Selective Thermal and Photocatalytic Decomposition of Aqueous Hydrazine to Produce H2 over Ag-Modified TiO2 Nanomaterial
Abstract
1. Introduction
2. Experimental Procedure
2.1. Chemicals
2.2. Synthesis of Ag-Modified TiO2 Nanomaterial
2.3. Photocatalytic Activity Test
2.4. Characterization of Synthesized Nanomaterials
3. Results and Discussion
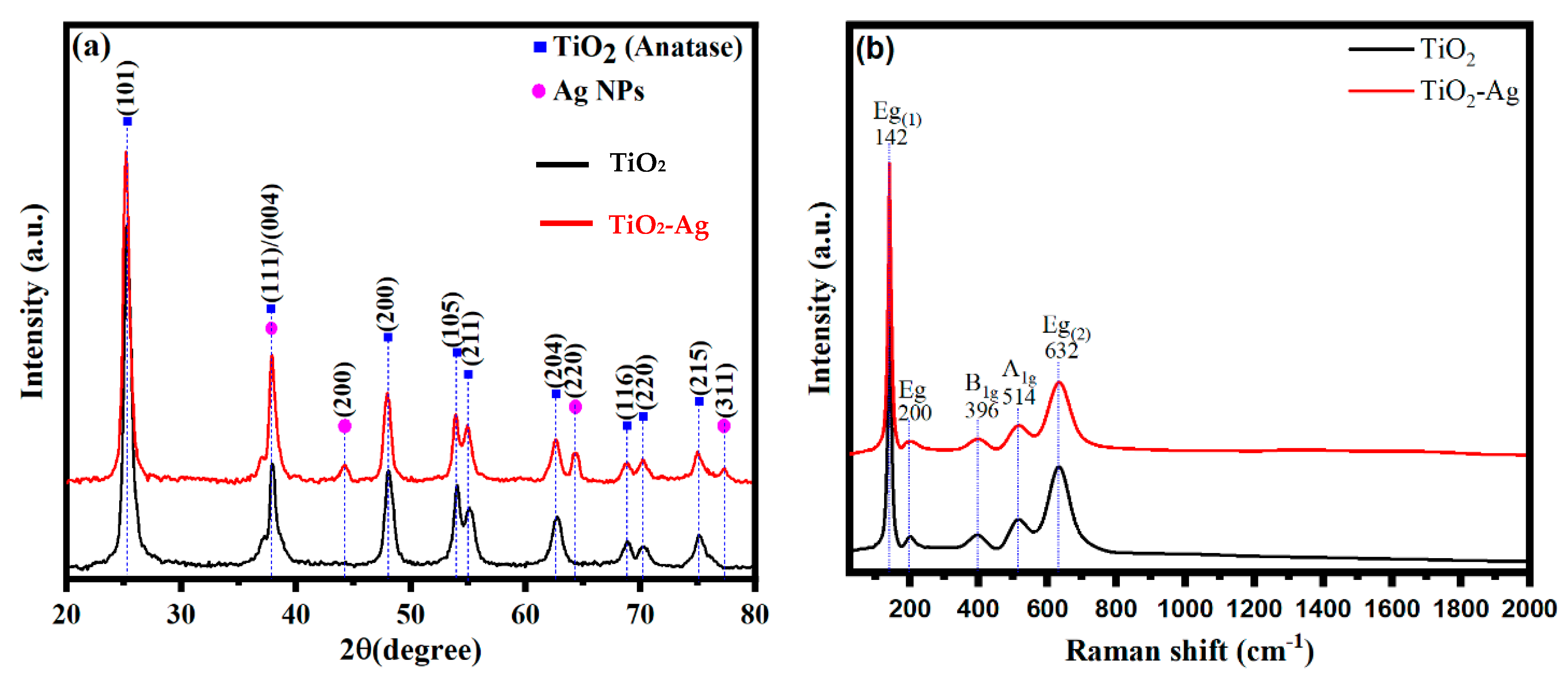
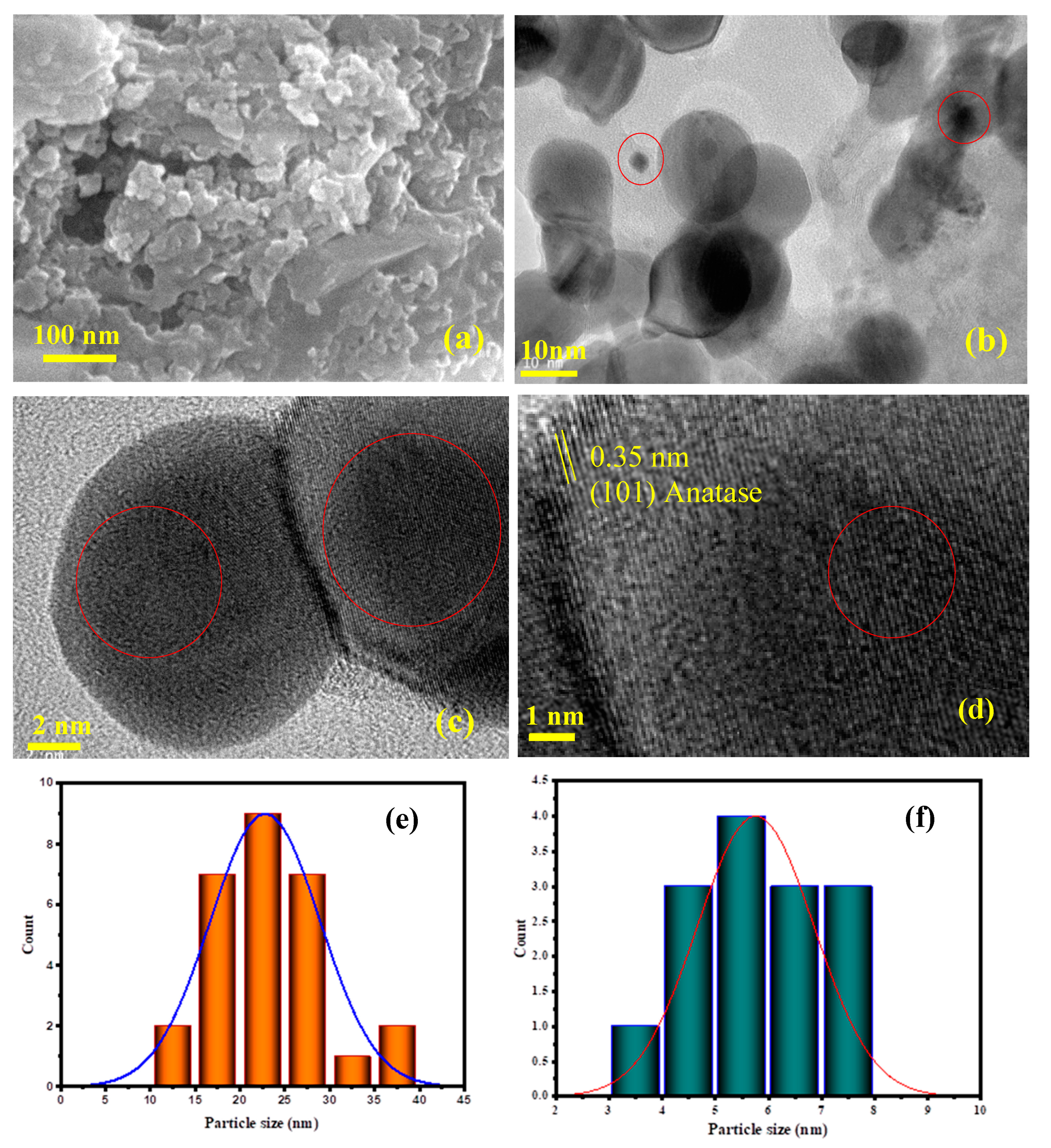
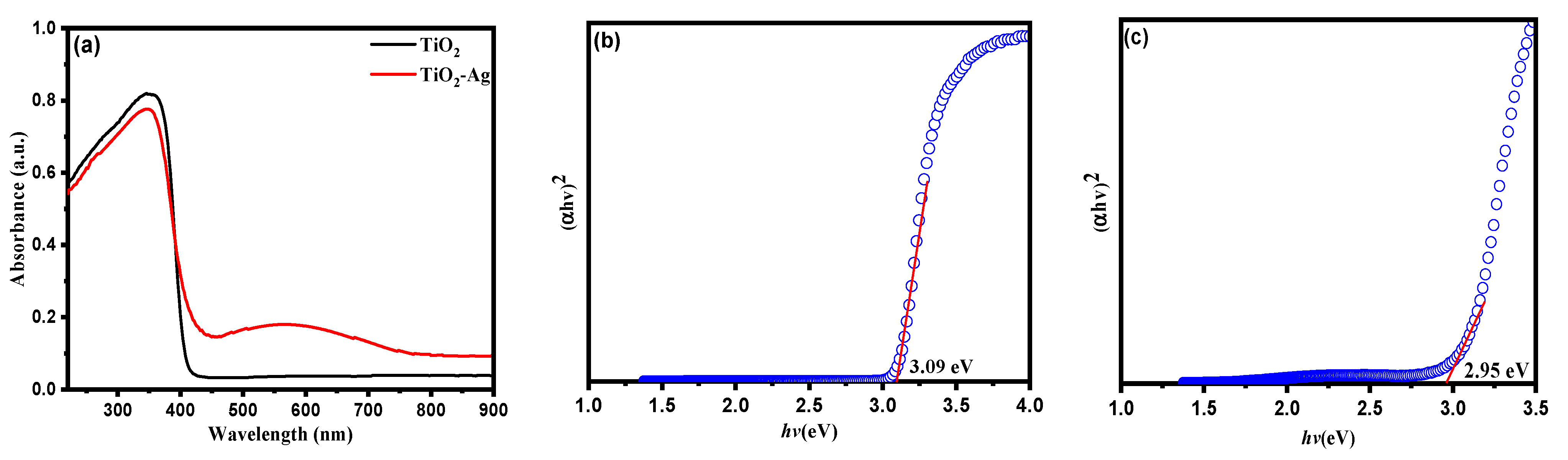
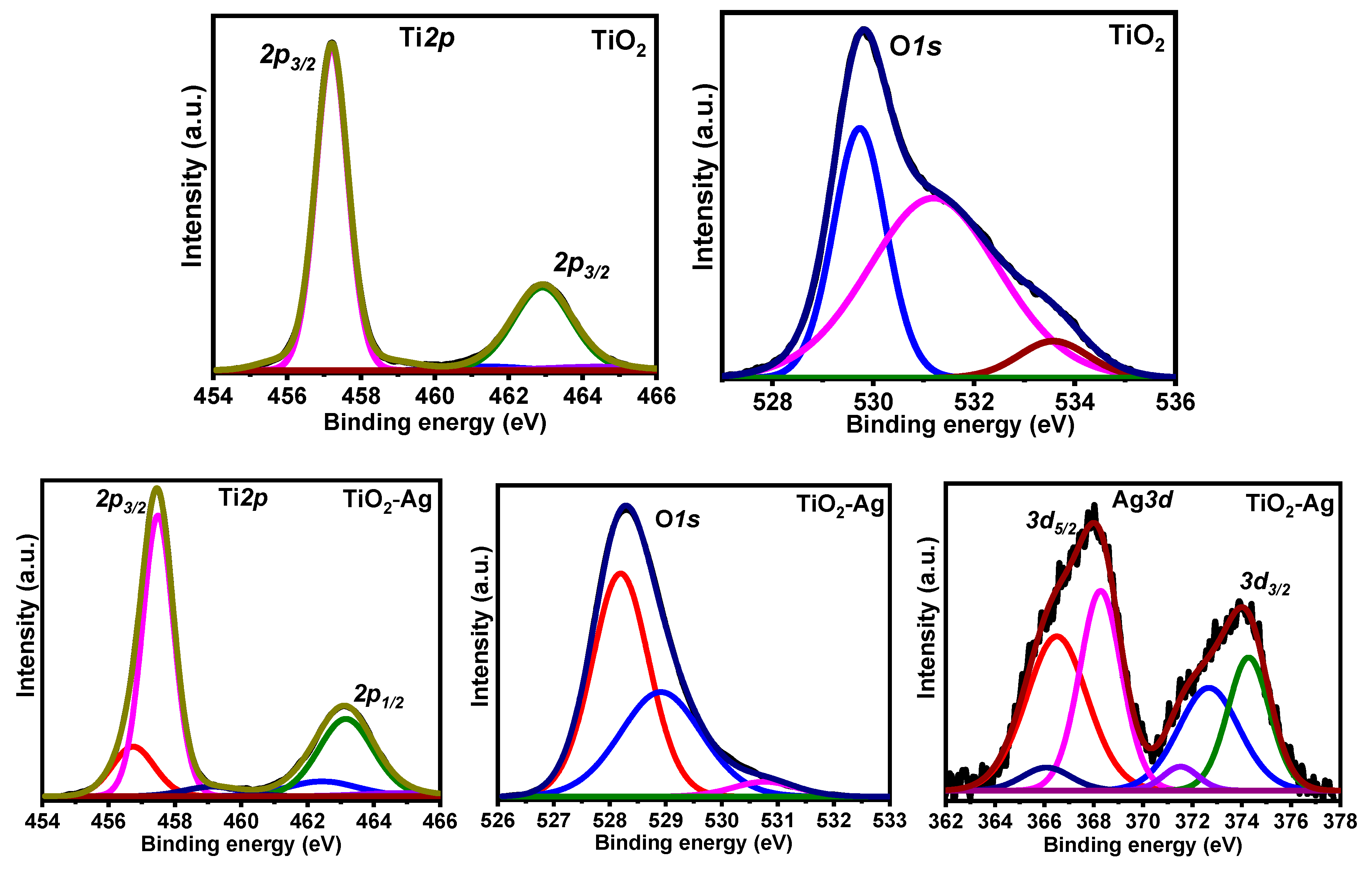
3.1. Catalysts Characterization
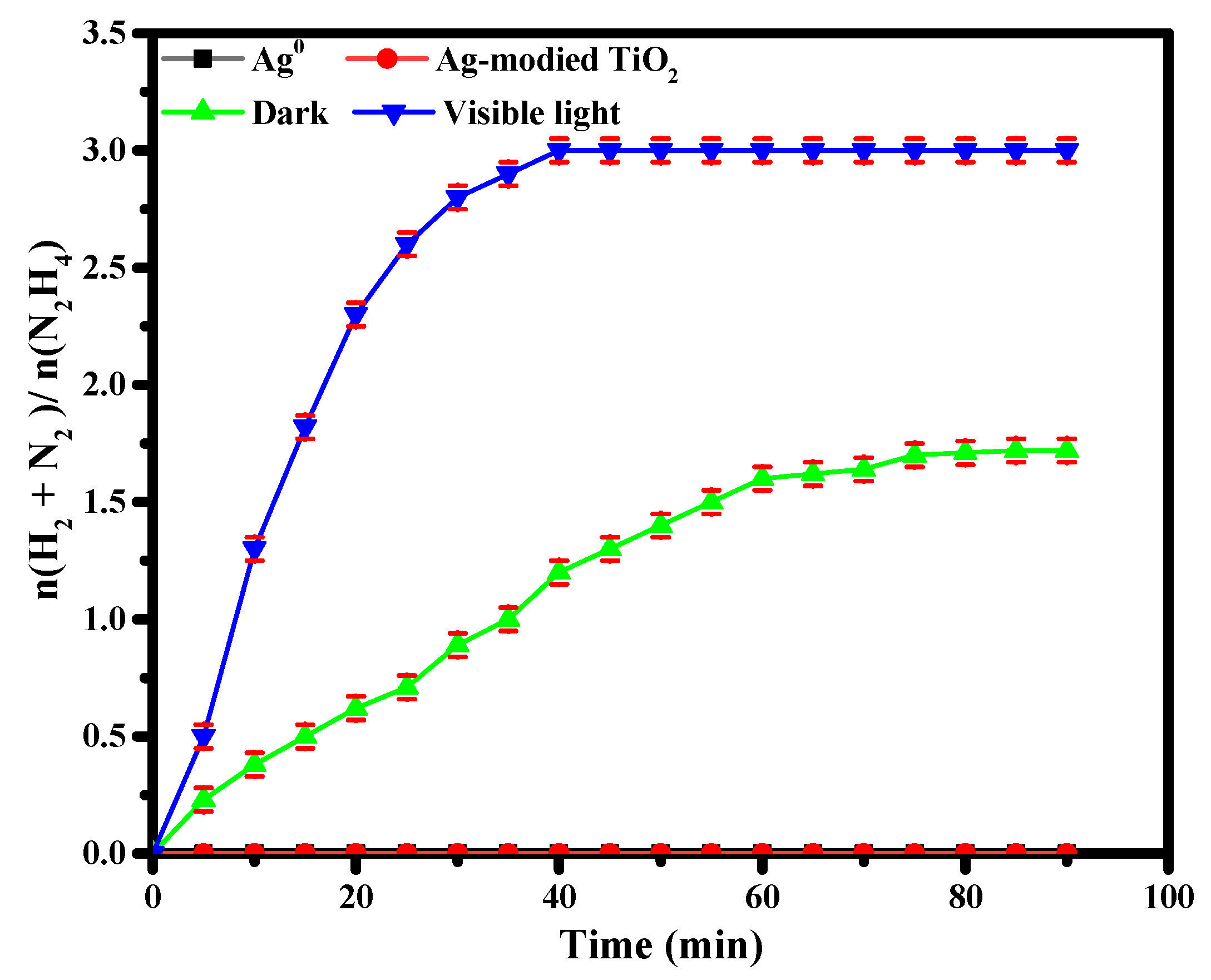
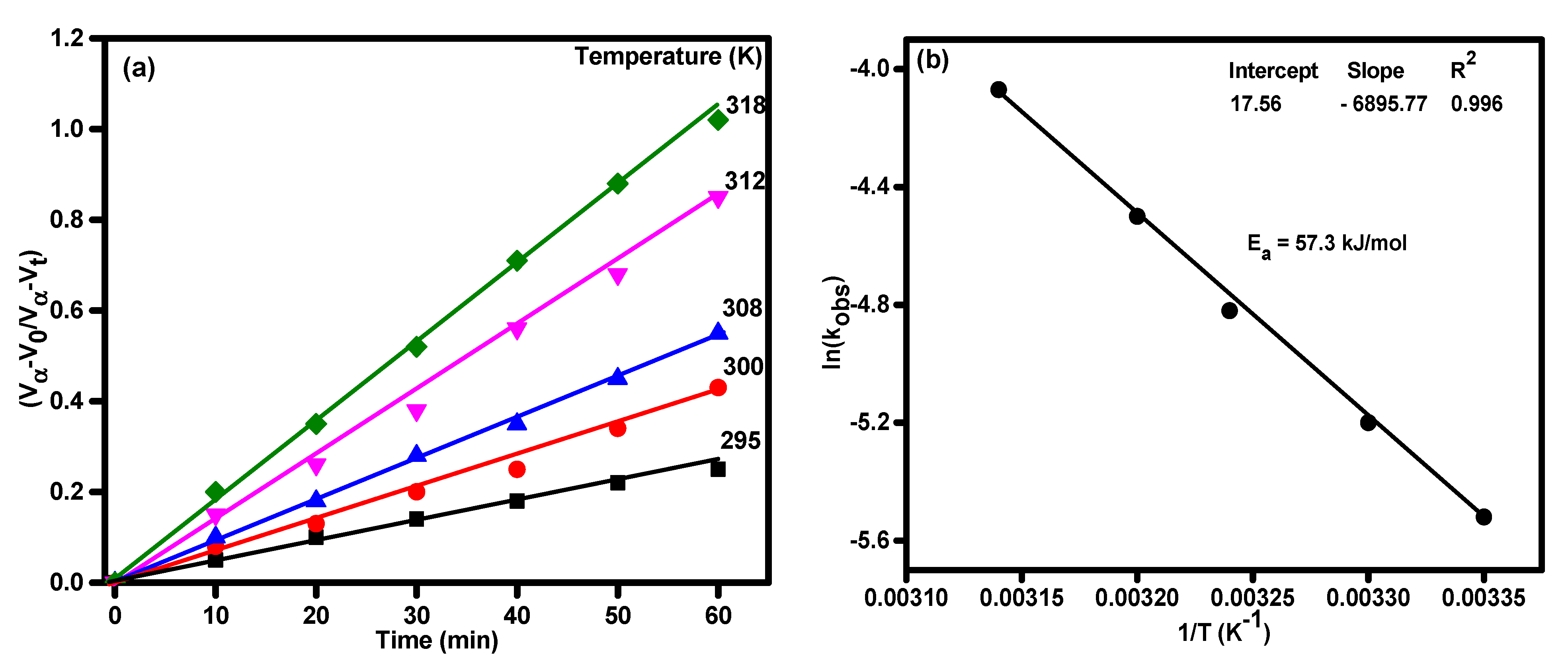
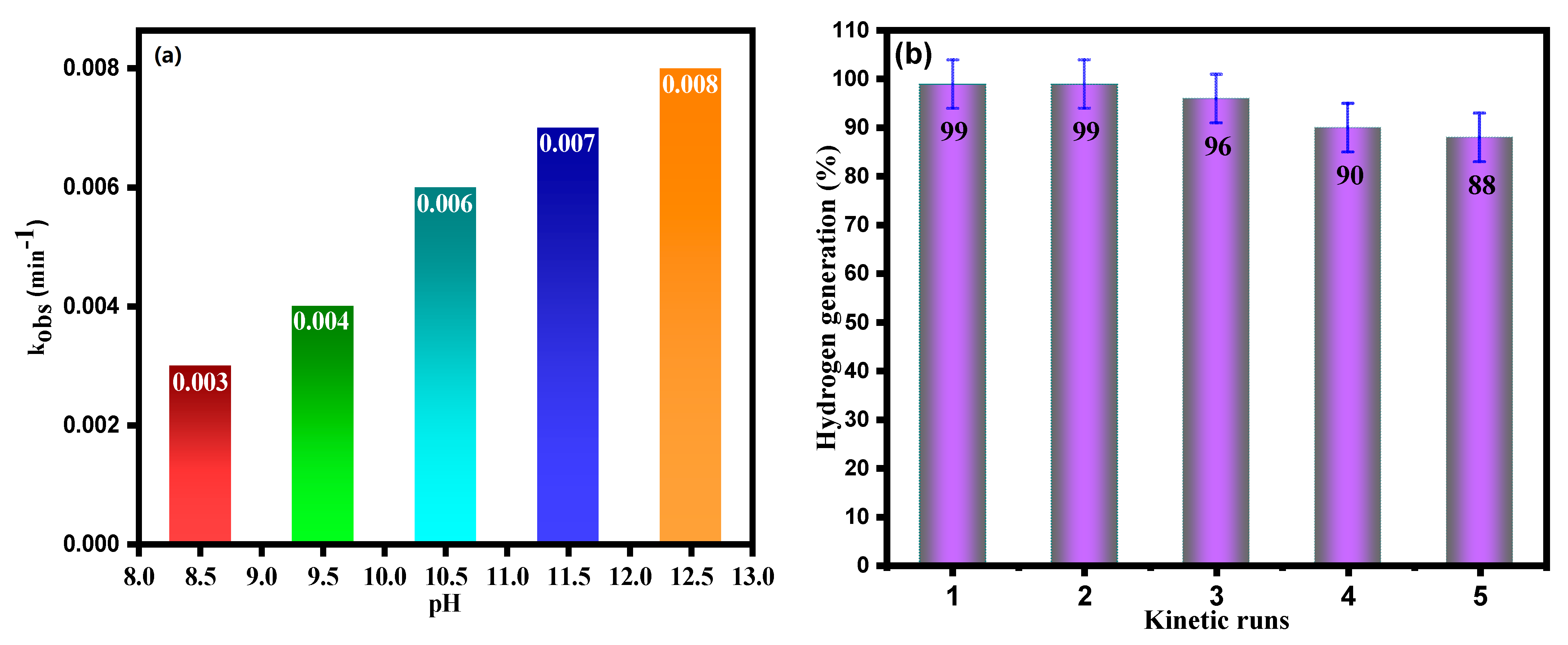
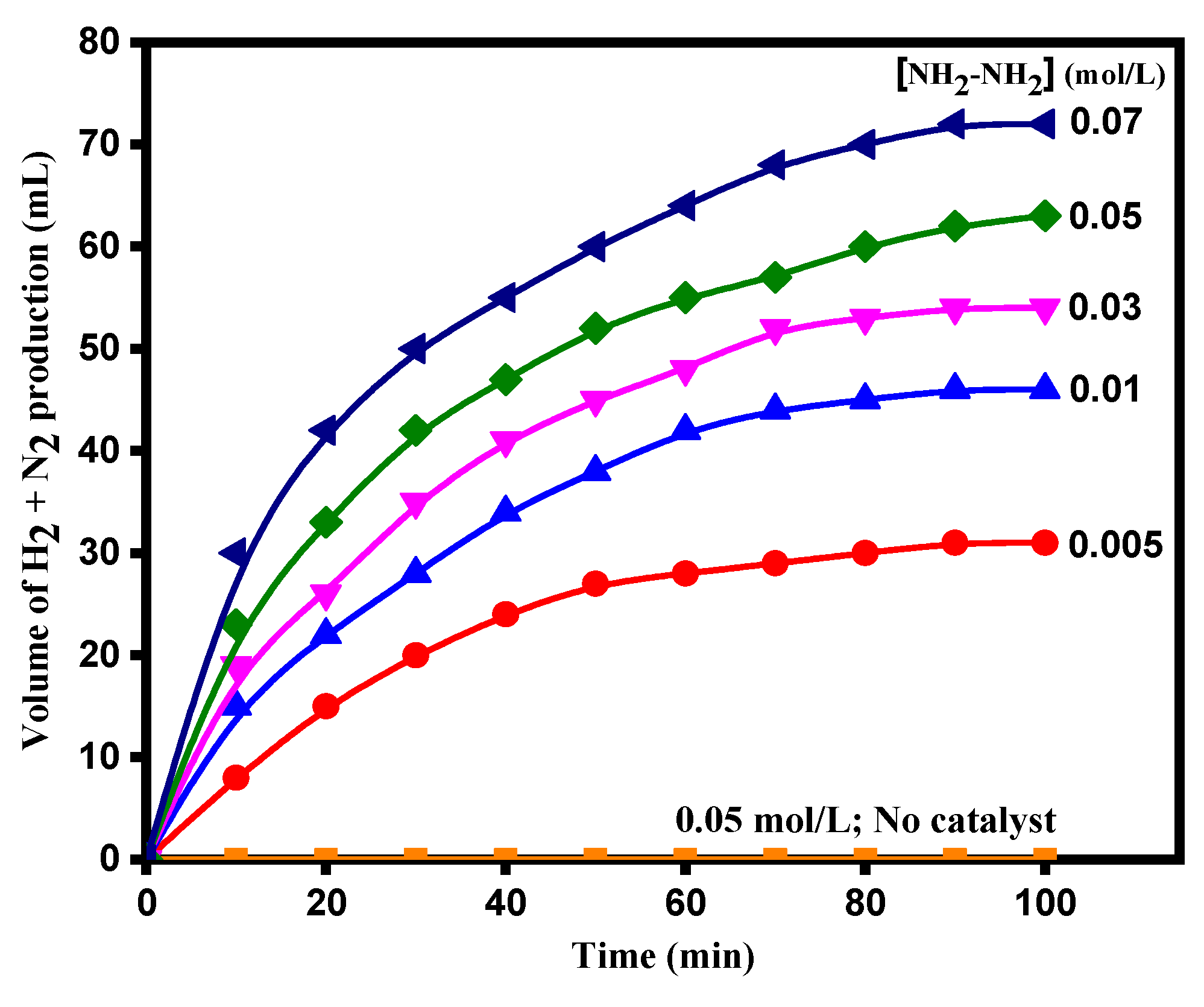
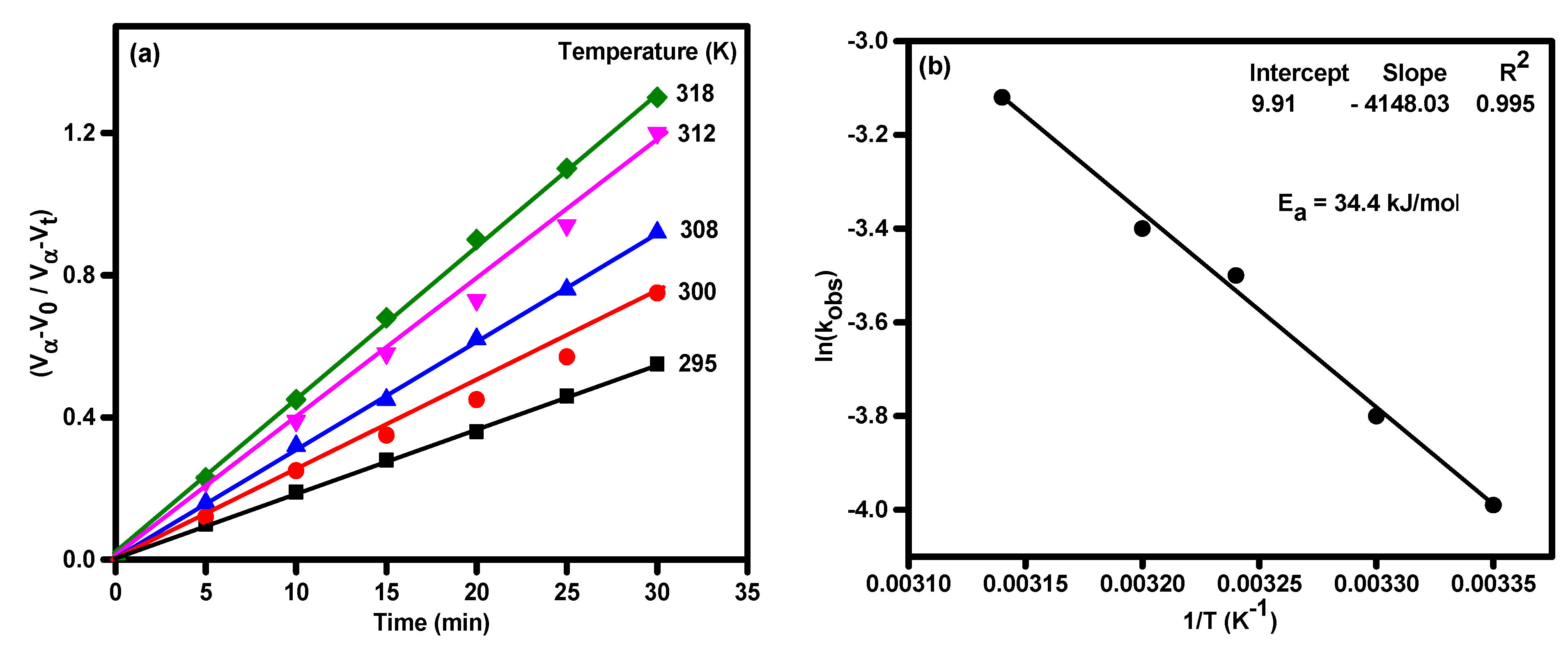
3.2. Catalytic Activity of TiO2 and Ag-Modified TiO2 Nanomaterials for Hydrazine Decomposition
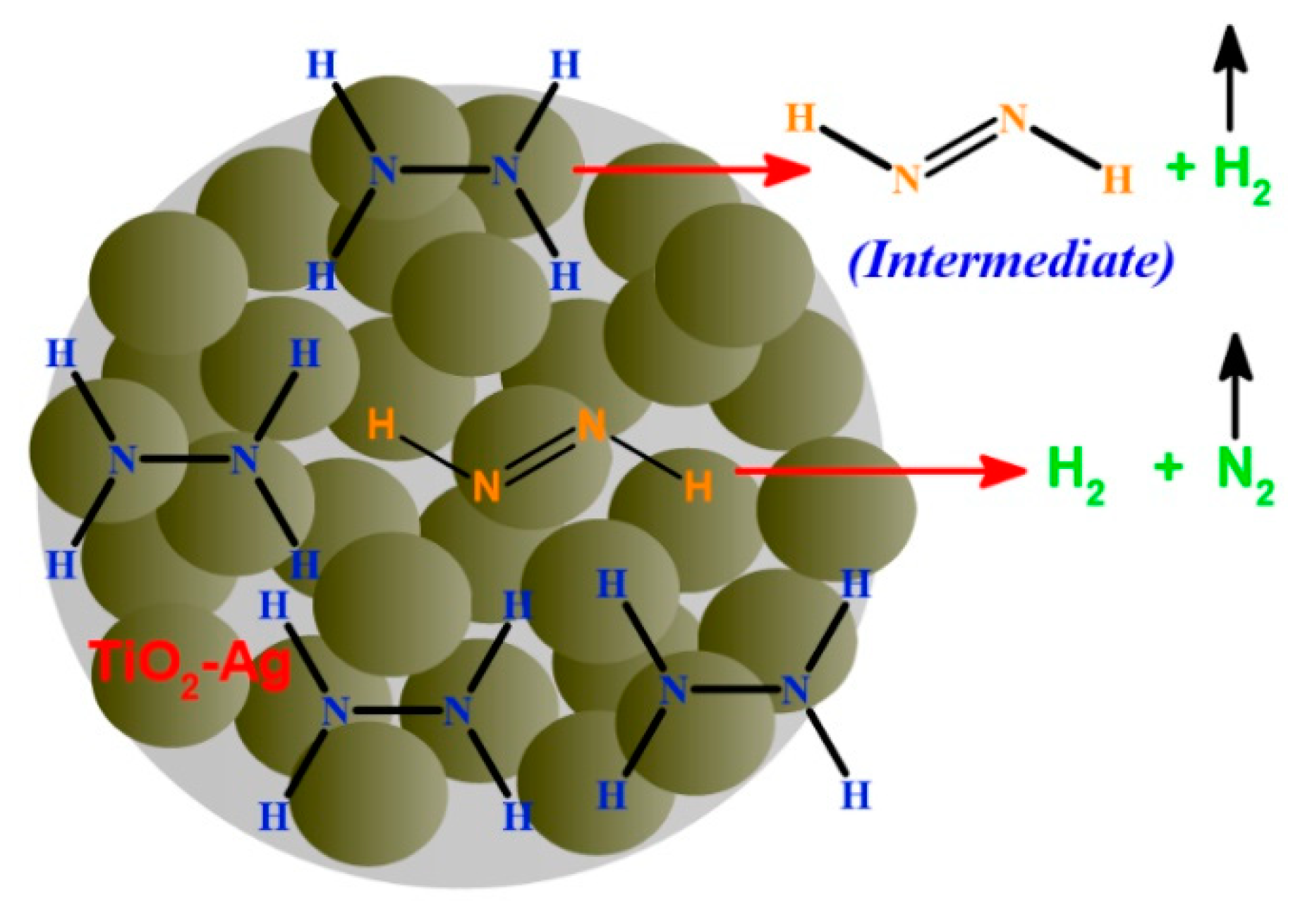
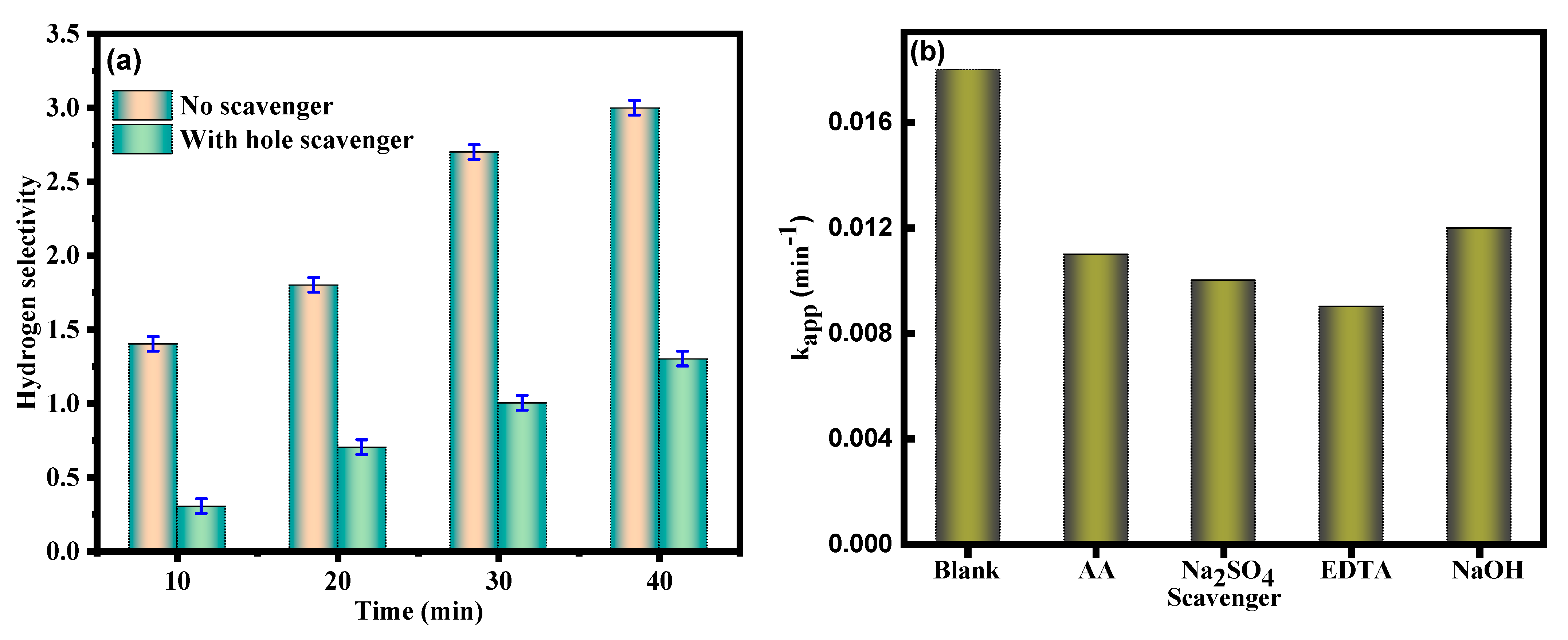
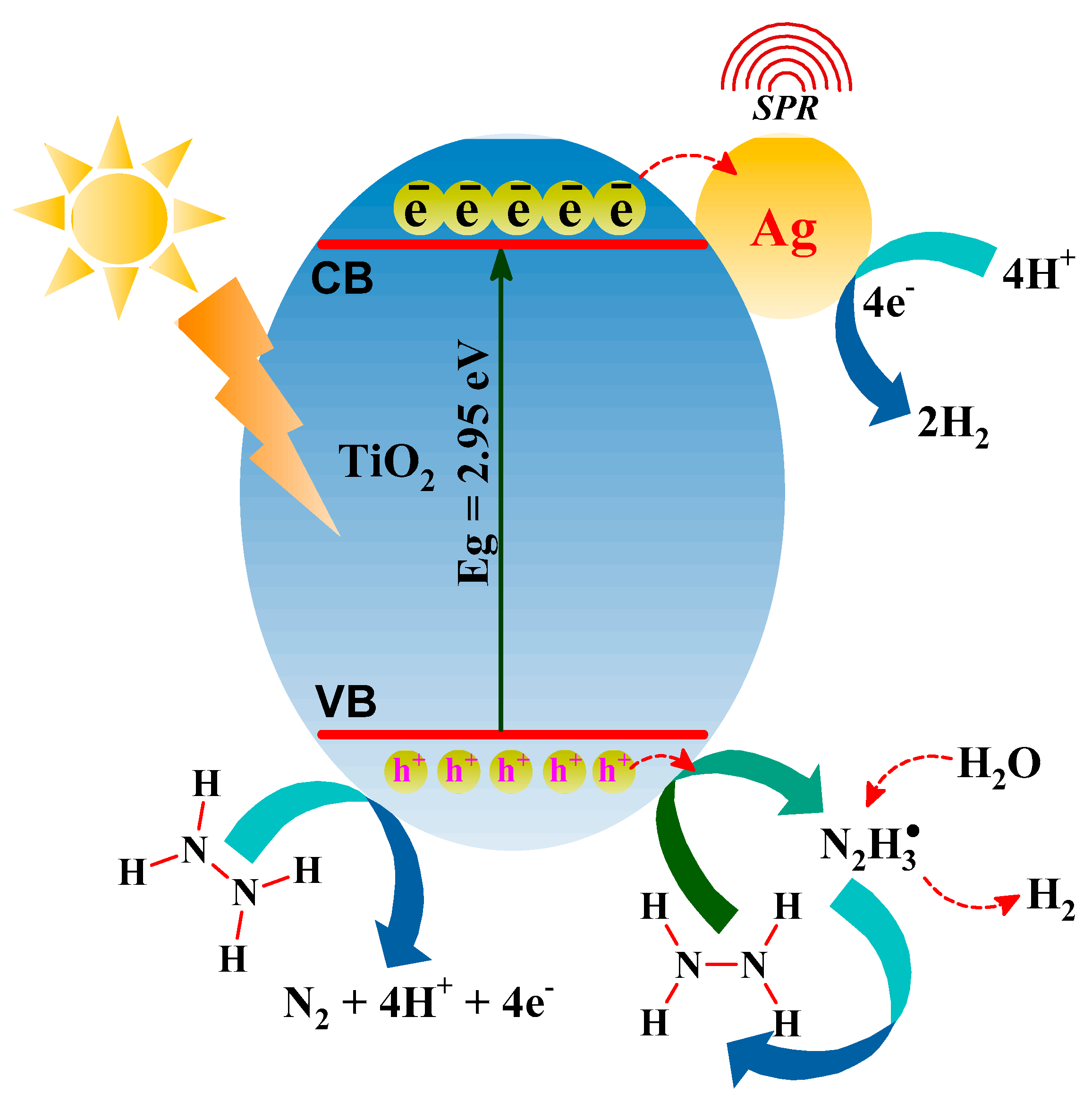
3.3. Mechanism of Photochemical Hydrazine Decomposition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Zou, Z.; Ye, J.; Sayama, K.; Arakawa, H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 2001, 414, 625–627. [Google Scholar] [CrossRef]
- Zhang, G.; Li, G.; Lan, Z.-A.; Lin, L.; Savateev, A.; Heil, T.; Zafeiratos, S.; Wang, X.; Antonietti, M. Optimizing optical absorption, exciton dissociation, and charge transfer of a polymeric carbon nitride with ultrahigh solar hydrogen production activity. Angew. Chem. Int. Ed. 2017, 129, 13630–13634. [Google Scholar] [CrossRef]
- Wei, R.-B.; Huang, Z.-L.; Gu, G.-H.; Wang, Z.; Zeng, L.; Chen, Y.; Liu, Z.-Q. Z-scheme CdS/WO3 on a carbon cloth, enabling effective hydrogen evolution. Appl. Catal. B 2018, 231, 101–107. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Singh, S.K.; Yan, J.-M.; Zhang, X.-B.; Xu, Q. Liquid-phase chemical hydrogen storage: Catalytic hydrogen generation under ambient conditions. ChemSusChem 2010, 3, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Liu, J.; Yun, H.; Li, D.; Cao, Q.; Hou, X.; Xu, Y. Hydrolysis hydrogen generation behavior of mechanico-chemical reaction Mg-M (M = Ni, Ce, and La) binary alloys- A feasible strategy for activating and improving environmental stability. J. Mater. Res. Technol. 2022, 21, 363–372. [Google Scholar] [CrossRef]
- Alberas, D.J.; Kiss, J.; Liu, Z.M.; White, J.M. Surface chemistry of hydrazine on Pt (111). Surf. Sci. 1992, 278, 51–61. [Google Scholar] [CrossRef]
