Electrochemical Investigations of Double Perovskite M2NiMnO6 (Where M = Eu, Gd, Tb) for High-Performance Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of the Double Perovskites and Their Characterization
2.2. Fabrication of the Catalytic Electrodes and Electrochemical Measurements
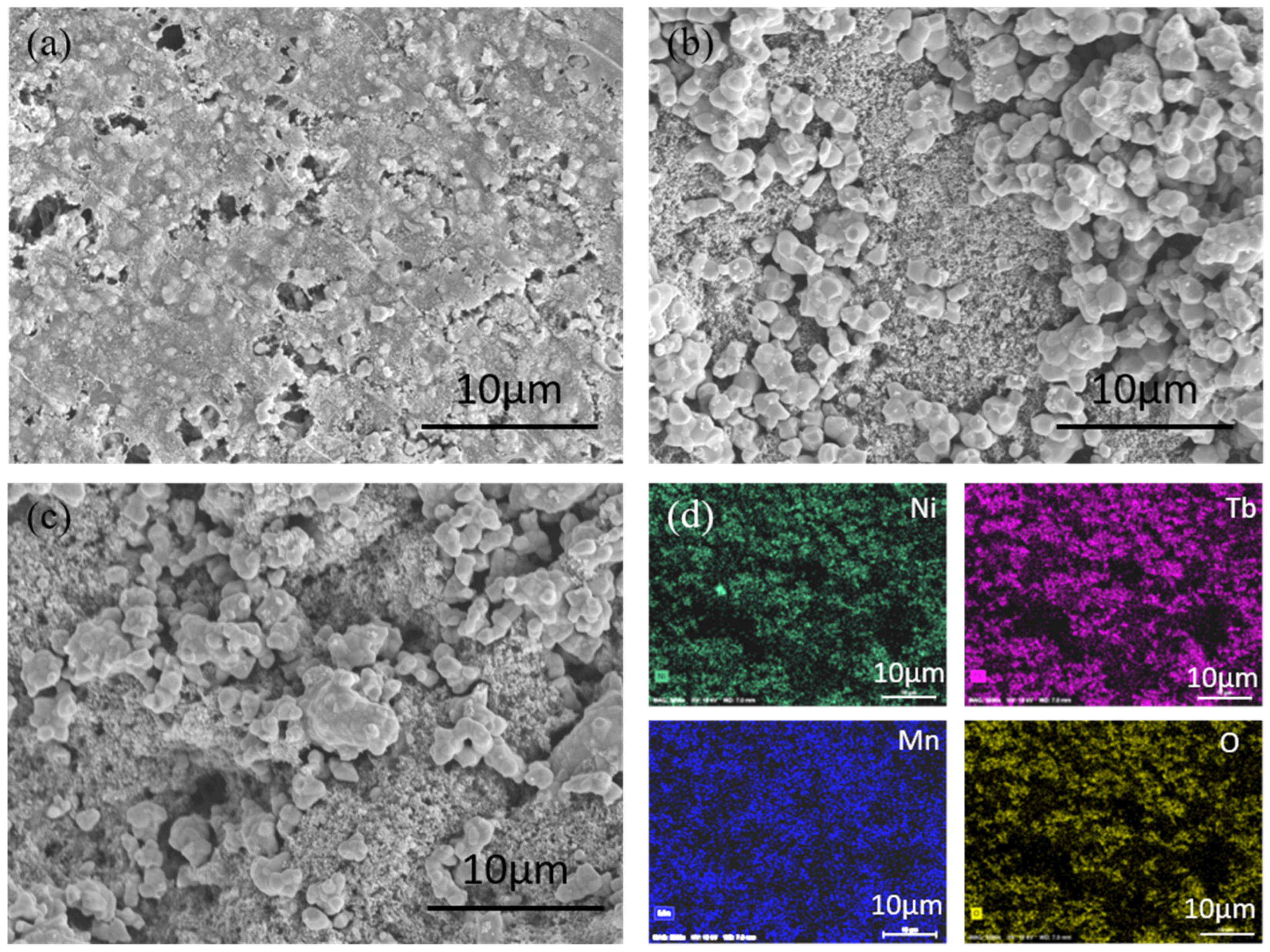
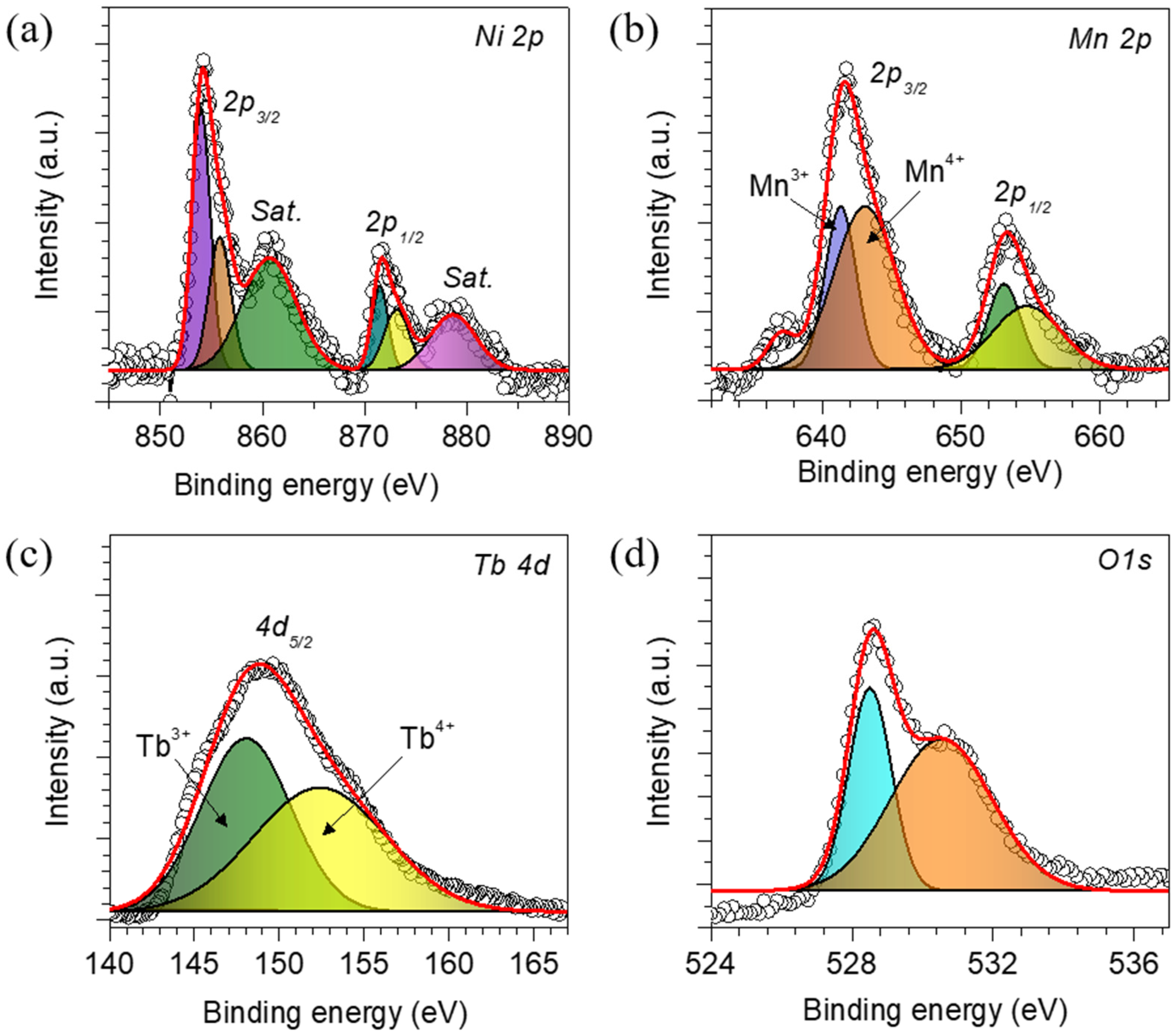
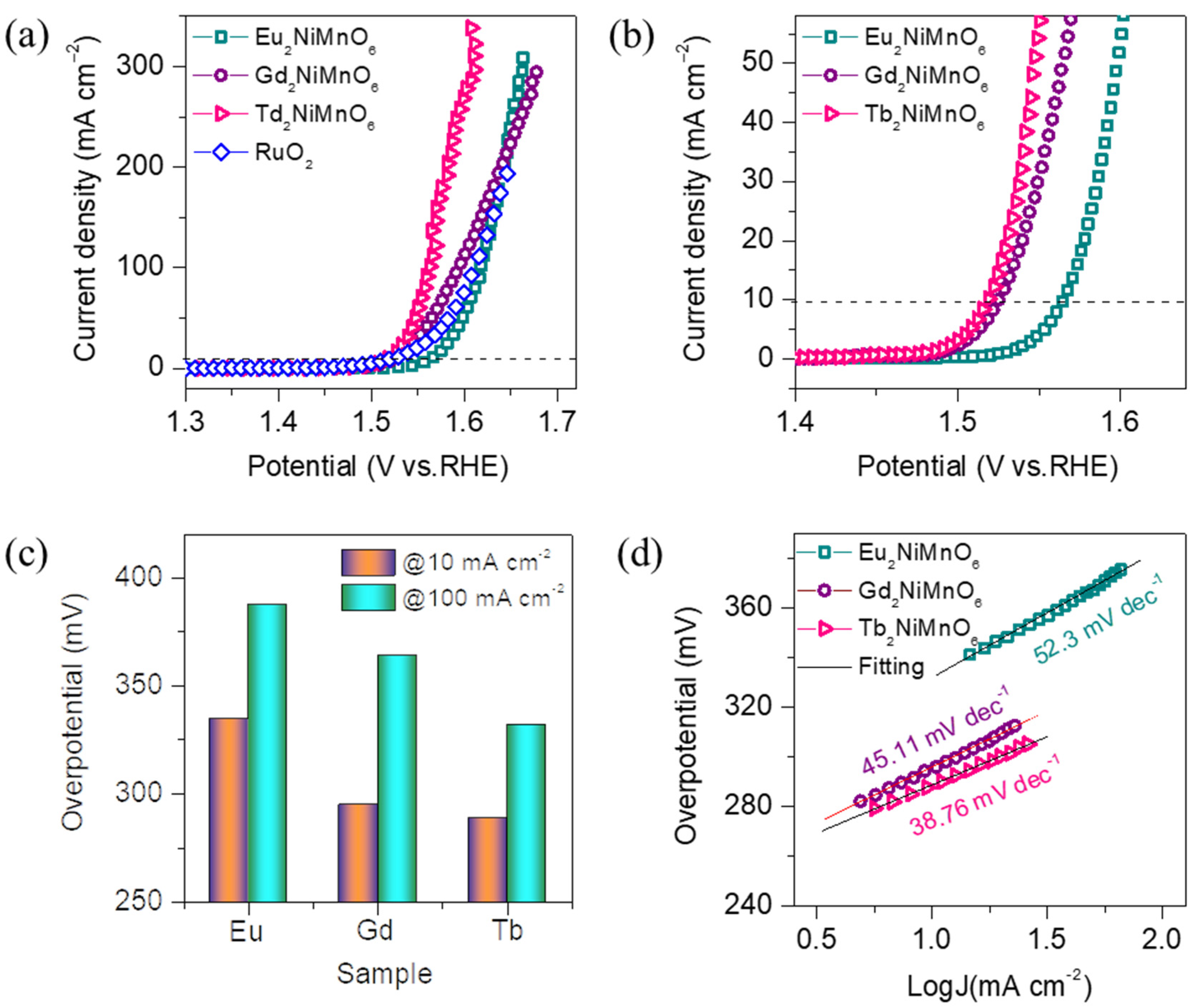
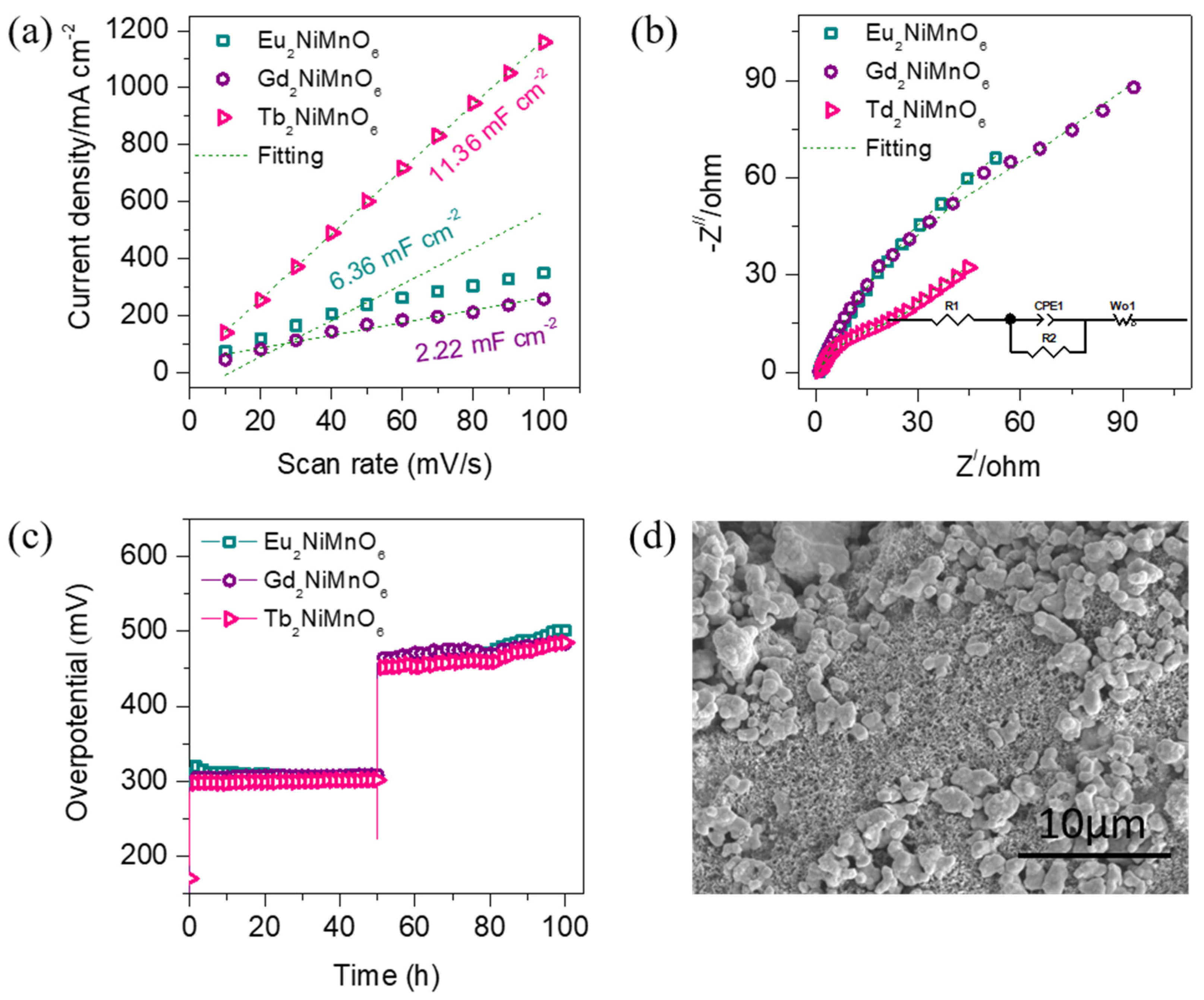
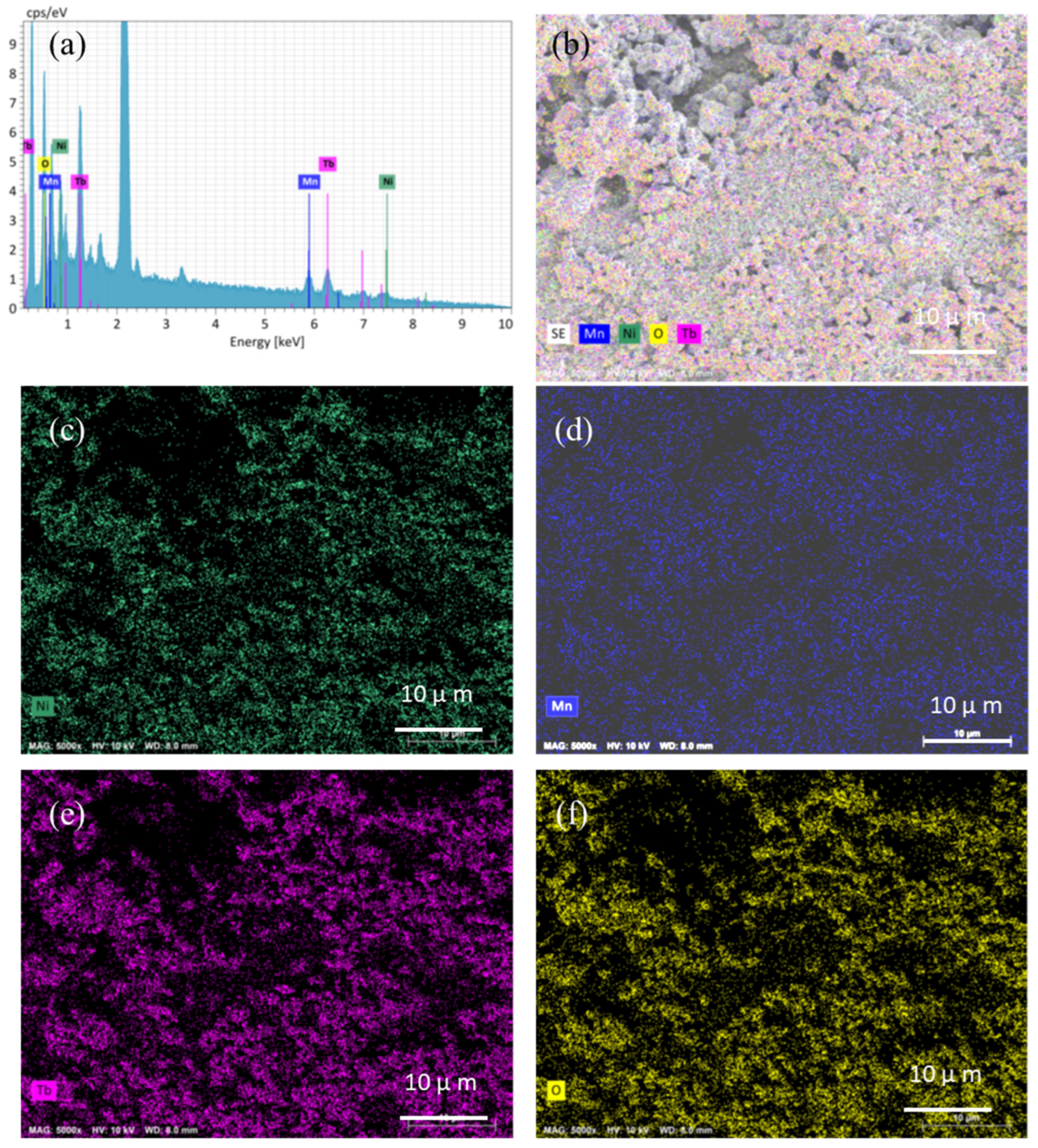
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, J.W.; Shi, Z.X.; Li, C.F.; Ren, Q.; Li, G.R. Regulation of Perovskite Surface Stability on the Electrocatalysis of Oxygen Evolution Reaction. ACS Mater. Lett. 2021, 3, 721–737. [Google Scholar] [CrossRef]
- Wang, P.; Luo, Y.; Zhang, G.; Chen, Z.; Ranganathan, H.; Sun, S.; Shi, Z. Interface Engineering of NixSy@MnOxHy Nanorods to Efficiently Enhance Overall-Water-Splitting Activity and Stability. Nano-Micro Lett. 2022, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Serra, J.M.; Borrás-Morell, J.F.; García-Baños, B.; Balaguer, M.; Plaza-González, P.; Santos-Blasco, J.; Catalán-Martínez, D.; Navarrete, L.; Catalá-Civera, J.M. Hydrogen production via microwave-induced water splitting at low temperature. Nat. Energy 2020, 5, 910–919. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Chavan, H.S.; Hou, B.; Lee, C.H.; Lee, S.U.; Cha, S.; Kim, H.; Im, H. A Robust Nonprecious CuFe Composite as a Highly Efficient Bifunctional Catalyst for Overall Electrochemical Water Splitting. Small 2020, 16, 1905884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Qi, Z.; Wu, Z.; Lui, Y.H.; Kim, T.H.; Tang, X.; Zhou, L.; Huang, W.; Hu, S. Defect-Rich 2D Material Networks for Advanced Oxygen Evolution Catalysts. ACS Energy Lett. 2019, 4, 328–336. [Google Scholar] [CrossRef]
