Solvent Effects in the Preparation of Catalysts Using Activated Carbon as a Carrier
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Catalysts
2.3. Characterization
2.4. Heterogeneous Catalytic Evaluation
3. Results and Discussion
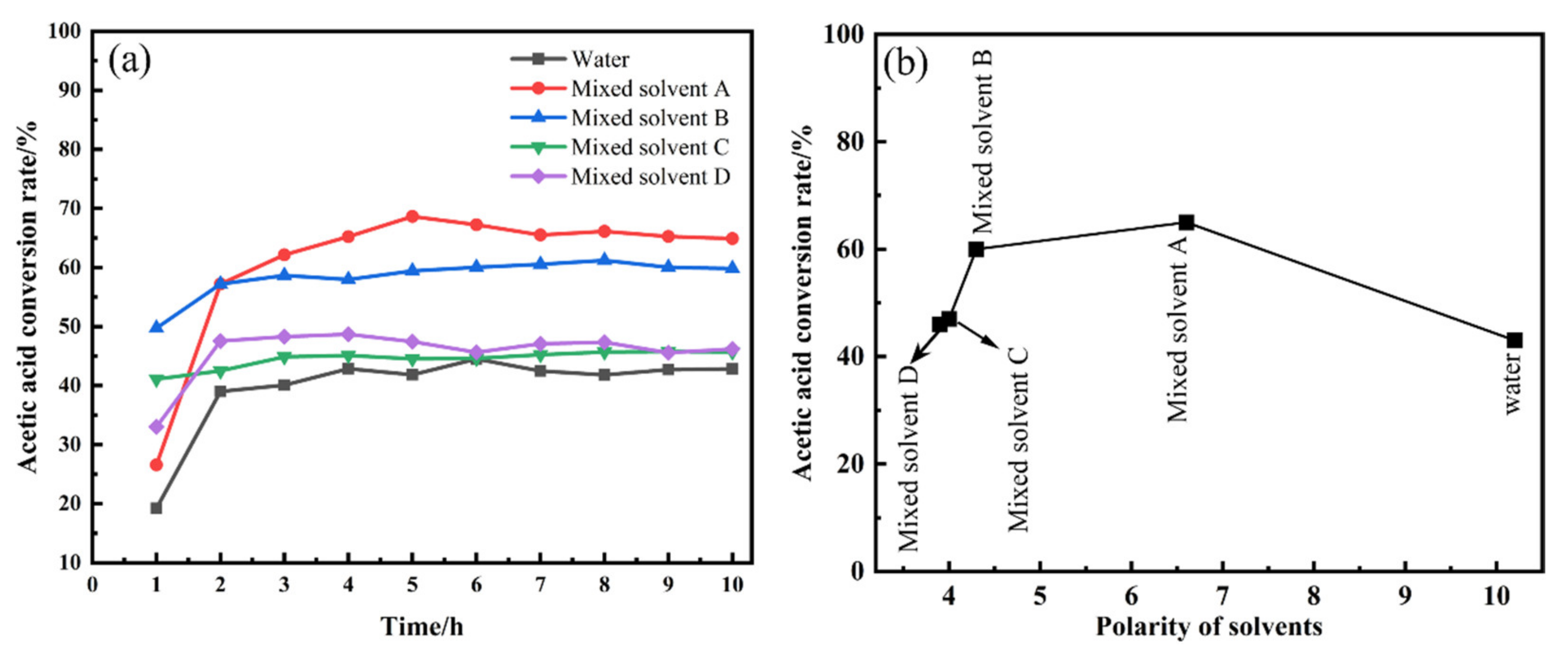
3.1. Performance Evaluation of Catalysts
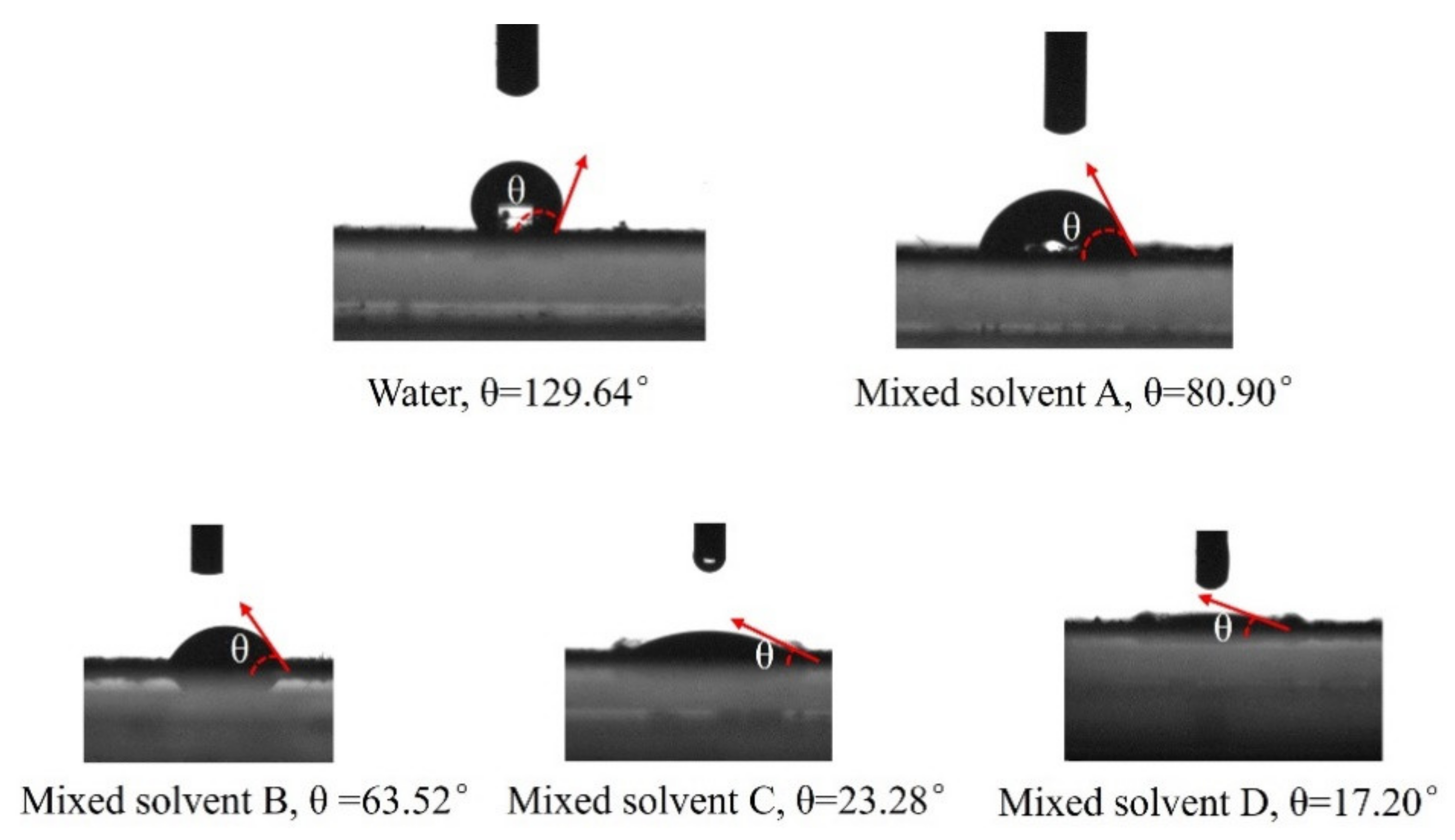
3.2. Analysis of Contact Angle
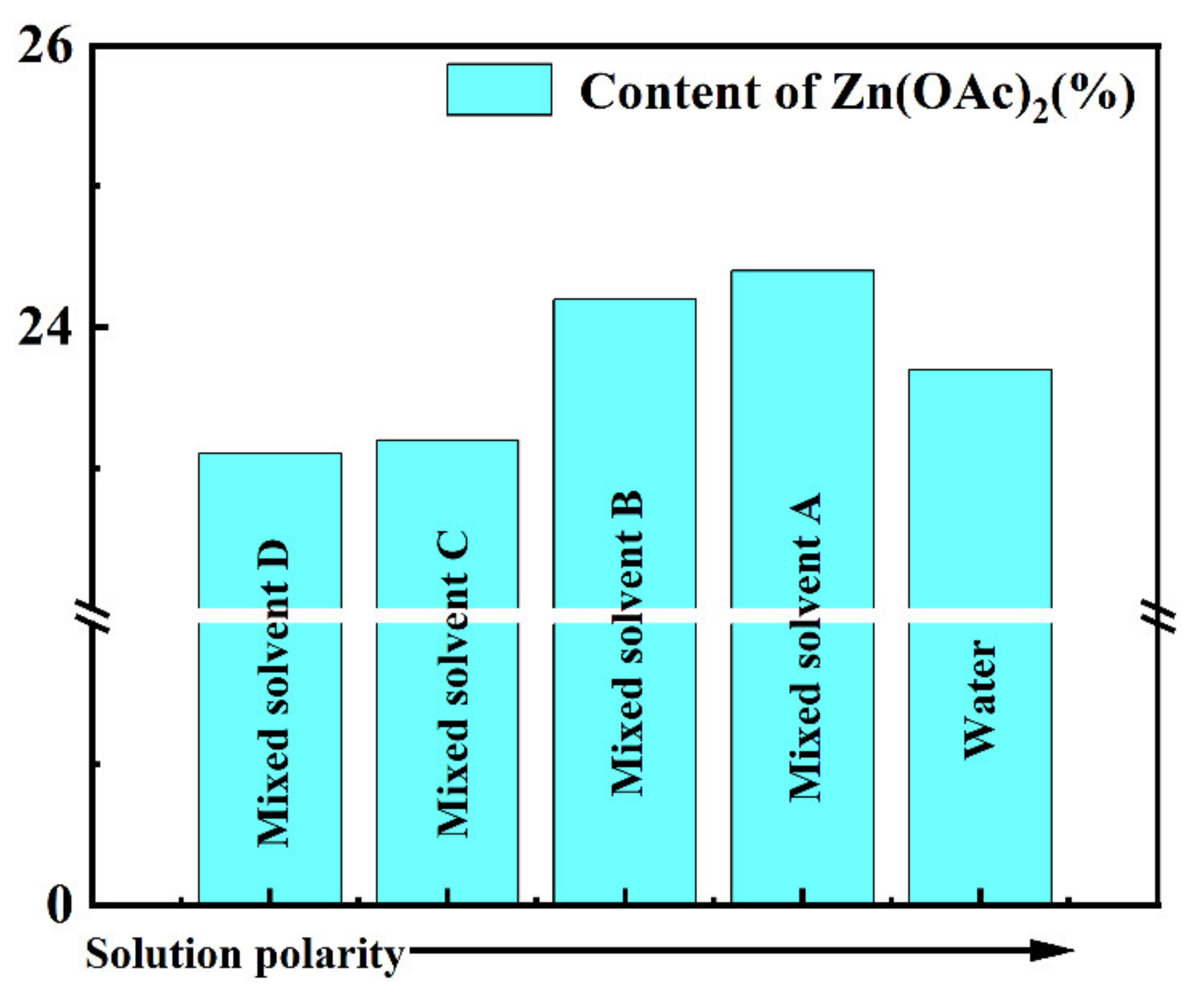
3.3. Relationship between Solvent Polarity and Active Components
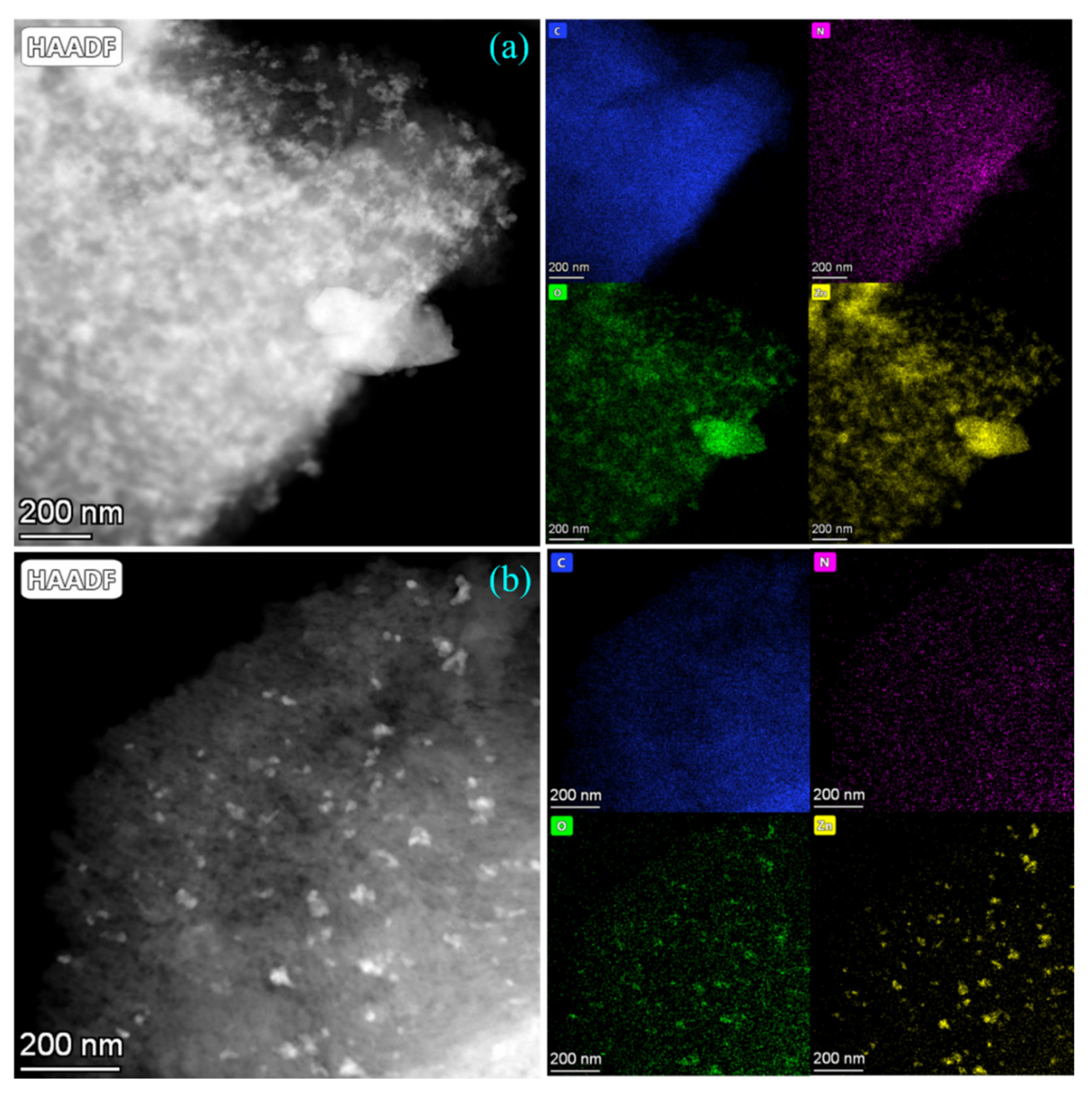
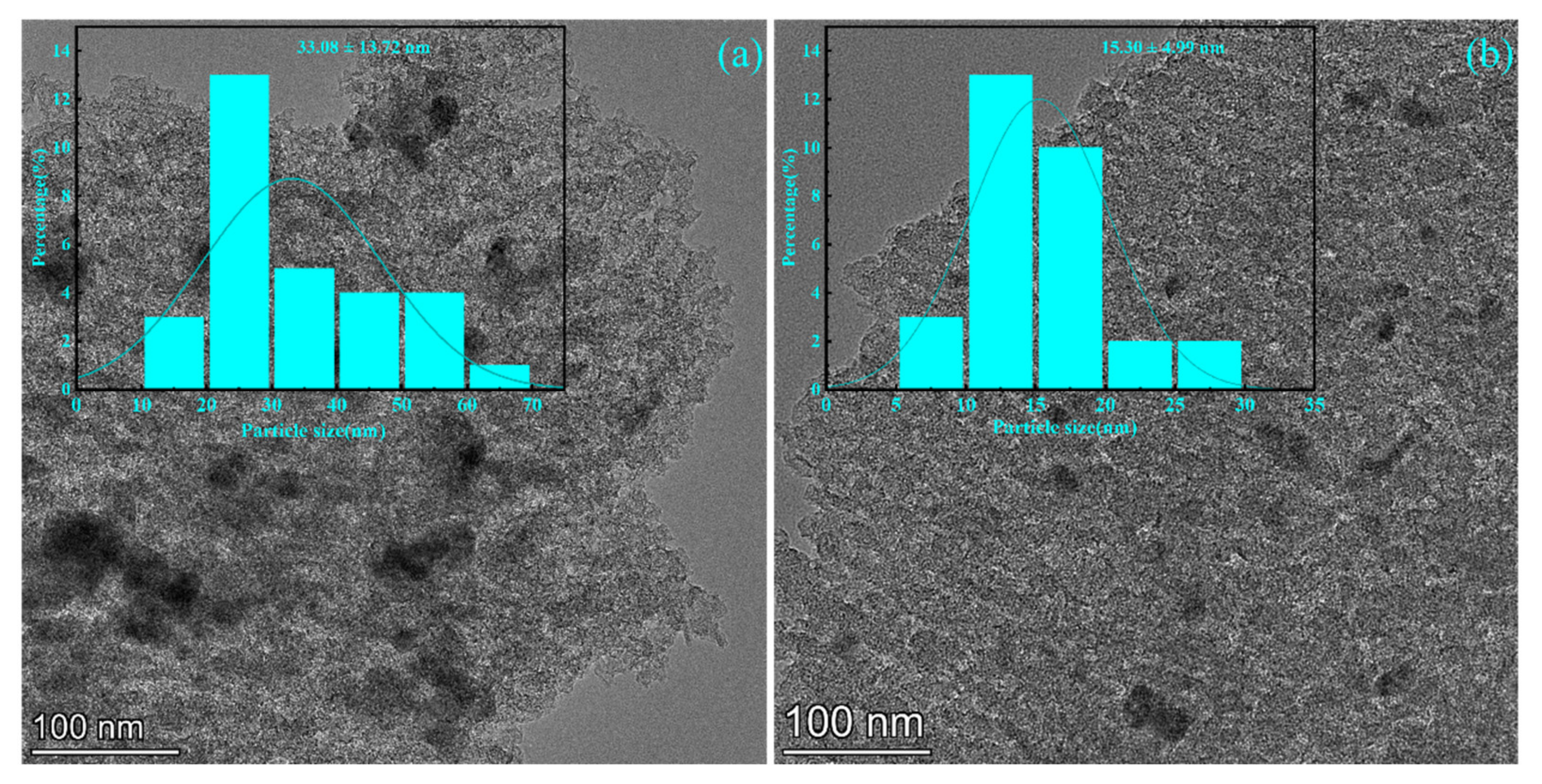
3.4. TEM Mapping Analysis
3.5. Analysis of BET Data
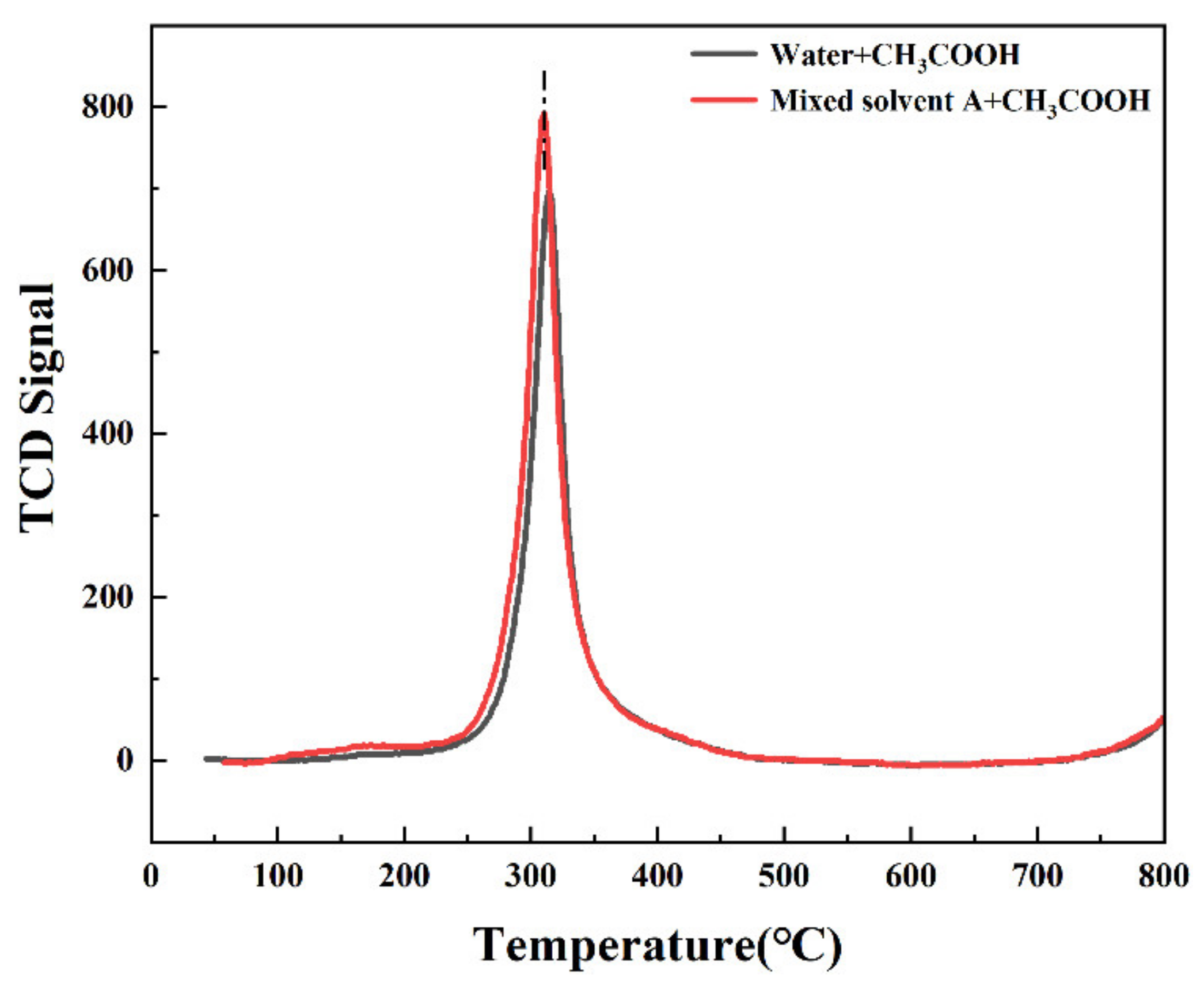
3.6. TPD Data Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ania, C.O.; Cabal, B.; Pevida, C.; Arenillas, A.; Parra, J.B.; Rubiera, F.; Pis, J.J. Removal of naphthalene from aqueous solution on chemically modified activated carbons. Water Res. 2007, 41, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Bayatpour, S.; Qian, X.; Frigo-Vaz, B.; Wang, P. Activated carbon fibers via reductive carbonization of cellulosic biomass for adsorption of nonpolar volatile organic compounds. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 612, 125908. [Google Scholar] [CrossRef]
