Key Strategies on Cu2O Photocathodes toward Practical Photoelectrochemical Water Splitting
Abstract
1. Introduction
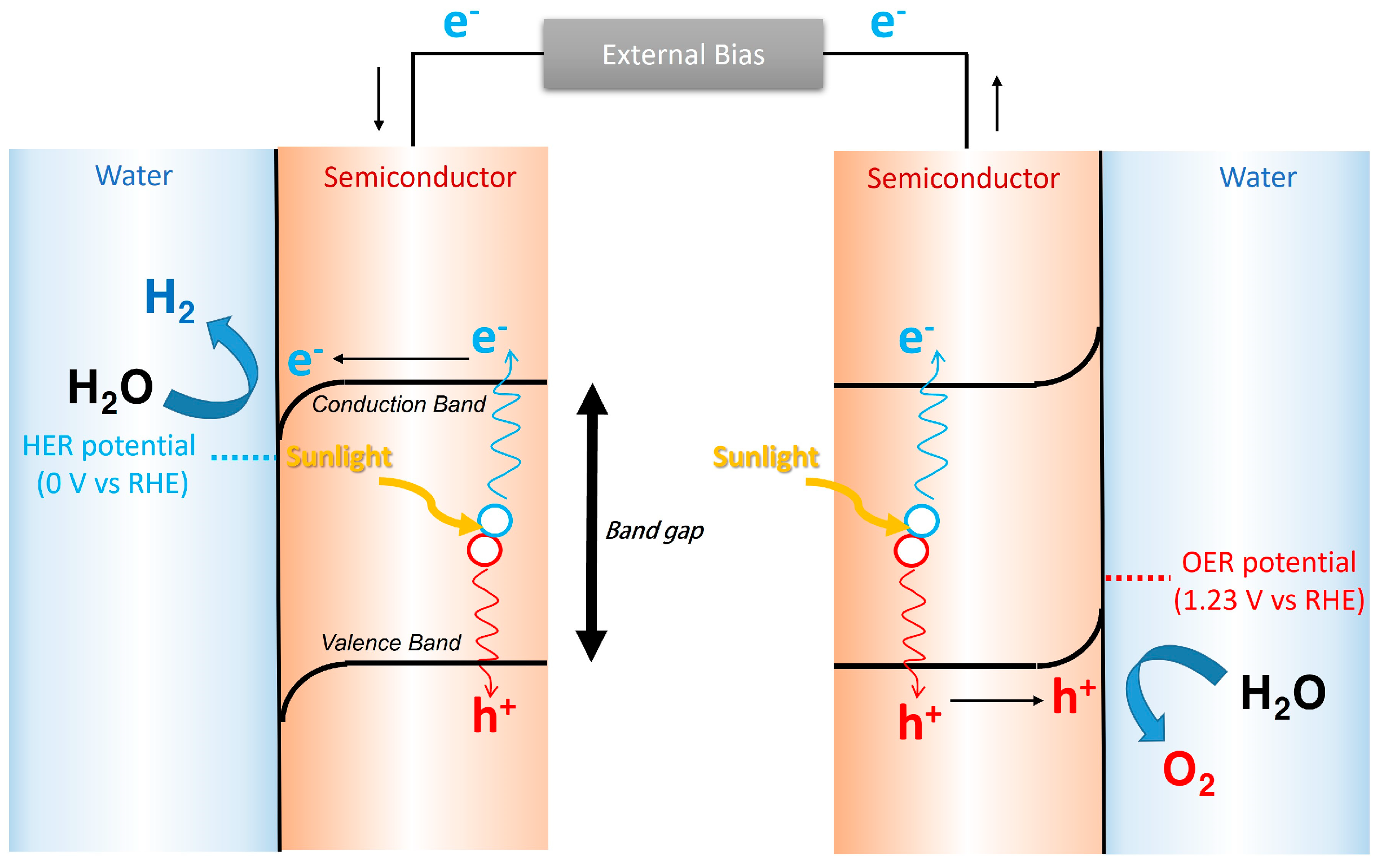
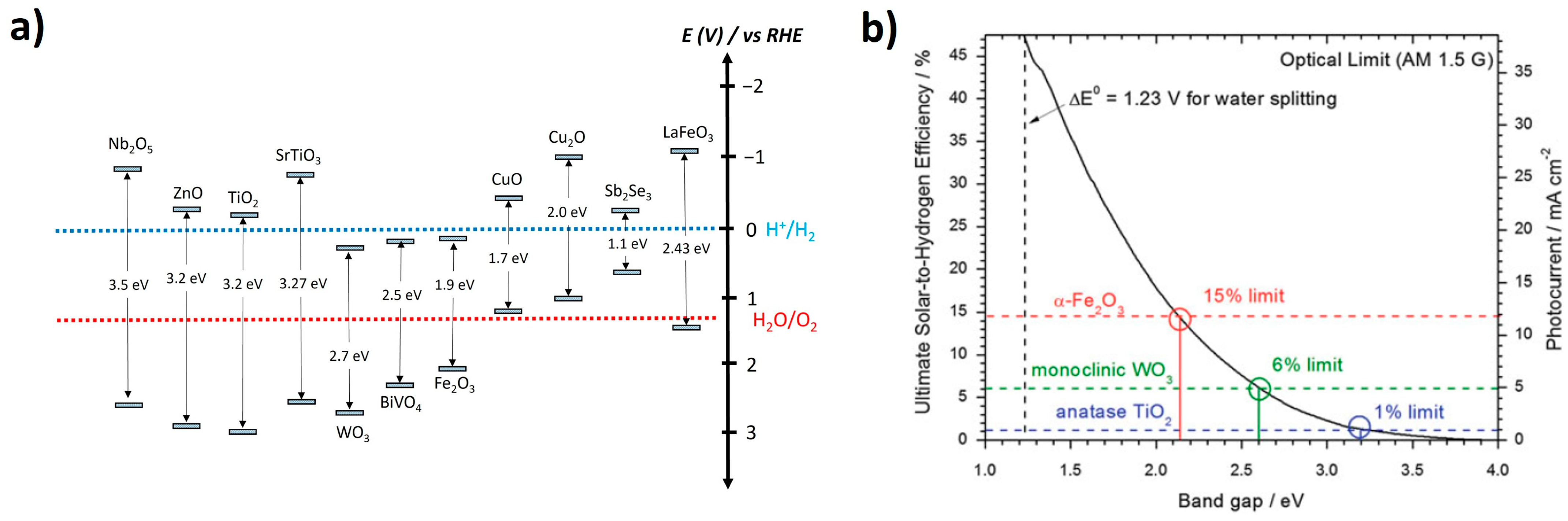
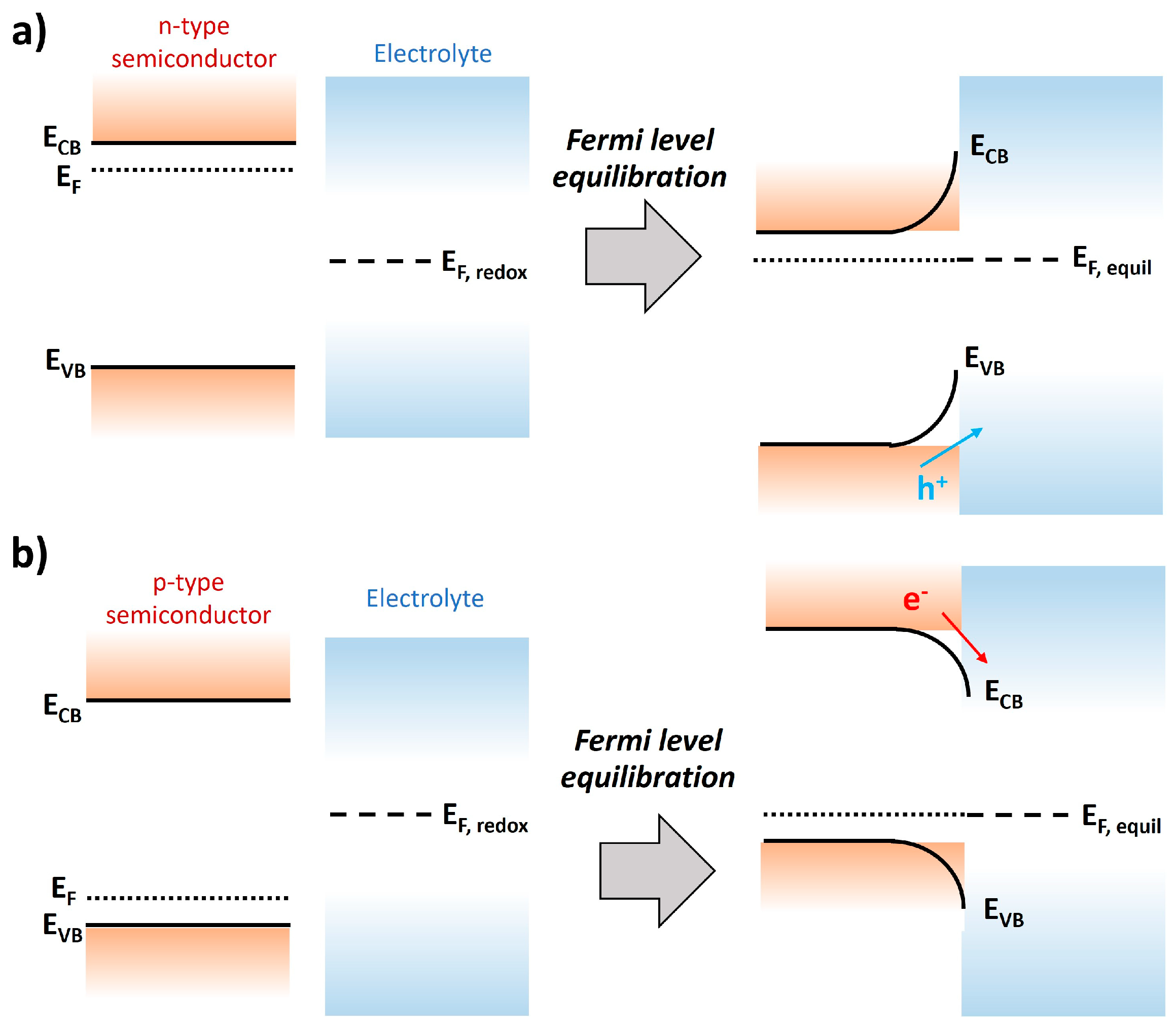
1.1. Basic Principles of Photoelectrochemical Water Splitting
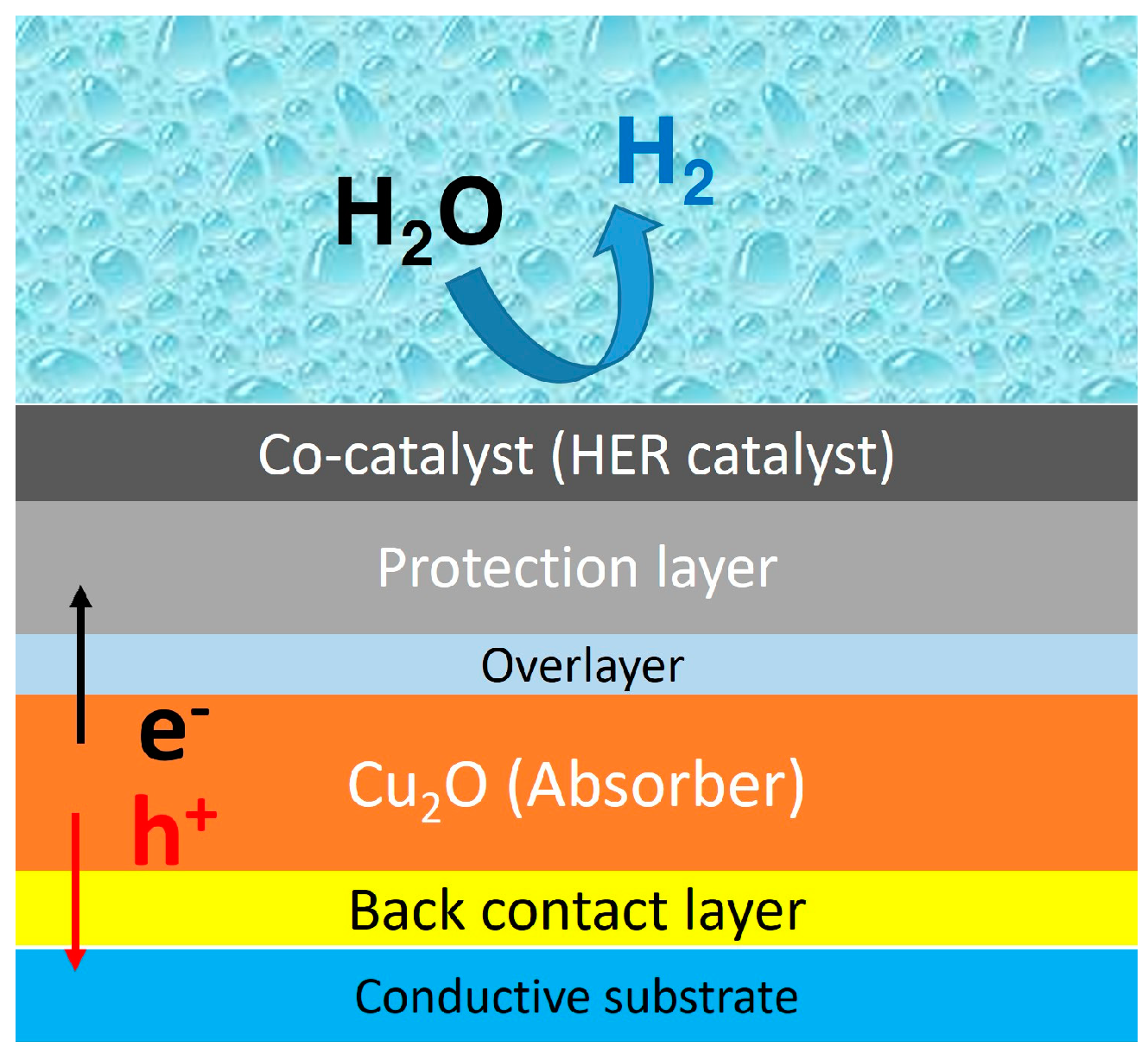
1.2. Cuprous Oxide Photocathode
2. Fundamentals and Research Progress
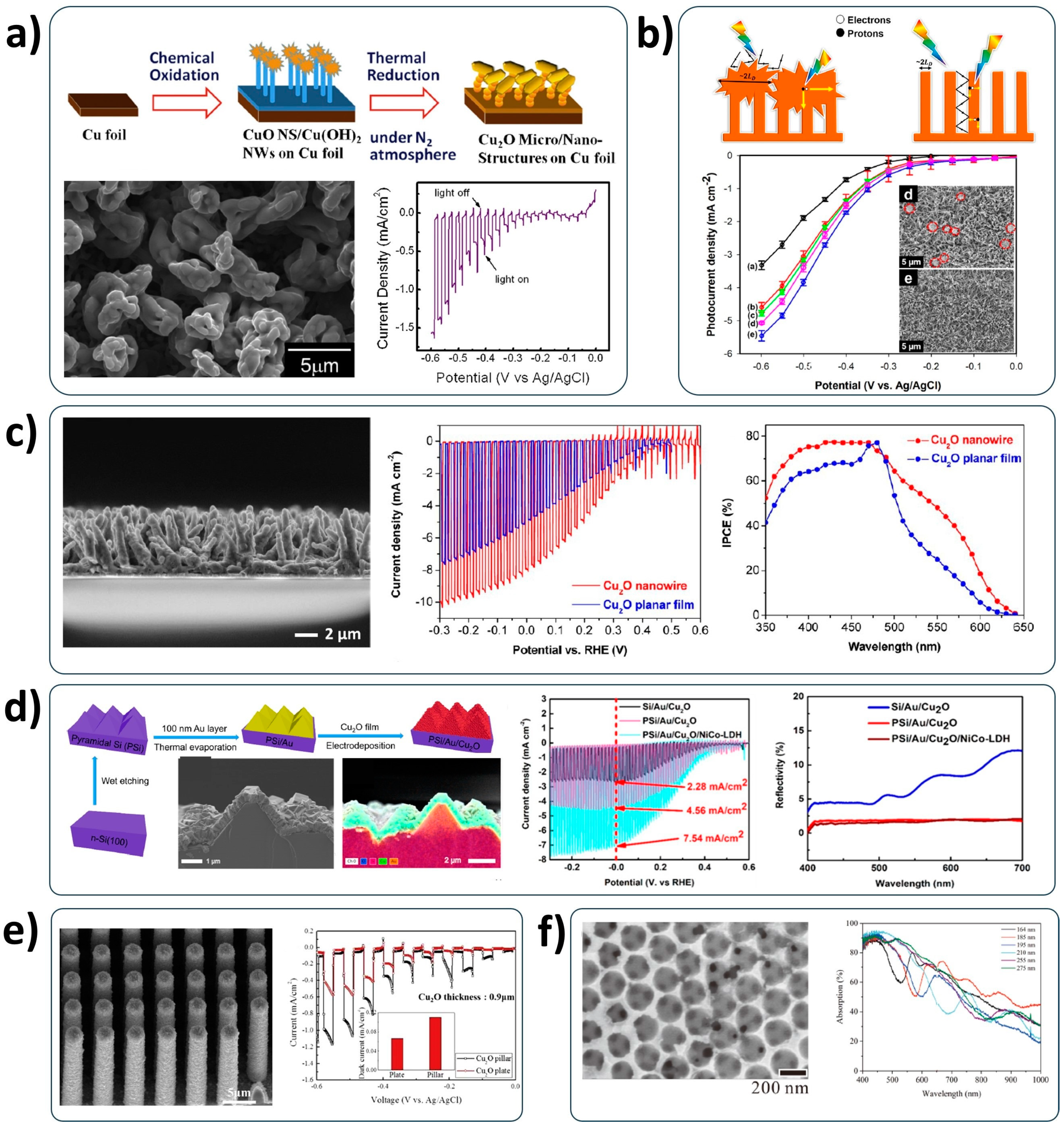
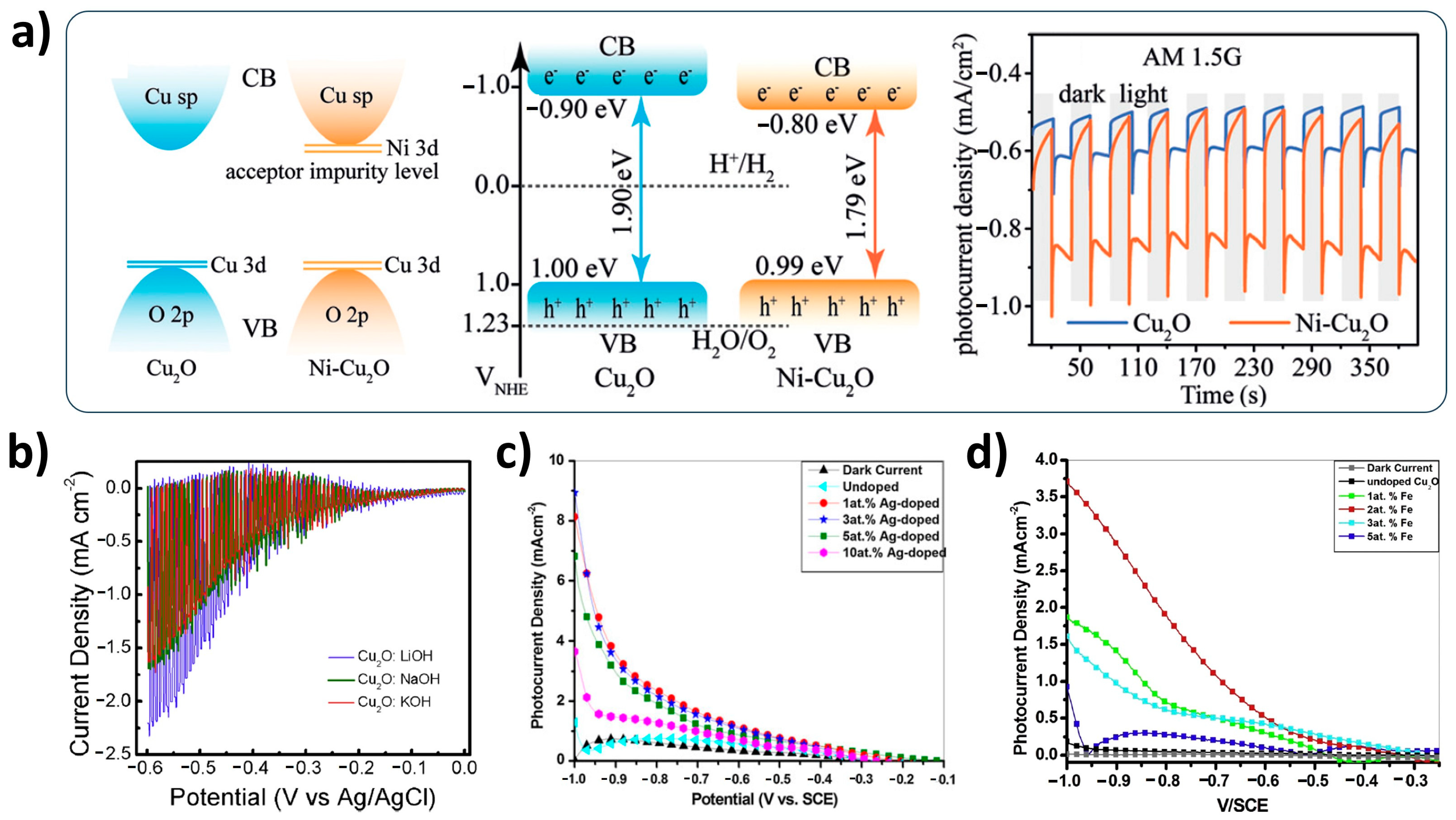
2.1. Cu2O Light Absorber
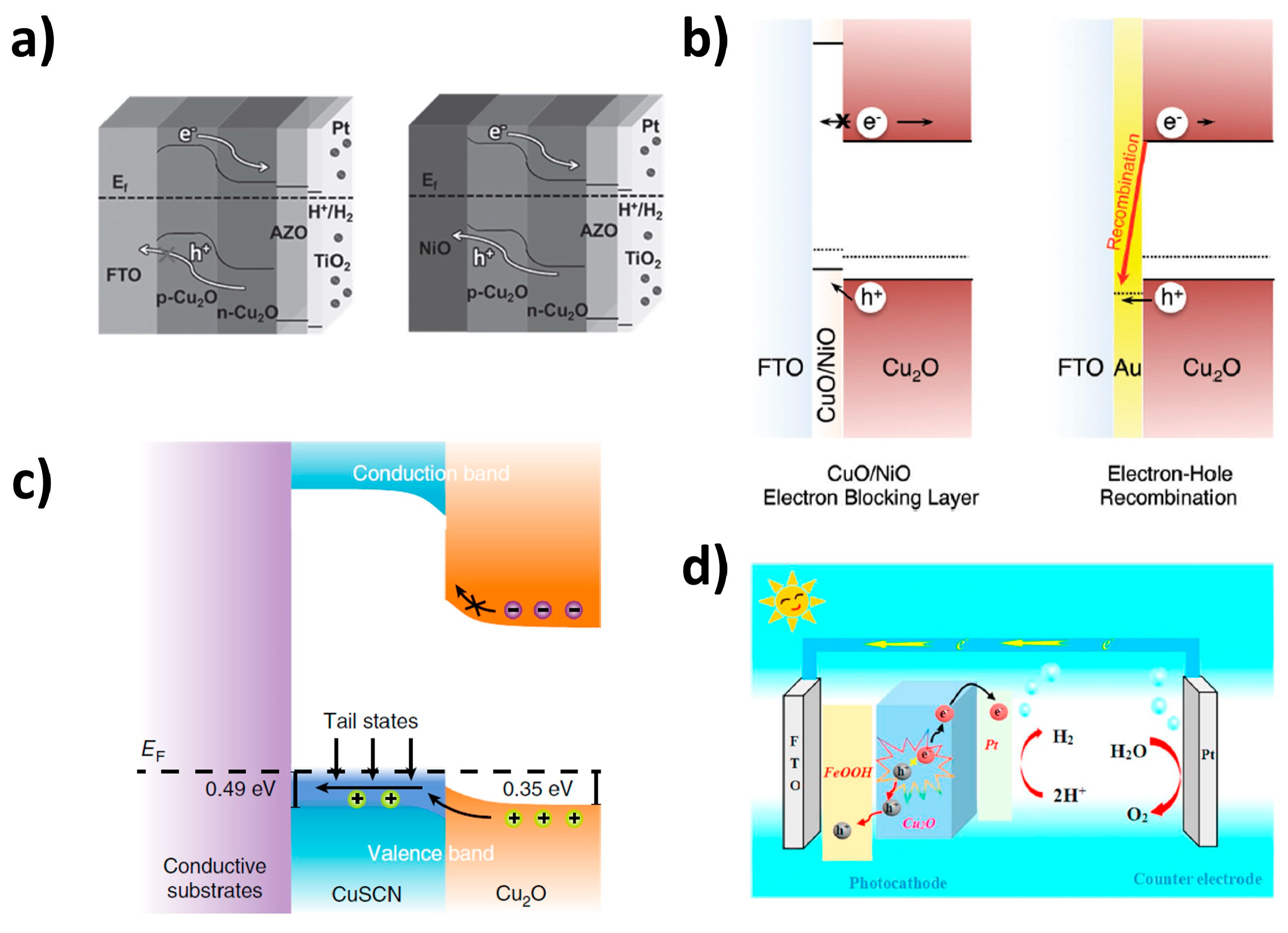
2.2. Back Contact Layer
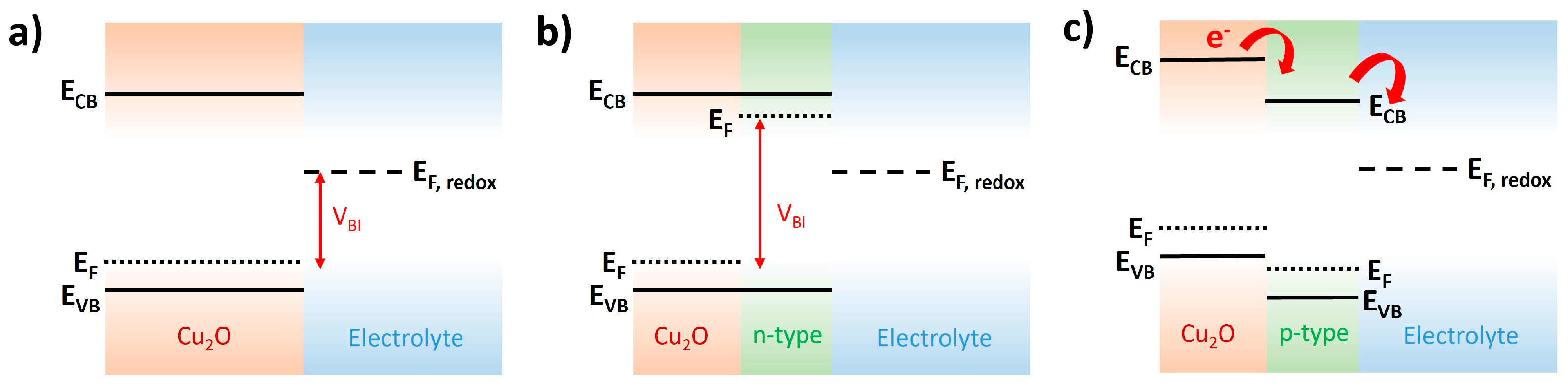
2.3. Overlayer
2.4. Protection Layer
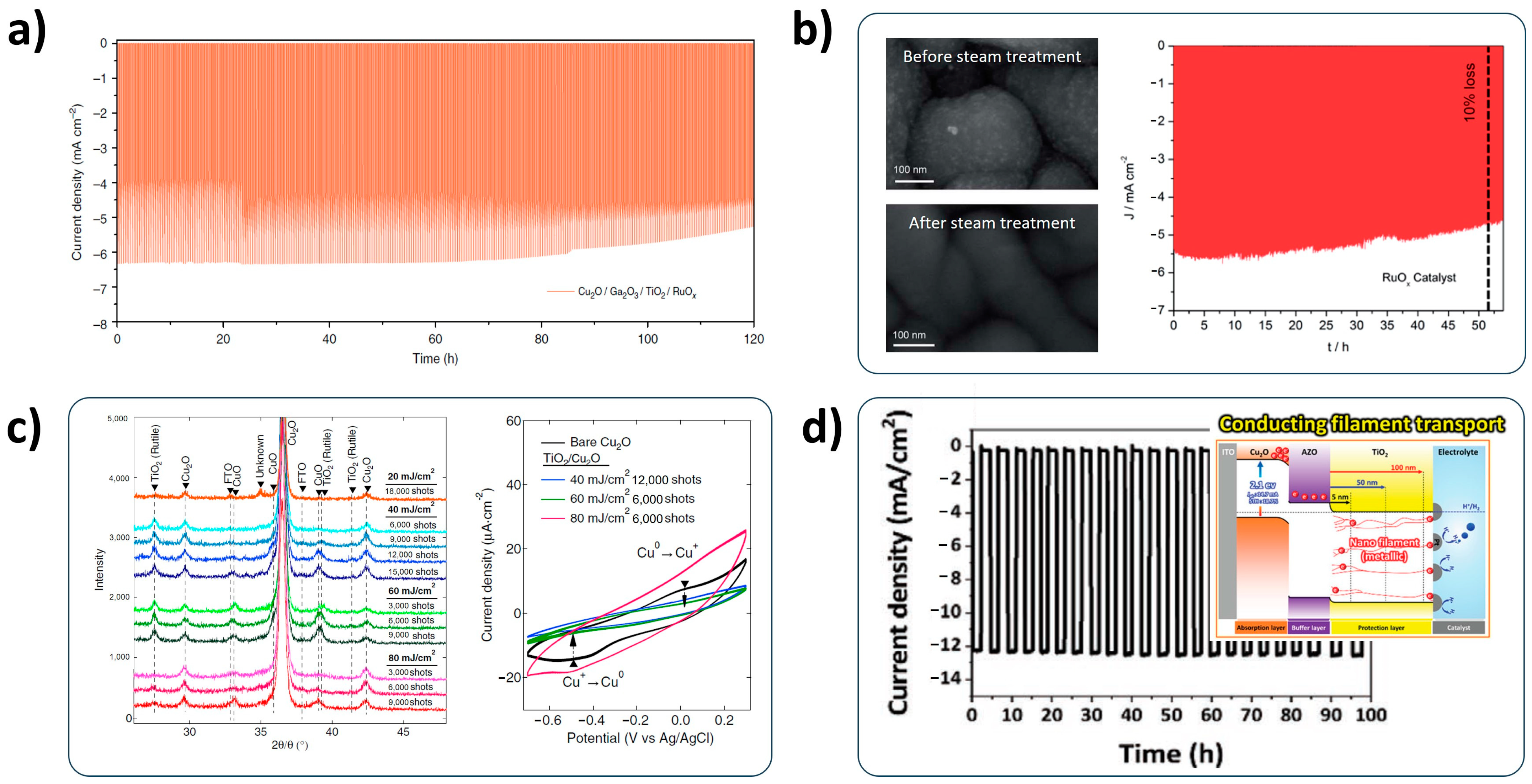
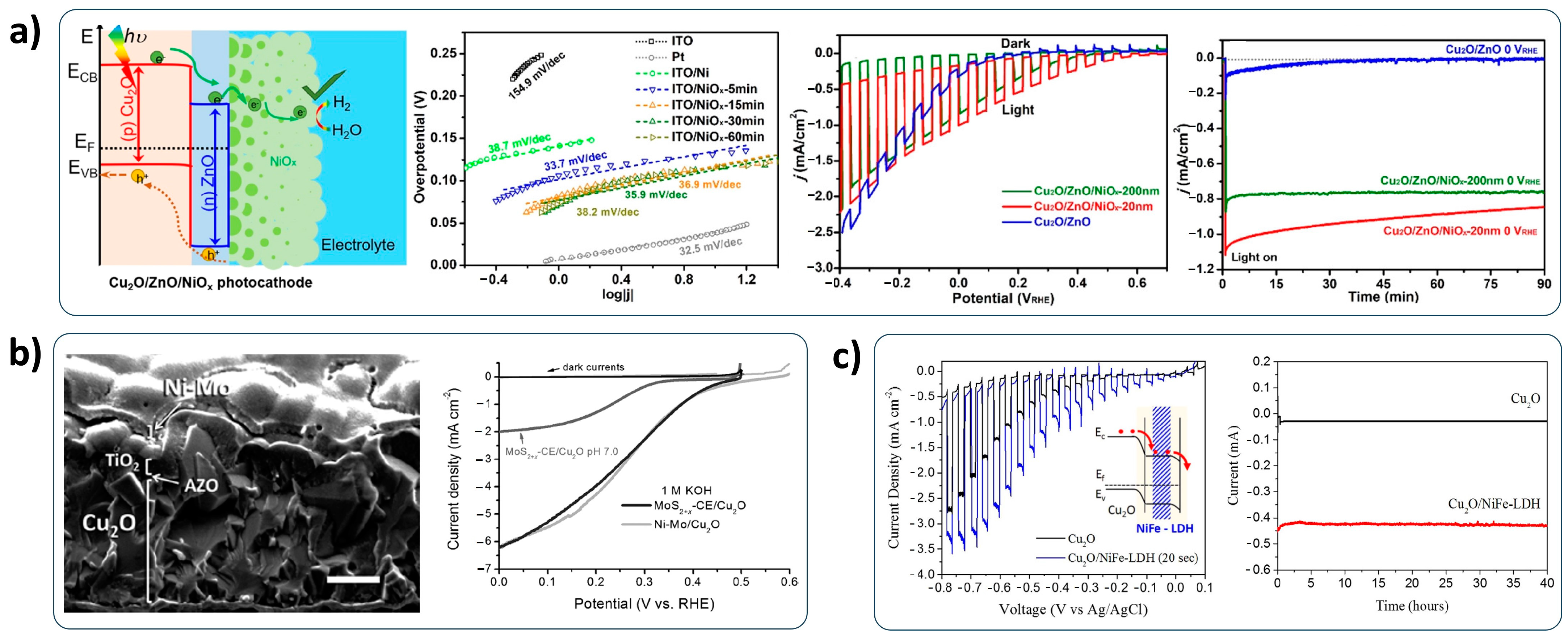
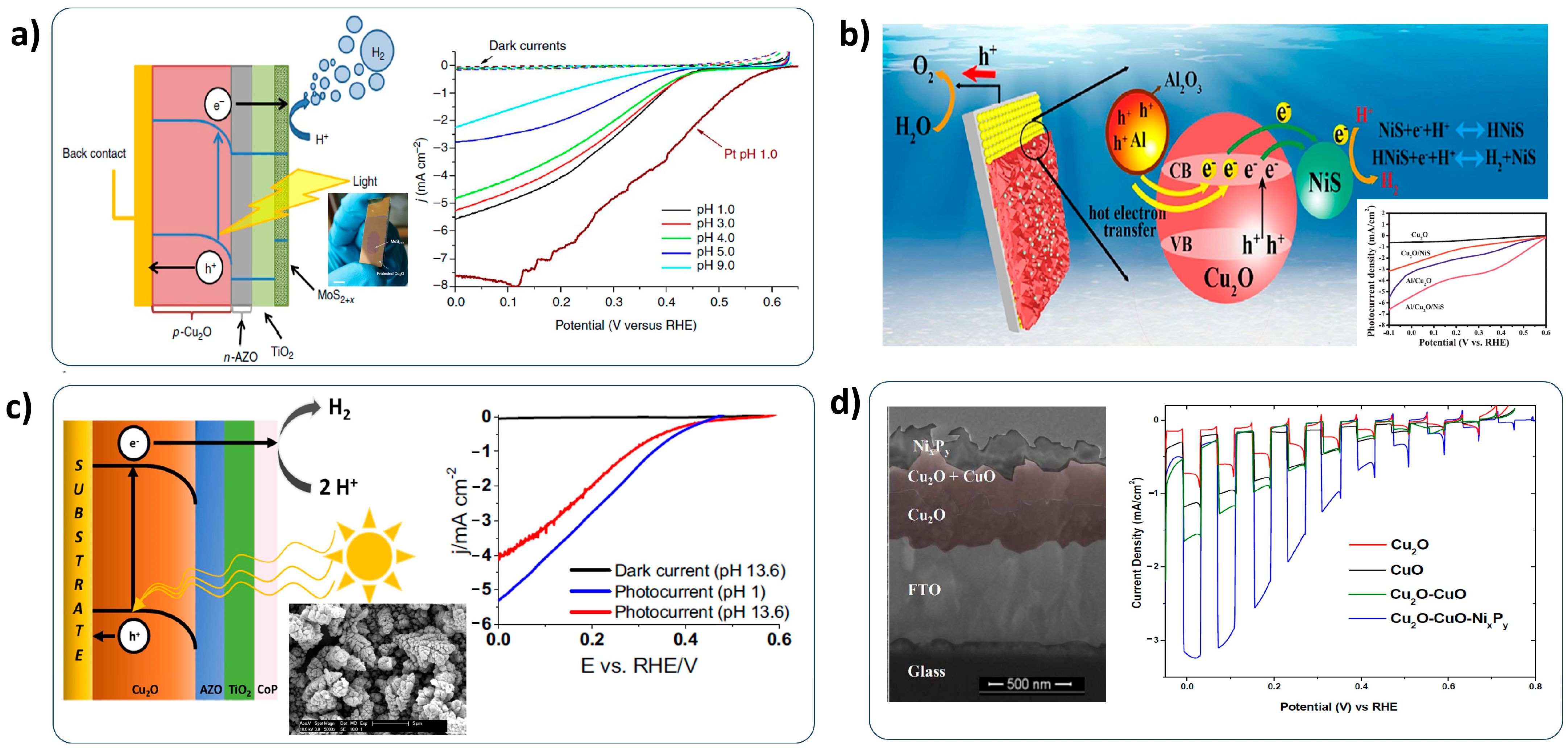
2.5. Co-Catalysts
3. Outlook and Future Research Directions
- Cu2O absorber: The further improvement of electron transport capability will be necessary. To this end, doping is the most efficient strategy. In the case of this strategy, the ionic radius of the dopant should be similar to that of Cu+ ions for reducing the defects on the Cu2O film. In