Cd2+ Sorption Alterations in Ultisol Soils Triggered by Different Engineered Nanoparticles and Incubation Times
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Nanoparticle Synthesis
2.2. Nanoparticles Synthesis
2.3. Characterization of ENPs
2.4. Soil Material
2.5. Sorption Experiments
3. Results and Discussion
3.1. Nanoparticle Characterization
3.2. Soil Characterization
3.3. Batch Experiment Results
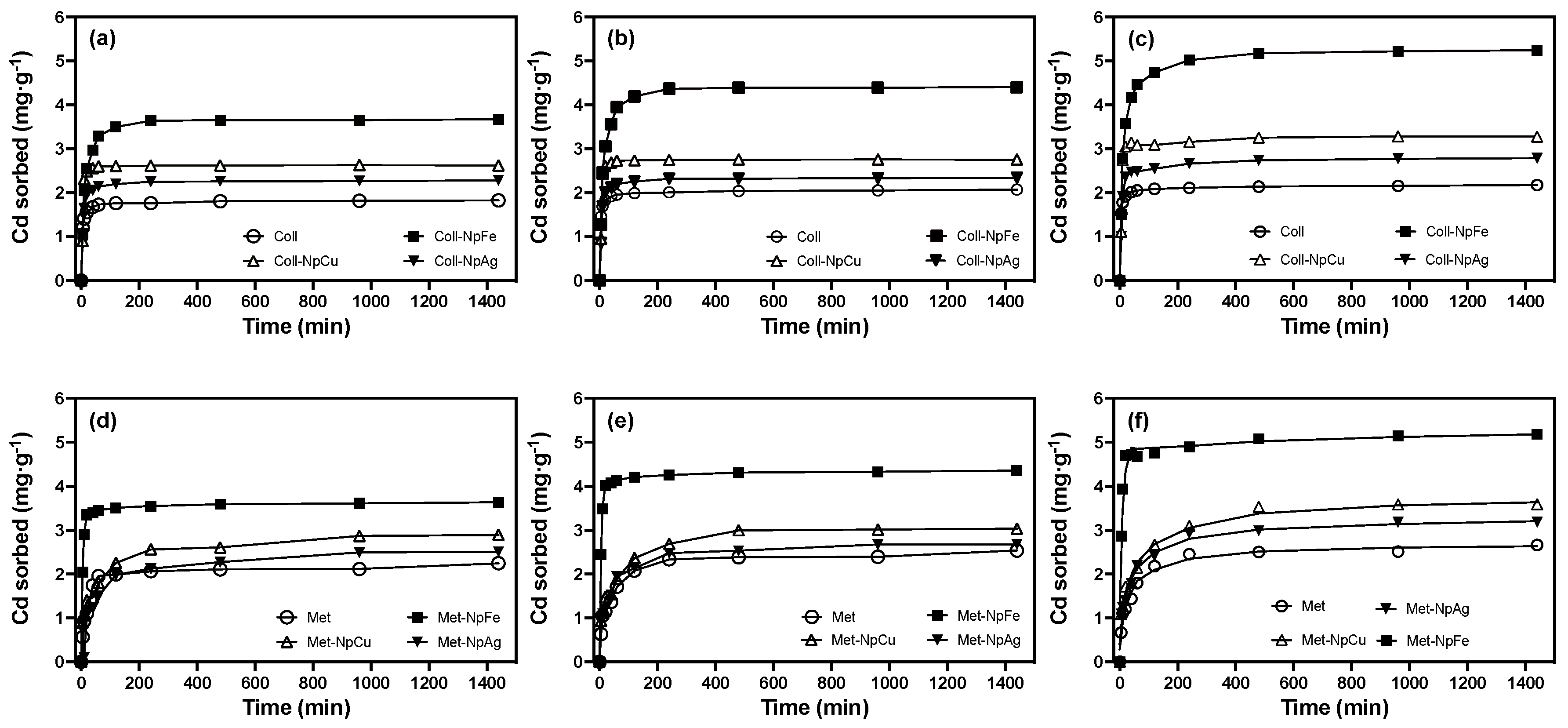
3.3.1. Sorption Kinetics
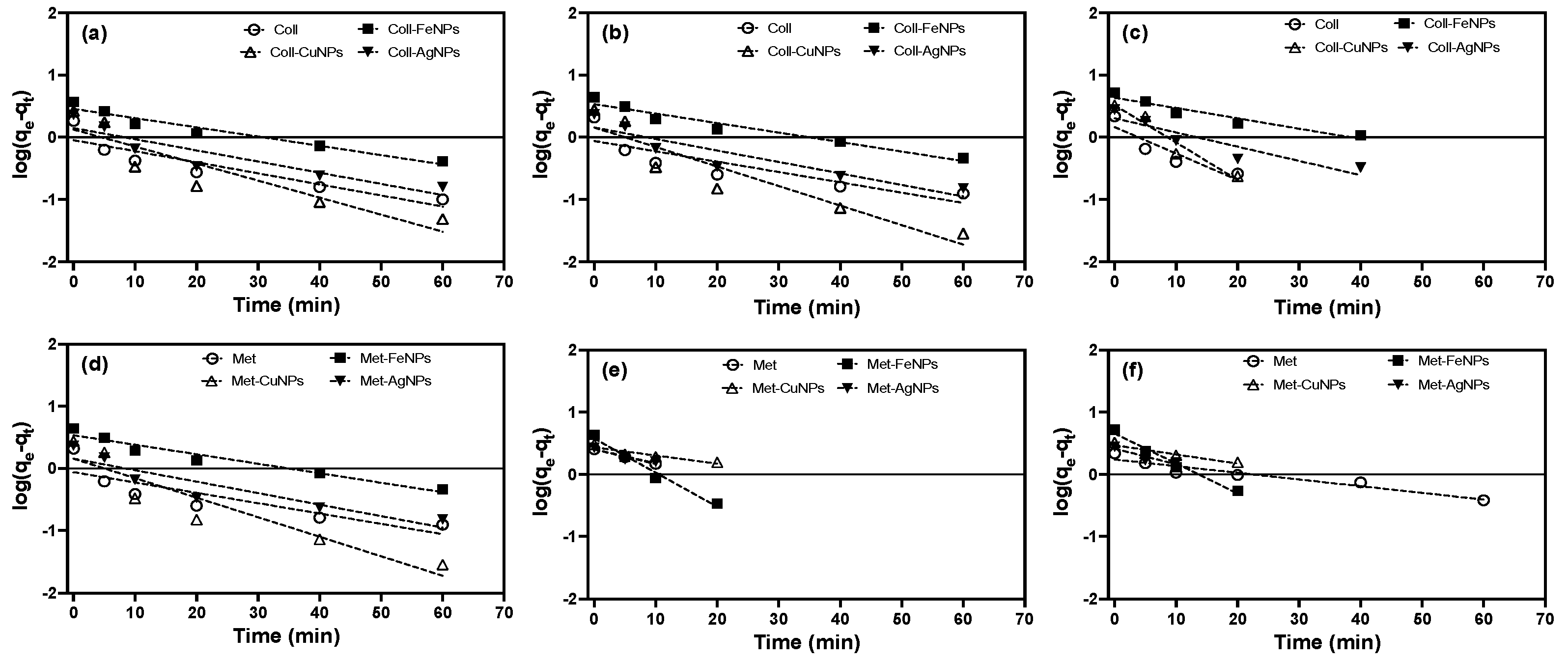
3.3.2. Kinetic Modelling: Pseudo-First-Order (PFO) and Pseudo-Second-Order (PSO) Models
3.3.3. Solute Transport Mechanism: Intraparticle Diffusion Kinetic Model
3.4. Cadmium Sorption: Role of Metallic Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stubbins, A.; Law, K.L.; Muñoz, S.E.; Bianchi, T.S.; Zhu, L. Plastics in the Earth System. Science 2021, 373, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Hochella, M.F.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. Natural, Incidental, and Engineered Nanomaterials and Their Impacts on the Earth System. Science 2019, 363, eaau8299. [Google Scholar] [CrossRef] [PubMed]
- Amundson, R.; Berhe, A.A.; Hopmans, J.W.; Olson, C.; Sztein, A.E.; Sparks, D.L. Soil and Human Security in the 21st Century. Science 2015, 348, 1261071. [Google Scholar] [CrossRef]
- Singh, A.K. Engineered Nanoparticles: Structure, Properties and Mechanisms of Toxicity; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780128014929. [Google Scholar]
- Avellan, A.; Simonin, M.; Anderson, S.M.; Geitner, N.K.; Bossa, N.; Spielman-Sun, E.; Bernhardt, E.S.; Castellon, B.T.; Colman, B.P.; Cooper, J.L.; et al. Differential Reactivity of Copper- And Gold-Based Nanomaterials Controls Their Seasonal Biogeochemical Cycling and Fate in a Freshwater Wetland Mesocosm. Environ. Sci. Technol. 2020, 54, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lamb, D.; Paneerselvam, P.; Choppala, G.; Bolan, N.; Chung, J.W. Role of Organic Amendments on Enhanced Bioremediation of Heavy Metal(Loid) Contaminated Soils. J. Hazard. Mater. 2011, 185, 549–574. [Google Scholar] [CrossRef]
- Ličina, V.; Akšić, M.F.; Tomić, Z.; Trajković, I.; Antić Mladenović, S.; Marjanović, M.; Rinklebe, J. Bioassessment of Heavy Metals in the Surface Soil Layer of an Opencast Mine Aimed for Its Rehabilitation. J. Environ. Manag. 2017, 186, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Lowry, G.V.; Avellan, A.; Gilbertson, L.M. Opportunities and Challenges for Nanotechnology in the Agri-Tech Revolution. Nat. Nanotechnol. 2019, 14, 517–522. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; ur Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in Agriculture: Current Status, Challenges and Future Opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Monreal, C.; Schnitzer, M.; Walsh, R.; Sultan, Y. Nanotechnology in Fertilizers. Nat. Nanotechnol. 2010, 5, 91. [Google Scholar] [CrossRef]
- Pradas del Real, A.E.; Vidal, V.; Carriere, M.; Castillo-Michel, H.A.; Levard, C.; Chaurand, P.; Sarret, G.; Carrière, M.; Castillo-Michel, H.A.; Levard, C.; et al. Ag Nanoparticles and Wheat Roots: A Complex Interplay. Environ. Sci. Technol. 2017, 51, 5774–5782. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P.; Ok, Y.S. Review on Nano Zerovalent Iron (NZVI): From Synthesis to Environmental Applications. Chem. Eng. J. 2016, 287, 618–632. [Google Scholar] [CrossRef]