- Roy, B.; Sebok, S.B.; Scott, E.M.; Fiordaliso, J.E.; Sørensen, A.; Bodin, D.B.; Trimarco, C.D.; Damsgaard, P.C.K.; Vesborg, O.; Hansen, I.E.L.; et al. Chorkendorff, Impact of nanoparticle size and lattice oxygen on water oxidation on NiFeOxHy. Nat. Catal. 2018, 1, 820–829. [Google Scholar] [CrossRef]
- Chavan, H.S.; Lee, C.; Inamdar, A.I.; Han, J.; Park, S.; Cho, S.; Shreshta, N.K.; Lee, S.; Hou, B.; Im, H.; et al. Designing and Tuning the Electronic Structure of Nickel–Vanadium Layered Double Hydroxides for Highly Efficient Oxygen Evolution Electrocatalysis. ACS Catal. 2022, 12, 3821–3831. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Chavan, H.S.; Seok, J.H.; Lee, C.H.; Shin, G.; Park, S.; Yeon, S.; Cho, S.; Park, Y.; Shrestha, N.K.; et al. Optimal rule-of-thumb design of NiFeMo layered double hydroxide nanoflakes for highly efficient and durable overall water-splitting at large currents. J. Mater. Chem. A 2022, 10, 20497–20508. [Google Scholar] [CrossRef]
- Qu, Y.; Yang, M.; Chai, J.; Tang, Z.; Shao, M.; Kwok, C.T.; Yang, M.; Wang, Z.; Chua, D.; Wang, S.; et al. Facile Synthesis of Vanadium-Doped Ni3S2 Nanowire Arrays as Active Electrocatalyst for Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 5959–5967. [Google Scholar] [CrossRef]
- Yu, X.Y.; Feng, Y.; Guan, B.Y.; Lou, X.W.; Paik, U. Carbon coated porous nickel phosphides nanoplates for highly efficient oxygen evolution reaction. Energy Environ. Sci. 2016, 9, 1246–1250. [Google Scholar] [CrossRef]
