Amphiphilic Bowl-Shaped Janus Particles Prepared via Thiol–Ene Click Reaction for Effective Oil–Water Separation
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
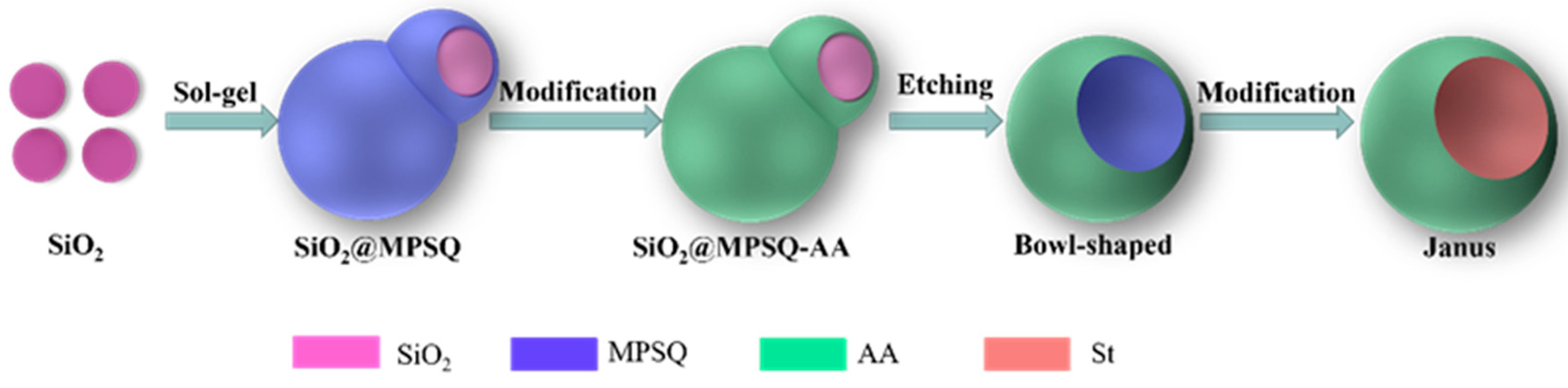
2.2. Synthesis of Snowman-like Composite Particles Based on Silica and Mercaptopropyl Polysilsesquioxane (SiO2@MPSQ)
2.3. Preparation of Bowl-Shaped Janus Composite Particles (AA-MPSQ-St)
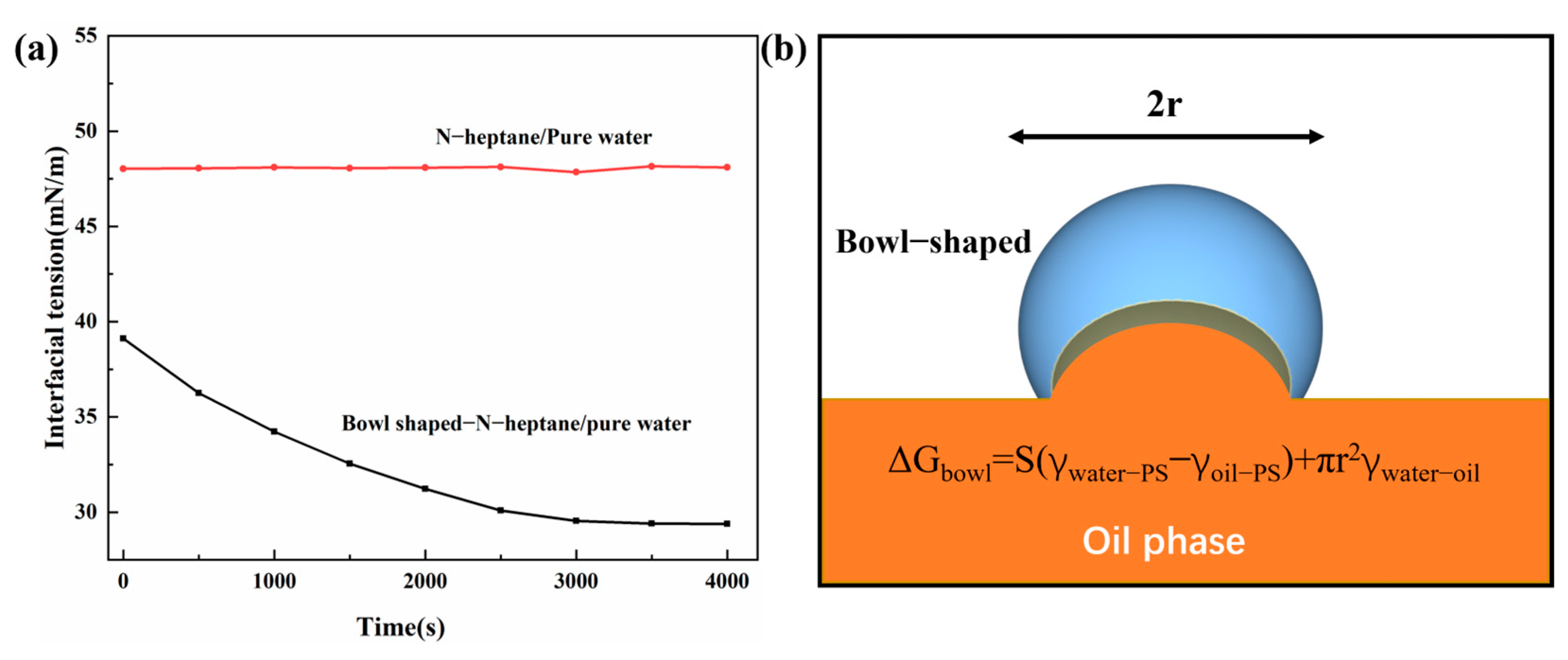
2.4. Preparation of Surfactant-Free n-Heptane-in-Water Emulsion and Oil–Water Separation Procedure
2.5. Characterization
3. Results and Discussion
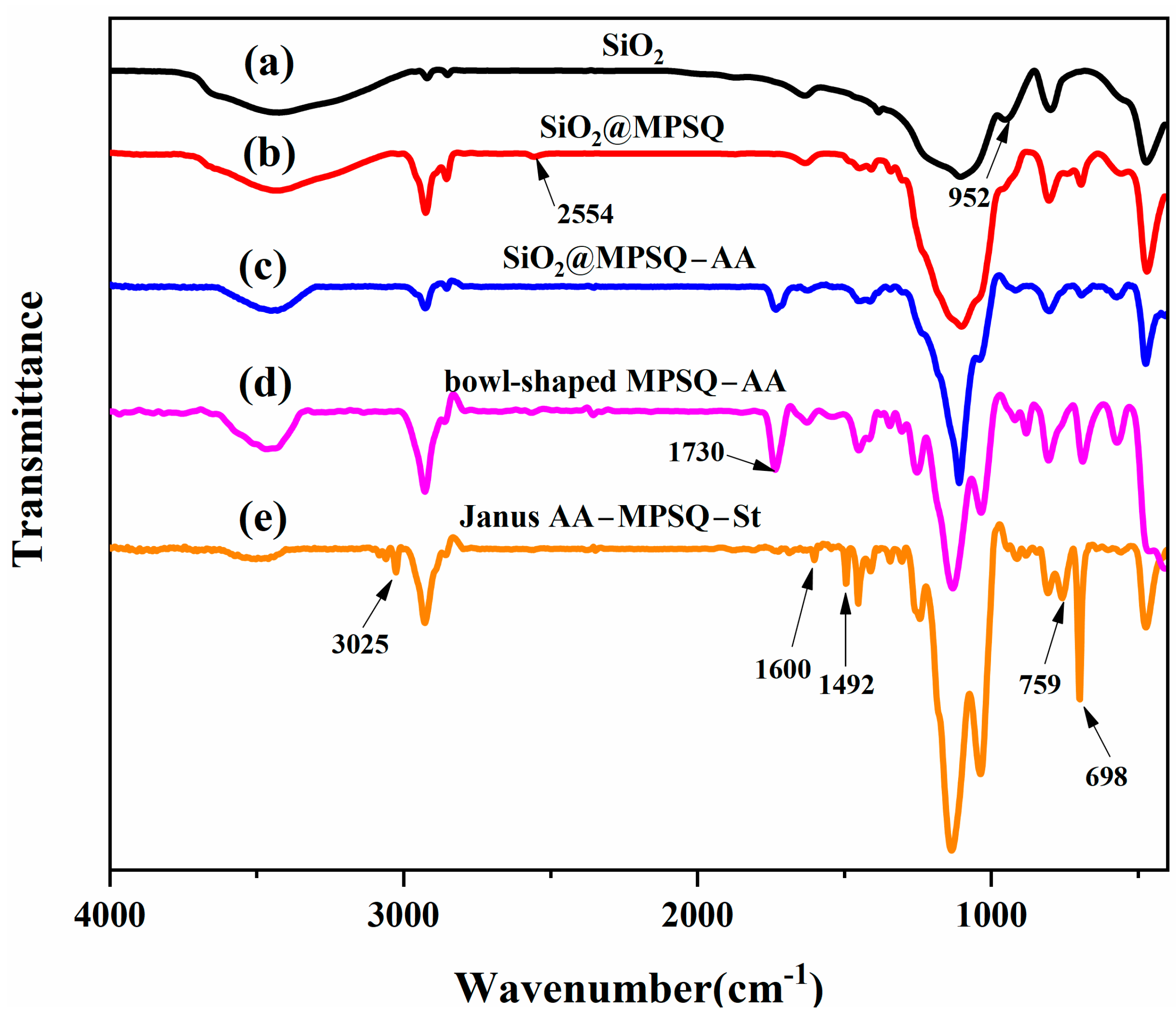
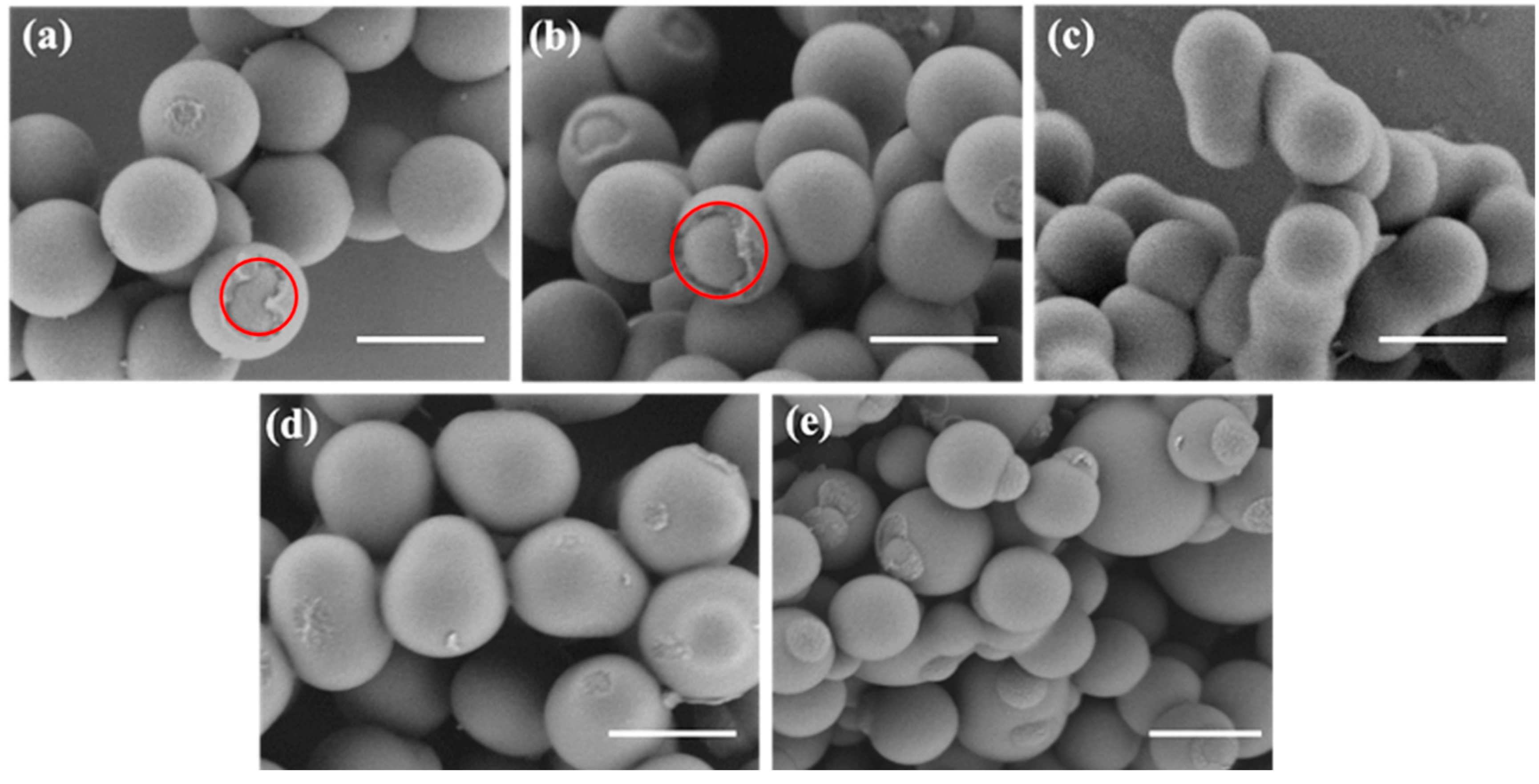
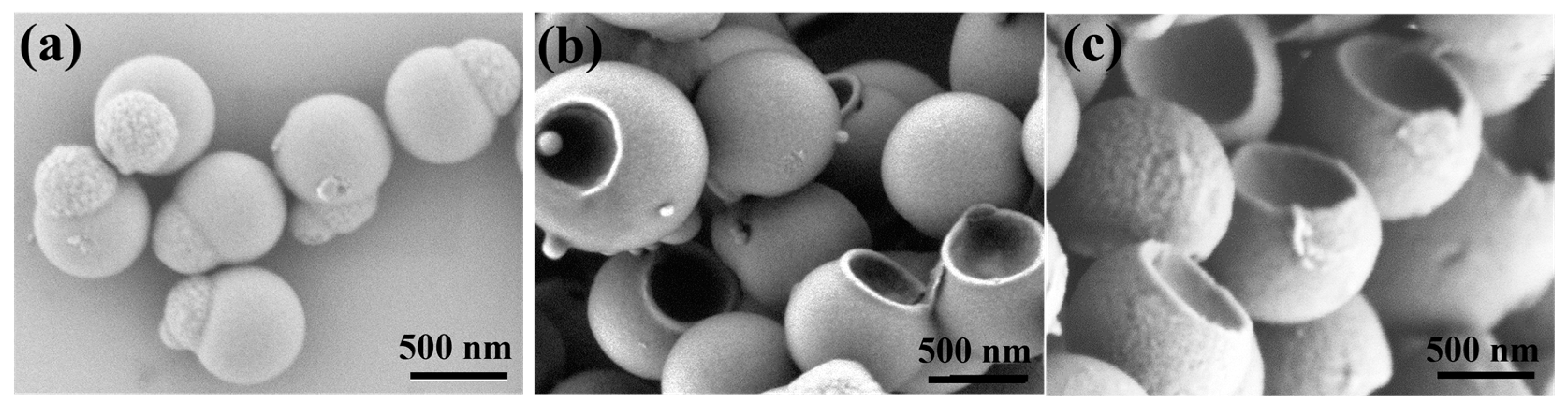
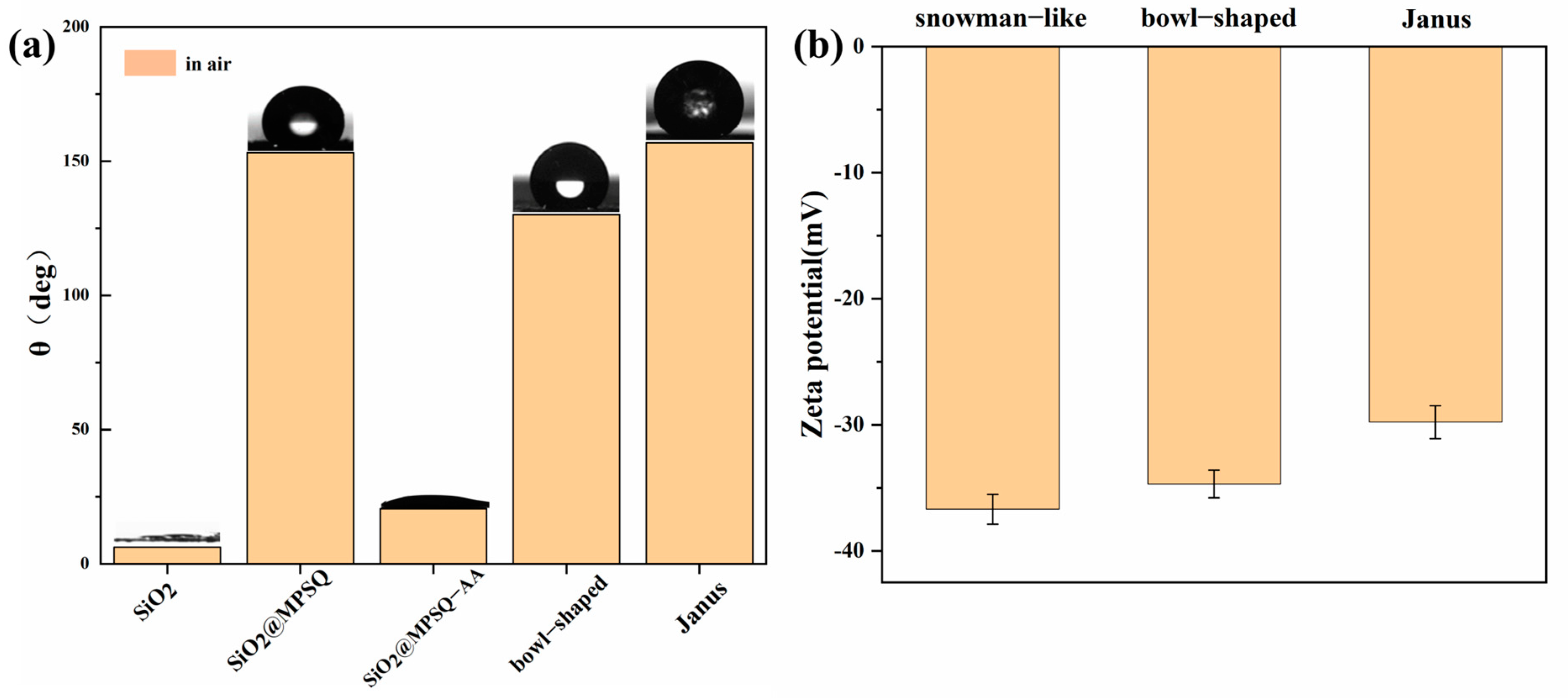
3.1. Preparation and Characterization of Snowman-like Particles
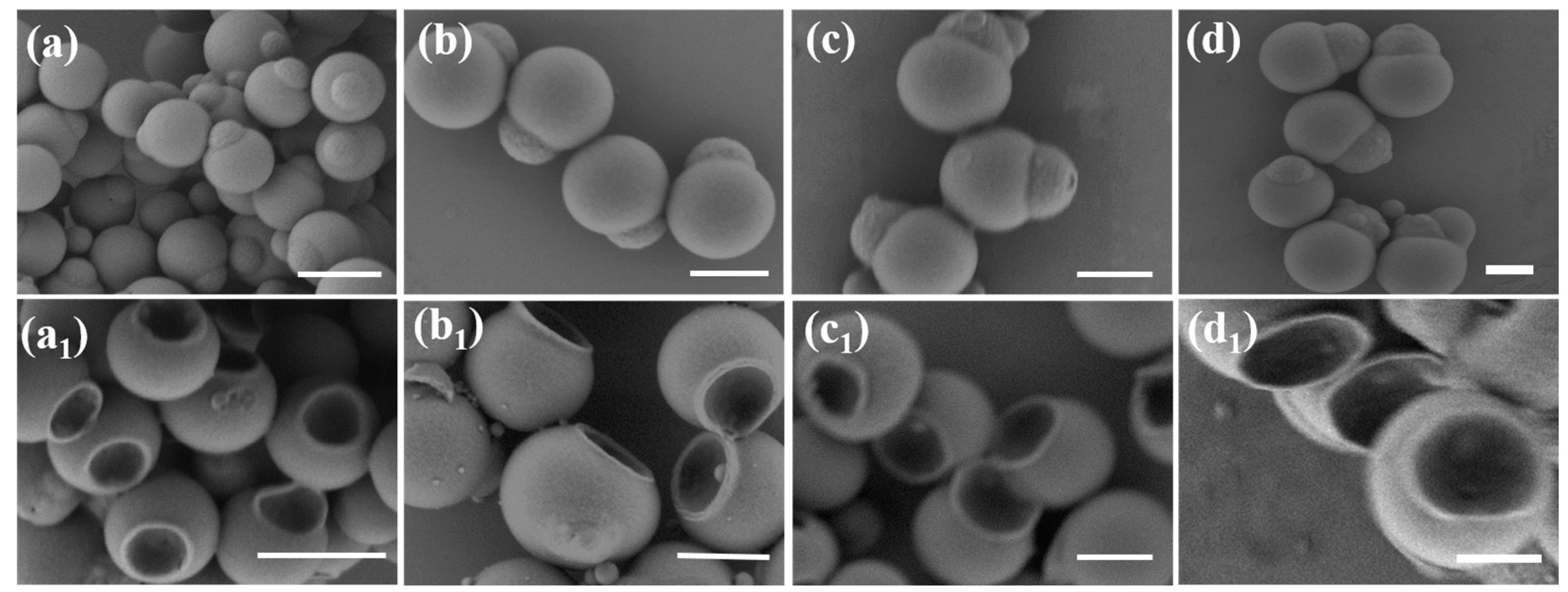
3.2. Characterization of Bowl-Shaped Composite Particles
3.3. Characterization of Janus Composite Particles
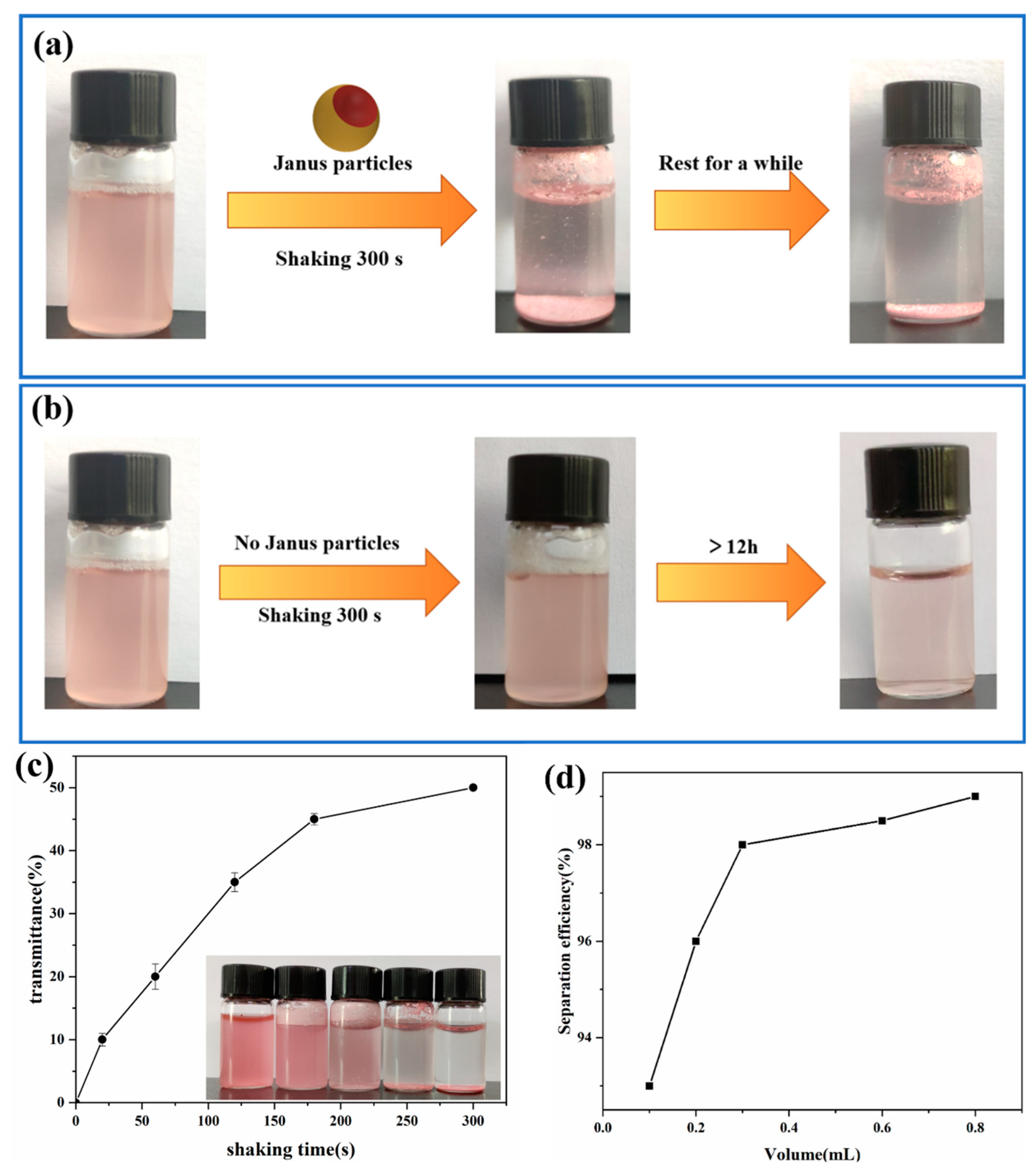
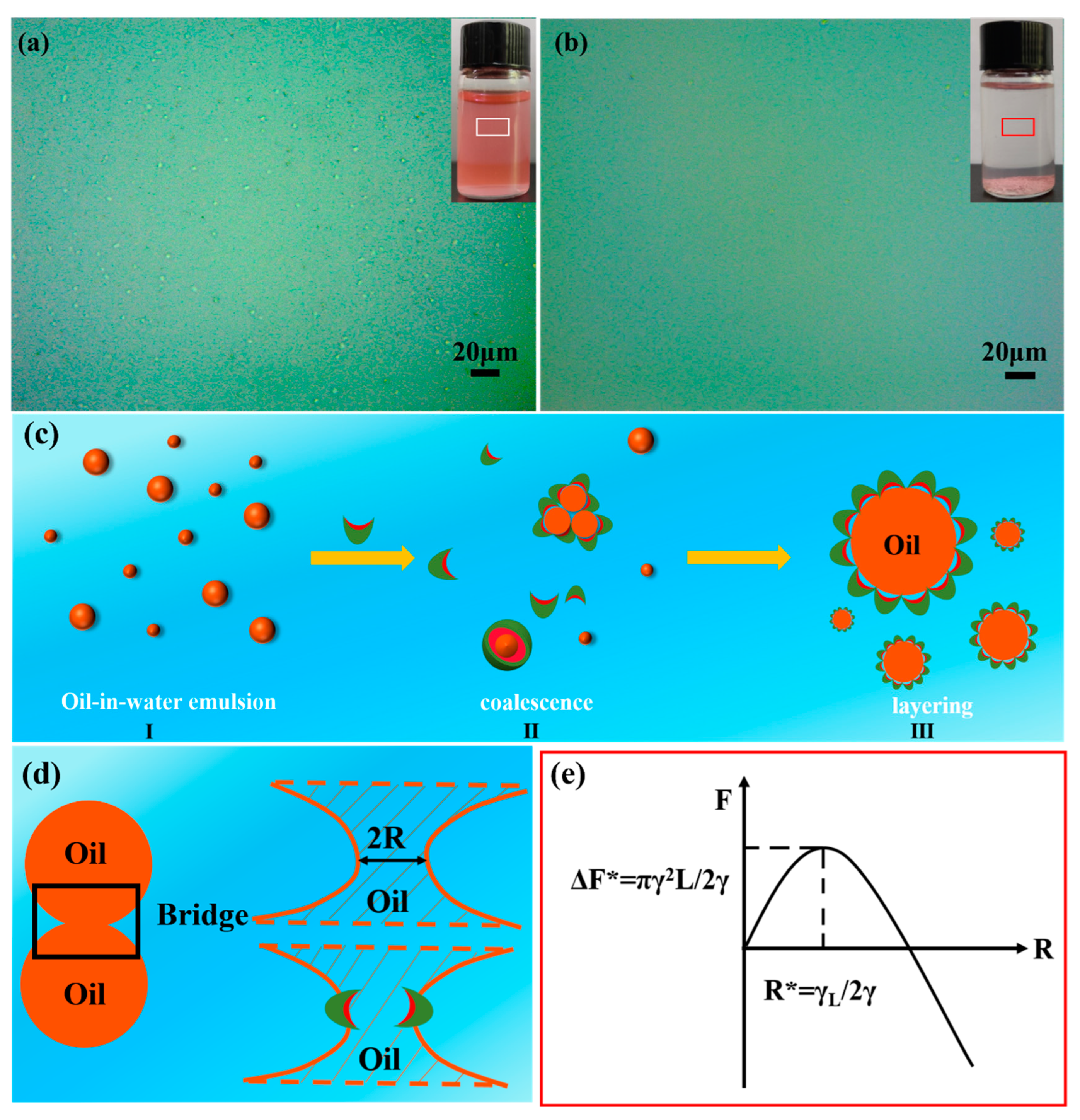
3.4. Oil–Water Separation of Amphiphilic Janus Particles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xue, Z.; Cao, Y.; Liu, N.; Feng, L.; Jiang, L. Special wettable materials for oil/water separation. J. Mater. Chem. A 2014, 2, 2445–2460. [Google Scholar] [CrossRef]