- Levard, C.; Hotze, E.M.; Lowry, G.V.; Brown, G.E. Environmental Transformations of Silver Nanoparticles: Impact on Stability and Toxicity. Environ. Sci. Technol. 2012, 46, 6900–6914. [Google Scholar] [CrossRef]
- Spielman-Sun, E.; Lombi, E.; Donner, E.; Howard, D.; Unrine, J.M.; Lowry, G. V Impact of Surface Charge on Cerium Oxide Nanoparticle Uptake and Translocation by Wheat (Triticum aestivum). Environ. Sci. Technol. 2017, 51, 7361–7368. [Google Scholar] [CrossRef]
- Phenrat, T.; Thongboot, T.; Lowry, G.V. Electromagnetic Induction of Zerovalent Iron (ZVI) Powder and Nanoscale Zerovalent Iron (NZVI) Particles Enhances Dechlorination of Trichloroethylene in Contaminated Groundwater and Soil: Proof of Concept. Environ. Sci. Technol. 2016, 50, 872–880. [Google Scholar] [CrossRef]
- Arancibia-Miranda, N.; Baltazar, S.E.; García, A.; Romero, A.H.; Rubio, M.A.; Altbir, D. Lead Removal by Nano-Scale Zero Valent Iron: Surface Analysis and PH Effect. Mater. Res. Bull. 2014, 59, 341–348. [Google Scholar] [CrossRef]
- Stegemeier, J.P.; Avellan, A.; Lowry, G. V Effect of Initial Speciation of Copper- and Silver-Based Nanoparticles on Their Long-Term Fate and Phytoavailability in Freshwater Wetland Mesocosms. Environ. Sci. Technol. 2017, 51, 12114–12122. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Shende, S.; Gupta, I.; Biswas, J.K.; Da Silva, S.S. Copper and Copper Nanoparticles: Role in Management of Insect-Pests and Pathogenic Microbes. Nanotechnol. Rev. 2018, 7, 303–315. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.B.; Hussain, I.; Singh, H. Effect of Biologically Synthesized Copper Oxide Nanoparticles on Metabolism and Antioxidant Activity to the Crop Plants Solanum lycopersicum and Brassica oleracea Var. Botrytis. J. Biotechnol. 2017, 262, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Kovačec, E.; Regvar, M.; van Elteren, J.T.; Arčon, I.; Papp, T.; Makovec, D.; Vogel-Mikuš, K. Biotransformation of Copper Oxide Nanoparticles by the Pathogenic Fungus Botrytis cinerea. Chemosphere 2017, 180, 178–185. [Google Scholar] [CrossRef]
- Kasana, R.C.; Panwar, N.R.; Kaul, R.K.; Kumar, P. Copper Nanoparticles in Agriculture: Biological Synthesis and Antimicrobial Activity. In Nanoscience in Food and Agriculture 3; Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 129–143. ISBN 978-3-319-48009-1. [Google Scholar]
- Monreal, C.M.; Derosa, M.; Mallubhotla, S.C.; Bindraban, P.S.; Dimkpa, C. Nanotechnologies for Increasing the Crop Use Efficiency of Fertilizer-Micronutrients. Biol. Fertil. Soils 2016, 52, 423–437. [Google Scholar] [CrossRef]
- Gao, X.; Rodrigues, M.; Spielman-Sun, E.; Lopes, S.; Rodrigues, S.; Zhang, Y.; Avellan, A.; Duarte, M.B.O.R.; Duarte, A.; Casman, E.A.; et al. Effect of Soil Organic Matter, Soil PH, and Moisture Content on Solubility and Dissolution Rate of CuO NPs in Soil. Environ. Sci. Technol. 2019, 53, 4959–4967. [Google Scholar] [CrossRef]