- Chen, J.; Zou, H.; Yao, Q.; Luo, M.; Li, X.; Lu, Z.-H. Cr2O3-modified NiFe nanoparticles as a noble-metal-free catalyst for complete dehydrogenation of hydrazine in aqueous solution. Appl. Surf. Sci. 2020, 501, 144247. [Google Scholar] [CrossRef]
- Motta, D.; Barlocco, I.; Bellomi, S.; Villa, A.; Dimitratos, N. Hydrous Hydrazine Decomposition for Hydrogen Production Using of Ir/CeO2: Effect of Reaction Parameters on the Activity. Nanomaterials 2021, 11, 1340. [Google Scholar] [CrossRef]
- Ding, Q.; Chen, T.; Li, Z.; Feng, Z.; Wang, X. Time-resolved infrared spectroscopic investigation of Ga2O3 photocatalysts loaded with Cr2O3-Rh cocatalysts for photocatalytic water splitting. Chin. J. Catal. 2021, 42, 808–816. [Google Scholar] [CrossRef]
- Fernando, K.A.; Sahu, S.; Liu, Y.; Lewis, W.K.; Guliants, E.A.; Jafariyan, A.; Wang, P.; Bunker, C.E.; Sun, Y.P. Carbon quantum dots and applications in photocatalytic energy conversion. ACS Appl. Mater. Interfaces 2015, 7, 8363–8376. [Google Scholar] [CrossRef] [PubMed]
- Escobedo Salas, S.; Serrano Rosales, B.; de Lasa, H. Quantum yield with platinum modified TiO2 photocatalyst for hydrogen production. Appl. Catal. B Environ. 2013, 140–141, 523–536. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Dozzi, M.V.; Selli, E. TiO2-based materials for photocatalytic hydrogen production. J. Energy Chem. 2017, 26, 250–258. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic Effect of MoS2 and Graphene as Cocatalysts for Enhanced Photocatalytic H2 Production Activity of TiO2 Nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Qin, J.; Shen, X.; Yu, R.; Ma, M.; Liu, R. Porous carbon doped TiO2 on TiC nanostructures for enhanced photocatalytic hydrogen production under visible light. J. Catal. 2017, 347, 36–44. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Volfová, L.; Pližingrová, E.; Ecorchard, P.; Motlochová, M.; Klementová, M.; Jánošíková, P.; Bezdička, P.; Kupčík, J.; Krýsa, J.; Lančok, A.; et al. Tailoring Photocatalytic Activity of TiO2 Nanosheets by 57Fe. J. Phys. Chem. C 2020, 124, 6669–6682. [Google Scholar] [CrossRef]
- Choi, W.Y.; Termin, A.; Hoffmann, M.R. The role of metal ion dopants in quantum sized TiO2: Correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Wessel, M.; Spohr, E. C-, N-, S-, and Fe-Doped TiO2 and SrTiO3 nanotubes for visible-light-driven photocatalytic water splitting: Prediction from first principles. J. Phys. Chem. C 2015, 119, 18686–18696. [Google Scholar]