- Du, J.; Zou, Z.; Liu, C.; Xu, C. Hierarchical Fe-doped Ni3Se4 ultrathin nanosheets as an efficient electrocatalyst for oxygen evolution reaction. Nanoscale 2018, 10, 5163–5170. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dai, J.; Zhou, C.; Guan, D.; Wu, X.; Zhou, W.; Shao, Z. Engineering Charge Redistribution within Perovskite Oxides for Synergistically Enhanced Overall Water Splitting. ACS Mater. Lett. 2021, 3, 1258–1265. [Google Scholar] [CrossRef]
- Zu, M.Y.; Wang, C.; Zhang, L.; Zheng, L.R.; Yang, H.G. Reconstructing bimetallic carbide Mo6Ni6C for carbon interconnected MoNi alloys to boost oxygen evolution electrocatalysis. Mater. Horiz. 2019, 6, 115–121. [Google Scholar] [CrossRef]
- Ma, W.; Wang, M.; Tan, C.; Wang, J.; Dai, Y.; Hu, L.; Lv, X.; Li, Q.; Dang, J. Formulating a heterolytic cleavage process of water on Ni3N nanosheets through single transition metal doping for ultra-efficient alkaline hydrogen evolution. Inorg. Chem. Front. 2023, 10, 5152–5160. [Google Scholar] [CrossRef]
- Hao, J.; Zhuang, Z.; Cao, K.; Gao, G.; Wang, C.; Lai, F.; Lu, S.; Ma, P.; Dong, W.; Liu, T.; et al. Unraveling the electronegativity-dominated intermediate adsorption on high-entropy alloy electrocatalysts. Nat. Commun. 2022, 13, 2662. [Google Scholar] [CrossRef] [PubMed]
- Shinde, K.P.; Lee, E.J.; Manawan, M.; Lee, A.; Park, S.-Y.; Jo, Y.; Ku, K.; Kim, J.M.; Park, J.S. Structural, magnetic, and magnetocaloric properties of R2NiMnO6 (R = Eu, Gd, Tb). Sci. Rep. 2021, 11, 20206. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Xu, J.; Yu, P.; Hu, X.; Wu, Y.; Zhang, Q.; Li, Y.; Qiao, L.; Zeng, Y.; Tian, H. Double perovskite La2CoMnO6 hollow spheres prepared by template impregnation for high-performance supercapacitors. Chem. Eng. J. 2020, 400, 125966. [Google Scholar] [CrossRef]