- Pradas del Real, A.E.; Castillo-Michel, H.; Kaegi, R.; Sinnet, B.; Magnin, V.V.V.; Findling, N.; Villanova, J.; Carrière, M.; Santaella, C.; Fernández-Martínez, A.; et al. Fate of Ag-NPs in Sewage Sludge after Application on Agricultural Soils. Environ. Sci. Technol. 2016, 50, 1759–1768. [Google Scholar] [CrossRef]
- Meier, C.; Voegelin, A.; Pradas Del Real, A.; Sarret, G.; Mueller, C.R.; Kaegi, R. Transformation of Silver Nanoparticles in Sewage Sludge during Incineration. Environ. Sci. Technol. 2016, 50, 3503–3510. [Google Scholar] [CrossRef] [PubMed]
- Bland, G.D.; Battifarano, M.; Pradas del Real, A.E.; Sarret, G.V.; Lowry, G. Distinguishing Engineered TiO2 Nanomaterials from Natural Ti Nanomaterials in Soil Using SpICP-TOFMS and Machine Learning. Environ. Sci. Technol 2021, 56, 2990–3001. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.; Venkatachalam, P.; Sahi, S.; Sharma, N. Silver and Titanium Dioxide Nanoparticle Toxicity in Plants: A Review of Current Research. Plant Physiol. Biochem. 2016, 107, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, M.; Kumar, A. Copper-Based Nanoparticles in the Soil-Plant Environment: Assessing Their Applications, Interactions, Fate and Toxicity. Chemosphere 2021, 281, 130940. [Google Scholar] [CrossRef] [PubMed]
- Manquián-Cerda, K.; Cruces, E.; Angélica Rubio, M.; Reyes, C.; Arancibia-Miranda, N. Preparation of Nanoscale Iron (Oxide, Oxyhydroxides and Zero-Valent) Particles Derived from Blueberries: Reactivity, Characterization and Removal Mechanism of Arsenate. Ecotoxicol. Environ. Saf. 2017, 145, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Kanel, S.R.; Manning, B.; Charlet, L.; Choi, H. Removal of Arsenic (III) from Groundwater by Nanoscale Zero-Valent Iron. Environ. Sci. Technol. 2005, 39, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Cadmium (Cd(2+)) Removal by Nano Zerovalent Iron: Surface Analysis, Effects of Solution Chemistry and Surface Complexation Modeling. Environ. Sci. Pollut. Res. Int. 2013, 20, 6210–6221. [Google Scholar] [CrossRef]
- Vítkov, M.; Puschenreiter, M.; Kom Arek, M.; Vítková, M.; Puschenreiter, M.; Komárek, M.; Vítkov, M.; Puschenreiter, M.; Kom Arek, M. Effect of Nano Zero-Valent Iron Application on As, Cd, Pb, and Zn Availability in the Rhizosphere of Metal(Loid) Contaminated Soils. Chemosphere 2018, 200, 217–226. [Google Scholar] [CrossRef]
- Tang, C.; Huang, Y.H.; Zeng, H.; Zhang, Z. Reductive Removal of Selenate by Zero-Valent Iron: The Roles of Aqueous Fe2+ and Corrosion Products, and Selenate Removal Mechanisms. Water Res. 2014, 67, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-H.H.; Liu, F.; Yi, S.; Chen, Y.-Z.Z.; Geng, X.; Zheng, C. Simultaneous Stabilization of Pb and Improvement of Soil Strength Using NZVI. Sci. Total Environ. 2019, 651, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, G.; Pang, L.; Doolette, C.; Kirby, J.K.; McLaughlin, M.J. Transport of Silver Nanoparticles in Saturated Columns of Natural Soils. Sci. Total Environ. 2013, 463–464, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Tourinho, P.S.; van Gestel, C.A.M.; Lofts, S.; Svendsen, C.; Soares, A.M.V.M.; Loureiro, S. Metal-Based Nanoparticles in Soil: Fate, Behavior, and Effects on Soil Invertebrates. Environ. Toxicol. Chem. 2012, 31, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Hofacker, A.F.; Voegelin, A.; Kaegi, R.; Weber, F.A.; Kretzschmar, R. Temperature-Dependent Formation of Metallic Copper and Metal Sulfide Nanoparticles during Flooding of a Contaminated Soil. Geochim. Cosmochim. Acta 2013, 103, 316–332. [Google Scholar] [CrossRef]