- Zhu, X.; He, M.; Sun, Y.; Xu, Z.; Wan, Z.; Hou, D.; Alessi, D.S.; Tsang, D.C. Insights into the adsorption of pharmaceuticals and personal care products (PPCPs) on biochar and activated carbon with the aid of machine learning. J. Hazard. Mater. 2022, 423, 127060. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yang, L.; Song, C. Chemical looping hydrogen production using activated carbon and carbon black as multi-function carriers. Int. J. Hydrogen Energy 2018, 43, 5501–5511. [Google Scholar] [CrossRef]
- Zhu, Z.; Ji, C.; Zhong, L.; Liu, S.; Cui, F.; Sun, H.; Wang, W. Magnetic Fe–Co crystal doped hierarchical porous carbon fibers for removal of organic pollutants. J. Mater. Chem. A 2017, 5, 18071–18080. [Google Scholar] [CrossRef]
- Berthold, J.; Rinaudo, M.; Salmeń, L. Association of water to polar groups; estimations by an adsorption model for ligno-cellulosic materials. Colloids Surf. A Physicochem. Eng. Asp. 1996, 112, 117–129. [Google Scholar] [CrossRef]
- Milescu, R.A.; Dennis, M.R.; McElroy, C.R.; Macquarrie, D.J.; Matharu, A.S.; Smith, M.W.; Clark, J.H.; Budarin, V.L. The role of surface functionality of sustainable mesoporous materials Starbon® on the adsorption of toxic ammonia and sulphur gasses. Sustain. Chem. Pharm. 2020, 15, 100230. [Google Scholar] [CrossRef]
