Composites of Montmorillonite and Titania Nanoparticles Prepared by Inverse Microemulsion Method: Physico-Chemical Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Electron Microscopy Analysis
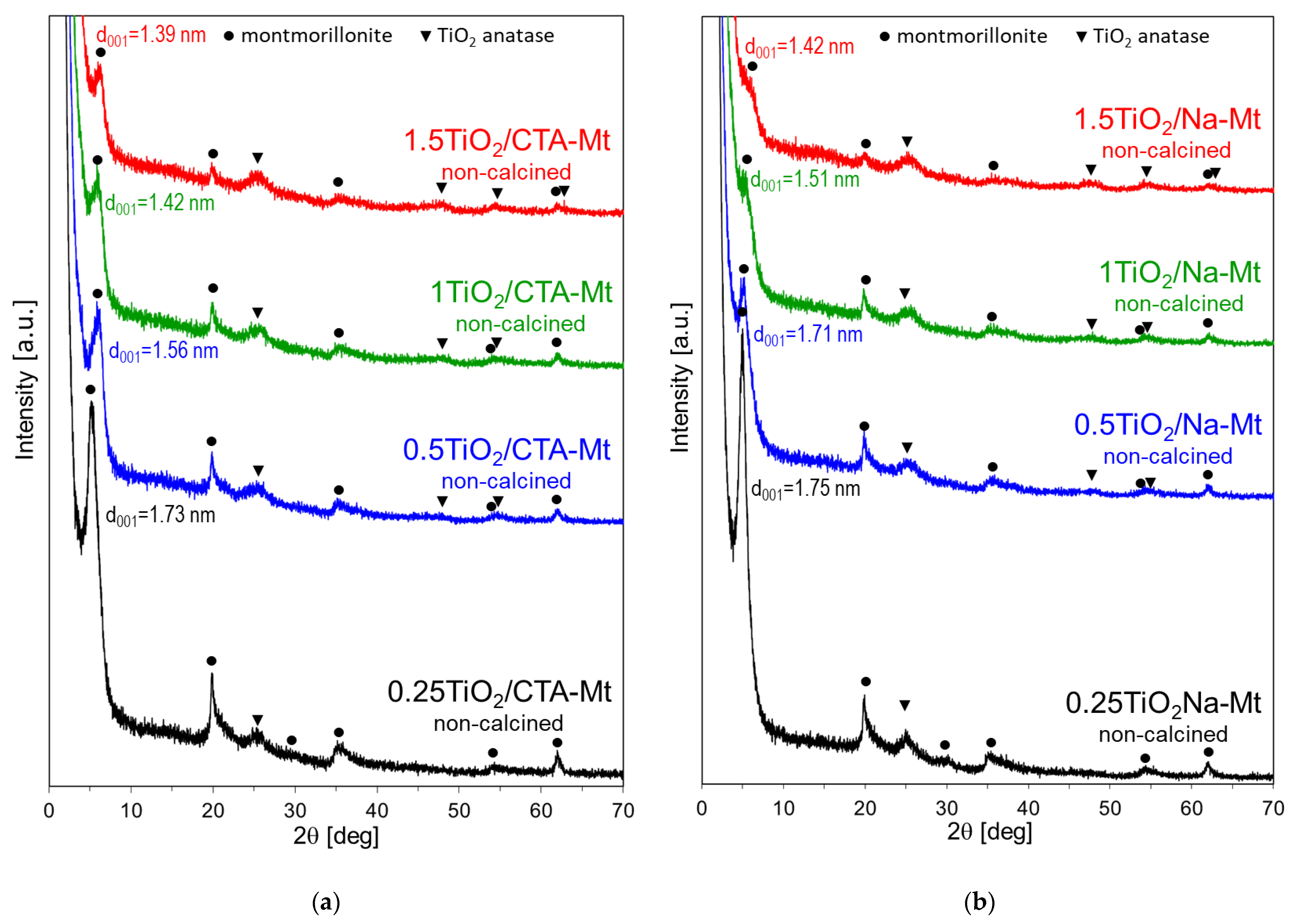
3.2. X-ray Diffraction Analysis
3.3. Thermal Analysis
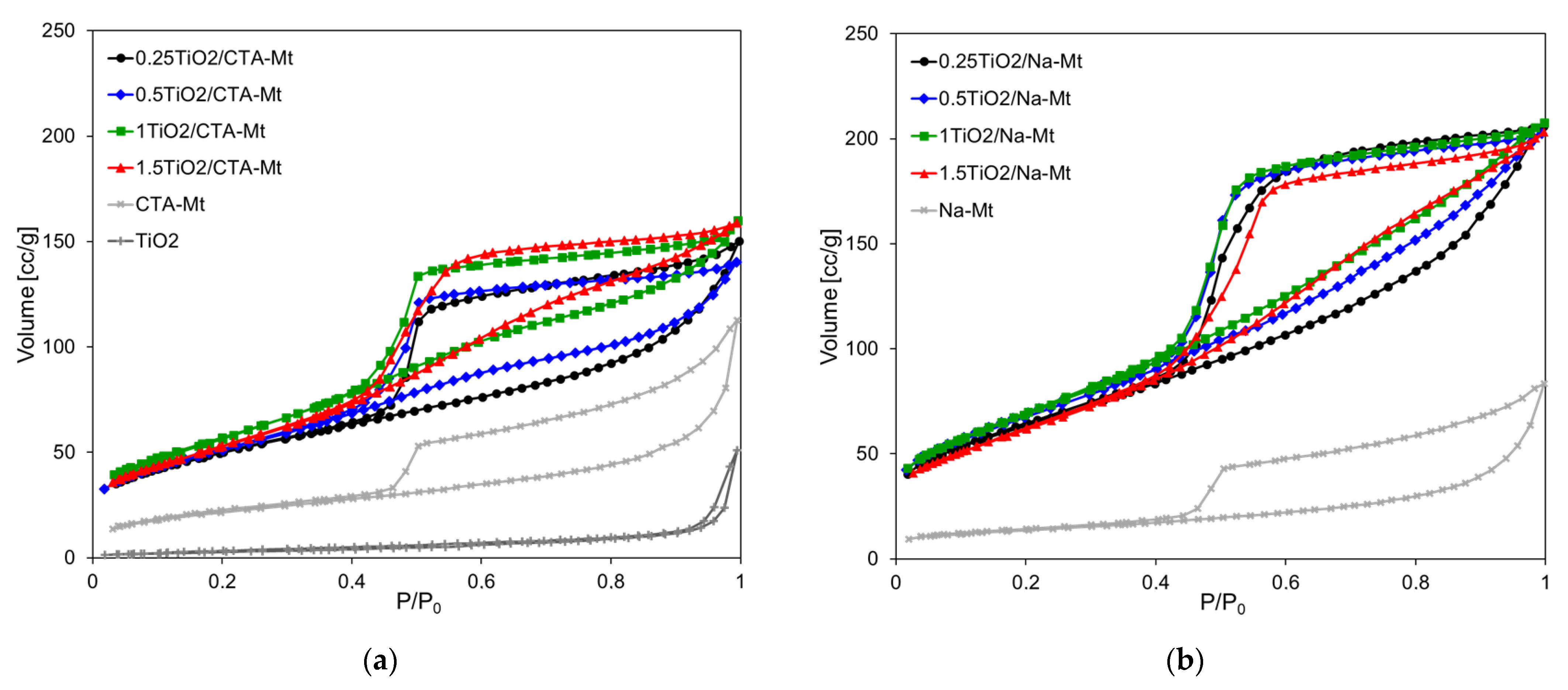
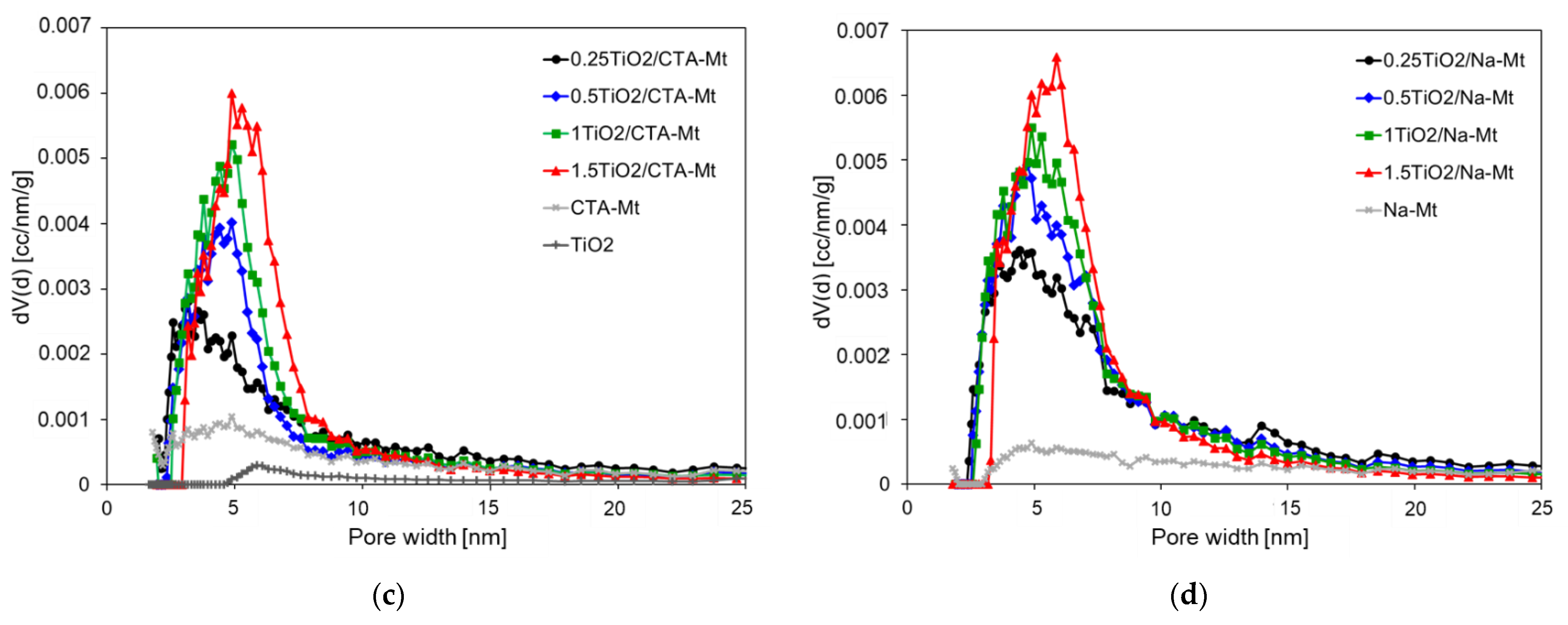
3.4. Textural Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serwicka, E.M. Titania-clay mineral composites for environmental catalysis and photocatalysis. Catalysts 2021, 11, 1087. [Google Scholar] [CrossRef]
- Fernández-García, M.; Rodriguez, J.A. Metal Oxide Nanoparticles. In Encyclopedia of Inorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Myakonkaya, O.; Hu, Z.; Nazar, M.F.; Eastoe, J. Recycling Functional Colloids and Nanoparticles. Chem. Eur. J. 2010, 16, 11784–11790. [Google Scholar] [CrossRef] [PubMed]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review. Appl. Catal. A Gen. 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Serwicka, E.M.; Bahranowski, K. Environmental catalysis by tailored materials derived from layered minerals. Catal. Today 2004, 90, 85–92. [Google Scholar] [CrossRef]
- Schoonheydt, R.A.; Johnston, C.T.; Bergaya, F. Clay minerals and their surfaces. In Developments in Clay Science; Schoonheydt, R., Johnston, C.T., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 9, pp. 1–21. [Google Scholar]
- Szczepanik, B. Photocatalytic degradation of organic contaminants over clay-TiO2 nanocomposites: A review. Appl. Clay Sci. 2017, 141, 227–239. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S. Clay supported TiO2 nanoparticles for photocatalytic degradation of environmental pollutants: A Review. J. Environ. Chem. Eng. 2018, 6, 6088–6107. [Google Scholar] [CrossRef]
