High-Efficiency Chemical-Mechanical Magnetorheological Finishing for Ultra-Smooth Single-Crystal Silicon
Abstract
1. Introduction
2. Samples Preparation and Characterizations
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mimura, H.; Handa, S.; Kimura, T.; Yumoto, H.; Yamakawa, D.; Yokoyama, H.; Matsuyama, S.; Inagaki, K.; Yamamura, K.; Sano, Y. Breaking the 10 nm barrier in hard-X-ray focusing. Nat. Phys. 2010, 6, 122–125. [Google Scholar] [CrossRef]
- Fujisawa, T.; Inoue, K.; Oka, T.; Iwamoto, H.; Uruga, T.; Kumasaka, T.; Inoko, Y.; Yagi, N.; Yamamoto, M.; Ueki, T. Small-angle X-ray scattering station at the SPring-8 RIKEN beamline. J. Appl. Crystallogr. 2010, 33, 797–800. [Google Scholar] [CrossRef]
- Spiller, E. Soft X-ray Optics. Opt. Photonics. News. 1993, 7, 60. [Google Scholar]
- Siewert, F.; Buchheim, J.; Boutet, S.; Williams, G.J.; Signorato, R. Ultra-precise characterization of LCLS hard X-ray focusing mirrors by high resolution slope measuring deflectometry. Opt. Express 2012, 20, 4525–4536. [Google Scholar] [CrossRef] [PubMed]
- Pardini, T.; Cocco, D.; Hau-Riege, S.P. Effect of slope errors on the performance of mirrors for X-ray free electron laser applications. Opt. Express 2015, 23, 31889. [Google Scholar] [CrossRef]
- Shi, X.; Assoufid, L.; Reininger, R. How to specify super-smooth mirrors: Simulation studies on nano-focusing and wavefront preserving X-ray mirrors for next-generation light sources. In Proceedings of the 8th International Symposium on Advanced Optical Manufacturing and Testing Technology (AOMATT2016), Suzhou, China, 26–29 April 2016. [Google Scholar]
- Tatchyn, R.; Arthur, J.; Boyce, R.; Fasso, A.; Howells, M. X-ray optics design studies for the SLAC 1.5-15 Angstrom Linac Coherent Light Source (LCLS). Nucl. Instrum. Methods Phys. Res. 1999, 429, 397–406. [Google Scholar] [CrossRef]