- Ivshina, I.B.; Kuyukina, M.S.; Krivoruchko, A.V.; Elkin, A.A.; Makarov, S.O.; Cunningham, C.J.; Peshkur, T.A.; Atlas, R.M.; Philp, J.C. Oil spill problems and sustainable response strategies through new technologies. Environ. Sci.-Proc.Imp. 2015, 17, 1201–1219. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Weng, D.; Mahmood, A.; Chen, S.; Wang, J. Separation mechanism and construction of surfaces with special wettability for oil/water separation. ACS Appl. Mater. Interfaces 2019, 11, 11006–11027. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, M.; Ahmad, A.; Ismail, N.; Rafatullah, M. Environmental Remediation through Carbon Based Nano Composites; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–18. [Google Scholar]
- Song, Y.; Zhou, J.; Fan, J.; Zhai, W.; Meng, J.; Wang, S. Hydrophilic/oleophilic magnetic Janus particles for the rapid and efficient oil-water separation. Adv. Funct. Mater. 2018, 28, 1802493. [Google Scholar] [CrossRef]
- Li, C.; Wu, L.; Yu, C.; Dong, Z.; Jiang, L. Peristome-mimetic curved surface for spontaneous and directional separation of micro water-in-oil drops. Angew. Chem. 2017, 129, 13811–13816. [Google Scholar] [CrossRef]