- Deepracha, S.; Vibulyaseak, K.; Ogawa, M. Chapter 2.1: Complexation of TiO2 with clays and clay minerals for hierarchically designed functional hybrids. In Advanced Supramolecular Nanoarchitectonics, 1st ed.; Ariga, K., Aono, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 125–150. [Google Scholar]
- Ruiz-Hitzky, E.; Aranda, P.; Akkari, M.; Khaorapapong, N.; Ogawa, M. Photoactive nanoarchitectures based on clays incorporating TiO2 and ZnO nanoparticles. Beilstein J. Nanotechnol. 2019, 10, 1140–1156. [Google Scholar] [CrossRef]
- Dlamini, M.C.; Maubane-Nkadimeng, M.S.; Moma, J.A. The use of TiO2/clay heterostructures in the photocatalytic remediation of water containing organic pollutants: A review. J. Environ. Chem. Eng. 2021, 9, 106546. [Google Scholar] [CrossRef]
- Napruszewska, B.D.; Michalik-Zym, A.; Rogowska, M.; Bielańska, E.; Rojek, W.; Gaweł, A.; Wójcik-Bania, M.; Bahranowski, K.; Serwicka, E.M. Novel montmorillonite/TiO2/MnAl-mixed oxide composites prepared from inverse microemulsions as combustion catalysts. Materials 2017, 10, 1326. [Google Scholar] [CrossRef] [Green Version]
- Napruszewska, B.D.; Michalik-Zym, A.; Dula, R.; Bielańska, E.; Rojek, W.; Machej, T.; Socha, R.P.; Lityńska-Dobrzyńska, L.; Bahranowski, K.; Serwicka, E.M. Composites derived from exfoliated Laponite and Mn-Al hydrotalcite prepared in inverse microemulsion: A new strategy for design of robust VOCs combustion catalysts. Appl. Catal. B Environ. 2017, 211, 46–56. [Google Scholar] [CrossRef]
- Bahranowski, K.; Gaweł, A.; Klimek, A.; Michalik-Zym, A.; Napruszewska, B.D.; Nattich-Rak, M.; Rogowska, M.; Serwicka, E.M. Influence of purification method of Na-montmorillonite on textural properties of clay mineral composites with TiO2 nanoparticles. Appl. Clay Sci. 2017, 140, 75–80. [Google Scholar] [CrossRef]