- Weber, F.-A.; Voegelin, A.; Kaegi, R.; Kretzschmar, R. Contaminant Mobilization by Metallic Copper and Metal Sulphide Colloids in Flooded Soil. Nat. Geosci. 2009, 2, 267–271. [Google Scholar] [CrossRef]
- Milne, C.J.; Lapworth, D.J.; Gooddy, D.C.; Elgy, C.N.; Valsami-Jones, E. Role of Humic Acid in the Stability of Ag Nanoparticles in Sub-Oxic Conditions. Environ. Sci. Technol. 2017, 51, 6063–6070. [Google Scholar] [CrossRef]
- Coutris, C.; Joner, E.J.; Oughton, D.H. Aging and Soil Organic Matter Content Affect the Fate of Silver Nanoparticles in Soil. Sci. Total Environ. 2012, 420, 327–333. [Google Scholar] [CrossRef]
- Baalousha, M.; Cornelis, G.; Kuhlbusch, T.A.J.; Lynch, I.; Nickel, C.; Peijnenburg, W.; Van Den Brink, N.W. Modeling Nanomaterial Fate and Uptake in the Environment: Current Knowledge and Future Trends. Environ. Sci. Nano 2016, 3, 323–345. [Google Scholar] [CrossRef]
- Cornelis, G.; Hund-Rinke, K.; Kuhlbusch, T.; van den Brink, N.; Nickel, C. Fate and Bioavailability of Engineered Nanoparticles in Soils: A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2720–2764. [Google Scholar] [CrossRef]
- Conway, J.R.; Beaulieu, A.L.; Beaulieu, N.L.; Mazer, S.J.; Keller, A.A. Environmental Stresses Increase Photosynthetic Disruption by Metal Oxide Nanomaterials in a Soil-Grown Plant. ACS Nano 2015, 9, 11737–11749. [Google Scholar] [CrossRef]
- Fulda, B.; Voegelin, A.; Maurer, F.; Christl, I.; Kretzschmar, R. Copper Redox Transformation and Complexation by Reduced and Oxidized Soil Humic Acid. 1. X-ray Absorption Spectroscopy Study. Environ. Sci. Technol. 2013, 47, 10903–10911. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jones, A.; Waite, T.D.; Chen, Y.; Huang, X.; Rosso, K.M.; Kappler, A.; Mansor, M.; Tratnyek, P.G.; Zhang, H. Fe (II) Redox Chemistry in the Environment. Chem. Rev. 2020, 121, 8161–8233. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, L.M.; Pourzahedi, L.; Laughton, S.; Gao, X.; Zimmerman, J.B.; Theis, T.L.; Westerhoff, P.; Lowry, G.V. Guiding the Design Space for Nanotechnology to Advance Sustainable Crop Production. Nat. Nanotechnol. 2020, 15, 801–810. [Google Scholar] [CrossRef]
- Hooda, P.S.; Truesdale, V.W.; Edwards, A.C.; Withers, P.J.A.; Aitken, M.N.; Miller, A.; Rendell, A.R. Manuring and Fertilization Effects on Phosphorus Accumulation in Soils and Potential Environmental Implications. Adv. Environ. Res. 2001, 5, 13–21. [Google Scholar] [CrossRef]
- Molina, M.; Aburto, F.; Calderón, R.; Cazanga, M.; Escudey, M. Trace Element Composition of Selected Fertilizers Used in Chile: Phosphorus Fertilizers as a Source of Long-Term Soil Contamination. Soil Sediment Contam. 2009, 18, 497–511. [Google Scholar] [CrossRef]
