An In Situ Chemotherapy Drug Combined with Immune Checkpoint Inhibitor for Chemoimmunotherapy
Abstract
:1. Introduction
2. Materials and Methods
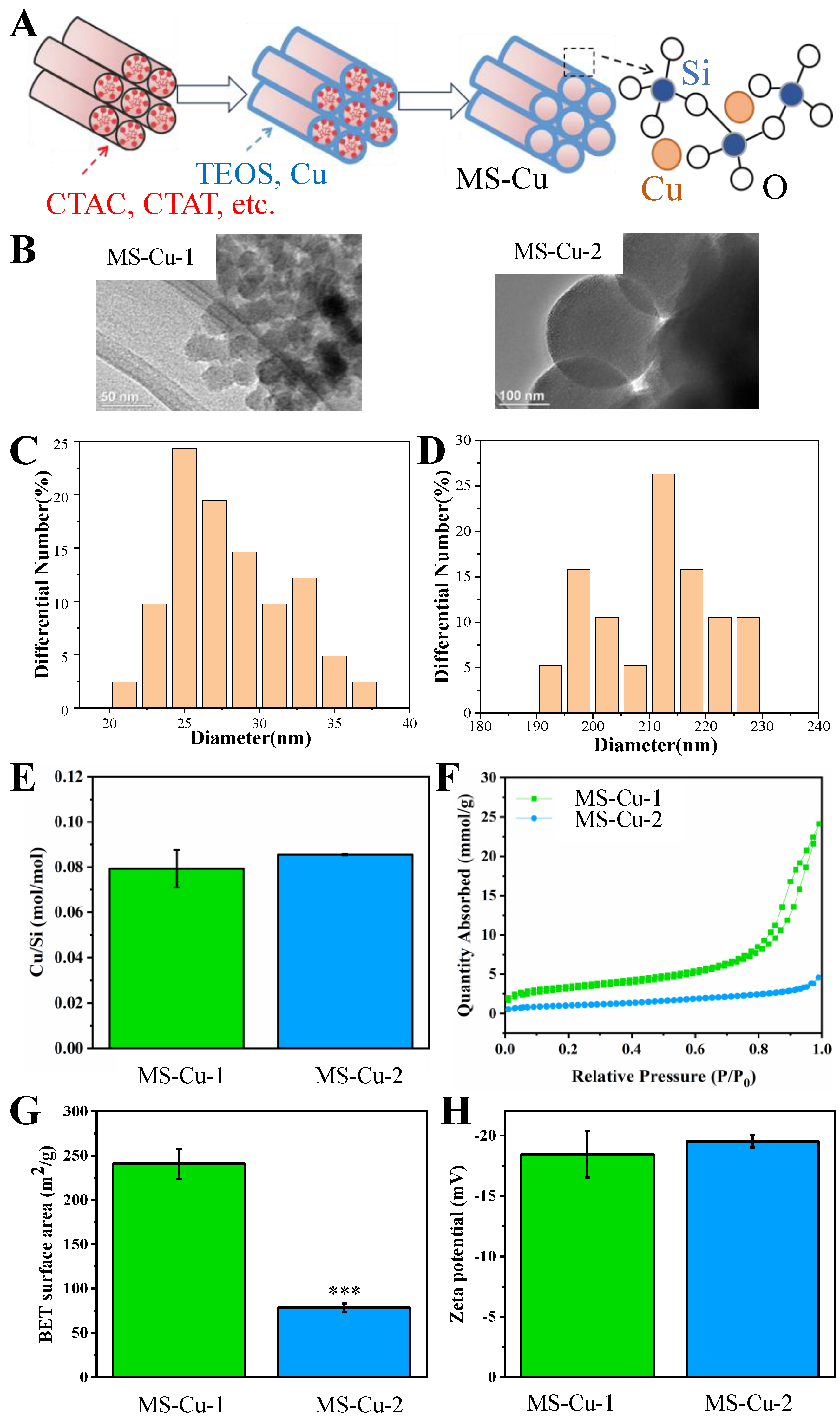
2.1. Preparation of MS-Cu Nanoparticles
2.2. Preparation of DSF/MS-Cu Composites
2.3. Characterization of MS-Cu Nanoparticles
2.4. In Vitro Cytotoxicity
2.5. Intracellular ROS Generation Induced by MS-Cu Nanoparticles
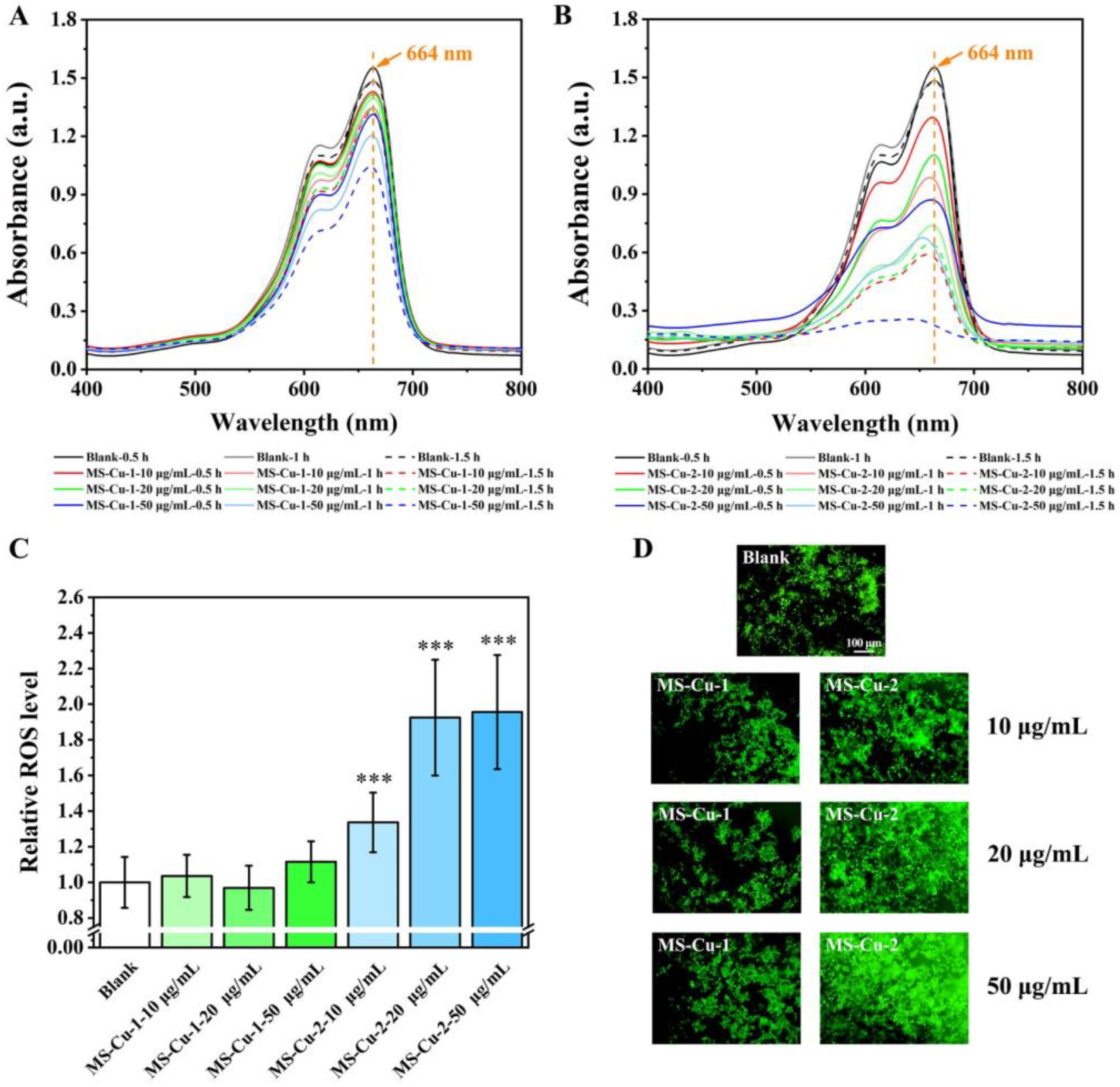
2.6. Extracellular ROS Generation Induced by MS-Cu Nanoparticles
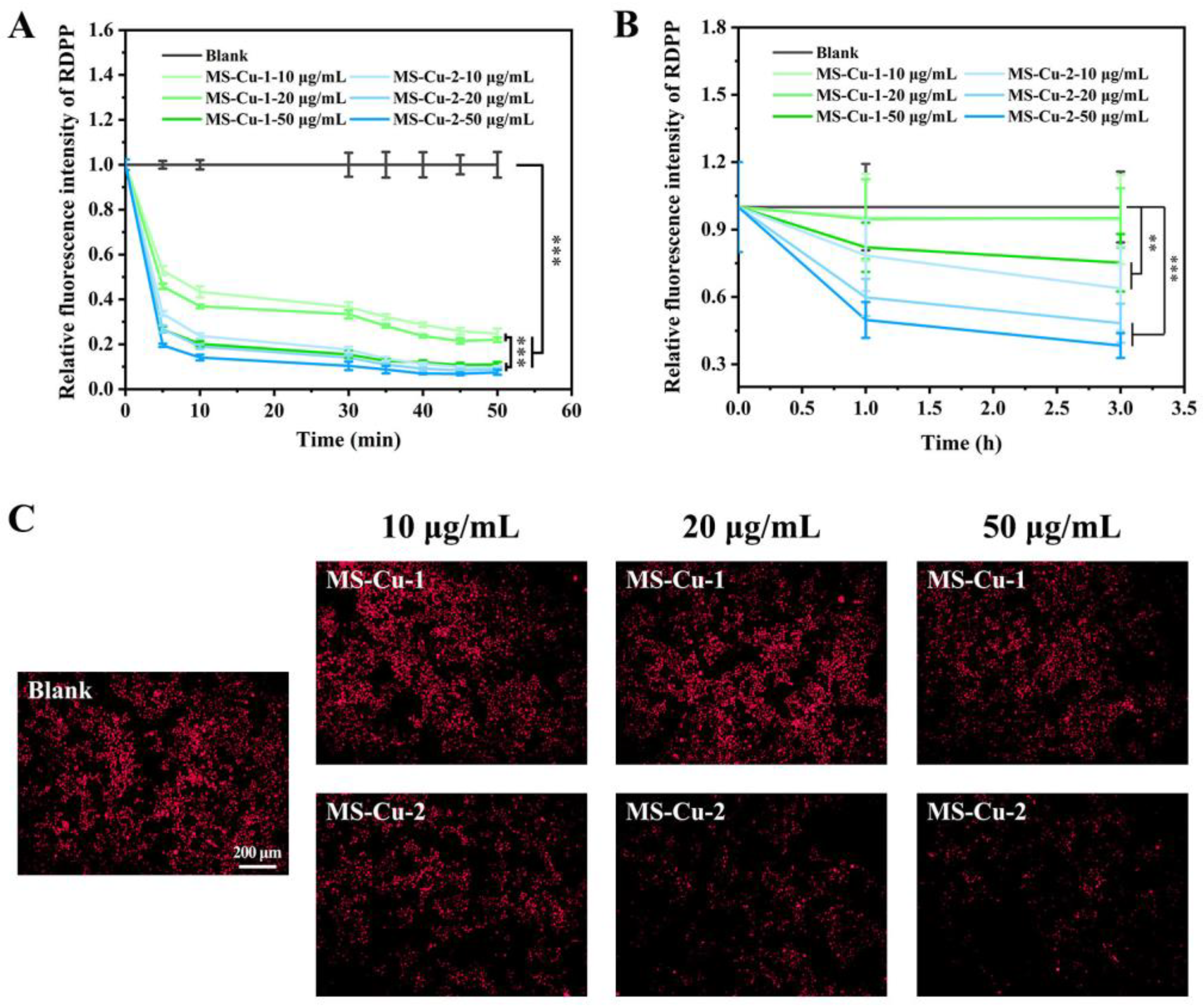
2.7. Intracellular Oxygenation Induced by MS-Cu Nanoparticles
2.8. Extracellular Oxygenation Induced by MS-Cu Nanoparticles
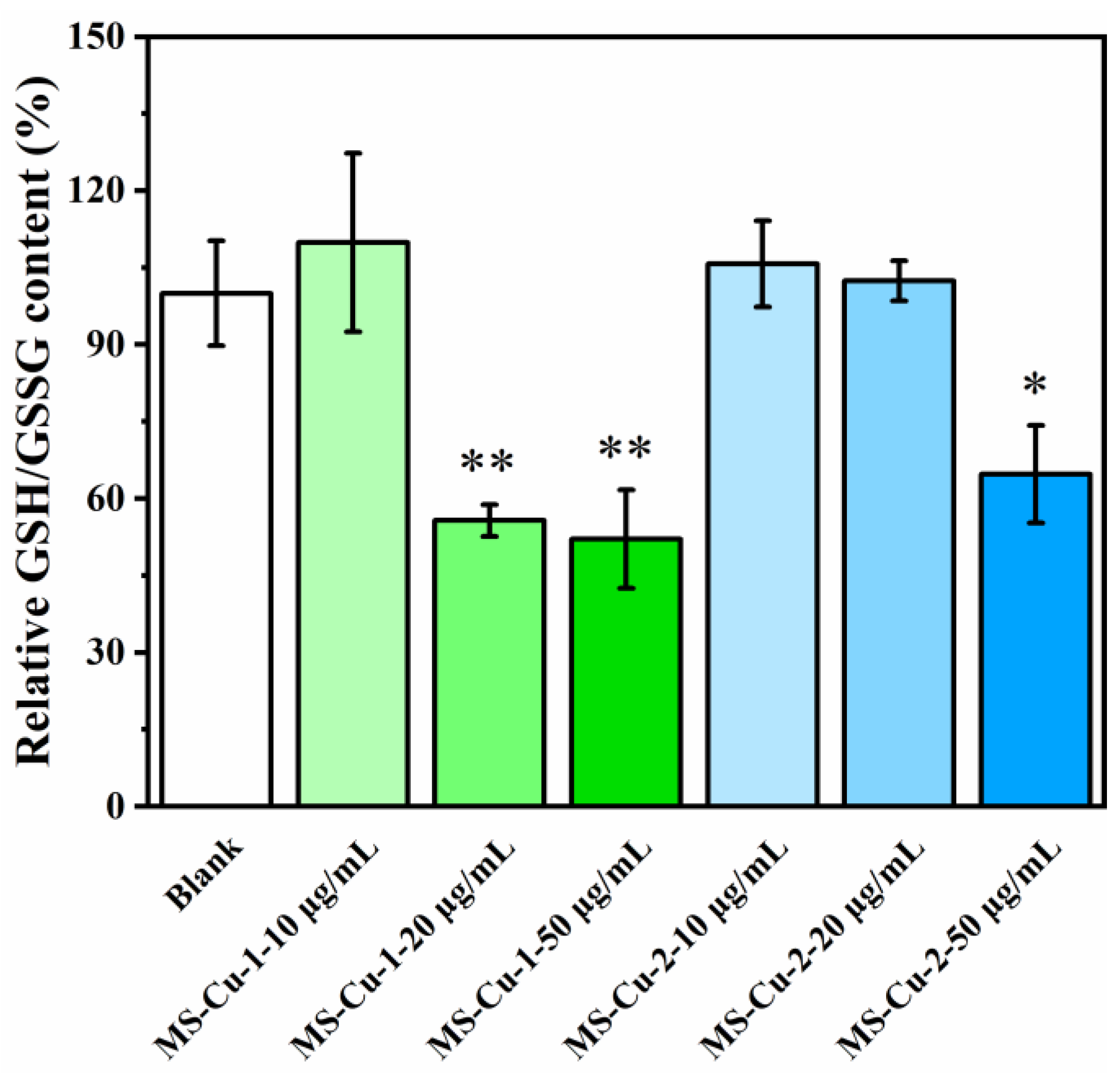
2.9. Intracellular GSH Depletion of MS-Cu Nanoparticles
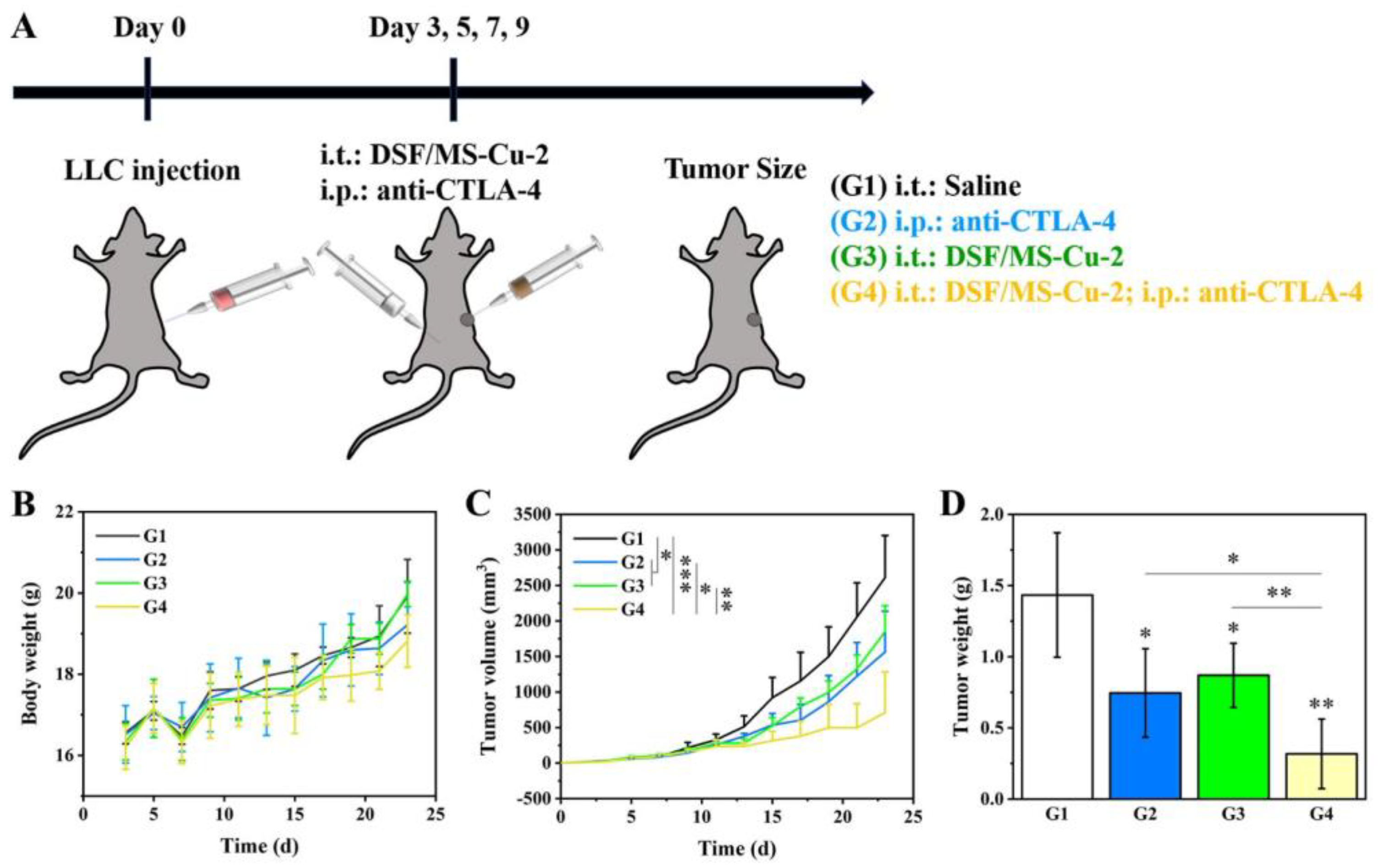
2.10. In Vivo Synergistic Antitumor Effects of DSF/MS-Cu–2 Combined with Immune Checkpoint Inhibitor
2.11. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization
3.2. In Vitro Cytotoxicity
3.3. ROS Generation
3.4. Oxygenation
3.5. Intracellular GSH Depletion
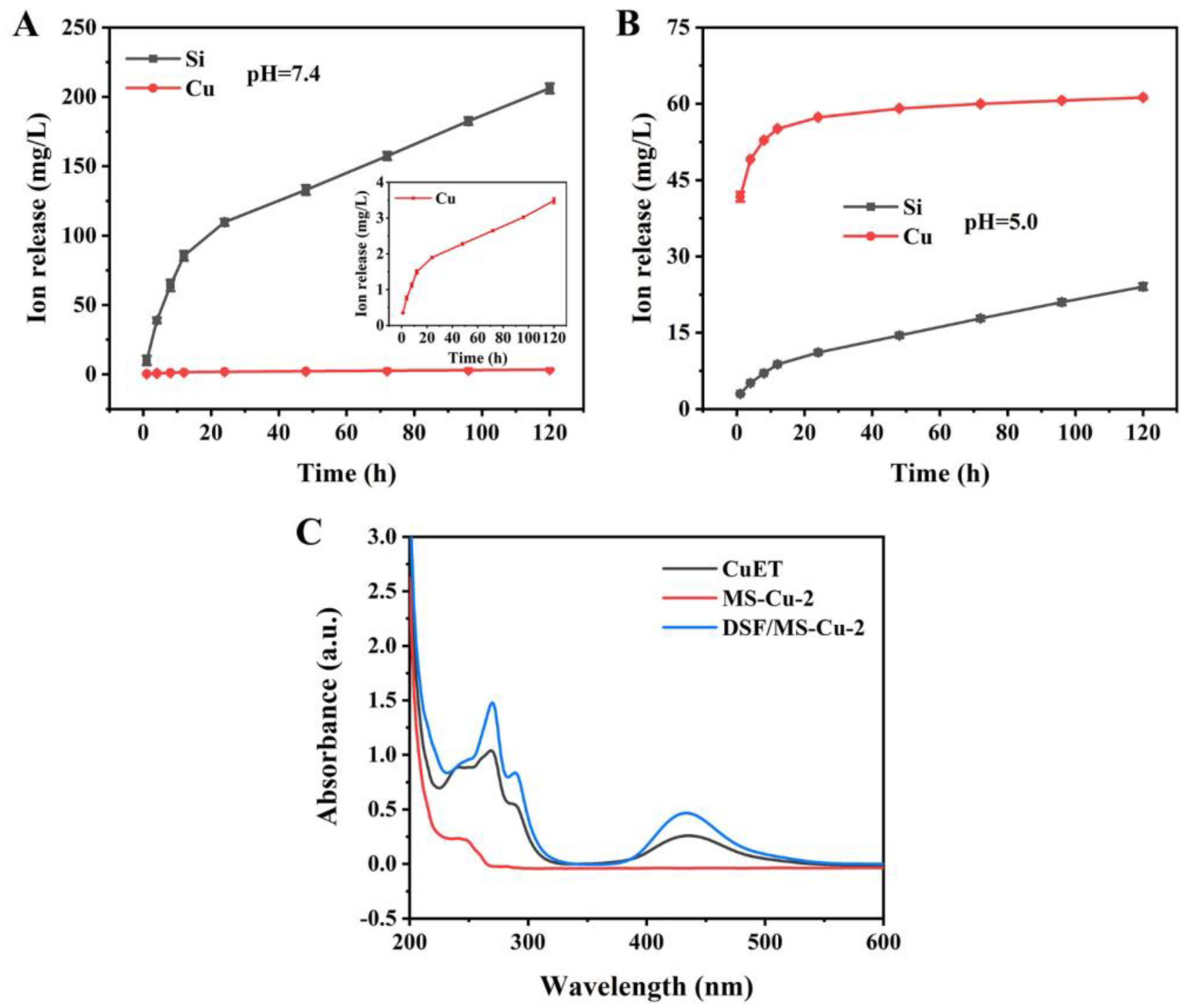
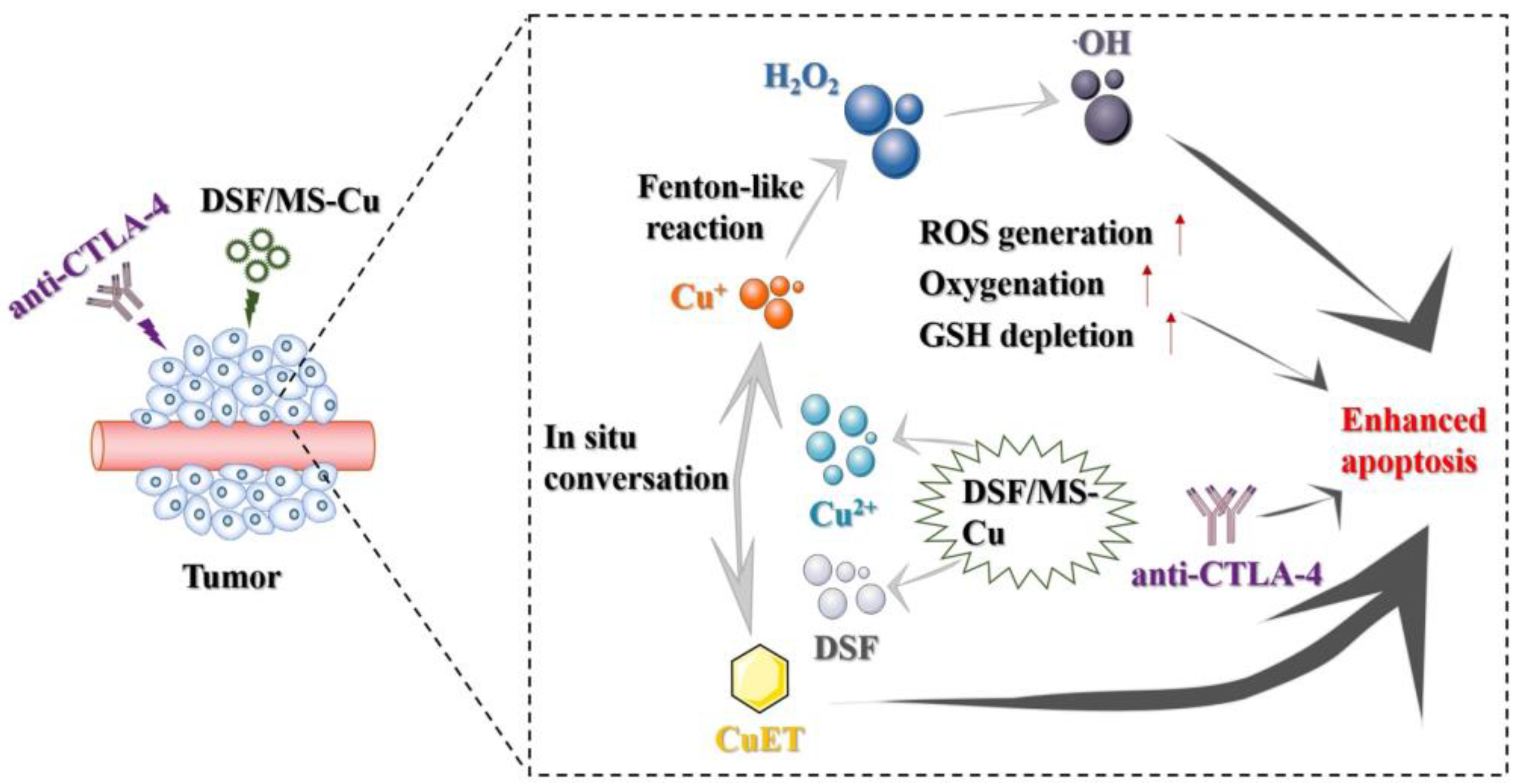
3.6. Ion Release and the Generation of CuET
3.7. In Vivo Anti-Tumor Efficacy of DSF/MS-Cu–2 in Combination with the Anti-CTLA–-4 Antibody
