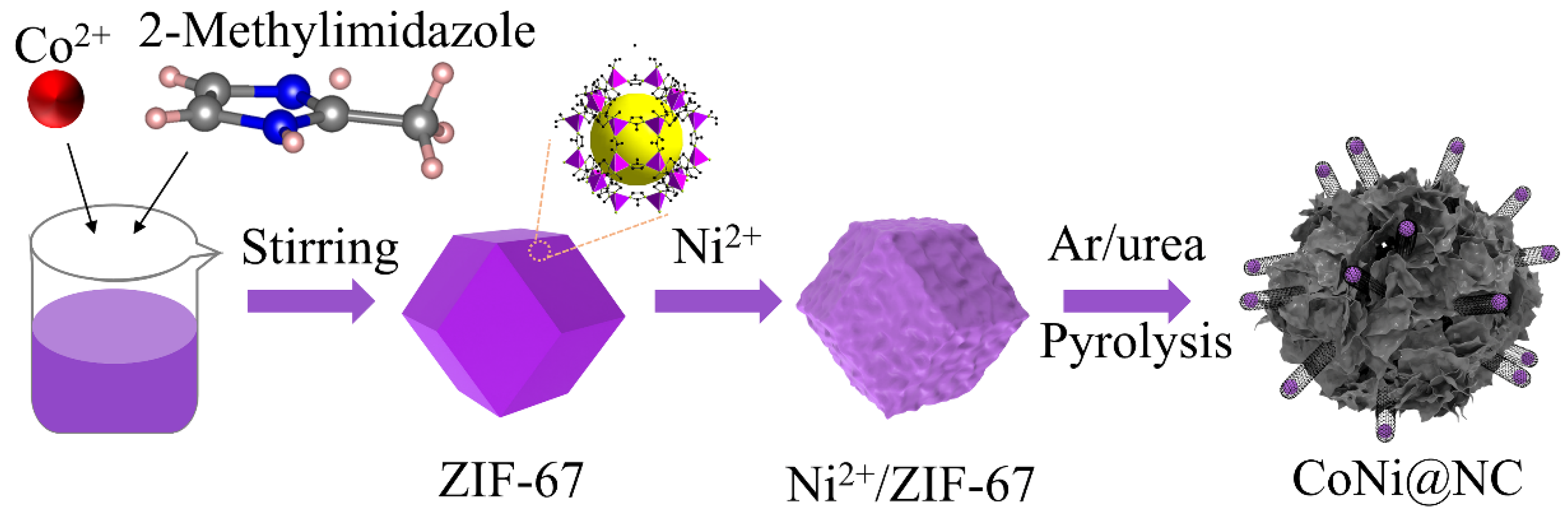
2.2. Preparation of CoNi@NC-
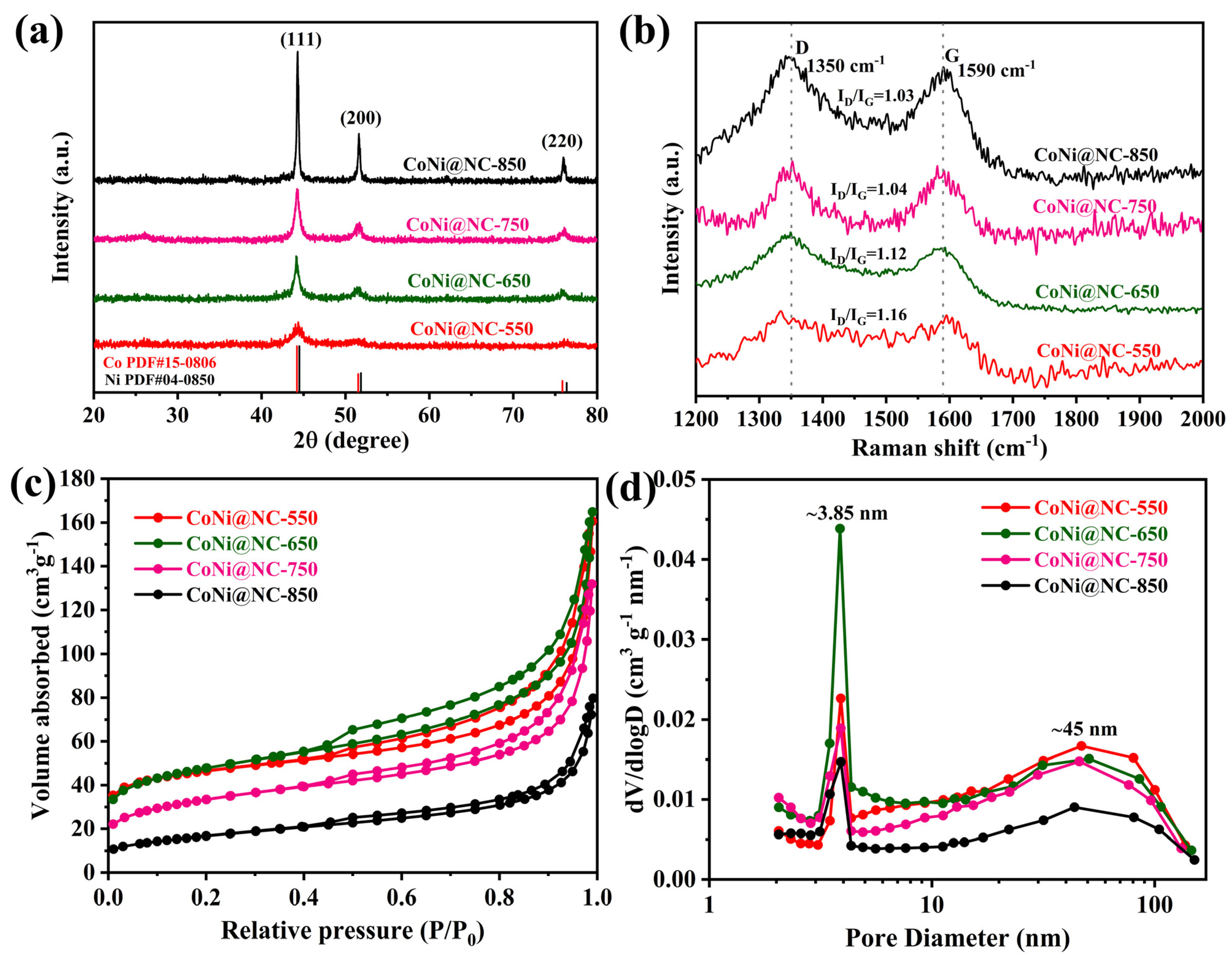
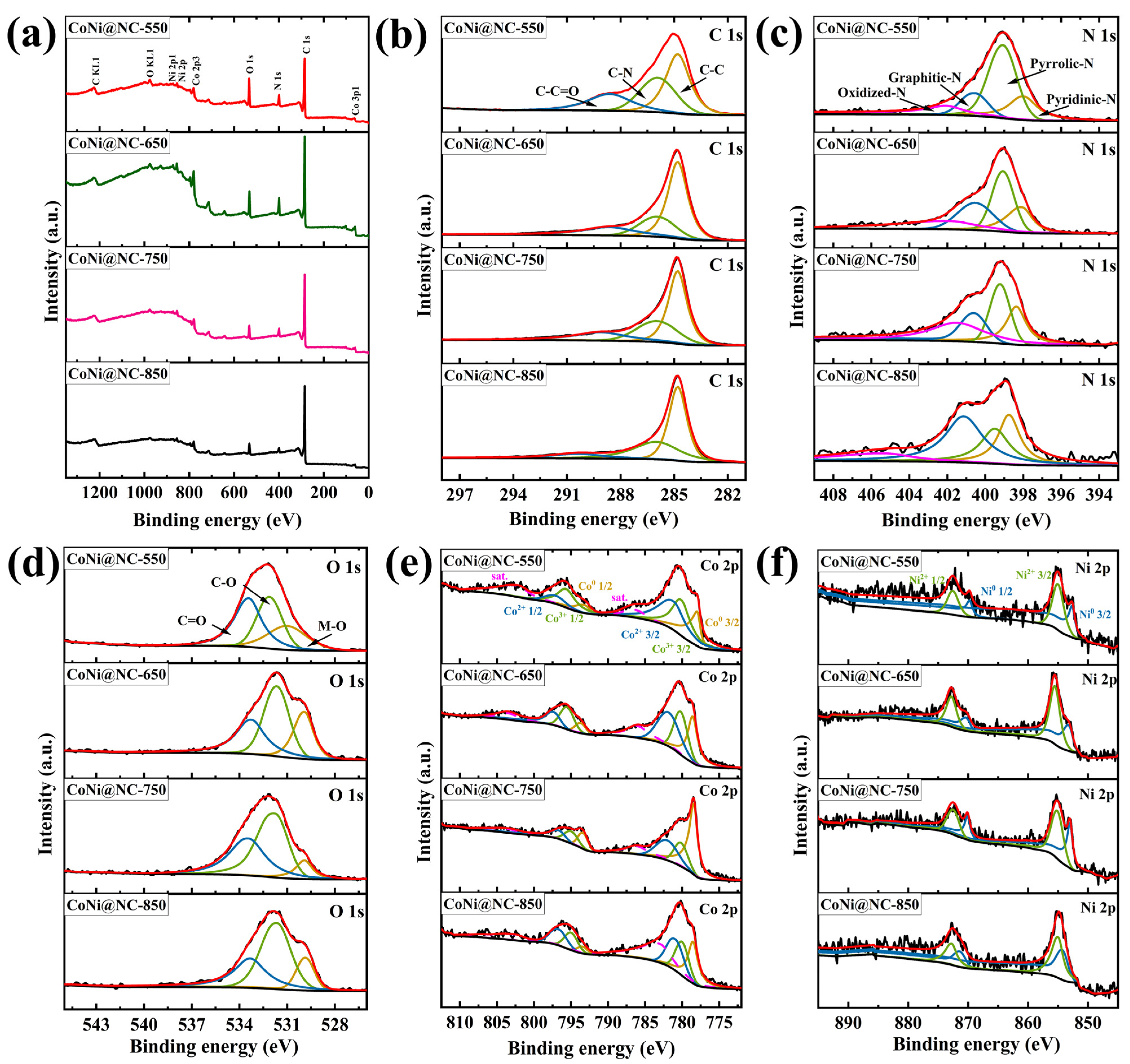
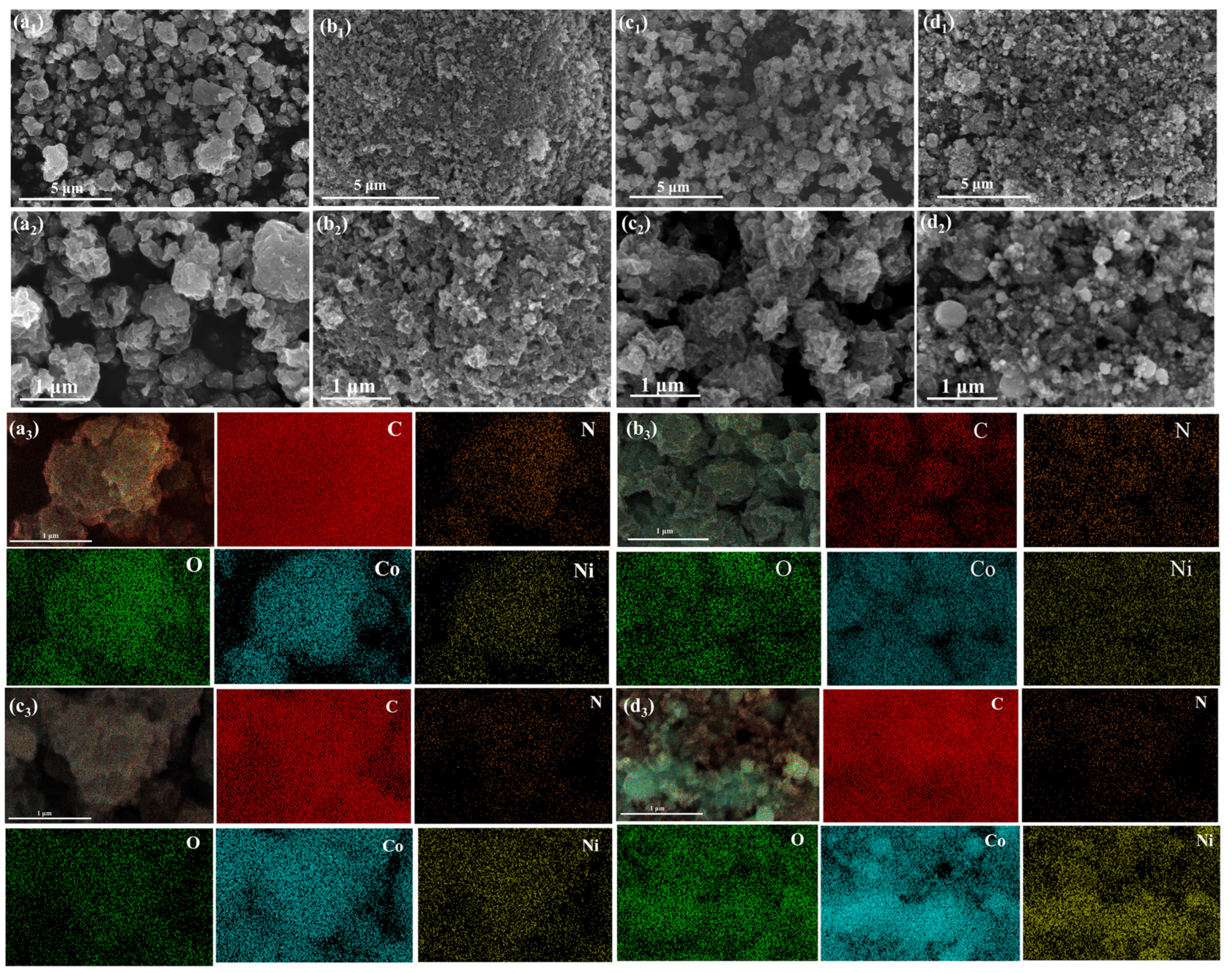
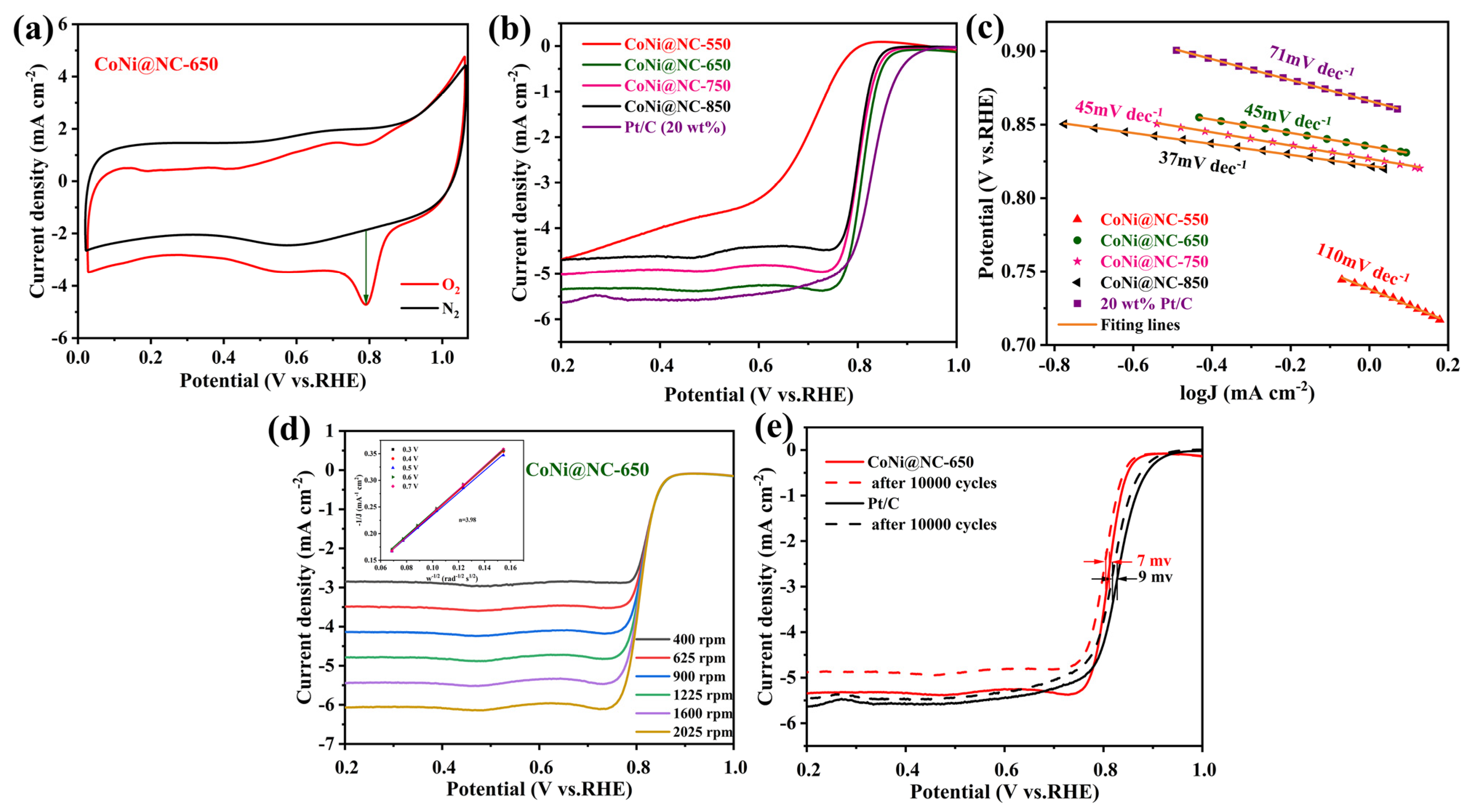
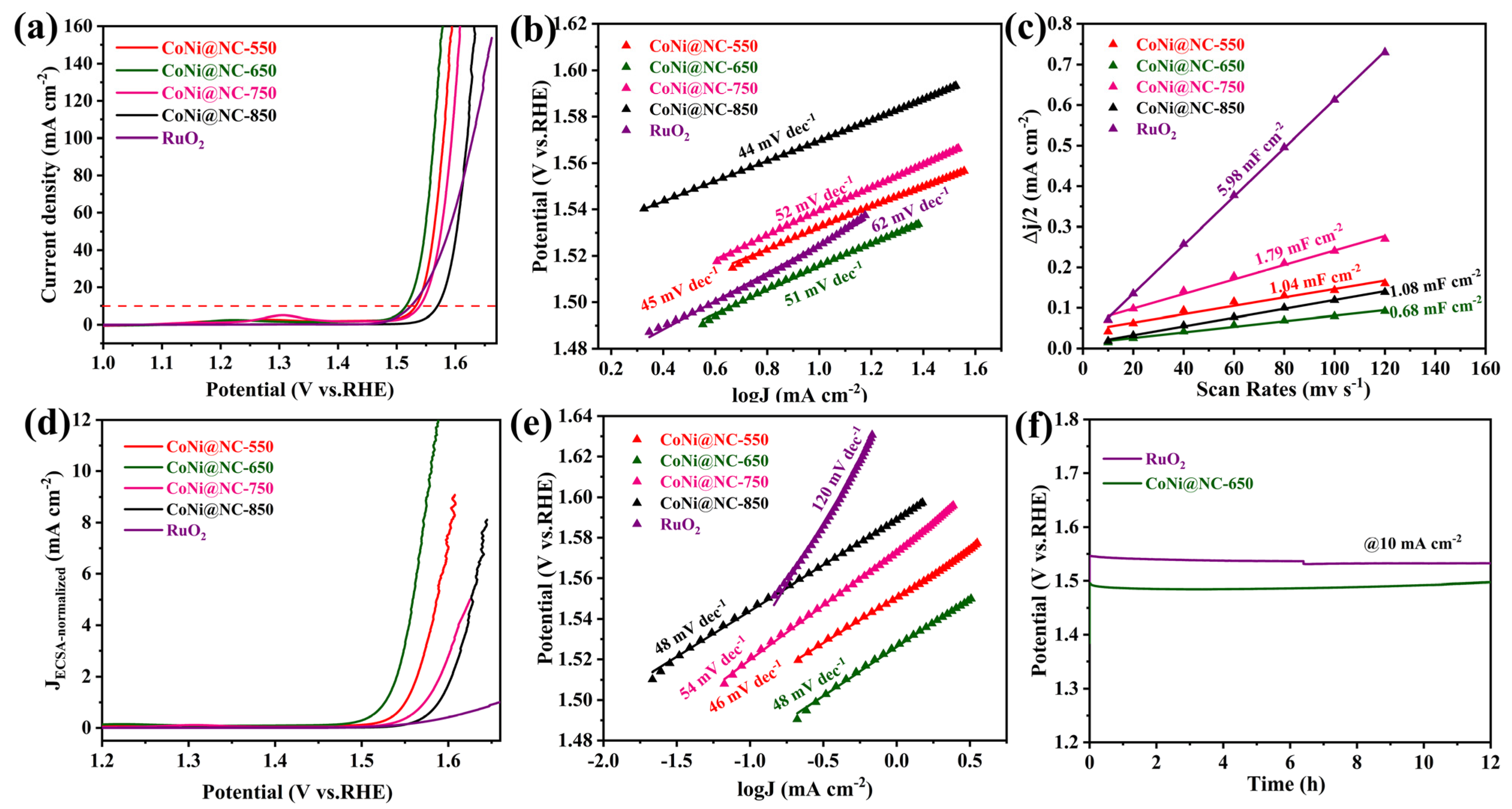
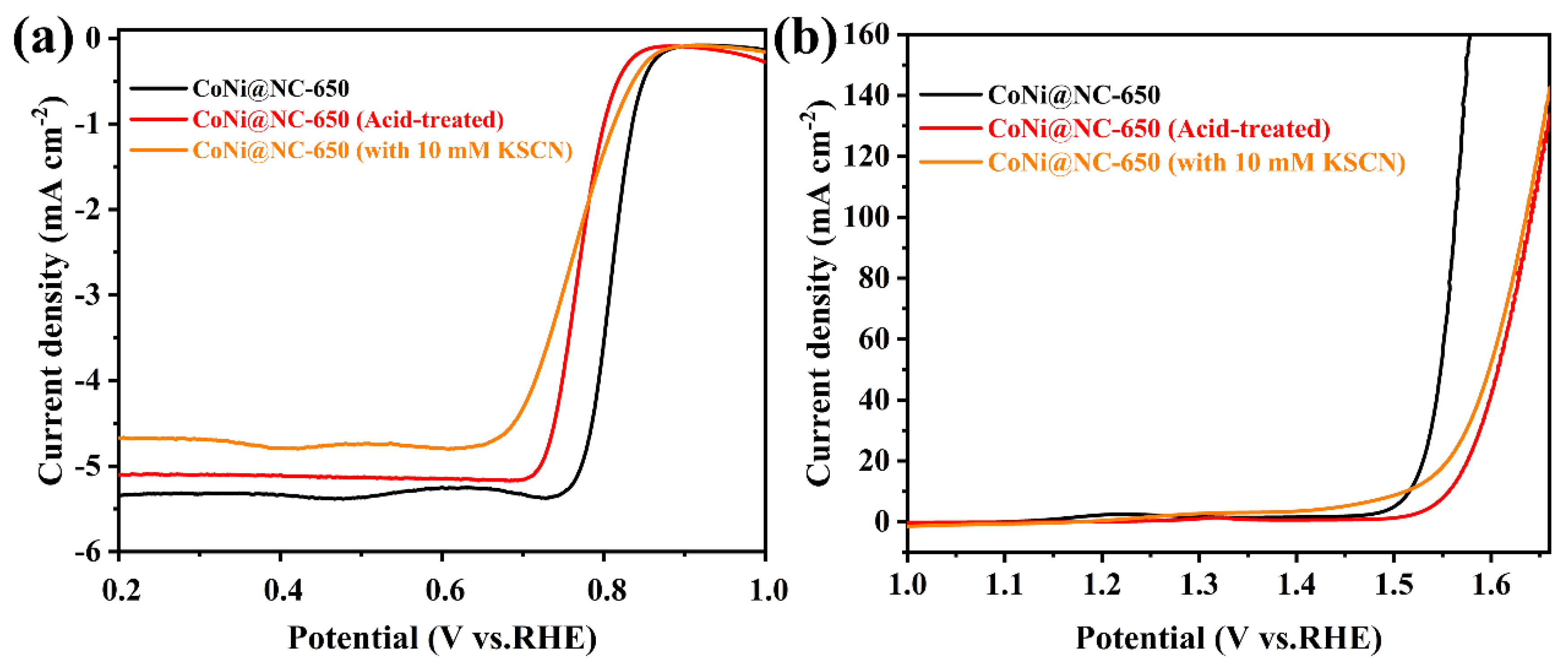
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, L.; Hu, Y.; Liu, R.; Mao, Y.; Balogun MS, J.T.; Tong, Y. Co-based MOF-derived Co/CoN/Co2P ternary composite embedded in N- and P-doped carbon as bifunctional nanocatalysts for efficient overall water splitting. Int. J. Hydrogen Energy 2019, 44, 11402–11410. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, W.; Dong, J.; Lu, X.F.; Lou, X.W.D. Intramolecular electronic coupling in porous iron cobalt (oxy) phosphide nanoboxes enhances the electrocatalytic activity for oxygen evolution. Energy Environ. Sci. 2019, 12, 3348–3355. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, B.; Li, H.; Ni, B.; Wang, K.; Zhang, Q.; Wang, X. Trimetallic Sulfide Mesoporous Nanospheres as Superior Electrocatalysts for Rechargeable Zn–Air Batteries. Adv. Energy Mater. 2018, 8, 1801839. [Google Scholar] [CrossRef]
- Singh, S.K.; Kashyap, V.; Manna, N.; Bhange, S.N.; Soni, R.; Boukherroub, R.; Szunerits, S.; Kurungot, S. Efficient and durable oxygen reduction electrocatalyst based on CoMn alloy oxide nanoparticles supported over N-doped porous graphene. ACS Catal. 2017, 7, 6700–6710. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, J.; Adimi, S.; Shen, H.; Yang, M. Zirconium nitride catalysts surpass platinum for oxygen reduction. Nat. Mater. 2020, 19, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Chisaka, M.; Yamamoto, Y.; Itagaki, N.; Hattori, Y. Active Site Formation for Oxygen Reduction Reaction on Carbon-Support-Free Titanium Oxynitride with Boosted Activity in Acidic Media. ACS Appl. Energy Mater. 2017, 1, 211–219. [Google Scholar] [CrossRef]