- Guigay, J.P.; Morawe, C.; Mocella, V.; Ferrero, C. An analytical approach to estimating aberrations in curved multilayer optics for hard x-rays: 1. Derivation of caustic shapes. Opt. Express 2008, 16, 12050–12059. [Google Scholar] [CrossRef] [PubMed]
- Morawe, C.; Guigay, J.P.; Mocella, V.; Ferrero, C. An analytical approach to estimating aberrations in curved multilayer optics for hard X-rays: 2. Interpretation and application to focusing experiments. Opt. Express 2008, 16, 16138–16150. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.; Golini, D.; Hsu, Y.; Puchebner, B.; Strafford, D.; Prokhorov, I.; Fess, E.; Pietrowski, D.; Kordonski, W. Magnetorheological finishing: A deterministic process for optics manufacturing. In Proceedings of the International Conferences on Optical Fabrication and Testing and Applications of Optical Holography, Tokyo, Japan, 2 August 1995. [Google Scholar]
- Kordonski, W.; Gorodkin, S. Material removal in magnetorheological finishing of optics. Appl. Opt. 2011, 50, 1984–1994. [Google Scholar] [CrossRef] [PubMed]
- Dumas, P.; McFee, C. Novel MRF fluid for Ultra-Low Roughness optical surfaces. In Proceedings of the 7th International Symposium on Advanced Optical Manufacturing and Testing Technologies: Advanced Optical Manufacturing Technologies (AOMATT 2014), Harbin, China, 6 August 2014. [Google Scholar]
- Dumas, P.; Golini, D.; Tricard, M. Improvement of figure and finish of diamond turned surfaces with magneto-rheological finishing (MRF). In Proceedings of the Window and Dome Technologies and Materials IX, Orlando, FL, USA, 18 May 2005. [Google Scholar]