- Basheer, C.; Kamran, M.; Ashraf, M.; Lee, H.K. Enhancing liquid-phase microextraction efficiency through chemical reactions. Trends Anal. Chem. 2019, 118, 426–433. [Google Scholar] [CrossRef]
- Zhu, Q.; Xuan, Y.; Zhang, K.; Chang, K. Enhancing photocatalytic CO2 reduction performance of g-C3N4-based catalysts with non-noble plasmonic nanoparticles. Appl. Catal. B Environ. 2021, 297, 120440. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Mahdi, A.B. Synthesis highly active surface of ZnO/AC nanocomposite for removal of pollutants from aqueous solutions: Thermodynamic and kinetic study. Appl. Nanosci. 2021, 1–14. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Liang, H.; Li, L.; Zhang, J. High-efficiency catalysis of Ru-based catalysts assisted by triazine-based ligands containing different heteroatoms (N, O, S) for acetylene hydrochlorination. Mol. Catal. 2022, 519, 112142. [Google Scholar] [CrossRef]
- Wu, C.; Dai, X.; Sun, X.; Zhang, J. Preparation and characterization of fluoroalkyl activated carbons/PVDF composite membranes for water and resources recovery by membrane distillation. Sep. Purif. Technol. 2023, 305, 122519. [Google Scholar] [CrossRef]