addition, the doping level should be optimized for improving the electron transport in the Cu2O photocathodes, because the excessive doping has a negative influence on the PEC performance. The fabrication of a high-quality Cu2O film with less defects or grain boundaries is also advantageous to improve the electron transport in the Cu2O photocathode. Furthermore, the development of transparent Cu2O photocathodes with efficient PEC performance paves the way for developing the efficient PEC-PEC or PEC-PV water-splitting system with short-band-gap materials;
- Back contact layer: The development of an alternative back contact layer to the expensive Au back contact layer is a main goal of this component. In the case of metal, its work function should be higher than that of Cu2O. Moreover, control of the opacity is necessary for the development of transparent Cu2O photocathodes. A semiconductor with a huge energy barrier is a good option because it efficiently hinders the electron recombination at the interface. In this case, the suitable deposition method of Cu2O should be considered on the semiconductor-based back contact layer;
- Overlayer: In the case of n-type overlayers, the created photovoltage in contact with Cu2O should be considered because it motivates the charge separation in the p-n junction with Cu2O. In the case of p-type overlayers, the exploration on the alternative material to the CuO overlayer with the proper energy level for enhancing the electron transfer into the water interface is a good strategy for the future research direction;
- Protection layer: Although the amorphous TiO2 protection layer is highly efficient for protecting a Cu2O photocathode against the corrosion, it is still not sufficient due to its pinholes or defects. Hence, the reduction in pinholes or defect of the amorphous TiO2 protection layer is useful for further improvement of its protection capability. The crystallization method of the TiO2 protection layer without damaging the Cu2O photocathode is also feasible to improve the stability of the Cu2O photocathode without a decreased PEC performance. The technique to form the hydrophobic surface on the Cu2O photocathode is a promising strategy to improve the stability of Cu2O photocathodes;
- Co-catalysts: The development of non-noble HER catalysts and the alleviation of noble components in HER catalysts is essential for the low-cost PEC water-splitting system. Although various HER catalysts have recently been developed [123], the deposition method should be considered for successfully applying to the Cu2O photocathode. Furthermore, the bonding of the HER catalyst with a Cu2O photocathode should be concerned for the durable Cu2O photocathode because it is directly related to the stability;
- Upscaling: The reported high PEC performance of Cu2O photocathodes is normally based on a small scale below 1 cm2. In general, it is significantly reduced in the large-scale Cu2O photocathodes [124]. Therefore, the research on maintaining its high PEC performance in the large-scale Cu2O photocathodes is necessary, such as a novel design. Although a few groups have recently reported their works on the large-scale Cu2O photocathode [124,125], more vigorous efforts on this are still essential for realizing the mass production of hydrogen via the PEC water-splitting system based on the Cu2O photocathode in the future.