- Priebe, J.B.; Radnik, J., Jr.; Lennox, A.J.; Pohl, M.-M.; Karnahl, M.; Hollmann, D.; Grabow, K.; Bentrup, U.; Junge, H.; Beller, M. Solar hydrogen production by plasmonic Au–TiO2 catalysts: Impact of synthesis protocol and TiO2 phase on charge transfer efficiency and H2 evolution rates. ACS Catal. 2015, 5, 2137–2148. [Google Scholar] [CrossRef]
- Brady, M.D.; Sampaio, R.N.; Wang, D.; Meyer, T.J.; Meyer, G.J. Dye-sensitized hydrobromic acid splitting for hydrogen solar fuel production. J. Am. Chem. Soc. 2017, 139, 15612–15615. [Google Scholar] [CrossRef]
- Abutalib, M.M.; Alghamdi, H.M.; Rajeh, A.; Nur, O.; Hezma, A.M.; Mannaa, M.A. Fe3O4/Co3O4-TiO2 S-scheme photocatalyst for degradation of organic pollutants and H2 production under natural sunlight. J. Mater. Res. Technol. 2022, 20, 1043–1056. [Google Scholar] [CrossRef]
- Zhao, X.G.; Huang, L.Q. Iridium, carbon, and nitrogen multiple-doped TiO2 nanoparticles with enhanced photocatalytic activity. Ceram. Int. 2017, 43, 3975–3980. [Google Scholar] [CrossRef]
- Díaz, L.; Rodríguez, V.D.; González-Rodríguez, M.; Rodríguez-Castellón, E.; Algarra, M.; Núñez, P.; Moretti, E. M/TiO2 (M = Fe, Co, Ni, Cu, Zn) catalysts for photocatalytic hydrogen production under UV and visible light irradiation. Inorg. Chem. Front. 2021, 8, 3491–3500. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Mamiyev, Z.; Dillert, R.; Zheng, N.; Bahnemann, D.W. Rh/TiO2-Photocatalyzed Acceptorless Dehydrogenation of N-Heterocycles upon Visible-Light Illumination. ACS Catal. 2020, 10, 5542–5553. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Zheng, N.; Dillert, R.; Bahnemann, D.W. Visible-Light-Mediated Photocatalytic Aerobic Dehydrogenation of N-heterocycles by Surface-Grafted TiO2 and 4-amino-TEMPO. ACS Catal. 2019, 9, 10694–10704. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, A.; Queffelec, C.; Gudat, D.; Wang, Q.; Jain, S.L.; Boukherroub, R.; Szunerits, S. Visible light assisted hydrogen generation from complete decomposition of hydrous hydrazine using rhodium modified TiO2 photocatalysts. Photochem. Photobiol. Sci. 2017, 16, 1036–1042. [Google Scholar] [CrossRef]
- Luo, F.; Miao, X.; Chu, W.; Wu, P.; Tong, D.G. Preparation of face-centered-cubic indium nanocubes and their superior dehydrogenation activity towards aqueous hydrazine with the assistance of light. J. Mater. Chem. A 2016, 4, 17665–17672. [Google Scholar] [CrossRef]