- Kim, M.K.; Moon, J.Y.; Choi, H.Y.; Oh, S.H.; Lee, N.; Choi, Y.J. Effects of different annealing atmospheres on magnetic properties in La2CoMnO6 single crystals. Curr. Appl. Phys. 2015, 15, 776–779. [Google Scholar] [CrossRef]
- Yin, W.J.; Weng, B.C.; Ge, J.; Sun, Q.D.; Li, Z.Z.; Yan, Y.F. Oxide perovskites, double perovskites and derivatives for electrocatalysis, photocatalysis, and photovoltaics. Energy Environ. Sci. 2019, 12, 442–462. [Google Scholar] [CrossRef]
- Alam, M.; Karmakar, K.; Pal, M.; Mandal, K. Electrochemical supercapacitor based on double perovskite Y2NiMnO6 nanowires. RSC Adv. 2016, 6, 114722–114726. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, A. Facile wet chemical synthesis and electrochemical behavior of La2FeCoO6 nano-crystallites. Mater. Sci. Semicond. Process. 2019, 99, 8–13. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Kumar, A. Energy storage properties of double perovskites Gd2NiMnO6 for electrochemical supercapacitor application. Solid State Sci. 2020, 105, 106252. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Li, Y.; Qian, Q.; Li, Z.; Zhu, Y.; Zhang, G. Manipulating dehydrogenation kinetics through dual-doping Co3N electrode enables highly efficient hydrazine oxidation assisting self-powered H2 production. Nat. Commun. 2020, 11, 1853. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Sun, Y.; Dong, P.; Smith, W.; Sun, Z.; Ge, Y.; Pei, Y.; Cao, Z.; Ajayan, P.M.; Shen, J.; et al. Revisiting the Role of Active Sites for Hydrogen Evolution Reaction through Precise Defect Adjusting. Adv. Funct. Mater. 2019, 29, 1901290. [Google Scholar] [CrossRef]