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Prevot, M.S.; Sivula, K. Photoelectrochemical tandem cells for solar water splitting. J. Phys. Chem. C 2013, 117, 17879–17893. [Google Scholar] [CrossRef]
- Ager, J.W.; Schaner, M.R.; Walczak, K.A.; Sharp, I.D.; Ardo, S. Experimental demonstrations of spontaneous, solar-driven photoelectrochemical water splitting. Energy Environ. Sci. 2015, 8, 2811–2824. [Google Scholar] [CrossRef]
- Chang, J.-H.; Kumar, M.; Shen, S.-Y. Fundamentals of photoelectrochemical water splitting. In Nanostructured Materials for Photoelectrochemical Water Splitting; Chang, J.-H., Kumar, M., Nayak, A.K., Eds.; IOP Publishing: London, UK, 2021; pp. 1-1–1-20. [Google Scholar]
- Peter, L.M.; Wijayantha, K.G.U. Photoelectrochemical water splitting at semiconductor electrodes: Fundamental problems and new perspectives. ChemPhysChem 2014, 15, 1983–1995. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hansora, D.; Sharma, P.; Jang, J.-W.; Lee, J.S. Toward practical solar hydrogen production—An artificial photosynthetic leaf-to-farm challenge. Chem. Soc. Rev. 2019, 48, 1908–1971. [Google Scholar] [CrossRef]
- Sivula, K.; van de Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Maggard, P.A. Capturing metastable oxide semiconductors for applications in solar energy conversion. Acc. Chem. Res. 2021, 54, 3160–3171. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Li, D.; Jiang, X.; Zhang, Y.; Zhang, B. A novel route to ZnO/TiO2 heterojunction composite fibers. J. Mater. Res. 2013, 28, 507–512. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, H.; Inoue, T.; Yao, Q. Band gap engineering of SrTiO3 for water splitting under visible light irradiation. Int. J. Hydrogen Energy 2014, 39, 12507–12514. [Google Scholar] [CrossRef]
- Nunes, B.N.; Lopes, O.F.; Patrocinio, A.O.T.; Bahnemann, D.W. Recent advances in niobium-based materials for photocatalytic solar fuel production. Catalysts 2020, 10, 126. [Google Scholar] [CrossRef]
- Chen, Z.; Dinh, H.N.; Miller, E. (Eds.) Introduction. In Photoelectrochemical Water Splitting: Standards, Experimental Methods, and Protocols; Springer: New York, NY, USA, 2013; pp. 1–6. [Google Scholar]
- Lee, M.G.; Park, J.S.; Jang, H.W. Solution-processed metal oxide thin film nanostructures for water splitting photoelectrodes: A review. J. Korean Ceram. Soc. 2018, 55, 185–202. [Google Scholar] [CrossRef]
- Dias, P.; Schreier, M.; Tilley, S.D.; Luo, J.; Azevedo, J.; Andrade, L.; Bi, D.; Hagfeldt, A.; Mendes, A.; Grätzel, M.; et al. Transparent cuprous oxide photocathode enabling a stacked tandem cell for unbiased water splitting. Adv. Energy Mater. 2015, 5, 1501537. [Google Scholar] [CrossRef]
- Wheeler, G.P.; Choi, K.-S. Photoelectrochemical properties and stability of nanoporous p-type LaFeO3 photoelectrodes prepared by electrodeposition. ACS Energy Lett. 2017, 2, 2378–2382. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.S. Elaborately modified BiVO4 photoanodes for solar water splitting. Adv. Mater. 2019, 31, 1806938. [Google Scholar] [CrossRef]
- Abdi, F.F.; Firet, N.; van de Krol, R. Efficient BiVO4 thin film photoanodes modified with cobalt phosphate catalyst and W-doping. ChemCatChem 2013, 5, 490–496. [Google Scholar] [CrossRef]
- Trzesniewski, B.J.; Smith, W.A. Photocharged BiVO4 photoanodes for improved solar water splitting. J. Mater. Chem. A 2016, 4, 2919–2926. [Google Scholar] [CrossRef]
- Tacca, A.; Meda, L.; Marra, G.; Savoini, A.; Caramori, S.; Cristino, V.; Bignozzi, C.A.; Pedro, V.G.; Boix, P.P.; Gimenez, S.; et al. Photoanodes based on nanostructured WO3 for water splitting. ChemPhysChem 2012, 13, 3025–3034. [Google Scholar] [CrossRef]
- Liu, X.; Wang, F.; Wang, Q. Nanostructure-based WO3 photoanodes for photoelectrochemical water splitting. Phys. Chem. Chem. Phys. 2012, 14, 7894–7911. [Google Scholar] [CrossRef] [PubMed]
- Dias, P.; Lopes, T.; Meda, L.; Andrade, L.; Mendes, A. Photoelectrochemical water splitting using WO3 phtoanodes: The substrate and temperature roles. Phys. Chem. Chem. Phys. 2016, 18, 5232–5243. [Google Scholar] [CrossRef] [PubMed]
- Steier, L.; Luo, J.; Schreier, M.; Mayer, M.T.; Sajavaara, T.; Grätzel, M. Low-temperature atomic layer deposition of crystalline and photoactive ultrathin hematite films for solar water splitting. ACS Nano 2015, 9, 11775–11783. [Google Scholar] [CrossRef] [PubMed]
- Sivula, K.; Formal, F.L.; Grätzel, M. Solar water splitting: Progress using hematite (α-Fe2O3) photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [CrossRef] [PubMed]
- Dias, P.; Andrade, L.; Mendes, A. Hematite-based photoelectrode for solar water splitting with very high photovoltage. Nano Energy 2017, 38, 218–231. [Google Scholar] [CrossRef]
- Cao, Q.; Yu, J.; Cao, Y.; Delaunay, J.-J.; Che, R. Unusual effects of vacuum annealing on large-area Ag3PO4 microcrystalline thin film photoanode boosting cocatalyst- and scavenger-free water splitting. J. Mater. 2021, 7, 929–939. [Google Scholar]
- Masudy-Panah, S.; Moakhar, R.S.; Chua, C.S.; Tan, H.R.; Wong, T.I.; Chi, D.; Dalapati, G.K. Nanocrystal engineering of sputter-grown CuO photocathode for visible-light-driven electrochemical water splitting. ACS Appl. Mater. Interfaces 2016, 8, 1206–1213. [Google Scholar] [CrossRef]
- Septina, W.; Prabhakar, R.R.; Wick, R.; Moehl, T.; Tilley, S.D. Stabilized solar hydrogen production with CuO/CdS heterojunction thin film photocathodes. Chem. Mater. 2017, 29, 1735–1743. [Google Scholar] [CrossRef]
- Li, Y.; Luo, K. Flexible cupric oxide photocathode with enhanced stability for renewable hydrogen energy production rom solar water splitting. RSC Adv. 2019, 9, 8350–8354. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Grätzel, M.; Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef]
- Paracchino, A.; Mathews, N.; Hisatomi, T.; Stefik, M.; Tilley, S.D.; Grätzel, M. Ultrathin films on copper(I) oxide water splitting photocathodes: A study on performance and stability. Energy Environ. Sci. 2012, 5, 8673–8681. [Google Scholar] [CrossRef]
- Bagal, I.V.; Chodankar, N.R.; Hassan, M.A.; Waseem, A.; Jahar, M.A.; Kim, D.-H.; Ryu, S.-W. Cu2O as an emerging photocathode for solar water splitting—A status review. Int. J. Hydrogen Energy 2019, 44, 21351–21378. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Li, C.; Chen, Q.; Zhen, Z.; Jiang, X.; Zhong, M.; Zhang, F.; Zhu, H. Scalable low-band-gap Sb2Se3 thin-film photocathodes for efficient visible-near-infrared solar hydrogen evolution. ACS Nano 2017, 11, 12753–12763. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kim, J.H.; Hutter, O.S.; Phillips, L.J.; Tan, J.; Park, J.; Lee, H.; Major, J.D.; Lee, J.S.; Moon, J. Benchmark performance of low-cost Sb2Se3 photocathodes for unassisted solar overall water splitting. Nat. Commun. 2020, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, S.; Zhang, Q.; Pan, S.; Zhao, Y.; Zhang, X. Controlling the crystallographic orientation of Sb2Se3 film for efficient photoelectrochemical water splitting. Sol. RLL 2022, 6, 2100798. [Google Scholar] [CrossRef]
- Pawar, G.S.; Tahir, A.A. Unbiased spontaneous solar fuel production using stable LaFeO3 photoelectrode. Sci. Rep. 2018, 5, 3501. [Google Scholar] [CrossRef] [PubMed]
- Son, M.-K.; Seo, H.; Watanabe, M.; Shiratani, M.; Ishihara, T. Characteristics of crystalline sputtered LaFeO3 thin films as photoelectrochemical water splitting photocathodes. Nanoscale 2020, 12, 9653–9660. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, J.; Xiao, Y.; Li, Y.; Delaunay, J.-J. Earth-abundant Cu-based metal oxide photocathodes for photoelectrochemical water splitting. Energy Environ. Sci. 2020, 13, 3269–3306. [Google Scholar] [CrossRef]