- Yue, X.; Li, Z.; Zhang, T.; Yang, D.; Qiu, F. Design and fabrication of superwetting fiber-based membranes for oil/water separation applications. Chem. Eng. J. 2019, 364, 292–309. [Google Scholar] [CrossRef]
- Ge, J.; Zong, D.; Jin, Q.; Yu, J.; Ding, B. Biomimetic and superwettable nanofibrous skins for highly efficient separation of oil-in-water emulsions. Adv. Funct. Mater. 2018, 28, 1705051. [Google Scholar] [CrossRef]
- Lin, X.; Hong, J. Recent advances in robust superwettable membranes for oil–water separation. Adv. Mater. Interfaces 2019, 6, 1900126. [Google Scholar] [CrossRef]
- Umar, K.; Yaqoob, A.A.; Ibrahim, M.N.M.; Parveen, T.; Safian, M.T.U. Environmental applications of smart polymer composites. In Smart Polymer Nanocomposites; Woodhead Publishing: Sawston, UK, 2021; pp. 295–312. [Google Scholar]
- Yin, T.; Yang, Z.; Zhang, F.; Lin, M.; Zhang, J.; Dong, Z. Assembly and mechanical response of amphiphilic Janus nanosheets at oil-water interfaces. J. Colloid Interface Sci. 2021, 583, 214–221. [Google Scholar] [CrossRef]