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Bedard, P.L.; Hyman, D.M.; Davids, M.S.; Siu, L.L. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet 2020, 395, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yamazaki, T.; Ebara, M.; Shirahata, N.; Hanagata, N. Hanagata. Nanoengineered coordination polymers boost cancer immunotherapy. Mater. Today 2023, 67, 127–150. [Google Scholar]
- Collins, F.S. Mining for therapeutic gold. Nat. Rev. Drug Discov. 2011, 10, 397. [Google Scholar] [CrossRef]
- Skrott, Z.; Mistrik, M.; Andersen, K.K.; Friis, S.; Majera, D.; Gursky, J.; Ozdian, T.; Bartkova, J.; Turi, Z.; Moudry, P.; et al. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature 2017, 552, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cui, Q.C.; Yang, H.; Dou, Q.P. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006, 66, 10425–10433. [Google Scholar] [CrossRef]
- Wu, W.; Yu, L.; Jiang, Q.; Huo, M.; Lin, H.; Wang, L.; Chen, Y.; Shi, J. Enhanced Tumor-Specific Disulfiram Chemotherapy by In Situ Cu(2+) Chelation-Initiated Nontoxicity-to-Toxicity Transition. J. Am. Chem. Soc. 2019, 141, 11531–11539. [Google Scholar] [CrossRef]
- Ren, X.; Li, Y.; Zhou, Y.; Hu, W.; Yang, C.; Jing, Q.; Zhou, C.; Wang, X.; Hu, J.; Wang, L.; et al. Overcoming the compensatory elevation of NRF2 renders hepatocellular carcinoma cells more vulnerable to disulfiram/copper-induced ferroptosis. Redox Biol. 2021, 46, 102122. [Google Scholar] [CrossRef]