- Li, C.; Cao, Q.; Wang, F.; Xiao, Y.; Li, Y.; Delaunay, J.-J.; Zhu, H. Engineering graphene and TMDs based van der Waals heterostructures for photovoltaic and photoelectrochemical solar energy conversion. Chem. Soc. Rev. 2018, 47, 4981–5037. [Google Scholar] [CrossRef]
- Cui, W.; Niu, W.; Wick, R.; Moehl, T.; Tilley, S.D. Operando deconvolution of photovoltaic and electrocatalytic performance in ALD TiO2 protected water splitting photocathodes. Chem. Sci. 2018, 9, 6062–6067. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Qin, C.; Lou, Z.-R.; Lu, Y.-F.; Zhu, L.-P. Cu2O photocathodes for unassisted solar water-splitting devices enabled by noble-metal cocatalysts simultaneously as hydrogen evolution catalysts and protection layers. Nanotechnology 2019, 30, 495407. [Google Scholar] [CrossRef] [PubMed]
- Kalanur, S.S.; Lee, Y.J.; Seo, H. Enhanced and stable photoelectrochemical H2 production using a engineered nano multijunction with Cu2O photocathode. Mater. Today Chem. 2022, 26, 101031. [Google Scholar] [CrossRef]
- Paracchino, A.; Brauer, J.C.; Moser, J.-E.; Thimsen, E.; Grätzel, M. Synthesis and characterization of high-photoactivity electrodeposited Cu2O solar absorber by photoelectrochemistry and ultrafast spectroscopy. J. Phys. Chem. C 2012, 116, 7341–7350. [Google Scholar] [CrossRef]
- Musselman, K.P.; Wisnet, A.; Iza, D.C.; Hesse, H.C.; Scheu, C.; MacManus-Driscoll, J.L.; Schmidt-Mende, L. Strong efficiency improvements in ultra-low-cost inorganic nanowire solar cells. Adv. Mater. 2010, 22, E254–E258. [Google Scholar] [CrossRef] [PubMed]
- Khiavi, N.D.; Katal, R.; Eshkalak, S.K.; Masudy-Panah, S.; Ramakrishna, S.; Jiangyong, H. Visible light driven heterojunction photocatalyst of CuO-Cu2O thin films for photocatalytic degradation of organic pollutants. Nanomaterials 2019, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Jongh, P.E.; Vanmaekelbergh, D.; Kelly, J.J. Cu2O: Electrodeposition and characterization. Chem. Mater. 1999, 11, 3512–3517. [Google Scholar] [CrossRef]
- Liu, Y.; Turley, H.K.; Tumbleston, J.R.; Samulski, E.T.; Lopez, R. Minority carrier transport length of electrodeposited Cu2O in ZnO/Cu2O heterojunction solar cells. Appl. Phys. Lett. 2011, 98, 162105. [Google Scholar] [CrossRef]
- Hsu, Y.-K.; Yu, C.-H.; Chen, Y.-C.; Lin, Y.-G. Synthesis of novel Cu2O micro/nanostructural photocathode for solar water splitting. Electrochim. Acta 2013, 105, 62–68. [Google Scholar] [CrossRef]
- Salehmin, M.N.I.; Minggu, L.J.; Mark-Lee, W.F.; Mohamed, M.A.; Arifin, K.; Jumali, M.H.H.; Kassim, M.B. Highly photoactive Cu2O nanowire film prepared with modified scalable synthesis method for enhanced photoelectrochemical performance. Sol. Energy Mater. Sol. Cells 2018, 182, 237–245. [Google Scholar] [CrossRef]
- Luo, J.; Steier, L.; Son, M.-K.; Schreier, M.; Mayer, M.T.; Grätzel, M. Cu2O nanowire photocathodes for efficient and durable solar water splitting. Nano Lett. 2016, 16, 1848–4857. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, W.; Wang, D.; Chen, H.; Zhou, G. Enhanced light trapping and charge separation via pyramidal Cu2O/NiCo-LDH photocathode for efficient water splitting. ACS Appl. Energy Mater. 2022, 5, 992–1001. [Google Scholar] [CrossRef]
- Yoon, S.; Lim, J.-H.; Yoo, B. Electrochemical synthesis of cuprous oxide on highly conducting metal micro-pillar arrays for water splitting. J. Alloys Compd. 2016, 677, 66–71. [Google Scholar] [CrossRef]
- Wu, Z.; Fu, M.; Liu, X.; Li, J.; Wei, C.; Zhang, Y.; Ning, Y.; He, D.; Wang, Y. Boosting the photocathode performances of protected Cu2O inverse opals using photonic-crystal heterostructures. Appl. Surf. Sci. 2024, 644, 158792. [Google Scholar] [CrossRef]
- Nishi, Y.; Miyata, T.; Minami, T. Electrochemically deposited Cu2O thin films on thermally oxidized Cu2O sheets for solar cell applications. Sol. Energy Mater. Sol. Cells 2016, 155, 405–410. [Google Scholar] [CrossRef]
- Nyborg, M.; Azarov, A.; Bergum, K.; Monakhov, E. Deposition and characterization of lithium doped direct current magnetron sputtered Cu2O films. Thin Solid Film. 2021, 722, 138573. [Google Scholar] [CrossRef]
- Wang, Y.; Kwok, C.K.G.; Xiao, D.; Zhu, J.; Shu, X.; Liu, C.P.; Yu, K.M. improving the p-type conductivity of Cu2O thin films by Ni doping and their heterojunction with n-ZnO. Appl. Surf. Sci. 2022, 590, 153047. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Abdel-wahab, M.S.; Elfayoumi, M.A.K.; Tawfik, W.Z. Highly efficient sputtered Ni-doped Cu2O photoelectrodes for solar hydrogen generation from water-splitting. Int. J. Hydrogen Energy 2023, 48, 1863–1876. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Xue, H.; Zhang, J.; Peng, S.; Han, X.; Deng, Y.; Hu, W. Acceptor-doping accelerated charge separation in Cu2O photocathode for photoelectrochemical water splitting: Theoretical and experimental studies. Angew. Chem. Int. Ed. 2020, 59, 18463–18467. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Hsiao, Z.-Q.; Hsu, Y.-K. Effect of alkaline-doping on photoelectrochemical activity of electrodeposited cuprous oxide films. Int. J. Hydrogen Energy 2018, 43, 13032–13038. [Google Scholar] [CrossRef]
- Upadhyay, S.; Sharma, D.; Singh, N.; Satsangi, V.R.; Shrivastav, R.; Waghmare, U.V.; Dass, S. Experimental and first-principles theoretical studies on Ag-doped cuprous oxide ad photocathode in photoelectrochemical splitting of water. J. Mater. Sci. 2014, 49, 868–876. [Google Scholar] [CrossRef]
- Upadhyay, S.; Sharma, D.; Singh, N.; Satsangi, V.R.; Shrivastav, R.; Waghmare, U.V.; Dass, S. Spray pyrolytically deposited Fe-doped Cu2O thin films for solar hydrogen generation: Experiments & first-principles analysis. Mater. Chem. Phys. 2015, 160, 32–39. [Google Scholar]
- Baek, S.K.; Kim, J.S.; Kim, Y.B.; Yoon, J.H.; Lee, H.-B.-R.; Cho, H.K. Dual role of Sb-incorporated buffer layers for high efficiency cuprous oxide photocathodic performance: Remarkably enhanced crystallinity and effective hole transport. ACS Sustain. Chem. Eng. 2017, 5, 8213–8221. [Google Scholar] [CrossRef]
- Qin, C.; Chen, X.; Liang, R.; Jiang, N.; Zheng, Z.; Ye, Z.; Zhu, L. Fabricating high-quality Cu2O photocathode by magnetron sputtering: Insight into defect states and charge carrier collection in Cu2O. ACS Appl. Energy Mater. 2022, 5, 14410–14422. [Google Scholar] [CrossRef]
- Yang, W.-Y.; Rhee, S.-W. Effect of electrode material on the resistance switching of Cu2O film. Appl. Phys. Lett. 2007, 91, 232907. [Google Scholar] [CrossRef]
- Singh, B.; Mehta, B.R. Relationship between nature of metal-oxide contacts and resistive switching properties of copper oxide thin film based devices. Thin Solid Film. 2014, 569, 35–43. [Google Scholar] [CrossRef]
- Ofuonye, B.; Lee, J.; Yan, M.; Sun, C.; Zuo, J.-M.; Adesida, I. Electrical and microstructural properties of thermally annealed Ni/Au and Ni/Pt/Au Schottky contacts on AlGaN/GaN heterostructures. Semicond. Sci. Technol. 2014, 29, 095005. [Google Scholar] [CrossRef]
- Shimura, K.; Yoshida, H. Heterogeneous photocatalytic hydrogen production from water and biomass derivatives. Energy Environ. Sci. 2011, 4, 2467–2481. [Google Scholar] [CrossRef]
- Lan, T.; Mundt, C.; Tran, M.; Padalkar, S. Effect of gold underlayer on copper(I) oxide photocathode performance. J. Mater. Res. 2017, 32, 1656–1664. [Google Scholar] [CrossRef]
- Visible, A.; Fracchia, M.; Baran, T.; Vertova, A.; Ghigna, P.; Ahlberg, E.; Rondinini, S.; Minguzzi, A. Electrodeposited cu thin layers as low cost and effective underlayers for Cu2O photocathodes in photoelectrochemical water electrolysis. J. Solid State Electrochem. 2020, 24, 339–355. [Google Scholar] [CrossRef]