- Sheikh, S.; Ghosh, D.; Dutta, A.; Bhattacharyya, S.; Sinha, T.P. Lead free double perovskite oxides Ln2NiMnO6(Ln=La, Eu, Dy, Lu), a new promising material for photovoltaic application. Mater. Sci. Eng. B 2017, 226, 10–17. [Google Scholar] [CrossRef]
- Klimkowicz, A.; Świerczek, K.; Zheng, K.; Baranowska, M.; Takasaki, A.; Dabrowski, B. Evaluation of BaY1−xPrxMn2O5+δ oxides for oxygen storage technology. Solid State Ion. 2014, 262, 659–663. [Google Scholar] [CrossRef]
- Zankowski, S.P.; Hoecke, L.V.; Mattelaer, F.; De Raedt, M.; Richard, O.; Detavernier, C.; Vereecken, P.M. Redox layer deposition of thin films of MnO2 on nanostructured substrates from aqueous solutions. Chem. Mater. 2019, 31, 4805–4816. [Google Scholar] [CrossRef]
- Masud, M.G.; Sakata, H.; Biswal, A.K.; Vishwakarma, P.N.; Chaudhuri, B.K. Structural, ac conductivity scaling and magnetodielectric behaviour of a partially disordered insulating ferromagnetic double perovskite Eu2NiMnO6. J. Phys. D Appl. Phys. 2015, 48, 375504. [Google Scholar] [CrossRef]
- Wang, T.; Wu, H.-Y.; Xing, R.; Sun, Y.-B.; Xv, B.; Zhao, J.-J. Physical Properties of Ca-Doped Double Perovskite La2NiMnO6. J. Low Temp. Phys. 2019, 196, 423–441. [Google Scholar]
- Zhang, W.; Shen, H.; Yin, M.; Lu, L.; Xu, B.; Li, D. Heterostructure Silicon Solar Cells with Enhanced Power Conversion Efficiency Based on Six/Ni3+ Self-Doped NiOx Passivating Contact. ACS Omega 2022, 7, 16494–16501. [Google Scholar] [CrossRef]