- Wang, X.; Oyane, A.; Inose, T.; Nakamura, M. In Situ Synthesis of a Tumor-Microenvironment-Responsive Chemotherapy Drug. Pharmaceutics 2023, 15, 1316. [Google Scholar] [CrossRef]
- Li, Y.; Fu, S.Y.; Wang, L.H.; Wang, F.Y.; Wang, N.N.; Cao, Q.; Wang, Y.T.; Yang, J.Y.; Wu, C.F. Copper improves the anti-angiogenic activity of disulfiram through the EGFR/Src/VEGF pathway in gliomas. Cancer Lett. 2015, 369, 86–96. [Google Scholar] [CrossRef]
- Araya, M.; Olivares, M.; Pizarro, F.; Méndez, M.A.; González, M.; Uauy, R. Supplementing copper at the upper level of the adult dietary recommended intake induces detectable but transient changes in healthy adults. J. Nutr. 2005, 135, 2367–2371. [Google Scholar] [CrossRef]
- Bagherpoor, A.J.; Shameem, M.; Luo, X.H.; Seelig, D.; Kassie, F. Inhibition of lung adenocarcinoma by combinations of sulfasalazine (SAS) and disulfiram-copper (DSF-Cu) in cell line models and mice. Carcinogenesis 2023, 44, 291–303. [Google Scholar] [CrossRef]