- Yin, X.; Guo, Y.; Xie, H.; Que, W.; Kong, L.B. Nickel oxide as efficient hole transport materials for perovskite solar cells. Sol. RRL 2019, 3, 1900001. [Google Scholar] [CrossRef]
- Girolamo, D.D.; Giacomo, F.D.; Matteocci, F.; Marrani, A.G.; Dini, D.; Abate, A. Progress, highlights and perspectives on NiO in perovskite photovoltaics. Chem. Sci. 2020, 11, 7746–7759. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Sato, A.; Ajiro, K.; Shiokawa, M.; Hashimoto, Y.; Maeda, T.; Sugiyama, M.; Gatanda, T.; Marumoto, K. Performance improvement mechanisms of perovskite solar cells by modification of NiOx hole-selective contacts with self-assembled-monolayers. Sol. Energy Mater. Sol. Cells 2023, 258, 112428. [Google Scholar] [CrossRef]
- Wei, Y.; Chang, X.; Wang, T.; Li, C.; Gong, J. A low-cost NiO Hole transfer layer for ohmic back contact to Cu2O for photoelectrochemical water splitting. Small 2017, 13, 1702007. [Google Scholar] [CrossRef] [PubMed]
- Son, M.-K.; Steier, L.; Schreier, M.; Mayer, M.T.; Luo, J.; Grätzel, M. A copper nickel mixed oxide hole selective layer for Au-free transparent cuprous oxide photocathodes. Energy Environ. Sci. 2017, 10, 912–918. [Google Scholar] [CrossRef]
- Pan, L.; Liu, Y.; Yao, L.; Ren, D.; Sivula, K.; Grätzel, M.; Hagfeldt, A. Cu2O photocathodes with band-tail states assisted hole transport for standalone solar water splitting. Nat. Commun. 2020, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Guo, Z.; Liu, Z. FeOOH as hole transfer layer to retard the photocorrosion of Cu2O for enhanced photoelectrochemical performance. Appl. Catal. B 2020, 260, 118213. [Google Scholar] [CrossRef]
- Cendula, P.; Mayer, M.T.; Luo, J.; Grätzel, M. Elucidation of photovoltage origin and charge transport in Cu2O heterojunctions for solar energy conversion. Sustain. Energy Fuels 2019, 3, 2633–2641. [Google Scholar] [CrossRef]
- Li, S.; Xu, W.; Meng, L.; Tian, W.; Li, L. Recent progress on semiconductor heterojunction-based photoanodes for photoelectrochemical water splitting. Small Sci. 2022, 2, 2100112. [Google Scholar] [CrossRef]
- Siripala, W.; Ivanovskaya, A.; Jaramillo, T.F.; Baeck, S.-H.; McFarland, E.W. A Cu2O/TiO2 heterojunction thin film cathode for photoelectrocatalysis. Sol. Energy Mater. Sol. Cells 2003, 77, 229–237. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.; Huan, Y.; Nie, T.; Ji, Z.; Bai, Z.; Cheng, X.; Xi, J.; Yan, X. The effect of composite catalyst on Cu2O/TiO2 heterojuction photocathodes for efficient water splitting. Appl. Surf. Sci. 2020, 526, 146700. [Google Scholar] [CrossRef]
- Qin, C.; Chen, X.; Jiang, N.; Liang, R.; Li, Z.; Zheng, Z.; Wu, J.; Chi, H.; Ye, Z.; Zhu, L. Surface densification strategy assisted Cu2O heterojunction photocathode for solar water splitting. Mater. Today Nano 2023, 21, 100294. [Google Scholar] [CrossRef]
- Lee, H.H.; Kim, D.S.; Choi, J.H.; Kim, Y.B.; Jung, S.H.; Sarker, S.; Deshpande, N.G.; Suh, H.W.; Cho, H.K. Optimal n-type Al-doped ZnO overlayers for charge transport enhancement in p-type Cu2O photocathodes. Micromachines 2021, 12, 338. [Google Scholar] [CrossRef]
- Minami, T.; Nishi, Y.; Miyata, T. High-efficiency Cu2O-based heterojunction solar cells fabricated using a Ga2O3 thin film as n-type layer. Appl. Phys. Express 2013, 6, 044101. [Google Scholar] [CrossRef]
- Li, C.; Hisatomi, T.; Watanabe, O.; Nakabayashi, M.; Shibata, N.; Domen, K.; Delaunay, J.-J. Simultaneous enhancement of photovoltage and charge transfer in Cu2O-based photocathode using buffer and protective layers. Appl. Phys. Lett. 2016, 109, 033902. [Google Scholar] [CrossRef]
- Pan, L.; Kim, J.H.; Mayer, M.T.; Son, M.-K.; Ummadisingu, A.; Lee, J.S.; Hagfeldt, A.; Luo, J.; Grätzel, M. Boosting the performance of Cu2O photocathodes for unassisted solar water splitting devices. Nat. Catal. 2018, 1, 412–420. [Google Scholar] [CrossRef]
- Mayer, M.T. Photovoltage at semiconductor-electrolyte junctions. Curr. Opin. Electrochem. 2017, 2, 104–110. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, D.; Wu, Q.; Diao, P. Cu2O/CuO bilayered composite as a high-efficiency photocathode for photoelectrochemical hydrogen evolution reaction. Sci. Rep. 2016, 6, 35158. [Google Scholar] [CrossRef]
- Du, F.; Chen, Q.-Y.; Wang, Y.-H. Effect of annealing process on the heterostructure CuO/Cu2O as a highly efficient photocathode for photoelectrochemical water reduction. J. Phys. Chem. Solids 2017, 104, 139–144. [Google Scholar] [CrossRef]
- Seo, Y.J.; Arunachalam, M.; Ahn, K.-S.; Kang, S.H. Integrating heteomixtured Cu2O/CuO photcathode interface through a hydrogen treatment for photoelectrochemical hydrogen evolution reaction. Appl. Surf. Sci. 2021, 551, 149375. [Google Scholar]
- Jeong, D.; Jo, W.; Jeong, J.; Kim, T.; Han, S.; Son, M.-K.; Jung, H. Characterization of Cu2O/CuO heterostructure photocathode by tailoring CuO thickness for phtoelectrochemical water splitting. RSC Adv. 2022, 12, 2632–2640. [Google Scholar] [CrossRef]
- Jamali, S.; Moshaii, A.; Mohammadian, N. Improvement of photoelectrochemical and stability properties of electrodeposited Cu2O thin films by annealing processes. Phys. Status Solidi A 2017, 214, 1700380. [Google Scholar] [CrossRef]
- John, S.; Roy, S.C. CuO/Cu2O nanoflake/nanowire heterostructure photocathode with enhanced surface area for photoelectrochemical solar energy conversion. Appl. Surf. Sci. 2020, 509, 144703. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.-W. Thermodynamic oxidation and reduction potentials of photocatalytic semiconductors in aqueous solution. Chem. Mater. 2012, 24, 3659–3666. [Google Scholar] [CrossRef]
- Son, M.-K.; Pan, L.; Mayer, M.T.; Hagfeldt, A.; Grätzel, M.; Luo, J. Structural and compositional investigations on the stability of cuprous oxide nanowire photocathodes for photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2021, 13, 55080–55091. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, J.; Steier, L.; Dias, P.; Stefik, M.; Sousa, C.T.; Araujo, J.P.; Mendes, A.; Graetzel, M.; Tilley, S.D. On the stability enhancement of cuprous oxide water splitting photocathodes by low temperature steam annealing. Energy Environ. Sci. 2014, 7, 4044–4052. [Google Scholar] [CrossRef]
- Mei, B.; Pedersen, T.; Malacrida, P.; Bae, D.; Frydendal, R.; Hansen, O.; Vesborg, P.C.K.; Chorkendorff, I. Crystalline TiO2: A generic and effective electron-conducting protection layer for photoanodes and -cathodes. J. Phys. Chem. C 2015, 119, 10519–10527. [Google Scholar] [CrossRef]
- Nishikawa, M.; Fukuda, M.; Nakabayashi, Y.; Saito, N.; Ogawa, N.; Nakajima, T.; Shinoda, K.; Tsuchiya, T.; Nosaka, Y. A method to give chemically stabilities of photoelectrodes for water splitting: Compositing of a highly crystalized TiO2 layer on a chemically unstable Cu2O photocathode using laser-induced crystallization process. Appl. Surf. Sci. 2016, 363, 173–180. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, Y.B.; Choi, J.H.; Suh, H.W.; Lee, H.H.; Lee, K.W.; Jung, S.H.; Kim, J.J.; Deshpande, N.G.; Cho, H.K. Toward simultaneous achievement of outstanding durability and photoelectrochemical reaction in Cu2O photocathodes via electrochemically designed resistive switching. Adv. Energy Mater. 2021, 11, 2101905. [Google Scholar] [CrossRef]
- Le, H.V.; Tran, P.D.; Mai, H.V.; Ung, T.T.D.; Nguyen, L.Q. Gold protective layer decoration and pn homojunction creation as novel strategies to improve photocatalytic activity and stability of the H2-evolving copper(I) oxide photocathode. Int. J. Hydrogen Energy 2018, 43, 21209–21218. [Google Scholar] [CrossRef]