- Xie, J.-X.; Cao, J.-P.; Zhao, X.-Y.; Jiang, W.; Zhao, L.; Zhao, M.; Bai, H.-C. Selective cleavage of the diphenyl ether C–O bond over a Ni catalyst supported on AC with different pore structures and hydrophilicities. Energy Fuels 2021, 35, 9599–9608. [Google Scholar] [CrossRef]
- Li, W.-J.; Kuo, J.-H.; Yang, R.-X.; Wey, M.-Y. Effect of preparation solvent and calcination atmosphere on Ni@ SiO2 catalyst for simultaneous production of hydrogen and carbon nanotubes from simulated plastic waste syngas. Energy Technol. 2019, 7, 1800586. [Google Scholar] [CrossRef]
- Jang, M.-S.; Cho, E.H.; Koo, K.Y.; Yoon, W.L.; Ko, C.H. Facile preparation of egg-shell-type pellet catalysts using immiscibility between hydrophobic solvent and hydrophilic solution: Enhancement of catalytic activity due to position control of metallic nickel inside alumina pellet. Appl. Catal. A Gen. 2017, 530, 211–216. [Google Scholar] [CrossRef]
- Mu, X.; Gu, J.; Feng, F.; Xiao, Z.; Chen, C.; Liu, S.; Mu, S. RuRh bimetallene nanoring as high-efficiency pH-universal catalyst for hydrogen evolution reaction. Adv. Sci. 2021, 8, 2002341. [Google Scholar] [CrossRef]
- Cavuoto, D.; Ravasio, N.; Scotti, N.; Gervasini, A.; Campisi, S.; Marelli, M.; Cappelletti, G.; Zaccheria, F. A green solvent diverts the hydrogenation of γ–valerolactone to 1, 4-pentandiol over Cu/SiO2. Mol. Catal. 2021, 516, 111936. [Google Scholar] [CrossRef]