- Catrin, R.; Neauport, J.; Taroux, D.; Cormont, P.; Maunier, C.; Lambert, S. Magnetorheological finishing for removing surface and subsurface defects of fused silica optics. Opt. Eng. 2014, 53, 7. [Google Scholar] [CrossRef]
- Campbell, J.H.; Hawley-Fedder, R.; Stolz, C.J.; Menapace, J.A.; Borden, M.R. NIF optical materials and fabrication technologies: An overview. In Proceedings of the Optical Engineering at the Lawrence Livermore National Laboratory II: The National Ignition Facility, San Jose, CA, USA, 28 May 2004. [Google Scholar]
- Ghosh, G.; Sidpara, A.; Bandyopadhyay, P.P. Experimental and theoretical investigation into surface roughness and residual stress in magnetorheological finishing of OFHC copper. J. Mater. Process. Technol. 2021, 288, 116899. [Google Scholar] [CrossRef]
- Thiess, H.; Lasser, H.; Siewert, F. Fabrication of X-ray mirrors for synchrotron applications. Nucl. Instrum. Methods Phys. Res. 2010, 616, 157–161. [Google Scholar] [CrossRef]
- Seifert, A. New Products for Synchrotron Application Based On Novel Surface Processing Developments. In Proceedings of the Synchrotron radiation instrumention: 9th International Conference on Synchrotron Radiation Instrumentation, Daegu, Republic of Korea, 28 May–2 June 2006. [Google Scholar]
- Liu, S.; Wang, H.; Hou, J.; Zhang, Q.; Chen, X.; Zhong, B.; Zhang, M. Combined processing strategy based on magnetorheological finishing for monocrystalline silicon x-ray mirrors. Appl. Opt. 2022, 61, 5575–5584. [Google Scholar] [CrossRef]
- Zhong, Y.; Dai, Y.; Xiao, H.; Shi, F. Experimental study on surface integrity and subsurface damage of fused silica in ultra-precision grinding. Int. J. Adv. Manuf. Technol. 2021, 115, 4021–4033. [Google Scholar] [CrossRef]