- Hu, E.; Yu, X.Y.; Chen, F.; Wu, Y.; Hu, Y.; Lou, X.W.D. Graphene Layers-Wrapped Fe/Fe5C2 Nanoparticles Supported on N-doped Graphene Nanosheets for Highly Efficient Oxygen Reduction. Adv. Energy Mater. 2018, 8, 1702476. [Google Scholar] [CrossRef]
- Wang, X.; Na, Z.; Yin, D.; Wang, C.; Wang, L. Phytic Acid-Assisted Formation of Hierarchical Porous CoP/C Nanoboxes for Enhanced Lithium Storage and Hydrogen Generation. ACS Nano 2018, 12, 12238–12246. [Google Scholar] [CrossRef] [PubMed]
- Gui, F.; Jin, Q.; Xiao, D.; Jin, Z.; Zhang, Y.; Cao, Y.; Yang, M.; Tan, Q.; Zhang, C.; Siahrostami, S.; et al. High-performance zinc–air batteries enabled by hybridizing atomically dispersed FeN2 with Co3O4 nanoparticles. J. Mater. Chem. A 2023, 11, 1312–1323. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Shi, Z.; Wang, X.; Yang, J.; Xiao, M.; Ge, J.; Xing, W.; Liu, C. Recent developments of iridium-based catalysts for the oxygen evolution reaction in acidic water electrolysis. J. Mater. Chem. A 2022, 10, 13170–13189. [Google Scholar] [CrossRef]
- Yin, S.; Li, G.; Qu, X.M.; Zhang, J.; Sun, S.G. Self-Template Synthesis of Atomically Dispersed Fe/N-Codoped Nanocarbon as Efficient Bifunctional Alkaline Oxygen Electrocatalyst. ACS Appl. Energy Mater. 2019, 3, 625–634. [Google Scholar] [CrossRef]
- Su, C.Y.; Cheng, H.; Li, W.; Liu, Z.Q.; Li, N.; Hou, Z.; Bai, F.Q.; Zhang, H.X.; Ma, T.Y. Atomic Modulation of FeCo–Nitrogen–Carbon Bifunctional Oxygen Electrodes for Rechargeable and Flexible All-Solid-State Zinc–Air Battery. Adv. Energy Mater. 2017, 7, 1602420. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Wang, C.; Wu, Z.Y.; Xiong, Y.; Xu, Q.; Yu, S.H.; Jiang, H.L. From Bimetallic Metal-Organic Framework to Porous Carbon: High Surface Area and Multicomponent Active Dopants for Excellent Electrocatalysis. Adv. Mater. 2015, 27, 5010–5016. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, G.; Wang, Y.; Han, X.; Liu, P. MOFs derived magnetic porous carbon microspheres constructed by core-shell Ni@C with high-performance microwave absorption. J. Mater. Sci. Technol.-Shenyang 2021, 88, 56–65. [Google Scholar] [CrossRef]
- Liu, P.; Gao, S.; Wang, Y.; Huang, Y.; Zhou, F.; Liu, P. Magnetic porous N-doped carbon composites with adjusted composition and porous microstructure for lightweight microwave absorbers. Carbon 2021, 173, 655–666. [Google Scholar] [CrossRef]
- Liu, W.; Duan, P.; Mei, C.; Wan, K.; Zhang, B.; Su, H.; Zhang, X.; Wang, J.; Zou, Z. Melamine-induced formation of carbon nanotubes assembly on metal–organic framework-derived Co/C composites for lightweight and broadband microwave absorption. Dalton Trans. 2021, 50, 6222–6231. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Liu, X.; Pu, Z.; Li, W.; Mu, S. From 3D ZIF Nanocrystals to Co-Nx/C Nanorod Array Electrocatalysts for ORR, OER and Zn-air Batteries. Adv. Funct. Mater. 2018, 28, 1704638. [Google Scholar] [CrossRef]
- Qian, Y.; An, T.; Birgersson, K.E.; Liu, Z.; Zhao, D. Web-like interconnected carbon networks from NaCl-assisted pyrolysis of ZIF-8 for highly efficient oxygen reduction catalysis. Small 2018, 14, 1704169. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hu, L.; Wang, N.; Li, Y.; Zhao, D.; Li, L.; Peng, X.; Cui, Z.; Ma, L.-J.; Tian, Y. Surface confinement assisted synthesis of nitrogen-rich hollow carbon cages with Co nanoparticles as breathable electrodes for Zn-air batteries. Appl. Catal. B Environ. 2019, 254, 55–65. [Google Scholar] [CrossRef]