- Liu, P.; Li, X.; Yu, H.; Niu, L.; Yu, L.; Ni, D.; Zhang, Z. Functional janus-SiO2 nanoparticles prepared by a novel “cut the gordian knot” method and their potential application for enhanced oil recovery. ACS Appl. Mater. Interfaces 2020, 12, 24201–24208. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, D.; Zhang, T.; Qiu, F.; Dai, Y.; Xu, J.; Jing, Z. Synthesis of MnO2/poly (n-butylacrylate-co-butyl methacrylate-co-methyl methacrylate) hybrid resins for efficient oils and organic solvents absorption. J. Clean. Prod. 2017, 148, 398–406. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, F.; Zhang, C.; Yang, D.; Qiu, F.; Peng, X. A novel multi-wall carbon nanotubes/poly (n-butylacrylate-co-butyl methacrylate) hybrid resin: Synthesis and oil/organic solvents absorption. Fibers Polym. 2017, 18, 1865–1873. [Google Scholar] [CrossRef]
- Zhang, T.; Kong, L.; Dai, Y.; Yue, X.; Rong, J.; Qiu, F.; Pan, J. Enhanced oils and organic solvents absorption by polyurethane foams composites modified with MnO2 nanowires. Chem. Eng. J. 2017, 309, 7–14. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, T.; Qiu, F.; Yue, X.; Yang, D.; Li, P.; Zhu, Y. Facile one-step fabrication of highly hydrophobic, renewable and mechanically flexible sponge with dynamic coating for efficient oil/water separation. J. Taiwan Inst. Chem. Eng. 2019, 95, 515–524. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, H.; Liu, Y.; Craig, V.S.J.; Lai, Z. Selective separation of oil and water with mesh membranes by capillarity. Adv. Colloid Interface Sci. 2016, 235, 46–55. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, T.; Guo, Q.; Qiu, F.; Yang, D.; Ou, Z. Recyclable biomass carbon@ SiO2@ MnO2 aerogel with hierarchical structures for fast and selective oil-water separation. Chem. Eng. J. 2018, 351, 622–630. [Google Scholar] [CrossRef]
- Duan, Y.; Zhao, X.; Sun, M.; Hao, H. Research Advances in the Synthesis, Application, Assembly, and Calculation of Janus Materials. Ind. Eng. Chem. Res. 2021, 60, 1071–1095. [Google Scholar] [CrossRef]
- Poggi, E.; Gohy, J.F. Janus particles: From synthesis to application. Colloid Polym. Sci. 2017, 295, 2083–2108. [Google Scholar] [CrossRef]
- Yan, W.; Pan, M.; Yuan, J.; Liu, G.; Cui, L.; Zhang, G.; Zhu, L. Raspberry-like patchy particles achieved by decorating carboxylated polystyrene cores with snowman-like poly (vinylidene fluoride)/poly (4-vinylpyridiene) Janus particles. Polymer 2017, 122, 139–147. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, F.; Qu, X.; Wang, Q.; Yang, Z. Robust reactive Janus composite particles of snowman shape. Macromolecules 2015, 48, 2715–2722. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.R.; Wang, F.W. Facile fabrication of snowman-like Janus particles with asymmetric fluorescent properties via seeded emulsion polymerization. Colloid Polym. Sci. 2013, 291, 2993–3003. [Google Scholar] [CrossRef]
- Wang, Q.; Xiao, A.; Shen, Z.; Fan, X. Janus particles with tunable shapes prepared by asymmetric bottlebrush block copolymers. Polym. Chem. 2019, 10, 372–378. [Google Scholar] [CrossRef]
- Sun, X.; Guo, R.; Wang, D.; Wei, Y.; Yang, C.; Xu, Z. Microfluidic preparation of polymer-lipid Janus microparticles with staged drug release property. J. Colloid Interface Sci. 2019, 553, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Liu, G. ABC triblock copolymer hamburger-like micelles, segmented cylinders, and Janus particles. Soft Matter 2010, 6, 3654–3661. [Google Scholar] [CrossRef]
- Yamagami, T.; Kitayama, Y.; Okubo, M. Preparation of stimuli-responsive “mushroom-like” Janus polymer particles as particulate surfactant by site-selective surface-initiated AGET ATRP in aqueous dispersed systems. Langmuir 2014, 30, 7823–7832. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, X.; He, Y.; Song, P.; Wang, R. One-pot synthesis of mushroom-shaped polymeric Janus particles by soap-free emulsion copolymerization. Eur. Polym. J. 2019, 113, 260–266. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, L.; Huang, H.; Deng, J. Optically Active Janus Particles Constructed by Chiral Helical Polymers through Emulsion Polymerization Combined with Solvent Evaporation-Induced Phase Separation. ACS Appl. Mater. Interfaces 2020, 12, 6319–6327. [Google Scholar] [CrossRef]
- Hou, Y.; Li, Y.; Wang, L.; Chen, D.; Bao, M.; Wang, Z. Amphiphilic Janus particles for efficient dispersion of oil contaminants in seawater. J. Colloid Interface Sci. 2019, 556, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Frank, B.D.; Perovic, M.; Djalali, S.; Antonietti, M.; Oschatz, M.; Zeininger, L. Synthesis of polymer Janus particles with tunable wettability profiles as potent solid surfactants to promote gas delivery in aqueous reaction media. ACS Appl. Mater. Interfaces 2021, 13, 32510–32519. [Google Scholar] [CrossRef]
- Franken, L.E.; Wei, Y.; Chen, J.; Boekema, E.J.; Zhao, D.; Stuart, M.C.A.; Feringa, B.L. Solvent mixing to induce molecular motor aggregation into bowl-shaped particles: Underlying mechanism, particle nature, and application to control motor behavior. J. Am. Chem. Soc. 2018, 140, 7860–7868. [Google Scholar] [CrossRef]