- Ganguli, A.K.; Ganguly, A.; Vaidya, S. Microemulsion-based synthesis of nanocrystalline materials. Chem. Soc. Rev. 2010, 39, 474–485. [Google Scholar] [CrossRef]
- Eastoe, J.; Hollamby, M.J.; Hudson, L. Recent advances in nanoparticle synthesis with reversed micelles. Adv. Colloid Interface Sci. 2006, 128–130, 5–15. [Google Scholar] [CrossRef]
- Asgari, S.; Saberi, A.H.; McClements, D.J.; Lin, M. Microemulsions as Nanoreactors for Synthesis of Biopolymer Nanoparticles. Trends Food Sci. Technol. 2019, 86, 118–130. [Google Scholar] [CrossRef]
- Kubacka, A.; Caudillo-Flores, U.; Barba-Nieto, I.; Muñoz-Batista, M.J.; Fernández-García, M. Microemulsion: A versatile synthesis tool for Photocatalysis. Curr. Opin. Colloid Interface Sci. 2020, 49, 42–59. [Google Scholar] [CrossRef]
- Šucha, V.; Kraus, I. Natural microporous materials of central Slovakia. In Natural Microporous Materials in Environmental Technology; Misaelides, P., Macasek, F., Pinnavaia, T.J., Colella, C., Eds.; Kluwer Academic Press: Dordrecht, The Netherlands, 1999; pp. 101–107. [Google Scholar]
- Ahmad, S.I.; Friberg, S. Catalysis in micellar and liquid-crystalline phases. I. System water-hexadecyltrimethylammonium bromide-hexanol. J. Am. Chem. Soc. 1972, 94, 5196–5199. [Google Scholar] [CrossRef]
- Tai, C.Y.; Hsiao, B.Y.; Chiu, H.Y. Preparation of spherical hydrous-zirconia nanoparticles by low temperature hydrolysis in a reverse microemulsion. Colloids Surf. A Physicochem. Eng. Asp. 2004, 237, 105–111. [Google Scholar] [CrossRef]
- Sterte, J. Synthesis and properties of titanium oxide cross-linked montmorillonite. Clays Clay Miner. 1986, 34, 658–664. [Google Scholar] [CrossRef]