- Terashima, Y.; Toda, E.; Itakura, M.; Otsuji, M.; Yoshinaga, S.; Okumura, K.; Shand, F.H.W.; Komohara, Y.; Takeda, M.; Kokubo, K.; et al. Targeting FROUNT with disulfiram suppresses macrophage accumulation and its tumor-promoting properties. Nat. Commun. 2020, 11, 609. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, X.; Huo, M.F.; Wang, L.Y.; Chen, Y.; Chen, W.; Wang, B.L. Tumor-responsive copper-activated disulfiram for synergetic nanocatalytic tumor therapy. Nano Res. 2021, 14, 205–211. [Google Scholar] [CrossRef]
- Swartz, M.A.; Iida, N.; Roberts, E.W.; Sangaletti, S.; Wong, M.H.; Yull, F.E.; Coussens, L.M.; Declerck, Y.A. Tumor microenvironment complexity: Emerging roles in cancer therapy. Cancer Res. 2012, 72, 2473–2480. [Google Scholar] [CrossRef]
- Liang, K.; Sun, H.; Yang, Z.; Yu, H.; Shen, J.; Wang, X.; Chen, H. Breaking the Redox Homeostasis: An Albumin-Based Multifunctional Nanoagent for GSH Depletion-Assisted Chemo-/Chemodynamic Combination Therapy. Adv. Funct. Mater. 2021, 31, 2100355. [Google Scholar] [CrossRef]
- Qian, C.; Yu, J.; Chen, Y.; Hu, Q.; Xiao, X.; Sun, W.; Wang, C.; Feng, P.; Shen, Q.; Gu, Z. Light-Activated Hypoxia-Responsive Nanocarriers for Enhanced Anticancer Therapy. Adv. Mater. 2016, 28, 3313–3320. [Google Scholar] [CrossRef] [PubMed]
- Chiu, D.K.; Tse, A.P.; Xu, I.M.; Di Cui, J.; Lai, R.K.; Li, L.L.; Koh, H.Y.; Tsang, F.H.; Wei, L.L.; Wong, C.M.; et al. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat. Commun. 