- Sun, X.; Dawson, S.R.; Parmentier, T.E.; Malta, G.; Davies, T.E.; He, Q.; Lu, L.; Morgan, D.J.; Carthey, N.; Johnston, P.; et al. Facile synthesis of precious-metal single-site catalysts using organic solvents. Nat. Chem. 2020, 12, 560–567. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Amjad, S.; Teixeira, P.; Lopes, J.M.; Henriques, C. Boosting Ni dispersion on zeolite-supported catalysts for CO2 methanation: The influence of the impregnation solvent. Energy Fuels 2020, 34, 14656–14666. [Google Scholar] [CrossRef]
- Zhang, M.; Zhuang, J.; Wu, X.; Yu, Y. Experimental and theoretical insights into the cyclotrimerization of acetylene during vinyl acetate synthesis. Chem. Eng. J. 2019, 378, 122183. [Google Scholar] [CrossRef]
- Hu, L.B.; Yu, F.; Wang, F.; Yang, S.C.; Peng, B.H.; Chen, L.; Wang, G.; Hou, J.; Dai, B.; Tian, Z.Q. Overwhelming electrochemical oxygen reduction reaction of zinc-nitrogen-carbon from biomass resource chitosan via a facile carbon bath method. Chin. Chem. Lett. 2020, 31, 1207–1212. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, X.; Li, M.; Wang, X.; Zhu, M.; Dai, B. A highly active in situ Zn(CH3COO)2-NC catalyst for the acetoxylation of acetylene. Ind. Eng. Chem. Res. 2022, 61, 1313–1321. [Google Scholar] [CrossRef]