- Zhu, T.T.; Zhou, C.H.; Kabwe, F.B.; Wu, Q.Q.; Li, C.S.; Zhang, J.R. Exfoliation of montmorillonite and related properties of clay/polymer nanocomposites. Appl. Clay Sci. 2019, 169, 48–66. [Google Scholar] [CrossRef]
- Lagaly, G.; Ogawa, M.; Dékány, I. Clay mineral–organic interactions; chapter 10.3. In Handbook of Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 435–505. [Google Scholar]
- Xie, H.; Li, N.; Liu, B.S.; Yang, J.J.; Zhao, X.J. Role of sodium ion on TiO2 photocatalyst: Influencing crystallographic properties or serving as the recombination center of charge carriers? J. Phys. Chem. C 2016, 120, 10390–10399. [Google Scholar] [CrossRef]
- Hedley, C.; Yuan, G.; Theng, B. Thermal analysis of montmorillonites modified with quaternary phosphonium and ammonium surfactants. Appl. Clay Sci. 2007, 35, 180–188. [Google Scholar] [CrossRef]
- Pálková, H.; Madejová, J.; Zimowska, M.; Bielańska, E.; Olejniczak, Z.; Lityńska-Dobrzyńska, L.; Serwicka, E.M. Laponite-derived porous clay heterostructures: I. Synthesis and physicochemical characterization. Micropor. Mesopor. Mater. 2010, 127, 228–236. [Google Scholar] [CrossRef]
- Andrunik, M.; Bajda, T. Modification of Bentonite with Cationic and Nonionic Surfactants: Structural and Textural Features. Materials 2019, 12, 3772. [Google Scholar] [CrossRef] [PubMed]
- Yariv, S. The role of charcoal on DTA curves of organo-clay complexes: An overview. Appl. Clay Sci. 2004, 24, 225–236. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1052–1069. [Google Scholar] [CrossRef]
- Gil, A.; Vicente, M.A. Progress and perspectives on pillared clays applied in energetic and environmental remediation processes. Curr. Opin. Green Sustain. Chem. 2020, 21, 56–63. [Google Scholar] [CrossRef]