- Al-Thubaiti, K.S.; Khan, Z. Trimetallic nanocatalysts to enhanced hydrogen production from hydrous hydrazine: The role of metal centers. Int. J. Hydrogen Energy 2020, 45, 13960–13974. [Google Scholar] [CrossRef]
- Sandoval, A.; Aguilar, A.; Louis, C.; Traverse, A.; Zanella, R. Bimetallic Au–Ag/TiO2 catalyst prepared by deposition–precipitation: High activity and stability in CO oxidation. J. Cataly 2011, 281, 40–49. [Google Scholar] [CrossRef]
- Singh, S.K.; Xu, Q. Bimetallic Ni-Pt nanocatalysts for selective decomposition of hydrazine in aqueous solution to hydrogen at room temperature for chemical hydrogen storage. Inorg. Chem. 2010, 49, 6148–6152. [Google Scholar] [CrossRef]
- Khan, Z. Trimetallic nanoparticles: Synthesis, characterization and catalytic degradation of formic acid for hydrogen generation. Int. J. Hydrogen Energy 2019, 44, 11503–11513. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Pinjari, D.V.; Gogate, P.R.; Sonawane, S.H.; Pandit, A.B. Ultrasound assisted synthesis of doped TiO2 nanoparticles: Characterization and comparison of effectiveness for photocatalytic oxidation of dyestuff effluent. Ultrason. Sonochem. 2013, 20, 277–286. [Google Scholar] [CrossRef]
- Liu, E.; Kang, L.; Yang, Y.; Sun, T.; Hu, X.; Zhu, C.; Liu, H.; Wang, Q.; Li, X.; Fan, J. Plasmonic Ag deposited TiO2 nano-sheet film for enhanced photocatalytic hydrogen production by water splitting. Nanotechnology 2014, 25, 165401. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Sun, S.; Swart, H.C.; Gupta, R.K. Noble metals-TiO2 nanocomposites: From fundamental mechanisms to photocatalysis, surface-enhanced Raman scattering and antibacterial applications. Appl. Mater. Today 2018, 11, 82–135. [Google Scholar] [CrossRef]
- Mills, A.; Hill, G.; Stewart, M.; Graham, D.; Smith, W.E.; Hodgen, S.; Halfpenny, P.J.; Faulds, K.; Robertson, P. Characterization of novel Ag on TiO2 films for surface-enhanced Raman scattering. Appl. Spectrosc. 2004, 58, 922. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, X.; Ruan, W.; Yang, J.; Zhao, B.; Xu, W.; Lombardi, J.R. Charge-transfer-induced surface-enhanced Raman scattering on Ag–TiO2 nanocomposites. J. Phys. Chem. C 2009, 113, 16226–16231. [Google Scholar] [CrossRef]
- Yu, J.G.; Zhang, L.J.; Cheng, B.; Su, Y.R. Hydrothermal preparation and photocatalytic activity of hierarchically sponge-like macro-/mesoporous titania. J. Phys. Chem. C 2007, 111, 10582–10589. [Google Scholar] [CrossRef]