- Bao, Y.X.; Yan, Y.; Nan, L.; Hao, B.W.; Xin, W. A metal–organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy 2016, 1, 15006. [Google Scholar]
- Kundu, A.; Samanta, A.; Raj, C.R. Hierarchical Hollow MOF-Derived Bamboo-like N-doped Carbon Nanotube-Encapsulated Co0.25Ni0.75 Alloy: An Efficient Bifunctional Oxygen Electrocatalyst for Zinc-Air Battery. ACS Appl. Mater. Interfaces 2021, 13, 30486–30496. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Ghatak, A.; Bhattacharyya, S.; Raj, C.R. Transition metal alloy integrated tubular carbon hybrid nanostructure for bifunctional oxygen electrocatalysis. Electrochim. Acta 2020, 348, 136274. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, Y. Metal-organic framework-derived CoNi-embedded carbon nanocages as efficient electrocatalysts for oxygen evolution reaction. Ionics 2017, 24, 1773–1780. [Google Scholar] [CrossRef]
- Fu, J.; Hassan, F.M.; Zhong, C.; Lu, J.; Liu, H.; Yu, A.; Chen, Z. Defect engineering of chalcogen-tailored oxygen electrocatalysts for rechargeable quasi-solid-state zinc–air batteries. Adv. Mater. 2017, 29, 1702526. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, Z.; Cai, J.; Liu, B.; Zhang, Y.; Gong, X.; Sui, X.; Yu, A.; Zhao, L.; Wang, Z.; et al. Template-guided synthesis of Co nanoparticles embedded in hollow nitrogen doped carbon tubes as a highly efficient catalyst for rechargeable Zn-air batteries. Nano Energy 2020, 71, 104592. [Google Scholar] [CrossRef]
- Cui, X.; Yang, S.; Yan, X.; Leng, J.; Shuang, S.; Ajayan, P.M.; Zhang, Z. Pyridinic-nitrogen-Dominated graphene aerogels with Fe–N–C coordination for highly efficient oxygen reduction reaction. Adv. Funct. Mater. 2016, 26, 5708–5717. [Google Scholar] [CrossRef]