- Yin, L.; Lin, Z.; Hu, H.; Dai, Y. Rapid polishing process for the X ray reflector. Appl. Opt. 2022, 61, 7991–7998. [Google Scholar] [CrossRef] [PubMed]
- Burge, J.H.; Kim, D.W.; Martin, H.M. Process optimization for polishing large aspheric mirrors. In Proceedings of the Advances in Optical and Mechanical Technologies for Telescopes and Instrumentation, Montréal, QC, Canada, 28 July 2014. [Google Scholar]
- Menapace, J.A. Developing magnetorheological finishing (MRF) technology for the manufacture of large-aperture optics in megajoule class laser systems. In Proceedings of the Laser Damage Symposium XLII: Annual Symposium on Optical Materials for High Power Lasers, Boulder, CO, USA, 3 December 2010. [Google Scholar]
- Messner, W.J.; Hall, C. High removal rate MRF[TM]. In Proceedings of the Optifab, Rochester, NY, USA, 28 October 2021. [Google Scholar]
- Johnson, J.S.; Grobsky, K.; Bray, D.J. Rapid fabrication of lightweight silicon carbide mirrors. In Proceedings of the International Symposium on Optical Science and Technology, Seattle, WA, USA, 9 September 2002. [Google Scholar]
- Kai, R.; Xiao, L.; Li, G.Z.; Yang, B.; Long, X.L. Belt-MRF for large aperture mirrors. Opt. Express 2014, 22, 19262–19276. [Google Scholar]
- Jung, B.; Jang, K.I.; Min, B.K.; Sang, J.L.; Seok, J. Magnetorheological finishing process for hard materials using sintered iron-CNT compound abrasives. Int. J. Mach. Tools Manuf. 2009, 49, 407–418. [Google Scholar] [CrossRef]
- Kyung, I.; Jang, E.N.; Chan, Y.L.; Jong, W. Mechanism of synergetic material removal by electrochemomechanical magnetorheological polishing. Int. J. Mach. Tools Manuf. 2013, 70, 88–92. [Google Scholar]