- Zhang, F.X.; Guan, N.J.; Li, Y.Z.; Zhang, X.; Chen, J.X.; Zeng, H.S. Control of morphology of silver clusters coated on titanium dioxide during photocatalysis. Langmuir 2003, 19, 8230–8234. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, J.; Chen, Y.; Qian, Y.; Xu, B.; Chu, W.; An, D. Recent progress of silver-containing photocatalysts for water disinfection under visible light irradiation: A review. Sci. Total Environ. 2022, 804, 150024. [Google Scholar] [CrossRef]
- Waterhouse, G.I.; Bowmaker, G.A.; Metson, J.B. The thermal decomposition of silver (I, III) oxide: A combined XRD, FT-IR and Raman spectroscopic study. Phys. Chem. Chem. Phys. 2001, 3, 3838–3845. [Google Scholar] [CrossRef]
- Makula, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV−Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ferrero, E.; Sakatani, Y.; Boissiere, C.; Grosso, D.; Fuertes, A.; Fraxedas, J.; Sanchez, C. Nanostructured titanium oxynitride porous thin films as efficient visible-active photocatalysts. Adv. Funct. Mater. 2007, 17, 3348–3354. [Google Scholar] [CrossRef]
- Knorr, F.J.C.; Mercado, C.; McHale, J.L. Trap-state distributions and carrier transport in pure and mixed-phase TiO2: Influence of contacting solvent and interfacial electron transfer. J. Phys. Chem. C 2008, 112, 12786–12794. [Google Scholar] [CrossRef]
- Yu, J.; Yue, L.; Liu, S.; Huang, B.; Zhang, X. Hydrothermal preparation and photocatalytic activity of mesoporous Au-TiO2 nanocomposite microspheres. J. Colloid. Interface Sci. 2009, 334, 58–64. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 Surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Hariharan, D.; Thangamuniyandi, P.; Christy, A.J.; Vasantharaja, R.; Selvakumar, P.; Sagadevan, S.; Pugazhendhi, A.; Nehru, L.C. Enhanced photocatalysis and anticancer activity of green hydrothermal synthesized Ag@TiO2 nanoparticles. J. Photochem. Photobiol. B Biol. 2020, 202, 11636. [Google Scholar] [CrossRef]
- Jones, T.E.; Rocha, T.C.R.; Knop-Gericke, A.; Stampfl, C.; Schlogl, R.; Piccinina, S. Thermodynamic and spectroscopic properties of oxygen on silver under an oxygen atmosphere. Phys. Chem. Chem. Phys. 2015, 17, 9288. [Google Scholar] [CrossRef]
- Jawhari, A.H.; Hasan, N.; Radini, I.A.; Malik, M.A.; Narasimharao, K. Pt-Ag/Ag3PO4-WO3 nanocomposites for photocatalytic H2 production from bioethanol. Fuel 2023, 344, 127998. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, D.; Yan, J.; Jia, X.; Li, Y.; Yu, P.; Zhang, T. The pore size distribution and its relationship with shale gas capacity in organic-rich mudstone of Wufeng-Longmaxi Formations, Sichuan Basin, China. J. Nat. Gas. Geosci. 2016, 1, 213–220. [Google Scholar] [CrossRef]