- Molina-Roco, M.; Escudey, M.; Antilén, M.; Arancibia-Miranda, N.; Manquián-Cerda, K. Distribution of Contaminant Trace Metals Inadvertently Provided by Phosphorus Fertilisers: Movement, Chemical Fractions and Mass Balances in Contrasting Acidic Soils. Environ. Geochem. Health 2018, 121, 8161–8233. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Abbas, T.; Zia-ur-Rehman, M.; Hannan, F.; Keller, C.; Al-Wabel, M.I.; Ok, Y.S. Cadmium Minimization in Wheat: A Critical Review. Ecotoxicol. Environ. Saf. 2016, 130, 43–53. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Smolders, E.; Zhao, F.J.; Grant, C.; Montalvo, D. Managing Cadmium in Agricultural Systems; Elsevier: Amsterdam, The Netherlands, 2021; Volume 166, ISBN 9783510654178. [Google Scholar]
- Baba, H.; Tsuneyama, K.; Yazaki, M.; Nagata, K.; Minamisaka, T.; Tsuda, T.; Nomoto, K.; Hayashi, S.; Miwa, S.; Nakajima, T.; et al. The Liver in Itai-Itai Disease (Chronic Cadmium Poisoning): Pathological Features and Metallothionein Expression. Mod. Pathol. 2013, 26, 1228–1234. [Google Scholar] [CrossRef]
- Manquián-Cerda, K.; Cruces, E.; Escudey, M.; Zúñiga, G.; Calderón, R. Interactive Effects of Aluminum and Cadmium on Phenolic Compounds, Antioxidant Enzyme Activity and Oxidative Stress in Blueberry (Vaccinium corymbosum L.) Plantlets Cultivated In Vitro. Ecotoxicol. Environ. Saf. 2018, 150, 320–326. [Google Scholar] [CrossRef]
- Manquián-Cerda, K.; Escudey, M.; Zúñiga, G.; Arancibia-miranda, N.; Molina, M.; Cruces, E. Ecotoxicology and Environmental Safety Effect of Cadmium on Phenolic Compounds, Antioxidant Enzyme Activity and Oxidative Stress in Blueberry (Vaccinium corymbosum L.) Plantlets Grown in Vitro. Ecotoxicol. Environ. Saf. 2016, 133, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Suazo-Hernández, J.; Arancibia-Miranda, N.; Mlih, R.; Cáceres-Jensen, L.; Bolan, N.; Mora, M. de la L. Impact on Some Soil Physical and Chemical Properties Caused by Metal and Metallic Oxide Engineered Nanoparticles: A Review. Nanomaterials 2023, 13, 572. [Google Scholar] [CrossRef] [PubMed]
- Qafoku, N.P. Chapter Two—Terrestrial Nanoparticles and Their Controls on Soil-/Geo-Processes and Reactions. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2010; Volume 107, pp. 33–91. ISBN 0065-2113. [Google Scholar]
- Dimkpa, C.O. Soil Properties Influence the Response of Terrestrial Plants to Metallic Nanoparticles Exposure. Curr. Opin. Environ. Sci. Health 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, Y.; Xue, Q.; Wu, X. Synthesis of Highly Stable Dispersions of Nanosized Copper Particles Using L-Ascorbic Acid. Green Chem. 2011, 13, 900–904. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and Thermodynamics of Cadmium Ion Removal by Adsorption onto Nano Zerovalent Iron Particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Escudey, M.; Galindo, G.; Förster, J.E.; Briceño, M.; Diaz, P.; Chang, A. Chemical Forms of Phosphorus of Volcanic Ash-Derived Soils in Chile. Commun. Soil Sci. Plant Anal. 2001, 32, 601–616. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Klumpp, E.; Arancibia-Miranda, N.; Poblete-Grant, P.; Jara, A.; Bol, R.; de La Luz Mora, M. Describing Phosphorus Sorption Processes on Volcanic Soil in the Presence of Copper or Silver Engineered Nanoparticles. Minerals 2021, 11, 373. [Google Scholar] [CrossRef]
- Pizarro, C.; Escudey, M.; Fabris, J.D. Influence of Organic Matter on the Iron Oxide Mineralogy of Volcanic Soils. Hyperfine Interact. 2003, 148–149, 53–59. [Google Scholar] [CrossRef]