- Wang, Y.; Zhang, Y.; Feng, Z. Analyzing and improving surface texture by dual-rotation magnetorheological finishing. Appl. Surf. Sci. 2016, 360, 224–233. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, F.; Fan, W.; Huang, W.; He, J. Research on magnetorheological finishing of aspheric optics for single-crystal silicon. In Proceedings of the Second Symposium on Novel Technology of X-ray Imaging, Hefei, China, 10 May 2019. [Google Scholar]
- Bentley, J.L.; Stoebenau, S.; Maloney, C.; Oswald, E.S.; Dumas, P. Fine figure correction and other applications using novel MRF fluid designed for ultra-low roughness. In Proceedings of the Optifab, Rochester, NY, USA, 11 October 2015. [Google Scholar]
- Sidpara, A.; Jain, V.K. Nano–level finishing of single crystal silicon blank using magnetorheological finishing process. Tribol. Int. 2012, 47, 159–166. [Google Scholar] [CrossRef]
- Estragnat, E.; Tang, G.; Liang, H.; Jahanmir, S.; Pei, P.; Martin, J.M. Experimental investigation on mechanisms of silicon chemical mechanical polishing. J. Electron. Mater. 2004, 33, 334–339. [Google Scholar] [CrossRef]
- Wang, Y.G.; Zhang, L.C.; Biddut, A. Chemical effect on the material removal rate in the CMP of silicon wafers. Wear 2011, 270, 312–316. [Google Scholar] [CrossRef]
- Su, J.X.; Guo, D.M.; Kang, R.K.; Jin, Z.J.; Li, X.J.; Tian, Y.B. Modeling and Analyzing on Nonuniformity of Material Removal in Chemical Mechanical Polishing of Silicon Wafer. Mater. Sci. Forum. 2004, 471–472, 26–31. [Google Scholar] [CrossRef]
- Hartmann, M.; Kullmann, S.; Keller, H. Wastewater Treatment with Heterogeneous Fenton-type Catalysts Based on Porous Materials. J. Mater. Chem. 2010, 20, 9002–9017. [Google Scholar] [CrossRef]