- Wang, M.; Chen, K.; Liu, J.; He, Q.; Li, G.; Li, F. Efficiently Enhancing Electrocatalytic Activity of α-MnO2 Nanorods/N-Doped Ketjenblack Carbon for Oxygen Reduction Reaction and Oxygen Evolution Reaction Using Facile Regulated Hydrothermal Treatment. Catalysts 2018, 8, 138. [Google Scholar] [CrossRef]
- Gupta, P.; Mahapatra, P.K.; Choudhary, R.N.P. TbFeO3Ceramic: An Exciting Colossal Dielectric with Ferroelectric Properties. Phys. Status Solidi B 2020, 257, 1900236. [Google Scholar] [CrossRef]
- Yi, K.; Tang, Q.; Wu, Z.; Zhu, X. Unraveling the Structural, Dielectric, Magnetic, and Optical Characteristics of Nanostructured La2NiMnO6 Double Perovskites. Nanomaterials 2022, 12, 979. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Morales, O.; Raaijman, S.; Kortlever, R.; Kooyman, P.J.; Wezendonk, T.; Gascon, J.; Fu, W.T.; Koper, M.T.M. Iridium-based double perovskites for efficient water oxidation in acid media. Nat. Commun. 2016, 7, 12363. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Awasthi, M.K.; Maji, P.; Pal, M.; Aziz, S.T.; Lahiri, G.K.; Dutta, A. Double Perovskite Oxides Bringing a Revelation in Oxygen Evolution Reaction Electrocatalyst Design. ChemElectroChem 2023, 10, 20220109. [Google Scholar] [CrossRef]
- Miao, X.; Wu, L.; Lin, Y.; Yuan, X.; Zhao, J.; Yan, W.; Zhou, S.; Shi, L. The role of oxygen vacancies in water oxidation for perovskite cobalt oxide electrocatalysts: Are more better? Chem. Commun. 2019, 55, 1442–1445. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Ding, X.; Shen, Z.; Cui, M.; Oropeza, F.E.; Gorni, G.; de la Peña O’Shea, V.A.; Li, W.; Qi, D.