- Chen, K.; Sun, Z.; Fang, R.; Shi, Y.; Cheng, H.M.; Li, F. Metal–organic frameworks (MOFs)-Derived nitrogen-doped porous carbon anchored on graphene with multifunctional effects for lithium–sulfur batteries. Adv. Funct. Mater. 2018, 28, 1707592. [Google Scholar] [CrossRef]
- Bagotzky, V.S.; Tarasevich, M.R.; Radyushkina, K.A.; Levina, O.A.; Andrusyova, S.I. Electrocatalysis of the oxygen reduction process on metal chelates in acid electrolyte. J. Power Sources 1978, 2, 233–240. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Wang, M.R.; Lai, Y.Q.; Li, X.Y. Nitrogen-doped microporous carbon: An efficient oxygen reduction catalyst for Zn-air batteries. J. Power Sources 2017, 359, 71–79. [Google Scholar] [CrossRef]
- Guo, D.; Tian, Z.; Wang, J.; Ke, X.; Zhu, Y. Co2N nanoparticles embedded N-doped mesoporous carbon as efficient electrocatalysts for oxygen reduction reaction. Appl. Surf. Sci. 2019, 473, 555–563. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, L.; Chen, Z.; Zhong, L.; Zhao, D.; Chi, X.; Zhao, X.; Li, L.; Lu, X.; Leng, K. Hierarchically porous carbon plates derived from wood as bifunctional ORR/OER electrodes. Adv. Mater. 2019, 31, 1900341. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sui, X.; Zhou, Q.Y.; Li, J.Z.; Zhang, J.J.; Huang, G.S.; Wang, Z.B. 1D N-doped hierarchically porous hollow carbon tubes derived from a supramolecular template as metal-free electrocatalysts for a highly efficient oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 6212–6219. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Z.; Gao, S.; Su, J.; Lun, Z.Y.; Xia, G.; Chen, J.; Zhang, R.; Chen, Q. Tuning Electronic Structures of Nonprecious Ternary Alloys Encapsulated in Graphene Layers for Optimizing Overall Water Splitting Activity. ACS Catal. 2016, 7, 469–479. [Google Scholar] [CrossRef]