- Thalmann, B.; Voegelin, A.; Sinnet, B.; Morgenroth, E.; Kaegi, R. Sulfidation Kinetics of Silver Nanoparticles Reacted with Metal Sulfides. Environ. Sci. Technol. 2014, 48, 4885–4892. [Google Scholar] [CrossRef]
- Liang, L.; Li, X.; Lin, Z.; Tian, C.; Guo, Y. The Removal of Cd by Sulfidated Nanoscale Zero-Valent Iron: The Structural, Chemical Bonding Evolution and the Reaction Kinetics. Chem. Eng. J. 2020, 382, 122933. [Google Scholar] [CrossRef]
- Yan, W.; Ramos, M.A.V.; Koel, B.E.; Zhang, W.X. As(III) Sequestration by Iron Nanoparticles: Study of Solid-Phase Redox Transformations with X-Ray Photoelectron Spectroscopy. J. Phys. Chem. C 2012, 116, 5303–5311. [Google Scholar] [CrossRef]
- Lowry, G.V.; Espinasse, B.P.; Badireddy, A.R.; Richardson, C.J.; Reinsch, B.C.; Bryant, L.D.; Bone, A.J.; Deonarine, A.; Chae, S.; Therezien, M.; et al. Long-Term Transformation and Fate of Manufactured Ag Nanoparticles in a Simulated Large Scale Freshwater Emergent Wetland. Environ. Sci. Technol. 2012, 46, 7027–7036. [Google Scholar] [CrossRef] [PubMed]
- Julich, D.; Gäth, S. Sorption Behavior of Copper Nanoparticles in Soils Compared to Copper Ions. Geoderma 2014, 235–236, 127–132. [Google Scholar] [CrossRef]
- Cáceres-Jensen, L.; Rodríguez-Becerra, J.; Parra-Rivero, J.; Escudey, M.; Barrientos, L.; Castro-Castillo, V. Sorption Kinetics of Diuron on Volcanic Ash Derived Soils. J. Hazard. Mater. 2013, 261, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Arancibia-Miranda, N.; Silva-Yumi, J.; Escudey, M. Effect of Cations in the Background Electrolyte on the Adsorption Kinetics of Copper and Cadmium and the Isoelectric Point of Imogolite. J. Hazard. Mater. 2015, 299, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Azizian, S. Kinetic Models of Sorption: A Theoretical Analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef]
- Serrano, S.; Garrido, F.; Campbell, C.G.; García-González, M.T. Competitive Sorption of Cadmium and Lead in Acid Soils of Central Spain. Geoderma 2005, 124, 91–104. [Google Scholar] [CrossRef]
- Shipley, H.J.; Engates, K.E.; Guettner, A.M. Study of Iron Oxide Nanoparticles in Soil for Remediation of Arsenic. J. Nanoparticle Res. 2011, 13, 2387–2397. [Google Scholar] [CrossRef]
- O’Carroll, D.; Sleep, B.; Krol, M.; Boparai, H.; Kocur, C. Nanoscale Zero Valent Iron and Bimetallic Particles for Contaminated Site Remediation. Adv. Water Resour. 2013, 51, 104–122. [Google Scholar] [CrossRef]
- Arancibia-Miranda, N.; Manquián-Cerda, K.; Pizarro, C.; Maldonado, T.; Suazo-Hernández, J.; Escudey, M.; Bolan, N.; Sarkar, B. Mechanistic Insights into Simultaneous Removal of Copper, Cadmium and Arsenic from Water by Iron Oxide-Functionalized Magnetic Imogolite Nanocomposites. J. Hazard. Mater. 2020, 398, 122940. [Google Scholar] [CrossRef]
- Martinis, E.M.; Denardin, J.C.; Calderón, R.; Flores, C.; Manquián, K. Enhanced Removal of Mercury and Lead by a Novel and Efficient Surface-Functionalized Imogolite with Nanoscale Zero-Valent Iron Material. Environ. Sci. Pollut. Res. 2021, 29, 20221–20233. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Qiu, H.; Lv, L.; Pan, B.; Zhang, Q.Q.; Zhang, W.; Zhang, Q.Q. Critical Review in Adsorption Kinetic Models. J. Zhejiang Univ. Sci. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