- Dai, H.; Zhong, Y.; Wang, P. Hydrogen generation from decomposition of hydrous hydrazine over Ni-Ir/CeO2 catalyst. Prog. Nat. Sci. Mater. Int. 2017, 27, 121–125. [Google Scholar] [CrossRef]
- Singh, S.K.; Zhang, X.-B.; Xu, Q. Room-temperature hydrogen generation from hydrous hydrazine for chemical hydrogen storage. J. Am. Chem. Soc. 2009, 131, 9894–9895. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.E.K.; Bouhfid, R. Recent progress on Ag/TiO2 photocatalysts: Photocatalytic and bactericidal behaviors. Environ. Sci. Pollut. Res. 2021, 28, 44638–44666. [Google Scholar] [CrossRef]
- Zaheer, Z. Chitosan capped noble metal doped CeO2 nanomaterial: Synthesis, and their enhanced catalytic activities. Int. J. Biol. Macromol. 2021, 166, 1258–1271. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Xu, Q. Nanocatalysts for hydrogen generation from hydrazine. Catal. Sci. Technol. 2013, 3, 1889–1900. [Google Scholar] [CrossRef]
- Liu, J.; Hurt, R.H. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef]
- Gaunt, H.; Wetton, E.A.M. The reaction between hydrazine and oxygen in water. J. Appl. Chem. 1966, 16, 171–176. [Google Scholar] [CrossRef]
- Xue, B.; Sun, T.; Wu, J.-K.; Mao, F.; Yang, W. AgI/TiO2 nanocomposites: Ultrasound-assisted preparation, visible-light-induced photocatalytic degradation of methyl orange and antibacterial activity. Ultrason. Sonochem. 2015, 22, 1–6. [Google Scholar] [CrossRef]
- Oliaee, S.N.; Zhang, C.; Hwang, S.Y.; Cheung, H.M.; Peng, Z. Hydrogen Production via Hydrazine Decomposition on Model Platinum−Nickel Nanocatalyst with a Single (111) Facet. J. Phys. Chem. C 2016, 120, 9764–9772. [Google Scholar] [CrossRef]
- Chen, L.; Huang, R.; Yin, S.F.; Luo, S.L.; Au, C.T. Flower-like Bi2O2CO3: Facile synthesis and their photocatalytic application in the treatment of dye-containing wastewater. Chem. Eng. J. 2012, 193–194, 123–130. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiang, D.; He, T.; Wu, Z.; Chen, M. In-situ ion exchange synthesis of hierarchical AgI/BiOI microsphere photocatalyst with enhanced photocatalytic properties. Cryst. Eng. Comm. 2013, 15, 7556–7563. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Santo, V.D. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ran, J. Facile preparaion and enhanced photocatalytic H2-production activity of Cu(OH)2 cluster modified TiO2. Energy Environ. Sci. 2011, 4, 1364–1371. [Google Scholar] [CrossRef]