- Belver, C.; Bedia, J.; Rodriguez, J.J. Titania–clay heterostructures with solar photocatalytic applications. Appl. Catal. B Environ. 2015, 176–177, 278–287. [Google Scholar] [CrossRef]
- Djellabi, R.; Ghorab, M.F.; Cerrato, G.; Morandi, S.; Gatto, S.; Oldani, V.; Di Michele, A.; Bianchi, C.L. Photoactive TiO2– montmorillonite composite for degradation of organic dyes in water. J. Photochem. Photobiol. A Chem. 2014, 295, 57–63. [Google Scholar] [CrossRef]
- Michalik-Zym, A.; Dula, R.; Duraczńyska, D.; Kryściak-Czerwenka, J.; Machej, T.; Socha, R.P.; Włodarczyk, W.; Gaweł, A.; Matusik, J.; Bahranowski, K.; et al. Active, selective and robust Pd and/or Cr catalysts supported on Ti-, Zr- or [Ti,Zr]-pillared montmorillonites for destruction of chlorinated volatile organic compounds. Appl. Catal. B-Environ. 2015, 174–175, 293–307. [Google Scholar] [CrossRef]
- Butman, M.F.; Ovchinnikov, N.L.; Karasev, N.S.; Kochkina, N.E.; Agafonov, A.V.; Vinogradov, A.V. Photocatalytic and adsorption properties of TiO2-pillared montmorillonite obtained by hydrothermally activated intercalation of titanium polyhydroxo complexes. Beilstein J. Nanotechnol. 2018, 9, 364–378. [Google Scholar] [CrossRef]
- Belessi, V.; Lambropoulou, D.; Konstantinou, I.; Katsoulidis, A.; Pomonis, P.; Petridis, D.; Albanis, T. Structure and photocatalytic performance of TiO2/clay nanocomposites for the degradation of dimethachlor. Appl. Catal. B Environ. 2007, 73, 292–299. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Li, G.; Nie, X.; An, T.; Zhang, S.; Zhao, H. Synthesis and characterization of novel SiO2 and TiO2 co-pillared montmorillonite composite for adsorption and photocatalytic degradation of hydrophobic organic pollutants in water. Catal. Today 2011, 164, 364–369. [Google Scholar] [CrossRef]