- Gewirth, A.A.; Varnell, J.A.; DiAscro, A.M. Nonprecious metal catalysts for oxygen reduction in heterogeneous aqueous systems. Chem. Rev. 2018, 118, 2313–2339. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Lu, X.F.; Xia, B.Y.; Lou, X. Advanced Electrocatalysts for the Oxygen Reduction Reaction in Energy Conversion Technologies. Joule 2020, 4, 45–68. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, C.; Yuan, H.; Zhang, W.; Zheng, H.; Cao, R. PVP-assisted transformation of a metal–organic framework into Co-embedded N-enriched meso/microporous carbon materials as bifunctional electrocatalysts. Chem. Commun. 2018, 54, 7519–7522. [Google Scholar] [CrossRef]
- Tang, X.; Cao, R.; Li, L.; Huang, B.; Zhai, W.; Yuan, K.; Chen, Y. Engineering efficient bifunctional electrocatalysts for rechargeable zinc–air batteries by confining Fe–Co–Ni nanoalloys in nitrogen-doped carbon nanotube@ nanosheet frameworks. J. Mater. Chem. A 2020, 8, 25919–25930. [Google Scholar] [CrossRef]
- Feng, X.; Bo, X.; Guo, L. CoM(M=Fe,Cu,Ni)-embedded nitrogen-enriched porous carbon framework for efficient oxygen and hydrogen evolution reactions. J. Power Sources 2018, 389, 249–259. [Google Scholar] [CrossRef]
- Liu, P.; Gao, S.; Wang, Y.; Huang, Y.; Wang, Y.; Luo, J. Core–shell CoNi@ graphitic carbon decorated on B, N-codoped hollow carbon polyhedrons toward lightweight and high-efficiency microwave attenuation. ACS Appl. Mater. Interfaces 2019, 11, 25624–25635. [Google Scholar] [CrossRef]
- Wen, S.; Yang, T.; Zhao, N.; Ma, L.; Liu, E. Ni-Co-Mo-O nanosheets decorated with NiCo nanoparticles as advanced electrocatalysts for highly efficient hydrogen evolution. Appl. Catal. B Environ. 2019, 258, 117953. [Google Scholar] [CrossRef]