- Duereh, A.; Guo, H.; Honma, T.; Hiraga, Y.; Sato, Y.; Lee Smith, R., Jr.; Inomata, H. Solvent polarity of cyclic ketone (cyclopentanone, cyclohexanone): Alcohol (methanol, ethanol) renewable mixed-solvent systems for applications in pharmaceutical and chemical processing. Ind. Eng. Chem. Res. 2018, 57, 7331–7344. [Google Scholar] [CrossRef]
- Yu, H.; Li, T.; Yang, X.; Li, C.; Mi, J.; Meng, H.; Jin, J. Hydrophobic carbon-based coating on metal tube with efficient and stable adsorption–desorption of CO2 from wet flue gas. Sep. Purif. Technol. 2023, 307, 122798. [Google Scholar] [CrossRef]
- Oka, K.; Shibue, T.; Sugimura, N.; Watabe, Y.; Winther-Jensen, B.; Nishide, H. Nonpolar water clusters: Proton nuclear magnetic resonance spectroscopic evidence for transformation from polar water to nonpolar water clusters in liquid state. J. Phys. Chem. Lett. 2021, 12, 276–279. [Google Scholar] [CrossRef]
- Hernández Mejía, C.; van Deelen, T.W.; de Jong, K.P. Activity enhancement of cobalt catalysts by tuning metal-support interactions. Nat. Commun. 2018, 9, 4459. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, H.; Liu, S.; Ye, D.; Qu, R.; Zheng, C.; Gao, X. Engineering nano-ordered of Ni nanoparticles on KIT-6 for enhanced catalytic hydrogenation of nitrobenzene. Appl. Surf. Sci. 2020, 525, 146382. [Google Scholar] [CrossRef]