- Zhai, W.; Song, Y.; Gao, Z.; Fan, J.; Wang, S. Precise Synthesis of Polymer Particles Spanning from Anisotropic Janus Particles to Heterogeneous Nanoporous Particles. Macromolecules 2019, 52, 3237–3243. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, X.; Du, Y.; Zhang, Z.; Zhang, X. Amphiphilic Bowl-Shaped Janus Particles Prepared via Thiol–Ene Click Reaction for Effective Oil–Water Separation. Nanomaterials 2023, 13, 455. https://doi.org/10.3390/nano13030455
Qi X, Du Y, Zhang Z, Zhang X. Amphiphilic Bowl-Shaped Janus Particles Prepared via Thiol–Ene Click Reaction for Effective Oil–Water Separation. Nanomaterials. 2023; 13(3):455. https://doi.org/10.3390/nano13030455
Chicago/Turabian StyleQi, Xian, Yaxian Du, Ziqiang Zhang, and Xu Zhang. 2023. "Amphiphilic Bowl-Shaped Janus Particles Prepared via Thiol–Ene Click Reaction for Effective Oil–Water Separation" Nanomaterials 13, no. 3: 455. https://doi.org/10.3390/nano13030455
APA StyleQi, X., Du, Y., Zhang, Z., & Zhang, X. (2023). Amphiphilic Bowl-Shaped Janus Particles Prepared via Thiol–Ene Click Reaction for Effective Oil–Water Separation. Nanomaterials, 13(3), 455. https://doi.org/10.3390/nano13030455