- Furube, A.; Hashimoto, S. Insight into plasmonic hot-electron transfer and plasmon molecular drive: New dimensions in energy conversion and nanofabrication. NPG Asia Mater. 2017, 9, e454. [Google Scholar] [CrossRef]
- Miao, X.; Chen, M.M.; Chu, W.; Wu, P.; Tong, D.G. Mesoporous face-centered-cubic In4Ni Alloy nanorices: Superior catalysts for hydrazine dehydrogenation in aqueous solution. ACS Appl. Mater. Interfaces 2016, 8, 25268–25278. [Google Scholar] [CrossRef]
- Guo, F.; Zou, H.; Yao, Q.; Huang, B.; Lu, Z.-H. Monodispersed bimetallic nanoparticles anchored on TiO2-decorated titanium carbide MXene for efficient hydrogen production from hydrazine in aqueous solution. Renew. Energy 2020, 155, 1293–1301. [Google Scholar] [CrossRef]


| Without Visible Light Radiation | |||||
|---|---|---|---|---|---|
| Hydrazine (mol/L) | Catalyst (g) | pH | Temperature (K) | kobs (min−1) | R2 |
| 1.0 × 10−2 | 0.0 | 9.5 | 295 | No degradation | |
| 1.0 × 10−2 | 0.02 | 9.5 | 295 | 0.002 | 0.996 |
| 3.0 × 10−2 | 0.02 | 9.5 | 295 | 0.004 | 0.993 |
| 5.0 × 10−2 | 0.02 | 9.5 | 295 | 0.007 | 0.997 |
| 7.0 × 10−2 | 0.02 | 9.5 | 295 | 0.009 | 0.991 |
| 3.0 × 10−2 | 0.03 | 9.5 | 295 | 0.005 | 0.995 |
| 3.0 × 10−2 | 0.04 | 9.5 | 295 | 0.008 | 0.992 |
| 3.0 × 10−2 | 0.04 | 8.5 | 295 | 0.003 | 0.992 |
| 3.0 × 10−2 | 0.02 | 10.5 | 295 | 0.006 | 0.992 |
| 3.0 × 10−2 | 0.02 | 11.5 | 295 | 0.007 | 0.991 |
| 3.0 × 10−2 | 0.04 | 12.5 | 295 | 0.008 | 0.992 |
| 3.0 × 10−2 | 0.02 | 9.5 | 300 | 0.006 | 0.996 |
| 3.0 × 10−2 | 0.02 | 9.5 | 308 | 0.007 | 0.994 |
| 3.0 × 10−2 | 0.02 | 9.5 | 312 | 0.009 | 0.994 |
| 3.0 × 10−2 | 0.02 | 9.5 | 318 | 0.017 | 0.998 |
| Under Visible Light Radiation | |||||
| 5.0 × 10−3 | 0.02 | 9.5 | 295 | 0.009 | 0.995 |
| 1.0 × 10−2 | 0.02 | 9.5 | 295 | 0.012 | 0.992 |
| 3.0 × 10−2 | 0.02 | 9.5 | 295 | 0.018 | 0.998 |
| 5.0 × 10−2 | 0.02 | 9.5 | 295 | 0.025 | 0.996 |
| 7.0 × 10−2 | 0.02 | 9.5 | 295 | 0.042 | 0.991 |
| 3.0 × 10−2 | 0.03 | 9.5 | 295 | 0.032 | 0.993 |
| 3.0 × 10−2 | 0.03 | 8.5 | 295 | 0.027 | 0.993 |
| 3.0 × 10−2 | 0.04 | 9.5 | 295 | 0.062 | 0.995 |
| 3.0 × 10−2 | 0.02 | 10.5 | 295 | 0.083 | 0.994 |
| 3.0 × 10−2 | 0.02 | 11.5 | 295 | 0.093 | 0.991 |
| 3.0 × 10−2 | 0.03 | 12.5 | 295 | 0.098 | 0.993 |
| 3.0 × 10−2 | 0.02 | 9.5 | 300 | 0.021 | 0.998 |
| 3.0 × 10−2 | 0.02 | 9.5 | 309 | 0.030 | 0.999 |
| 3.0 × 10−2 | 0.02 | 9.5 | 312 | 0.039 | 0.998 |
| 3.0 × 10−2 | 0.02 | 9.5 | 318 | 0.044 | 0.999 |
| Reaction Conditions | pH | Observations |