- Li, M.; Xu, Z.; Chen, Y.; Shen, G.; Wang, X.; Dai, B. MOFs-derived Zn-based catalysts in acetylene acetoxylation. Nanomaterials 2022, 12, 98. [Google Scholar] [CrossRef]
- Morrow, B. The initial mechanism of vinyl acetate synthesis from acetic acid and acetylene catalyzed by active carbon-zinc acetate. J. Catal. 1984, 86, 328–332. [Google Scholar] [CrossRef]
- Shen, Y.H.; Li, Y.M.; Liu, H.C. Base-free aerobic oxidation of glycerol on TiO2-supported bimetallic Au-Pt catalysts. J. Energy Chem. 2015, 24, 669–673. [Google Scholar] [CrossRef]
- Wu, X.; He, P.; Wang, X.; Dai, B. Zinc acetate supported on N-doped activated carbon as catalysts for acetylene acetoxylation. Chem. Eng. J. 2017, 309, 172. [Google Scholar] [CrossRef]
- Zhu, F.; Zhu, M.; Kang, L. B-doped activated carbon as a support for a high-performance Zn-based catalyst in acetylene acetoxylation. Green Energy Environ. 2020, 7, 221–228. [Google Scholar] [CrossRef]
- Dong, X.Q.; Wang, Y.C.; Yu, Y.Z.; Zhang, M.H. Density functional theory investigation on the synthesis mechanism of vinyl acetate from acetylene and acetic acid catalyzed by ordered mesoporous carbon-supported zinc acetate. Ind. Eng. Chem. Res. 2018, 57, 7363–7373. [Google Scholar] [CrossRef]