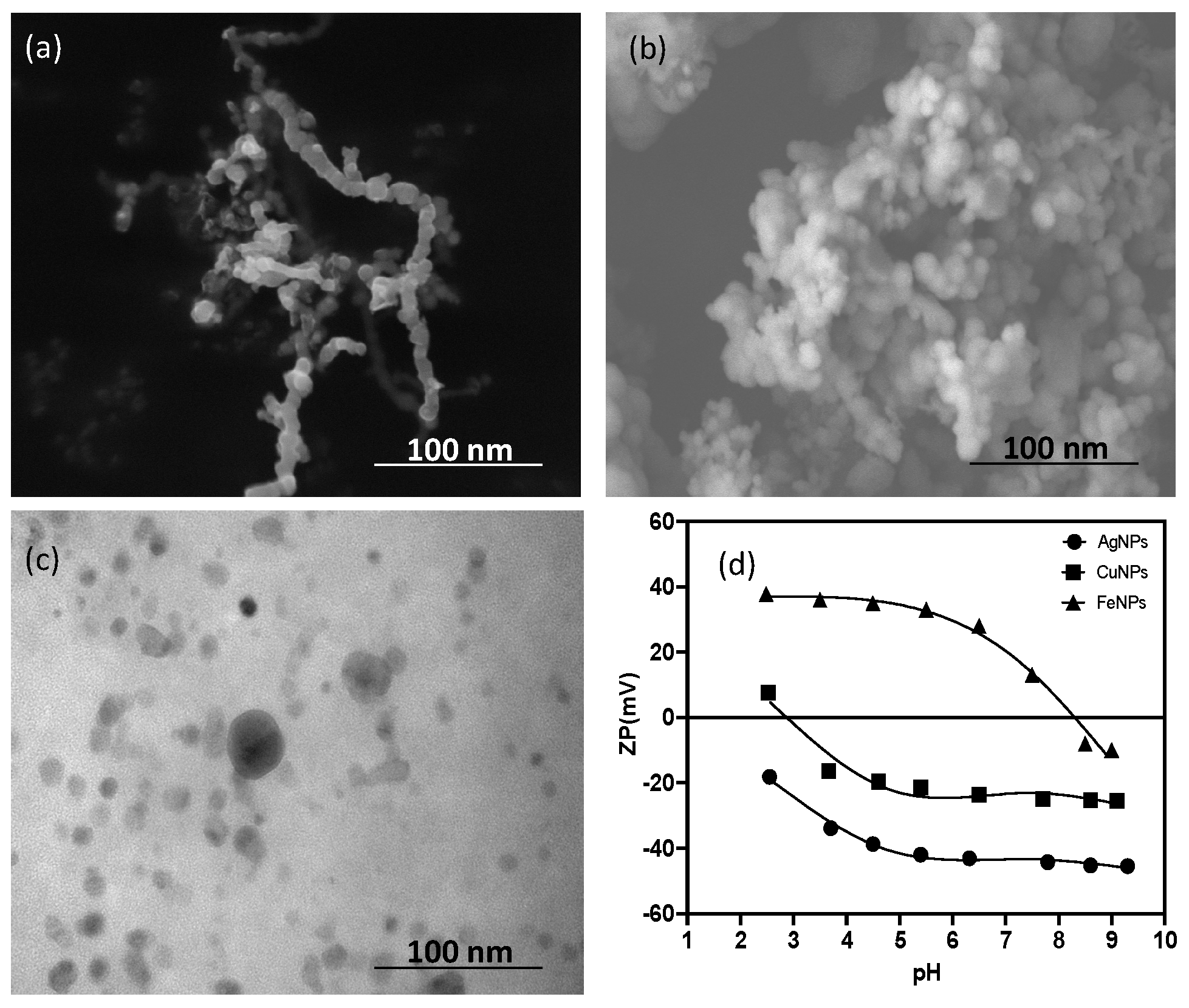


| Parameter | Collipilli | Metrenco |
|---|---|---|
| Soil Order or Source | Ultisol | Ultisol |
| Soil Class | Fine, mixed, | Fine, |
| thermic typic rhodoxeralf | mesic paleumult | |
| Sampling Location | ||
| Latitude | 38°58′ S | 38°34′ S |
| Longitude | 72°09′ W | 72°22′ W |
| Rainfall (m·year−1) | 120–400 | 100–300 |
| Mean annual temperature (°C) | 15.8 | 14.6 |
| Electrical conductivity (dSm−1) | 0.040 ± 0.001 | 0.050 ± 0.001 |
| Organic carbon (wt%) | 2.1 ± 0.2 | 2.8 ± 0.1 |
| Available P (mg·kg−1) | 4.0 ± 0.1 | 10 ± 0.2 |
| Total P (mg·kg−1) | 821 ± 8 | 807 ±15 |
| Available Cd (mg·kg−1) | <0.01 | <0.01 |
| Total Cd (mg·kg−1) | 0.030 ± 0.01 | 0.027 ± 0.01 |
| pH (H2O) | 6.2 ± 0.1 | 5.8 ± 0.1 |
| Exchangeable cations (cmol(+)·kg−1) | 6.0 ± 0.1 | 8.0 ± 0.1 |
| Calcium | 7.0 ± 0.1 | 10.8 ± 0.1 |
| Magnesium | 3.6 ± 0.1 | 3.2 ± 0.1 |
| Potassium | 0.55 ± 0.0 | 1.1 ± 0.0 |
| Sodium | 0.1 ± 0.0 | 0.2 ± 0.0 |
| IEP | 1.7 ± 0.2 | 0.18 ± 0.1 |
| Mineralogical composition > 50% | Kaolinite | Halloysite |
| Collipulli | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Month | 3 Month | 6 Month | ||||||||||
| Treatment | Control | AgNPs | CuNPs | FeNPs | Control | AgNPs | CuNPs | FeNPs | Control | AgNPs | CuNPs | FeNPs |
| qexp (mg·g−1) | 1.7 ± 0.1 | 2.2 ± 0.1 | 2.6 ± 0.1 | 3.5 ± 0.3 | 2.0 ± 0.1 | 2.3 ± 0.1 | 2.7 ± 0.3 | 3.9 ± 0.3 | 2.1 ± 0.3 | 2.6 ± 0.4 | 3.1 ± 0.3 | 3.9 ± 0.1 |
| qexp (%) | 44.2 ± 2.4 | 54.9 ± 3.2 | 65.1 ± 4.1 | 87.4 ± 1.3 | 49.8 ± 3.5 | 56.4 ± 4.6 | 68.4 ± 4.3 | 97.0 ± 2.6 | 52.2 ± 2.5 | 63.7 ± 3.5 | 77.3 ± 4.6 | 95.0 ± 3.7 |
| Parameters | 1 Month | 3 Month | 6 Month | |||||||||
| qe (mg·g−1) | 1.8 ± 0.1 | 2.3 ± 0.1 | 2.7 ± 0.1 | 3.3 ± 0.1 | 2.0 ± 0.0 | 2.4 ± 0.1 | 2.9 ± 0.1 | 4.2 ± 0.1 | 2.1 ± 0.1 | 2.8 ± 0.1 | 3.3 ± 0.1 | 4.0 ± 0.1 |
| k2 (×10−4 g·mg−1·min−1) | 0.2 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 |
| h (mg·g−1·min−1) | 0.7 ± 0.0 | 0.4 ± 0.0 | 0.6 ± 0.0 | 0.4 ± 0.0 | 1.0 ± 0.1 | 0.4 ± 0.0 | 0.7 ± 0.0 | 0.5 ± 0.0 | 1.0 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.0 | 0.5 ± 0.0 |
| r2 | 0.999 | 0.975 | 0.917 | 0.994 | 0.999 | 0.977 | 0.925 | 0.996 | 0.983 | 0.942 | 0.948 | 0.991 |
| χ2 | 0.019 | 0.115 | 0.072 | 0.013 | 0.015 | 0.098 | 0.087 | 0.012 | 0.01 | 0.071 | 0.479 | 0.115 |
| Metrenco | ||||||||||||
| Treatment | Control | AgNPs | CuNPs | FeNPs | Control | AgNPs | CuNPs | FeNPs | Control | AgNPs | CuNPs | FeNPs |
| qexp (mg·g−1) | 2.1 ± 0.2 | 2.3 ± 0.4 | 2.6 ± 0.4 | 3.6 ± 0.3 | 2.3 ± 0.4 | 2.5 ± 0.3 | 2.7 ± 0.2 | 4.1 ± 0.1 | 2.5 ± 0.3 | 3.0 ± 0.4 | 3.5 ± 0.3 | 4.1 ± 0.5 |
| qexp (%) | 57.3 ± 2.4 | 56.8 ± 1.9 | 65.3 ± 4.3 | 89.8 ± 7.5 | 58.4 ± 5.1 | 61.9 ± 3.9 | 67.9 ± 4.4 | 96.5 ± 4.9 | 62.5 ± 4.2 | 74.7 ± 4.9 | 88.3 ± 7.7 | 97.8 ± 2.1 |
| Parameters | 1 Month | 3 Month | 6 Month | |||||||||
| qe (mg·g−1) | 2.2 ± 0.1 | 2.5 ± 0.1 | 2.8 ± 0.1 | 3.7 ± 0.1 | 2.4 ± 0.1 | 2.5 ± 0.1 | 2.8 ± 0.3 | 4.2 ± 0.1 | 2.6 ± 0.1 | 3.1 ± 0.1 | 3.5 ± 0.2 | 4.3 ± 0.1 |
| k2 (×10−4 g·mg−1·min−1) | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.1 ± 0.0 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.1 ± 0.0 |