- Chen, D.; Du, G.; Zhu, Q.; Zhou, F. Synthesis and characterization of TiO2 pillared montmorillonites: Application for methylene blue degradation. J. Colloid Interface Sci. 2013, 409, 151–157. [Google Scholar] [CrossRef]
- Sang, L.; Zhao, Y.; Burda, C. TiO2 Nanoparticles as Functional Building Blocks. Chem. Rev. 2014, 114, 9283–9318. [Google Scholar] [CrossRef]
- Noman, M.T.; Ashraf, M.A.; Ali, A. Synthesis and applications of nano-TiO2: A review. Environ. Sci. Pollut. Res. 2019, 26, 3262–3291. [Google Scholar] [CrossRef]
- Yang, H.; Yang, B.; Chen, W.; Yang, J. Preparation and Photocatalytic Activities of TiO2-Based Composite Catalysts. Catalysts 2022, 12, 1263. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Padmanabhan, N.T.; Thomas, N.; Louis, J.; Mathew, D.T.; Ganguly, P.; John, H.; Pillai, S.C. Graphene coupled TiO2 photocatalysts for environmental applications: A review. Chemosphere 2021, 271, 129506. [Google Scholar] [CrossRef]
- Paul, S.; Rahman, M.A.; Sharif, S.B.; Kim, J.; Siddiqui, S.; Hossain, M.A.M. TiO2 as an anode of high-performance lithium-ion batteries: A comprehensive review towards practical application. Nanomaterials 2022, 12, 2034. [Google Scholar] [CrossRef]
- Javed, R.; Ain, N.U.; Gul, A.; Ahmad, M.A.; Guo, W.; Ao, Q.; Tian, S. Diverse biotechnological applications of multifunctional titanium dioxide nanoparticles: An up-to-date review. IET Nanobiotechnol. 2022, 16, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Hassan, T.; Salam, A.; Khan, A.; Khan, S.U.; Khanzada, H.; Wasim, M.; Khan, M.Q.; Kim, I.S. Functional nanocomposites and their potential applications: A review. J. Polym. Res. 2021, 28, 36. [Google Scholar] [CrossRef]

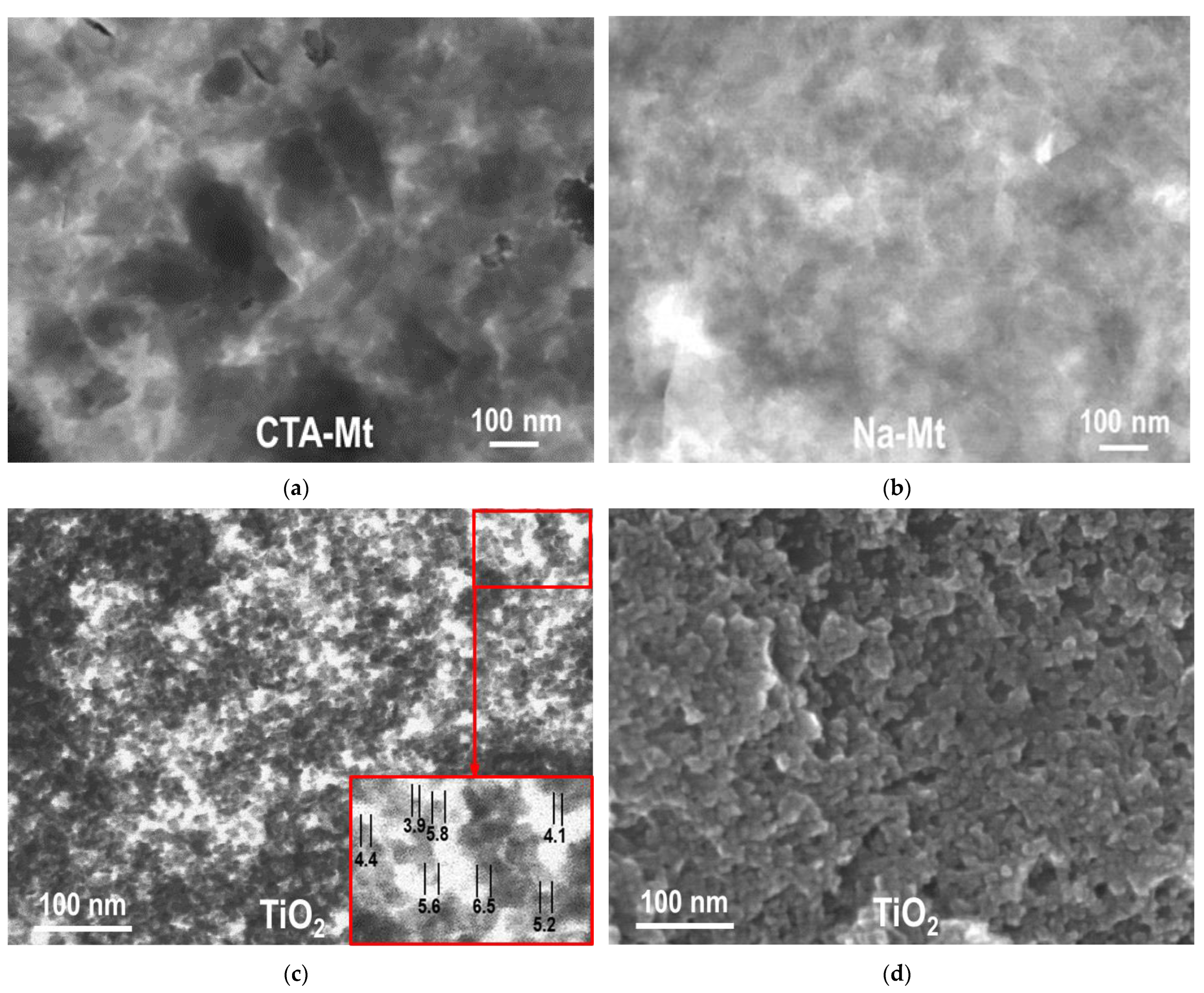
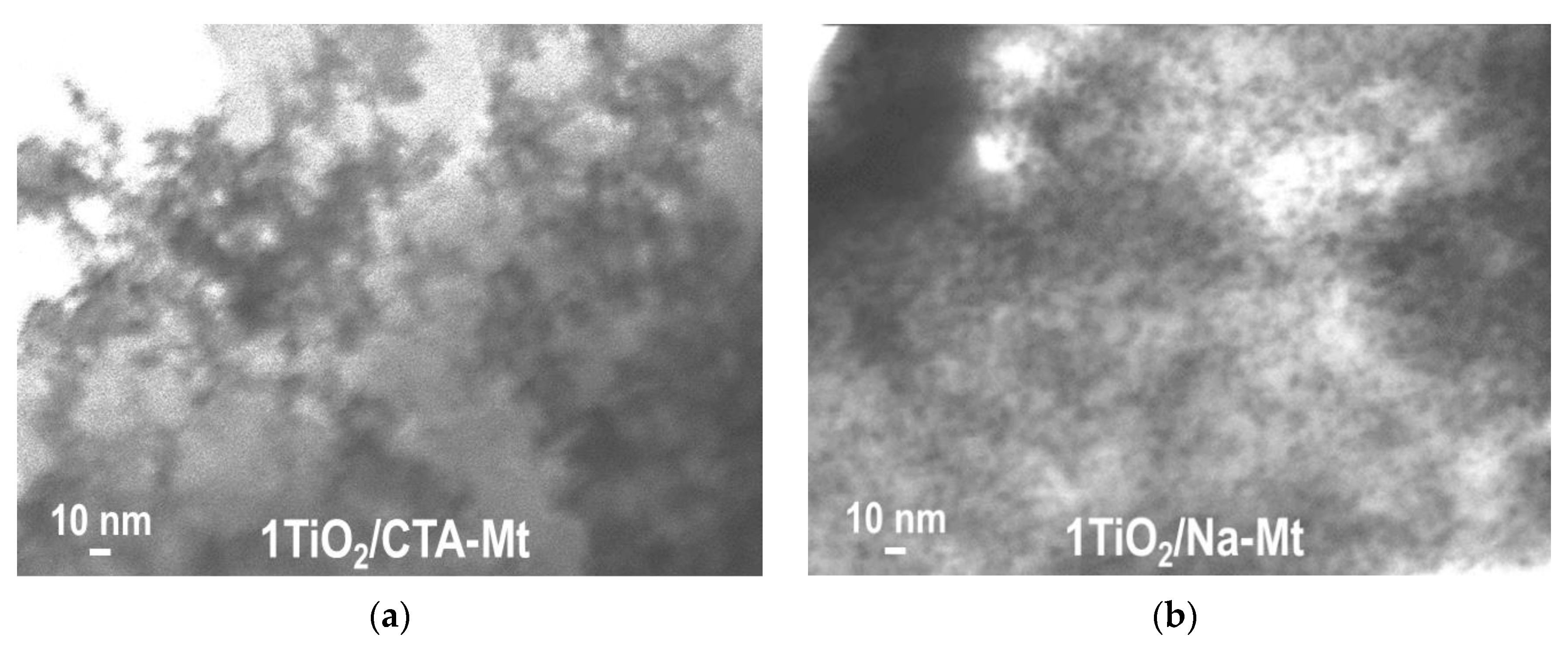
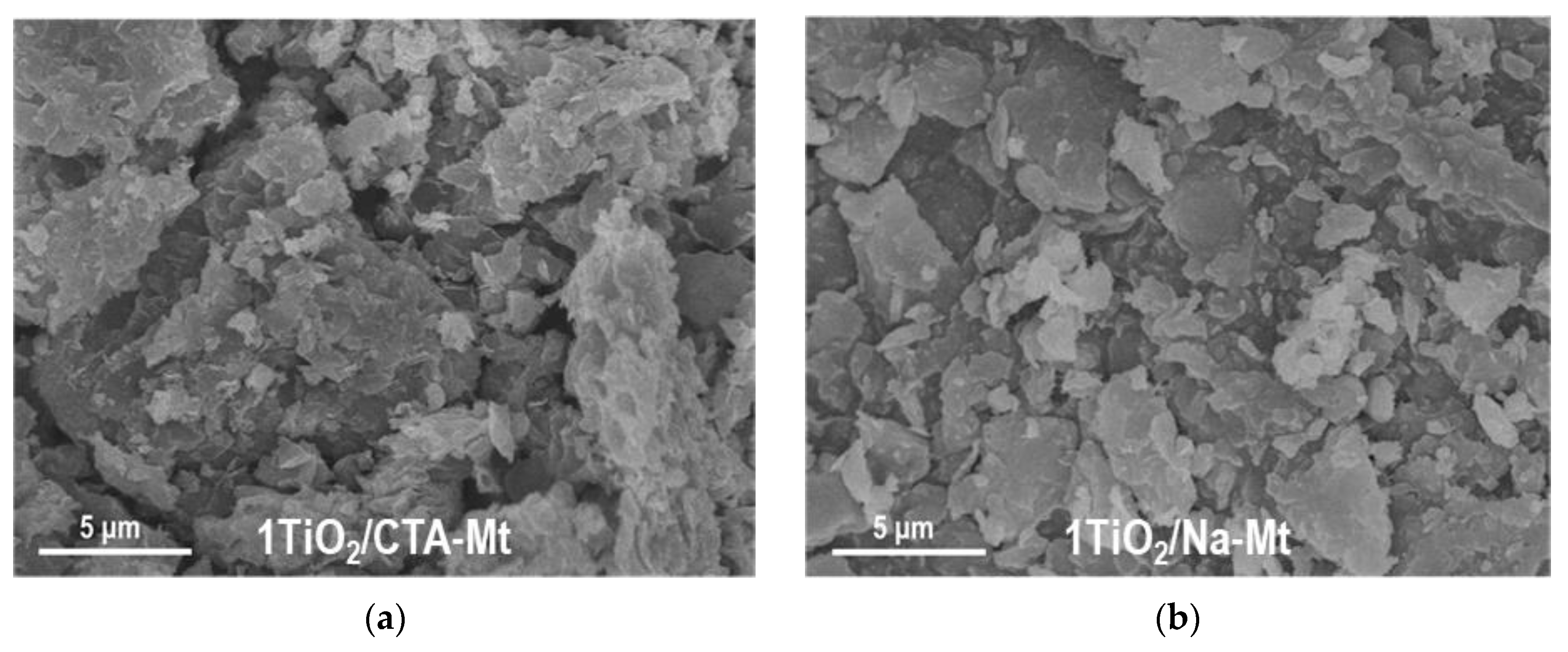