| Samples | BET Surface Area m2/g | Pore Volume cm3/g | Pore Size nm |
|---|---|---|---|
| F-water | 903.36 | 0.32 | 2.70 |
| U-water | 388.53 | 0.25 | 3.39 |
| F-mixed solvent A | 991.49 | 0.36 | 2.69 |
| U-mixed solvent A | 601.44 | 0.29 | 3.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Li, M.; Shen, G.; Chen, Y.; Lu, D.; Ren, P.; Jiang, H.; Wang, X.; Dai, B. Solvent Effects in the Preparation of Catalysts Using Activated Carbon as a Carrier. Nanomaterials 2023, 13, 393. https://doi.org/10.3390/nano13030393
Xu Z, Li M, Shen G, Chen Y, Lu D, Ren P, Jiang H, Wang X, Dai B. Solvent Effects in the Preparation of Catalysts Using Activated Carbon as a Carrier. Nanomaterials. 2023; 13(3):393. https://doi.org/10.3390/nano13030393
Chicago/Turabian StyleXu, Zhuang, Mengli Li, Guowang Shen, Yuhao Chen, Dashun Lu, Peng Ren, Hao Jiang, Xugen Wang, and Bin Dai. 2023. "Solvent Effects in the Preparation of Catalysts Using Activated Carbon as a Carrier" Nanomaterials 13, no. 3: 393. https://doi.org/10.3390/nano13030393
APA StyleXu, Z., Li, M., Shen, G., Chen, Y., Lu, D., Ren, P., Jiang, H., Wang, X., & Dai, B. (2023). Solvent Effects in the Preparation of Catalysts Using Activated Carbon as a Carrier. Nanomaterials, 13(3), 393. https://doi.org/10.3390/nano13030393