-C.; Zhang, K.H.L. Tailoring the Electronic Structures of the La2NiMnO6 Double Perovskite as Efficient Bifunctional Oxygen Electrocatalysis. Chem. Mater. 2021, 33, 2062–2071. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Y.; Chen, D.; Liu, J.; Zhang, Z.; Shao, Z.; Ciucci, F. Water Splitting with an Enhanced Bifunctional Double Perovskite. ACS Catal. 2018, 8, 364–371. [Google Scholar] [CrossRef]
- Zhu, J.; Guđmundsdóttir, J.B.; Strandbakke, R.; Both, K.G.; Aarholt, T.; Carvalho, P.A.; Sørby, M.H.; Jensen, I.J.T.; Guzik, M.N.; Norby, T.; et al. Double Perovskite Cobaltites Integrated in a Monolithic and Noble Metal-Free Photoelectrochemical Device for Efficient Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 20313–20325. [Google Scholar] [CrossRef]
- Sun, H.; Chen, G.; Sunarso, J.; Dai, J.; Zhou, W.; Shao, Z. Molybdenum and Niobium Codoped B-Site-Ordered Double Perovskite Catalyst for Efficient Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2018, 10, 16939–16942. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, X.; Hu, X.; Zhao, Q.; Li, L.; Yu, P.; Jing, C.; Zhang, Y.; Huang, G.; Jiang, B.; et al. Electrostatic adsorbing graphene quantum dot into nickel–based layered double hydroxides: Electron absorption/donor effects enhanced oxygen electrocatalytic activity. Nano Energy 2021, 84, 105932. [Google Scholar] [CrossRef]