- Li, Y.; Luo, K. Performance improvement of a p-Cu2O nanocrystal photocathode with an ultra-thin silver protective layer. Chem. Commun. 2019, 55, 9963–9966. [Google Scholar] [CrossRef]
- Kunturu, P.P.; Huskens, J. Efficient solar water splitting photocathodes comprising a copper oxide heterostructure protected by a thin carbon layer. ACS Appl. Energy Mater. 2019, 2, 7850–7860. [Google Scholar] [CrossRef]
- Das, C.; Ananthoju, B.; Dhara, A.K.; Aslam, M.; Sarkar, S.K.; Balasubramaniam, K.R. Electron-selective TiO2/CVD-graphene layers for photocorrosion inhibition in Cu2O photocathodes. Adv. Mater. Interfaces 2017, 4, 1700271. [Google Scholar] [CrossRef]
- Diao, L.; Zheng, L.; Zhang, R.; Chen, F.; Li, Y.; Wang, W.; Lu, F.; Chen, L.; Liu, H.; Dong, H.; et al. Titanium nitride protected cuprous oxide photocathode for stable and efficient water reduction. ACS Appl. Energy Mater. 2022, 5, 770–776. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, X.; Luo, K.; Shao, Z. A hydrophobic polymer stabilized p-Cu2O nanocrystal photocathode for highly efficient solar water splitting. J. Mater. Chem. A 2019, 7, 15593–15598. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, Y.; Tian, M.; Liu, Y.-G.; Hou, J.; Li, C.; Jiang, H.-Y.; Tang, J. Improvement of the photoelectrochemical stability of Cu2O photocathode by Ph-C≡C-Cu grafting. Adv. Mater. Interfaces 2023, 10, 2201380. [Google Scholar] [CrossRef]
- Hansen, J.N.; Prats, H.; Toudahl, K.K.; Secher, N.M.; Chan, K.; Kibsgaard, J.; Chorkendorff, I. Is there anything better than Pt for HER? ACS Energy Lett. 2021, 6, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Baek, J.-B. Recent advances in noble metal (Pt, Ru, and Ir)-based electrocatalysts for efficient hydrogen evolution reaction. ACS Omega 2020, 5, 31–40. [Google Scholar] [CrossRef]
- Cao, D.; Nasori, N.; Wang, Z.; Wen, L.; Xu, R.; Mi, Y.; Lei, Y. Facile surface treatment on Cu2O photocathodes for enhancing the photoelectrochemical response. Appl. Catal. B 2016, 198, 398–403. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Z.; Guo, Z.; Yan, W.; Xin, Y. Enhancing light harvesting and charge separation of Cu2O photocathodes with spatially separated noble-metal cocatalysts toward highly efficient water splitting. J. Mater. Chem. A 2018, 6, 20393–20401. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Z.; Han, C.; Ma, X.; Tong, Z.; Tan, B. Cu2O/CuO heterojunction formed by thermal oxidation and decorated with Pt co-catalyst as an efficient photocathode for photoelectrochemical water splitting. J. Nanopart. Res. 2021, 23, 268. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Yeh, H.-Y.; Popescu, R.; Gerthsen, D.; Hsu, Y.-K. Solution-processed Cu2O/ZnO/TiO2/Pt nanowire photocathode for efficient photoelectrochemical water splitting. J. Alloys Compd. 2022, 899, 163348. [Google Scholar] [CrossRef]
- Tilley, S.D.; Schreier, M.; Azevedo, J.; Stefik, M.; Graetzel, M. Ruthenium oxide hydrogen evolution catalysis on composite cuprous oxide water-splitting photocathodes. Adv. Funct. Mater. 2014, 24, 303–311. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lai, Y.-H.; Mersch, D.; Reisner, E. Cu2O/NiOx nanocomposite as an inexpensive photocathode in photoelectrochemical water splitting. Chem. Sci. 2012, 3, 3482–3487. [Google Scholar] [CrossRef]
- Jian, J.; Kumar, R.; Sun, J. Cu2O/ZnO p-n junction decorated with NiOx as a protection layer and cocatalyst for enhanced photoelectrochemical water splitting. ACS Appl. Energy Mater. 2020, 3, 10408–10414. [Google Scholar] [CrossRef]
- Morales-Guio, C.G.; Liardet, L.; Mayer, M.T.; Tilley, S.D.; Grätzel, M.; Hu, X. Photoelectrochemical hydrogen production in alkaline solutions using Cu2O coated with earth-abundant hydrogen evolution catalysts. Angew. Chem. Int. Ed. 2015, 54, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Wolfe, J.; Fichou, D.; Chen, Z. Cu2O photocathode for low bias photoelectrochemical water splitting enabled by LiFe-layered double hydroxide co-catalyst. Sci. Rep. 2016, 6, 30882. [Google Scholar] [CrossRef] [PubMed]
- Morales-Guio, C.G.; Tilley, S.D.; Vrubel, H.; Grätzel, M.; Hu, X. Hydrogen evolution from a copper(I) oxide photocathode coated with an amorphous molybdenum sulphide catalyst. Nat. Commun. 2014, 5, 3059. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, Z.; Guo, Z.; Yan, W.; Ruan, M. Decorating Cu2O photocathode with noble-metal free Al and NiS cocatalysts for efficient photoelectrochemical water splitting by light harvesting management and charge separation design. J. Chem. Eng. 2020, 381, 122655. [Google Scholar] [CrossRef]
- Stern, L.-A.; Liardet, L.; Mayer, M.T.; Morales-Guio, C.G.; Grätzel, M.; Hu, X. Photoelectrochemical deposition of CoP on cuprous oxide photocathodes for solar hydrogen production. Electrochim. Acta 2017, 235, 311–316. [Google Scholar] [CrossRef][Green Version]
- Chhetri, M.; Rao, C.N.R. Photoelectrochemical hydrogen generation employing a Cu2O-based photocathode with improved stability and activity by using NixPy as the cocatalyst. Phys. Chem. Chem. Phys. 2018, 20, 15300–15306. [Google Scholar] [CrossRef]
- Janani, G.; Choi, H.; Surendran, S.; Sim, U. Recent advances in rational design of efficient electrocatalyst for full water splitting across all pH conditions. MRS Bull. 2020, 45, 539–547. [Google Scholar] [CrossRef]
- Son, M.-K. Design and demonstration of large scale Cu2O photocathodes with metal grid structure for photoelectrochemical water splitting. Energies 2021, 14, 7422. [Google Scholar] [CrossRef]
- Panzeri, G.; Cristina, M.; Jagadeesh, M.S.; Bussetti, G.; Magagnin, L. Modification of large area Cu2O/CuO photocathode with CuS non-noble catalyst for improved photocurrent and stability. Sci. Rep. 2020, 10, 18730. [Google Scholar] [CrossRef] [PubMed]



| Metal | Au | Pt | Cu | Ni | Co | Pd | Ir |
|---|---|---|---|---|---|---|---|
| Work function (eV) | 5.1~5.2 | 5.35~5.65 | 5.1 | 5.15 | 5.0 | 5.12~5.30 | 5.25~5.27 |
| Overlayer | Device | Onset Potential (V versus RHE) | Photocurrent Density (mA/cm2, 0 V versus RHE) | Ref. |
|---|---|---|---|---|
| TiO2 | Cu2O/TiO2 | 0.0 (versus Ag/AgCl) | −0.8 (−1.0 V versus Ag/AgCl) | [81] |
| TiO2 | Cu2O | 0.37 | −0.52 | [82] |
| Cu2O/TiO2 | 0.42 | −1.40 | ||
| Cu2O/TiO2/NiFe | 0.50 | −2.60 | ||
| Cu2O/TiO2/rGO/NiFe | 0.54 | −3.71 | ||
| ZnO | Cu2O/ZnO/TiO2/Pt | 0.65 | −4.00 | [83] |
| AZO | Cu2O/ZnO | 0.50 | −1.60 | [84] |
| Cu2O/AZO | 0.63 | −2.90 | ||
| Ga2O3 | Cu2O | 0.50 | - | [86] |
| Cu2O/Ga2O3/Pt | 0.90 | −4.00 | ||
| Cu2O/Ga2O3/TiO2/Pt | 1.00 | −6.50 | ||
| Ga2O3 | Cu2O/AZO/TiO2/RuOx | 0.50 | −8.00 | [87] |
| Cu2O/Ga2O3/TiO2/RuOx | 1.00 | −9.60 | ||
| CuO | Cu2O | 0.45 | −0.21 | [89] |
| Cu2O/CuO | 0.80 | −2.47 | ||
| CuO | Cu2O | 0.0 (versus Ag/AgCl) | −0.06 (−0.3 V versus Ag/AgCl) | [90] |
| Cu2O/CuO | 0.1 (versus Ag/AgCl) | −0.26 (−0.3 V versus Ag/AgCl) | ||
| CuO | Cu2O/CuO | 0.60 | −2.70 | [91] |
| CuO | Cu2O | 0.50 | −0.15 | [92] |
| Cu2O/CuO | 0.50 | −1.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, M.-K. Key Strategies on Cu2O Photocathodes toward Practical Photoelectrochemical Water Splitting. Nanomaterials 2023, 13, 3142. https://doi.org/10.3390/nano13243142
Son M-K. Key Strategies on Cu2O Photocathodes toward Practical Photoelectrochemical Water Splitting. Nanomaterials. 2023; 13(24):3142. https://doi.org/10.3390/nano13243142
Chicago/Turabian StyleSon, Min-Kyu. 2023. "Key Strategies on Cu2O Photocathodes toward Practical Photoelectrochemical Water Splitting" Nanomaterials 13, no. 24: 3142. https://doi.org/10.3390/nano13243142
APA StyleSon, M.-K. (2023). Key Strategies on Cu2O Photocathodes toward Practical Photoelectrochemical Water Splitting. Nanomaterials, 13(24), 3142. https://doi.org/10.3390/nano13243142