| h (mg·g−1·min−1) | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 1.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 1.4 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 1.5 ± 0.0 |
| r2 | 0.983 | 0.971 | 0.948 | 0.972 | 0.97 | 0.982 | 0.968 | 0.975 | 0.971 | 0.988 | 0.941 | 0.949 |
| χ2 | 0.095 | 0.048 | 0.045 | 0.012 | 0.021 | 0.052 | 0.035 | 0.017 | 0.023 | 0.083 | 0.054 | 0.029 |
| Treatment | Control | AgNPs | CuNPs | FeNPs | Control | AgNPs | CuNPs | FeNPs | Control | AgNPs | CuNPs | FeNPs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | 1 Month | 3 Month | 6 Month | |||||||||
| qe-2 (mg·g−1) | 1.6 ± 0.3 | 2.0 ± 0.2 | 2.4 ± 0.1 | 2.6 ± 0.4 | 1.8 ± 0.2 | 2.0 ± 0.3 | 2.6 ± 0.1 | 3.1 ± 0.2 | 1.9 ± 0.2 | 2.4 ± 0.1 | 3.1 ± 0.1 | 3.6 ± 0.1 |
| kint-2 (mg·g−1·min1/2) | 0.2 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.2 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.2 ± 0.0 | 0.6 ± 0.0 | 0.8 ± 0.0 | 0.9 ± 0.0 |
| C2 (mg·g−1) | 0.9 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 1.1 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 1.2 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 |
| r2 | 0.964 | 0.859 | 0.819 | 0.922 | 0.941 | 0.883 | 0.799 | 0.922 | 0.94 | 0.859 | 0.915 | 0.948 |
| Metrenco | ||||||||||||
| Parameters | 1 Month | 3 Month | 6 Month | |||||||||
| qe-2 (mg·g−1) | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.4 ± 0.0 | 3.4 ± 0.3 | 1.1 ± 0.0 | 1.2 ± 0.1 | 1.5 ± 0.0 | 3.7 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.0 | 1.7 ± 0.0 | 4.0 ± 0.0 |
| kint-2 (mg·g−1·min1/2) | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.6 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.6 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.8 ± 0.0 |
| C2 (mg·g−1) | 0.1 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.9 ± 0.1 | 0.2 ± 0.0 | 0.7 ± 0.0 | 0.4 ± 0.0 | 1.0 ± 0.1 | 0.2 ± 0.0 | 0.8 ± 0.0 | 0.4 ± 0.0 | 1.2 ± 0.0 |
| r2 | 0.905 | 0.89 | 0.994 | 0.922 | 0.81 | 0.953 | 0.994 | 0.922 | 0.811 | 0.993 | 0.975 | 0.996 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manquián-Cerda, K.; Calderón, R.; Molina-Roco, M.; Maldonado, T.; Arancibia-Miranda, N. Cd2+ Sorption Alterations in Ultisol Soils Triggered by Different Engineered Nanoparticles and Incubation Times. Nanomaterials 2023, 13, 3115. https://doi.org/10.3390/nano13243115
Manquián-Cerda K, Calderón R, Molina-Roco M, Maldonado T, Arancibia-Miranda N. Cd2+ Sorption Alterations in Ultisol Soils Triggered by Different Engineered Nanoparticles and Incubation Times. Nanomaterials. 2023; 13(24):3115. https://doi.org/10.3390/nano13243115
Chicago/Turabian StyleManquián-Cerda, Karen, Raúl Calderón, Mauricio Molina-Roco, Tamara Maldonado, and Nicolás Arancibia-Miranda. 2023. "Cd2+ Sorption Alterations in Ultisol Soils Triggered by Different Engineered Nanoparticles and Incubation Times" Nanomaterials 13, no. 24: 3115. https://doi.org/10.3390/nano13243115
APA StyleManquián-Cerda, K., Calderón, R., Molina-Roco, M., Maldonado, T., & Arancibia-Miranda, N. (2023). Cd2+ Sorption Alterations in Ultisol Soils Triggered by Different Engineered Nanoparticles and Incubation Times. Nanomaterials, 13(24), 3115. https://doi.org/10.3390/nano13243115