|---|---|---|
| NH2-NH2 + water | 9.5 | No production of H2/N2, time = 1 h |
| NH2-NH2 + water + O2 | 9.5 | No production of H2/N2, time = 1 h |
| NH2-NH2 + water | 9.5 | No production of H2/N2, time = 1 h |
| NH2-NH2 + water + visible light | 9.5 | No production of H2/N2, time = 1 h |
| NH2-NH2 + water + O2 + TiO2 | 9.5 | No production of H2/N2, time = 1 h |
| NH2-NH2 + water + O2 + TiO2-Ag | 9.5 | No production of H2/N2, time = 1 h |
| NH2-NH2 + water + O2 + TiO2-Ag + visible light | 9.5 | No production of H2/N2, time = 1 h |
| NH2-NH2 + water + TiO2-Ag | 9.5 | 45% H2/N2 production, time = 1 h, kobs = 0.007 min−1 |
| NH2-NH2 + water + TiO2-Ag + visible light | 9.5 | 100% H2/N2 production, time = 40 min, kobs = 0.025 min−1 |
| Catalyst | Activation Energy at Different Conditions | Reference |
|---|---|---|
| Ag0 | No decomposition | Present work |
| TiO2 | No decomposition | Present work |
| Ag-doped TiO2 | Ea = 57.3 kJ/mol in the dark | Present work |
| Ag-doped TiO2 | Ea = 34.4 kJ/mol under visible light | Present work |
| In4Ni | Ea = 38.9 kJ/mol in the dark | [67] |
| Ni0.8Pt0.2/DT-Ti3C2Tx | Ea = 64.3 kJ/mol in the dark | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Althabaiti, S.A.; Khan, Z.; Narasimharao, K.; Bawaked, S.M.; Al-Sheheri, S.Z.; Mokhtar, M.; Malik, M.A. Selective Thermal and Photocatalytic Decomposition of Aqueous Hydrazine to Produce H2 over Ag-Modified TiO2 Nanomaterial. Nanomaterials 2023, 13, 2076. https://doi.org/10.3390/nano13142076
Althabaiti SA, Khan Z, Narasimharao K, Bawaked SM, Al-Sheheri SZ, Mokhtar M, Malik MA. Selective Thermal and Photocatalytic Decomposition of Aqueous Hydrazine to Produce H2 over Ag-Modified TiO2 Nanomaterial. Nanomaterials. 2023; 13(14):2076. https://doi.org/10.3390/nano13142076
Chicago/Turabian StyleAlthabaiti, Shaeel Ahmed, Zaheer Khan, Katabathini Narasimharao, Salem Mohamed Bawaked, Soad Zahir Al-Sheheri, Mohamed Mokhtar, and Maqsood Ahmad Malik. 2023. "Selective Thermal and Photocatalytic Decomposition of Aqueous Hydrazine to Produce H2 over Ag-Modified TiO2 Nanomaterial" Nanomaterials 13, no. 14: 2076. https://doi.org/10.3390/nano13142076
APA StyleAlthabaiti, S. A., Khan, Z., Narasimharao, K., Bawaked, S. M., Al-Sheheri, S. Z., Mokhtar, M., & Malik, M. A. (2023). Selective Thermal and Photocatalytic Decomposition of Aqueous Hydrazine to Produce H2 over Ag-Modified TiO2 Nanomaterial. Nanomaterials, 13(14), 2076. https://doi.org/10.3390/nano13142076