- Georges, O.N.; Guillaume, M.; Yuheng, W.; Andrea, L.; Foster, F. XANES Evidence for Rapid Arsenic(III) Oxidation at Magnetite and Ferrihydrite Surfaces by Dissolved O2 via Fe2+-Mediated Reactions. Environ. Sci. Technol. 2010, 44, 5416–5422. [Google Scholar]
- Chen, L.; Wang, Y.; Zhang, H.; Gao, Y. Goethite as an efficient heterogeneous Fenton catalyst for the degradation of methyl orange. Catal. Today 2015, 252, 107–112. [Google Scholar]
- Huang, R.; Fang, Z.; Yan, X.; Wen, C. Heterogeneous sono-Fenton catalytic degradation of bisphenol A by Fe3O4 magnetic nanoparticles under neutral condition. Chem. Eng. J. 2012, 197, 242–249. [Google Scholar] [CrossRef]
- Chen, C.H.; Xie, B.; Ren, Y. The mechanisms of affecting factors in treating wastewater by Fenton reagent. J. Environ. Sci. 2000, 21, 93–96. [Google Scholar]
- Wakaki, M.; Shibuya, T.; Kudo, K. Physical Properties and Data of Optical Materials, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 339–378. [Google Scholar]
- Lucovsky, G.; Mantini, M.J.; Srivastava, J.K.; Irene, E.A. Low-temperature growth of silicon dioxide films: A study of chemical bonding by ellipsometry and infrared spectroscopy. J. Vac. Sci. Technol. B 1987, 5, 530–537. [Google Scholar] [CrossRef]
- Kim, J.T.; Kim, M.C. Silicon wafer technique for infrared spectra of silica and solid samples (I). Korean J. Chem. Eng. 1986, 3, 45–51. [Google Scholar] [CrossRef]
- Cook, L.M. Chemical processes in glass polishing. J. Non. Cryst. Solids 1990, 120, 152–171. [Google Scholar] [CrossRef]
- MichalskeI, T.A.; Freiman, S.W. A Molecular Mechanism for Stress Corrosion in Vitreous Silica. J. Am. Ceram. Soc. 1983, 66, 284–288. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y. The molecular level dissolution mechanisms of quartz under different pH conditions. Geochimica 2009, 38, 549–557. [Google Scholar]