2017, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Patel, S.P.; Roszik, J.; Qin, Y. Hypoxia-Driven Immunosuppressive Metabolites in the Tumor Microenvironment: New Approaches for Combinational Immunotherapy. Front. Immunol. 2018, 9, 1591. [Google Scholar] [CrossRef]
- Yu, X.; Wang, X.; Yamazaki, A.; Li, X. Tumor microenvironment-regulated nanoplatforms for the inhibition of tumor growth and metastasis in chemo-immunotherapy. J. Mater Chem. B 2022, 10, 3637–3647. [Google Scholar] [CrossRef]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mooney, D.J. Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat. Mater. 2018, 17, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the extracellular matrix: Drivers of tumour metastasis. Nat. Rev. Cancer 2014, 14, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Qian, G.; Ito, A. Synergistical chemotherapy and cancer immunotherapy using dual drug-delivering and immunopotentiating mesoporous silica. Appl. Mater. Today 2019, 16, 102–111. [Google Scholar] [CrossRef]
- Wang, X.; Ihara, S.; Li, X.; Ito, A.; Sogo, Y.; Watanabe, Y.; Yamazaki, A.; Tsuji, N.M.; Ohno, T. Rod-Scale Design Strategies for Immune-Targeted Delivery System toward Cancer Immunotherapy. ACS Nano 2019, 13, 7705–7715. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Ito, A.; Sogo, Y.; Watanabe, Y.; Tsuji, N.M. Hollow ZnO nanospheres enhance anticancer immunity by promoting CD4+ and CD8+ T cell populations in vivo. Small 2017, 13, 1701816. [Google Scholar] [CrossRef]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef]
- Chae, Y.K.; Arya, A.; Iams, W.; Cruz, M.R.; Chandra, S.; Choi, J.; Giles, F. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC). J. Immunother. Cancer 2018, 6, 39. [Google Scholar] [CrossRef]
- Pfirschke, C.; Engblom, C.; Rickelt, S.; Cortez-Retamozo, V.; Garris, C.; Pucci, F.; Yamazaki, T.; Poirier-Colame, V.; Newton, A.; Redouane, Y.; et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity 2016, 44, 343–354. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.B.; Yang, Z.J.; Ding, Y.; Lu, C.H.; Li, D.X.; Xu, Z.Z. Preparation of Monodispersed Mesoporous Silica Spheres with Controllable Particle Size Under an Alkaline Condition. Int. J. Appl. Ceram. Technol. 2012, 9, 1112–1123. [Google Scholar] [CrossRef]
- Wang, X.P.; Li, X.; Ito, A.; Sogo, Y.; Ohno, T. Particle-size-dependent toxicity and immunogenic activity of mesoporous silica-based adjuvants for tumor immunotherapy. Acta Biomater. 2013, 9, 7480–7849. [Google Scholar] [CrossRef] [PubMed]
- Parlar, M.; Yortsos, Y. Percolation Theory of Vapor Adsorption Desorption Processes in Porous Materials. J. Colloid Interface Sci. 1988, 124, 162–176. [Google Scholar] [CrossRef]