- Li, Q.; Xu, P.; Gao, W.; Ma, S.; Zhang, G.; Cao, R.; Cho, J.; Wang, H.L.; Wu, G. Graphene/graphene-tube nanocomposites templated from cage-containing metal-organic frameworks for oxygen reduction in Li–O2 batteries. Adv. Mater. 2014, 26, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Begum, H.; Ahmed, M.S.; Jung, S. Template-free synthesis of polyacrylonitrile-derived porous carbon nanoballs on graphene for efficient oxygen reduction in zinc–air batteries. J. Mater. Chem. A 2021, 9, 9644–9654. [Google Scholar] [CrossRef]
- Huang, W.; Li, J.; Liao, X.; Lu, R.; Ling, C.; Liu, X.; Meng, J.; Qu, L.; Lin, M.; Hong, X. Ligand modulation of active sites to promote electrocatalytic oxygen evolution. Adv. Mater. 2022, 4, 2200270. [Google Scholar] [CrossRef]
- Huang, W.; Chen, C.; Ling, Z.; Li, J.; Qu, L.; Zhu, J.; Yang, W.; Wang, M.; Owusu, K.A.; Qin, L. Ni/Fe based bimetallic coordination complexes with rich active sites for efficient oxygen evolution reaction. Chem. Eng. J. 2021, 405, 126959. [Google Scholar] [CrossRef]
- Chung, H.T.; Won, J.H.; Zelenay, P. Active and stable carbon nanotube/nanoparticle composite electrocatalyst for oxygen reduction. Nat. Commun. 2013, 4, 1922. [Google Scholar] [CrossRef]
- Jaouen, F.; Serventi, A.M.; Lefèvre, M.; Dodelet, J.P.; Bertrand, P. Non-noble electrocatalysts for O2 reduction: How does heat treatment affect their activity and structure? Part II. Structural changes observed by electron microscopy, Raman, and mass spectroscopy. J. Phys. Chem. C 2007, 111, 5971–5976. [Google Scholar] [CrossRef]
- Li, L.; Dai, P.; Gu, X.; Wang, Y.; Yan, L.; Zhao, X. High oxygen reduction activity on a metal–organic framework derived carbon combined with high degree of graphitization and pyridinic-N dopants. J. Mater. Chem. A 2017, 5, 789–795. [Google Scholar] [CrossRef]
- Cui, X.; Ren, P.; Deng, D.; Deng, J.; Bao, X. Single layer graphene encapsulating non-precious metals as high-performance electrocatalysts for water oxidation. Energy Environ. Sci. 2016, 9, 123–129. [Google Scholar] [CrossRef]