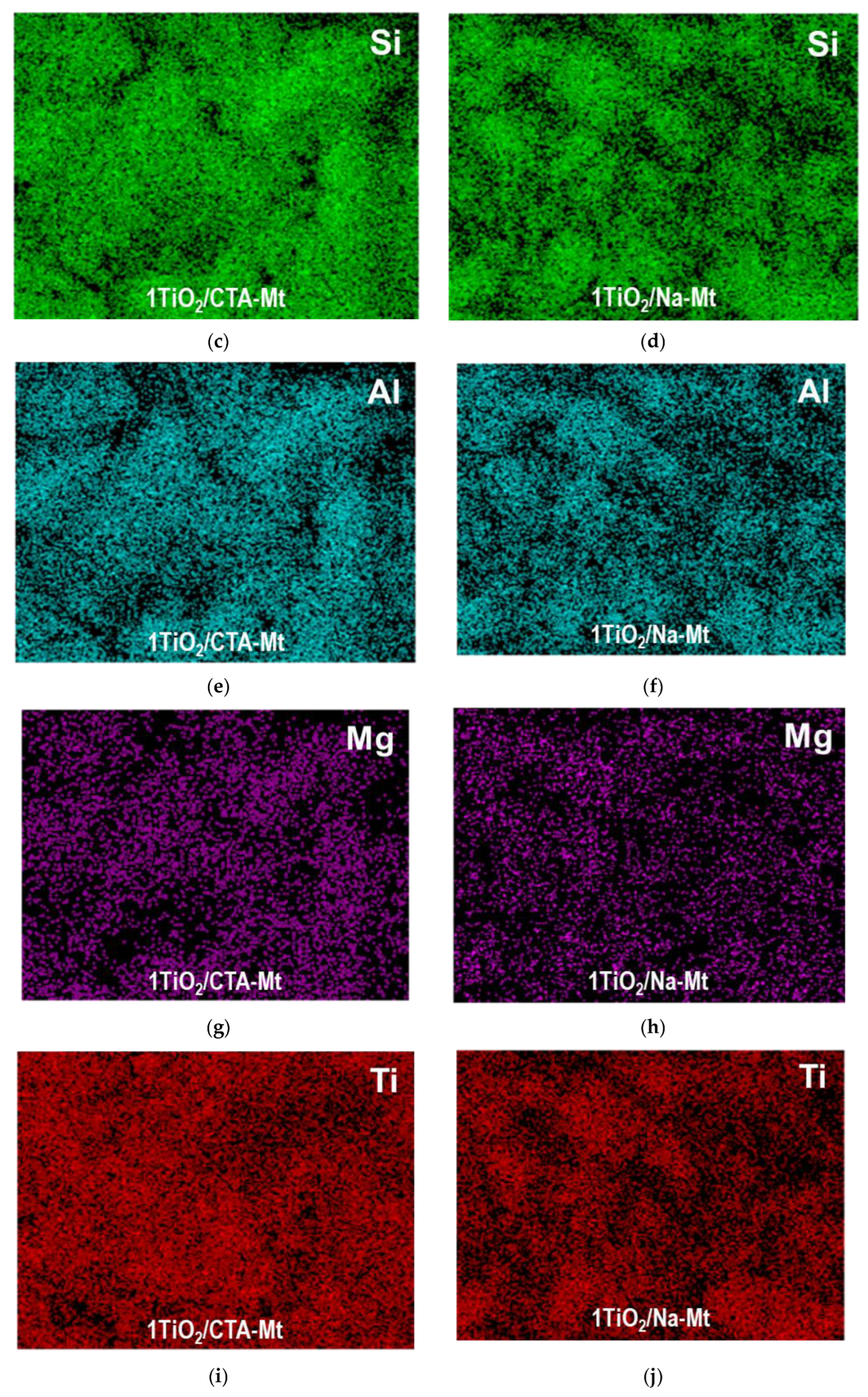


| Sample | TiO2 (wt. %) | SiO2 (wt. %) | Al2O3 (wt. %) | MgO (wt. %) | Fe2O3 (wt. %) | Na2O (wt. %) | SBET (m2g−1) | Vtot (cm3g−1) | Vmeso (cm3g−1) | Dav (nm) | Ddom (nm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.25TiO2/CTA-Mt | 16.4 | 56.3 | 21.6 | 3.8 | 1.9 | - | 174 | 0.233 | 0.174 | 5.3 | 3.8 |
| 0.5TiO2/CTA-Mt | 35.1 | 43.6 | 16.5 | 2.8 | 2.0 | - | 184 | 0.217 | 0.160 | 4.7 | 4.6 |
| 1TiO2/CTA-Mt | 45.2 | 36.5 | 14.4 | 2.2 | 1.7 | - | 208 | 0.247 | 0.189 | 4.8 | 4.9 |
| 1.5TiO2/CTA-Mt | 59.7 | 27.0 | 10.2 | 1.9 | 1.2 | - | 194 | 0.246 | 0.208 | 5.1 | 5.2 |
| 0.25TiO2/Na-Mt | 17.5 | 55.7 | 21.3 | 3.8 | 1.7 | - | 223 | 0.321 | 0.259 | 5.8 | 4.4 |
| 0.5TiO2/Na-Mt | 37.4 | 42.4 | 16.0 | 2.7 | 1.5 | - | 245 | 0.313 | 0.248 | 5.1 | 4.9 |
| 1TiO2/Na-Mt | 48.3 | 34.9 | 13.7 | 1.9 | 1.2 | - | 256 | 0.321 | 0.263 | 5.0 | 5.2 |
| 1.5TiO2/Na-Mt | 63.9 | 24.4 | 9.4 | 1.4 | 0.9 | - | 231 | 0.314 | 0.274 | 5.5 | 5.7 |
| calcined Na-Mt | - | 65.6 | 24.9 | 4.5 | 2.1 | 2.9 | 47 | 0.129 | 0.111 | 11.1 | 4.6 |
| calcined CTA-Mt | - | 66.5 | 25.8 | 4.9 | 2.8 | - | 92 | 0.168 | 0.136 | 7.3 | 4.0 |
| calcined TiO2 | 100.0 | - | - | - | - | - | 12 | 0.079 | 0.079 | 26.4 | 5.8 |
| Method of TiO2–Mt Composite Preparation | SBET (m2g−1) | Vtot (cm3g−1) | Reference |
|---|---|---|---|
| Pillaring of Mt with TiCl4 precursor, calcination 400 °C | 379 | 0.392 | [35] |
| Pillaring of Mt with TiCl4 precursor, hydrothermal treatment 115 °C calcination 500 °C | 135 | 0.303 | [36] |
| Pillaring of Mt with Ti(OC3H7)4 precursor, hydrothermal treatment 60 °C, calcination 500 °C | 183 | 0.19 | [37] |
| Pillaring of Mt with Ti(OC4H9)4 precursor, calcination 450 °C | 133 | 0.335 | [38] |