- Kuai, R.; Yuan, W.; Son, S.; Nam, J.; Xu, Y.; Fan, Y.; Schwendeman, A.; Moon, J.J. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci. Adv. 2018, 4, eaao1736. [Google Scholar] [CrossRef] [PubMed]
- Mathios, D.; Kim, J.E.; Mangraviti, A.; Phallen, J.; Park, C.-K.; Jackson, C.M.; Garzon-Muvdi, T.; Kim, E.; Theodros, D.; Polanczyk, M.; et al. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci. Transl. Med. 2016, 8, 370ra180. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.W.; et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.E.; Hockings, H.; Hilton, D.M.; Kermorgant, S. The role of MET in chemotherapy resistance. Oncogene 2021, 40, 1927–1941. [Google Scholar] [CrossRef] [PubMed]
- Rankin, E.B.; Giaccia, A.J. Hypoxic control of metastasis. Science 2016, 352, 175–180. [Google Scholar] [CrossRef]
- Cai, Y.-J.; Lu, J.-J.; Zhu, H.; Xie, H.; Huang, M.; Lin, L.-P.; Zhang, X.-W.; Ding, J. Salvicine triggers DNA double-strand breaks and apoptosis by GSH-depletion-driven H2O2 generation and topoisomerase II inhibition. Free. Radic. Biol. Med. 2008, 45, 627–635. [Google Scholar] [CrossRef]