- Deng, D.; Novoselov, K.S.; Fu, Q.; Zheng, N.; Tian, Z.; Bao, X. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 2016, 11, 218. [Google Scholar] [CrossRef]
- Song, L.; Wang, T.; Wang, Y.; Xue, H.; Fan, X.; Guo, H.; Xia, W.; Gong, H.; He, J. Porous iron-tungsten carbide electrocatalyst with high activity and stability toward oxygen reduction reaction: From the self-assisted synthetic mechanism to its active-species probing. ACS Appl. Mater. Interfaces 2017, 9, 3713–3722. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, J.; Tian, C.; Zhao, W.; Li, P.; Liu, W.; Song, L.; Liu, Y.; Wang, C.-A.; Xie, Z. MOF-Derived CoNi Nanoalloy Particles Encapsulated in Nitrogen-Doped Carbon as Superdurable Bifunctional Oxygen Electrocatalyst. Nanomaterials 2023, 13, 715. https://doi.org/10.3390/nano13040715
Wang L, Liu J, Tian C, Zhao W, Li P, Liu W, Song L, Liu Y, Wang C-A, Xie Z. MOF-Derived CoNi Nanoalloy Particles Encapsulated in Nitrogen-Doped Carbon as Superdurable Bifunctional Oxygen Electrocatalyst. Nanomaterials. 2023; 13(4):715. https://doi.org/10.3390/nano13040715
Chicago/Turabian StyleWang, Li, Jiewen Liu, Chuanjin Tian, Wenyan Zhao, Pengzhang Li, Wen Liu, Liang Song, Yumin Liu, Chang-An Wang, and Zhipeng Xie. 2023. "MOF-Derived CoNi Nanoalloy Particles Encapsulated in Nitrogen-Doped Carbon as Superdurable Bifunctional Oxygen Electrocatalyst" Nanomaterials 13, no. 4: 715. https://doi.org/10.3390/nano13040715
APA StyleWang, L., Liu, J., Tian, C., Zhao, W., Li, P., Liu, W., Song, L., Liu, Y., Wang, C.-A., & Xie, Z. (2023). MOF-Derived CoNi Nanoalloy Particles Encapsulated in Nitrogen-Doped Carbon as Superdurable Bifunctional Oxygen Electrocatalyst. Nanomaterials, 13(4), 715. https://doi.org/10.3390/nano13040715