| Pillaring of CTA-Mt with Ti(OC3H7)4 precursor, calcination 500 °C | 194 | 0.165 | [39] |
| Porous clay heterostructure synthesis with Ti(OC3H7)4 precursor and commercial organoclay Cloisite®30B, calcination 550 °C | 143 | 0.210 | [33] |
| Mt impregnated with TiCl4 precursor, calcination at 350 °C | 52 | 0.144 | [34] |
| TiCl4 precursor hydrolyzed within inverse micelles, mixed with exfoliated CTA-Mt, calcination 550 °C | 208 | 0.247 | this work |
| TiCl4 precursor hydrolyzed within inverse micelles, mixed with Na-Mt dispersed in inverse microemulsion, calcination 550 °C | 256 | 0.321 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalik, A.; Napruszewska, B.D.; Duraczyńska, D.; Walczyk, A.; Serwicka, E.M. Composites of Montmorillonite and Titania Nanoparticles Prepared by Inverse Microemulsion Method: Physico-Chemical Characterization. Nanomaterials 2023, 13, 686. https://doi.org/10.3390/nano13040686
Michalik A, Napruszewska BD, Duraczyńska D, Walczyk A, Serwicka EM. Composites of Montmorillonite and Titania Nanoparticles Prepared by Inverse Microemulsion Method: Physico-Chemical Characterization. Nanomaterials. 2023; 13(4):686. https://doi.org/10.3390/nano13040686
Chicago/Turabian StyleMichalik, Alicja, Bogna D. Napruszewska, Dorota Duraczyńska, Anna Walczyk, and Ewa M. Serwicka. 2023. "Composites of Montmorillonite and Titania Nanoparticles Prepared by Inverse Microemulsion Method: Physico-Chemical Characterization" Nanomaterials 13, no. 4: 686. https://doi.org/10.3390/nano13040686
APA StyleMichalik, A., Napruszewska, B. D., Duraczyńska, D., Walczyk, A., & Serwicka, E. M. (2023). Composites of Montmorillonite and Titania Nanoparticles Prepared by Inverse Microemulsion Method: Physico-Chemical Characterization. Nanomaterials, 13(4), 686. https://doi.org/10.3390/nano13040686