- Liu, J.J.; Wu, M.; Pan, Y.T.; Duan, Y.K.; Dong, Z.L.; Chao, Y.; Liu, Z.; Liu, B. Biodegradable Nanoscale Coordination Polymers for Targeted Tumor Combination Therapy with Oxidative Stress Amplification. Adv. Funct. Mater. 2020, 30, 1908865. [Google Scholar] [CrossRef]
- Zhang, J.L.; Sun, X.Y.; Zhao, X.F.; Liu, L.; Cheng, X.; Yang, C.R.; Hu, H.Y.; Qiao, M.X.; Chen, D.W.; Zhao, X.L. Watson-Crick Base Pairing-Inspired Laser/GSH Activatable miRNA-Coordination Polymer Nanoplexes for Combined Cancer Chemo-Immuno-Photothermal Therapy. ACS Appl. Mater. Interfaces 2022, 14, 20762–20777. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Wang, X. An In Situ Chemotherapy Drug Combined with Immune Checkpoint Inhibitor for Chemoimmunotherapy. Nanomaterials 2023, 13, 3144. https://doi.org/10.3390/nano13243144
Yuan X, Wang X. An In Situ Chemotherapy Drug Combined with Immune Checkpoint Inhibitor for Chemoimmunotherapy. Nanomaterials. 2023; 13(24):3144. https://doi.org/10.3390/nano13243144
Chicago/Turabian StyleYuan, Xinyuan, and Xiupeng Wang. 2023. "An In Situ Chemotherapy Drug Combined with Immune Checkpoint Inhibitor for Chemoimmunotherapy" Nanomaterials 13, no. 24: 3144. https://doi.org/10.3390/nano13243144
APA StyleYuan, X., & Wang, X. (2023). An In Situ Chemotherapy Drug Combined with Immune Checkpoint Inhibitor for Chemoimmunotherapy. Nanomaterials, 13(24), 3144. https://doi.org/10.3390/nano13243144