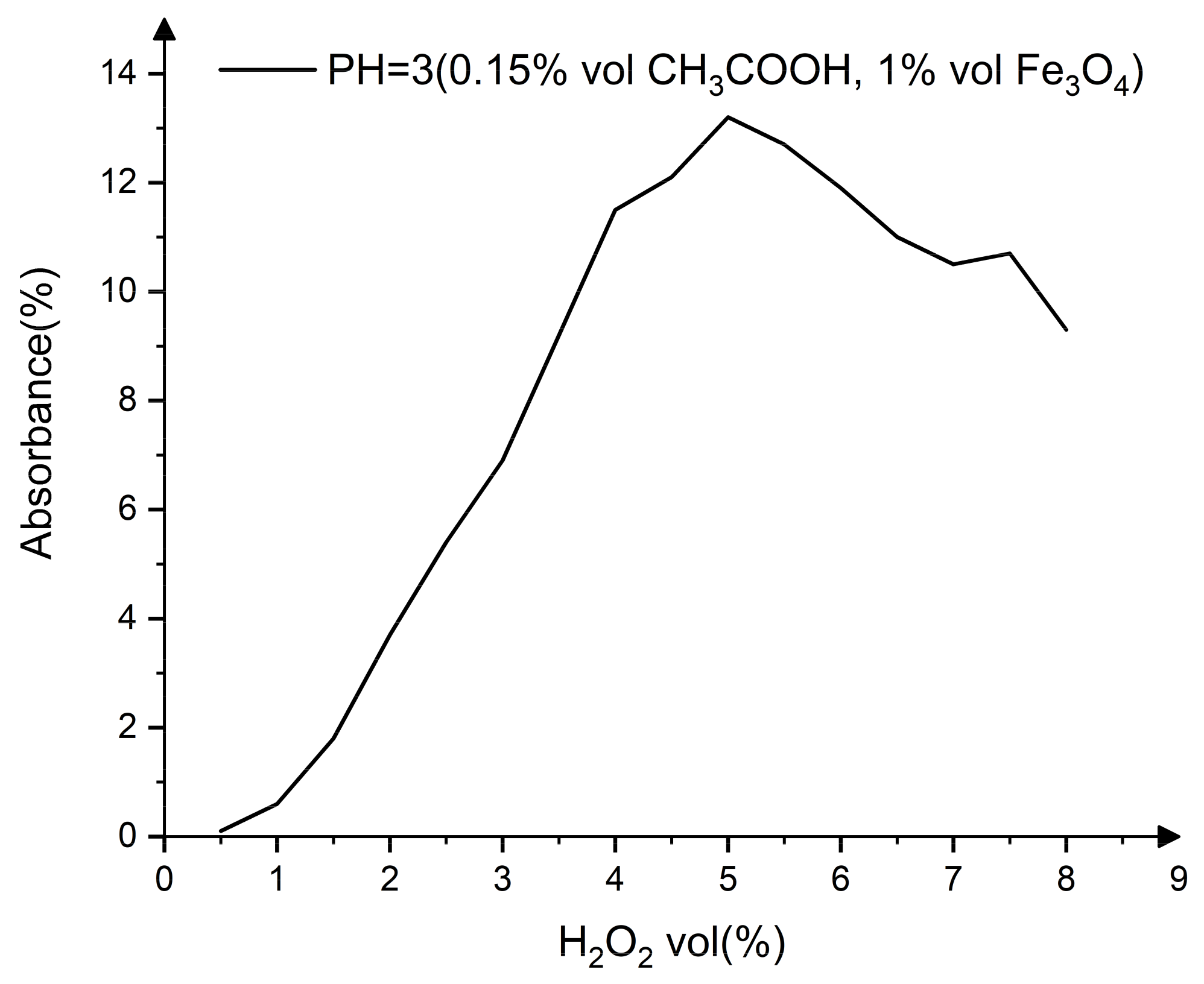
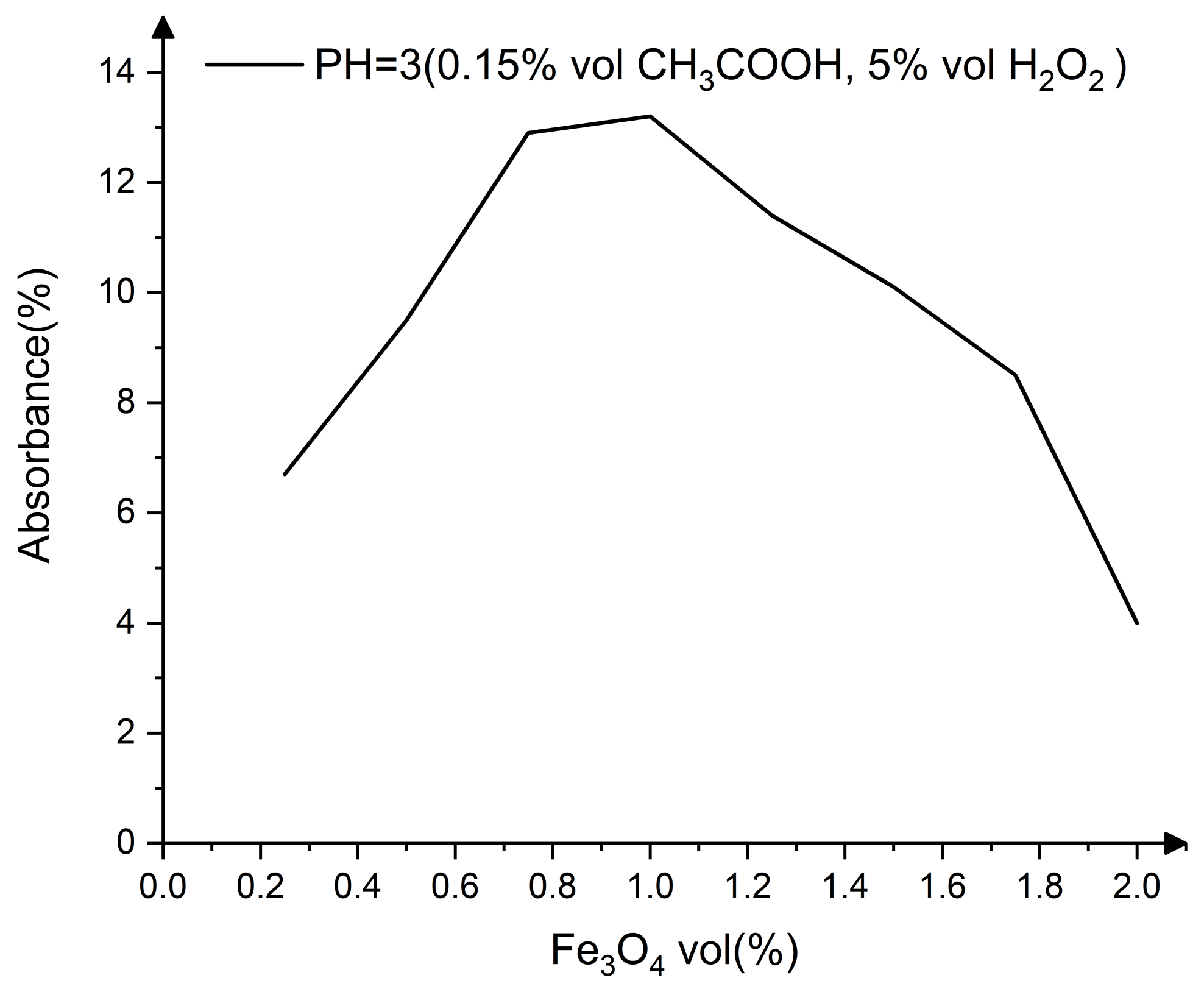


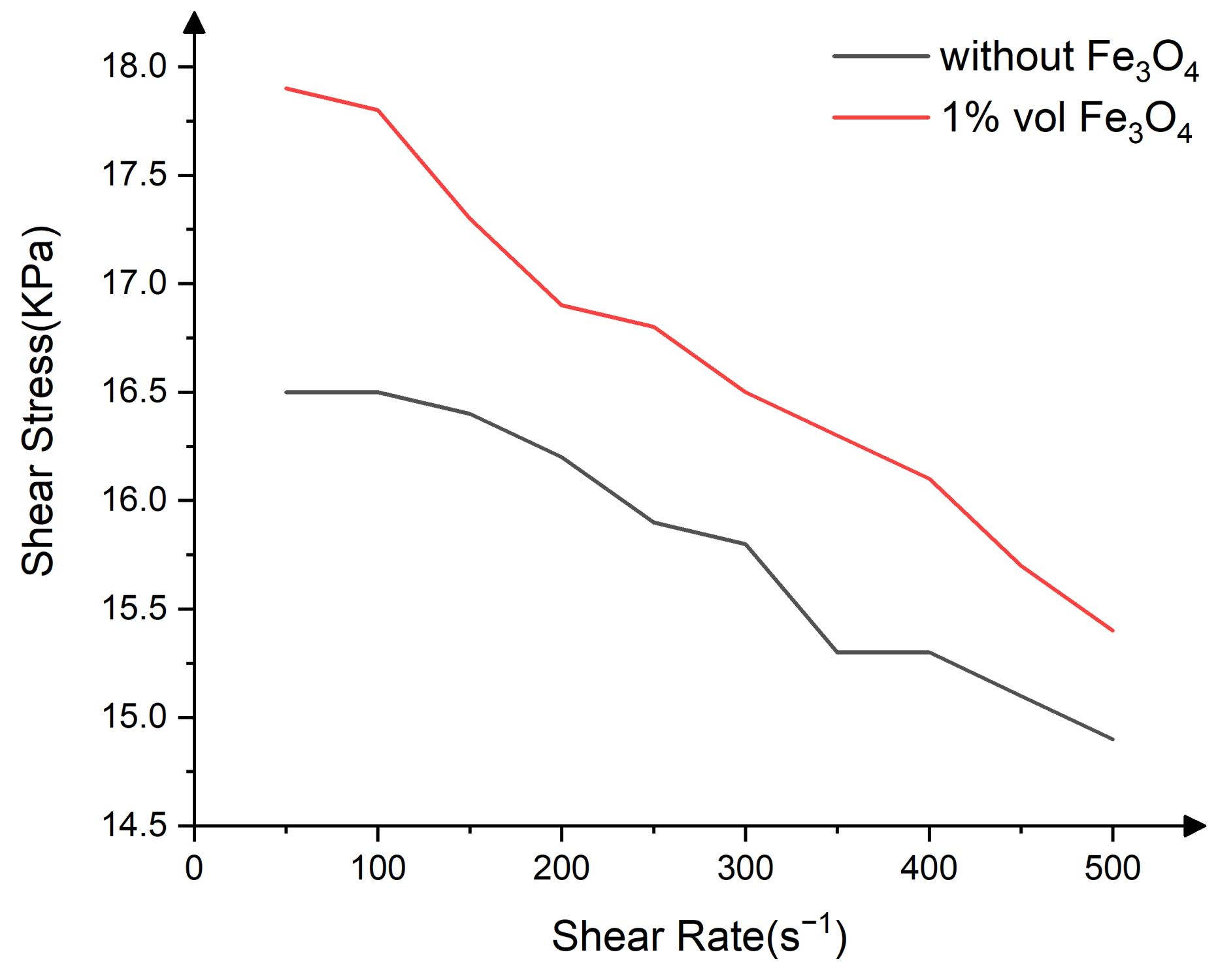


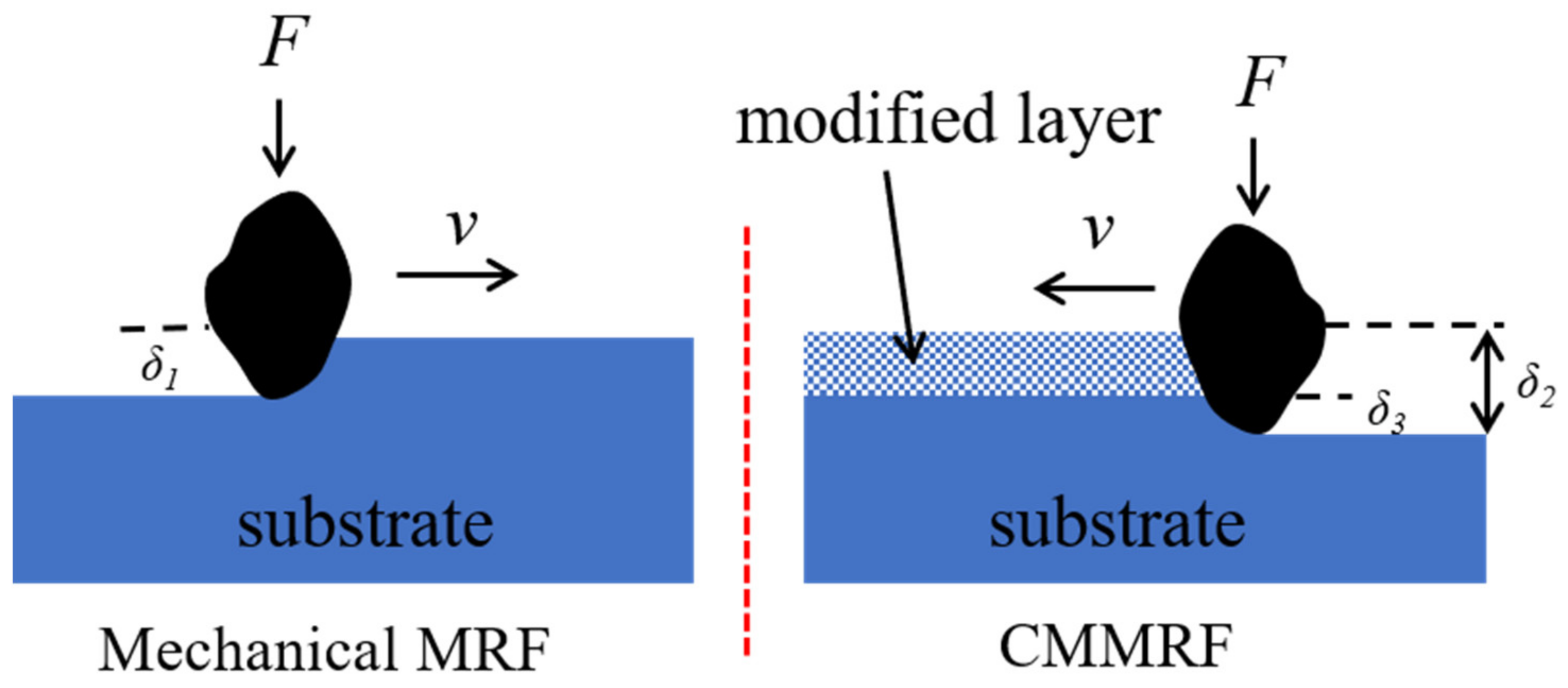
| Constituents | Purity | Supplier |
|---|---|---|
| CIP | - | BASF, Ludwigshafen, Germany |
| Nano-Fe3O4 | - | Delta, Xiamen, China |
| Diamond abrasive | - | Huanghe Whirlwind, Zhengzhou, China |
| H2O2 | AR | Sinopharm Chemical Reagent, Shanghai, China |
| CH3COOH | AR | Sinopharm Chemical Reagent, Shanghai, China |
| PEG200 | AR | OKA, Beijing, China |
| HCl | AR | Sinopharm Chemical Reagent, Shanghai, China |
| NaOH | AR | Sinopharm Chemical Reagent, Shanghai, China |
| Number | H2O2 (vol%) | Fe3O4 (vol%) | CH3COOH (vol%) | pH |
|---|---|---|---|---|
| 1 | 5 | - | - | 3 |
| 2 | 5 | 1 | - | 3 |
| 3 | 5 | 1 | 0.15 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Hu, H.; Dai, Y.; Zhong, Y.; Xue, S. High-Efficiency Chemical-Mechanical Magnetorheological Finishing for Ultra-Smooth Single-Crystal Silicon. Nanomaterials 2023, 13, 398. https://doi.org/10.3390/nano13030398
Lin Z, Hu H, Dai Y, Zhong Y, Xue S. High-Efficiency Chemical-Mechanical Magnetorheological Finishing for Ultra-Smooth Single-Crystal Silicon. Nanomaterials. 2023; 13(3):398. https://doi.org/10.3390/nano13030398
Chicago/Turabian StyleLin, Zhifan, Hao Hu, Yifan Dai, Yaoyu Zhong, and Shuai Xue. 2023. "High-Efficiency Chemical-Mechanical Magnetorheological Finishing for Ultra-Smooth Single-Crystal Silicon" Nanomaterials 13, no. 3: 398. https://doi.org/10.3390/nano13030398
APA StyleLin, Z., Hu, H., Dai, Y., Zhong, Y., & Xue, S. (2023). High-Efficiency Chemical-Mechanical Magnetorheological Finishing for Ultra-Smooth Single-Crystal Silicon. Nanomaterials, 13(3), 398. https://doi.org/10.3390/nano13030398