- Higareda, A.; Hernández-Arellano, D.L.; Ordoñez, L.C.; Barbosa, R.; Alonso-Vante, N. Advanced Electrocatalysts for the Oxygen Evolution Reaction: From Single- to Multielement Materials. Catalysts 2023, 13, 1346. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Chavan, H.S.; Pawar, S.M.; Kim, H.; Im, H. NiFeCo oxide as an efficient and sustainable catalyst for the oxygen evolution reaction. Int. J. Energy Res. 2020, 44, 1789–1797. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinde, K.P.; Chavan, H.S.; Salunke, A.S.; Oh, J.; Aqueel Ahmed, A.T.; Shrestha, N.K.; Im, H.; Park, J.; Inamdar, A.I. Electrochemical Investigations of Double Perovskite M2NiMnO6 (Where M = Eu, Gd, Tb) for High-Performance Oxygen Evolution Reaction. Nanomaterials 2023, 13, 3076. https://doi.org/10.3390/nano13233076
Shinde KP, Chavan HS, Salunke AS, Oh J, Aqueel Ahmed AT, Shrestha NK, Im H, Park J, Inamdar AI. Electrochemical Investigations of Double Perovskite M2NiMnO6 (Where M = Eu, Gd, Tb) for High-Performance Oxygen Evolution Reaction. Nanomaterials. 2023; 13(23):3076. https://doi.org/10.3390/nano13233076
Chicago/Turabian StyleShinde, Kiran P., Harish S. Chavan, Amol S. Salunke, Jeongseok Oh, Abu Talha Aqueel Ahmed, Nabeen K. Shrestha, Hyunsik Im, Joonsik Park, and Akbar I. Inamdar. 2023. "Electrochemical Investigations of Double Perovskite M2NiMnO6 (Where M = Eu, Gd, Tb) for High-Performance Oxygen Evolution Reaction" Nanomaterials 13, no. 23: 3076. https://doi.org/10.3390/nano13233076
APA StyleShinde, K. P., Chavan, H. S., Salunke, A. S., Oh, J., Aqueel Ahmed, A. T., Shrestha, N. K., Im, H., Park, J., & Inamdar, A. I. (2023). Electrochemical Investigations of Double Perovskite M2NiMnO6 (Where M = Eu, Gd, Tb) for High-Performance Oxygen Evolution Reaction. Nanomaterials, 13(23), 3076. https://doi.org/10.3390/nano13233076






