Evaluating Gelatin-Based Films with Graphene Nanoparticles for Wound Healing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Graphene Nanoparticle Characterization
2.3. In Vitro Biocompatibility
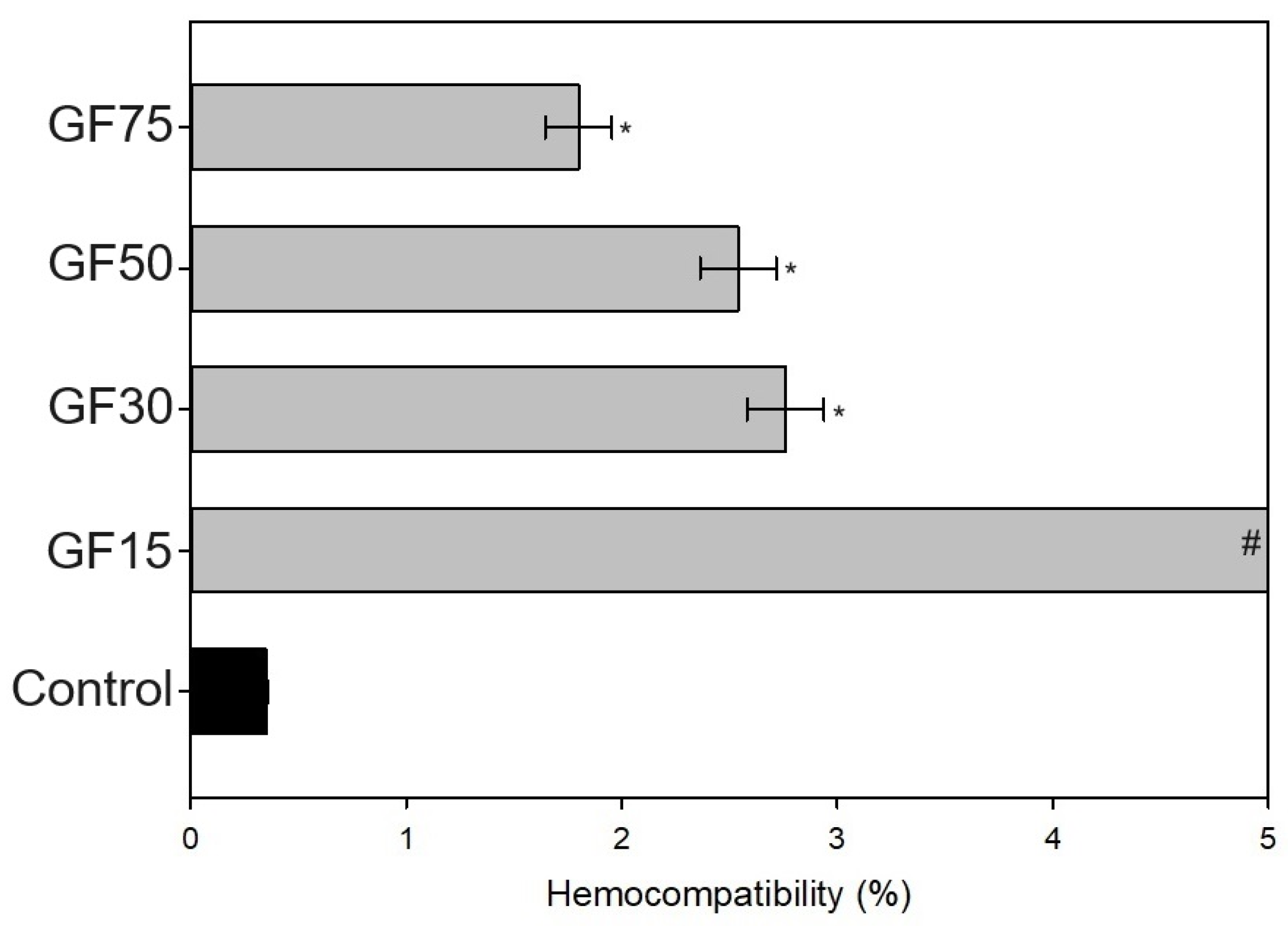
2.3.1. Hemocompatibility
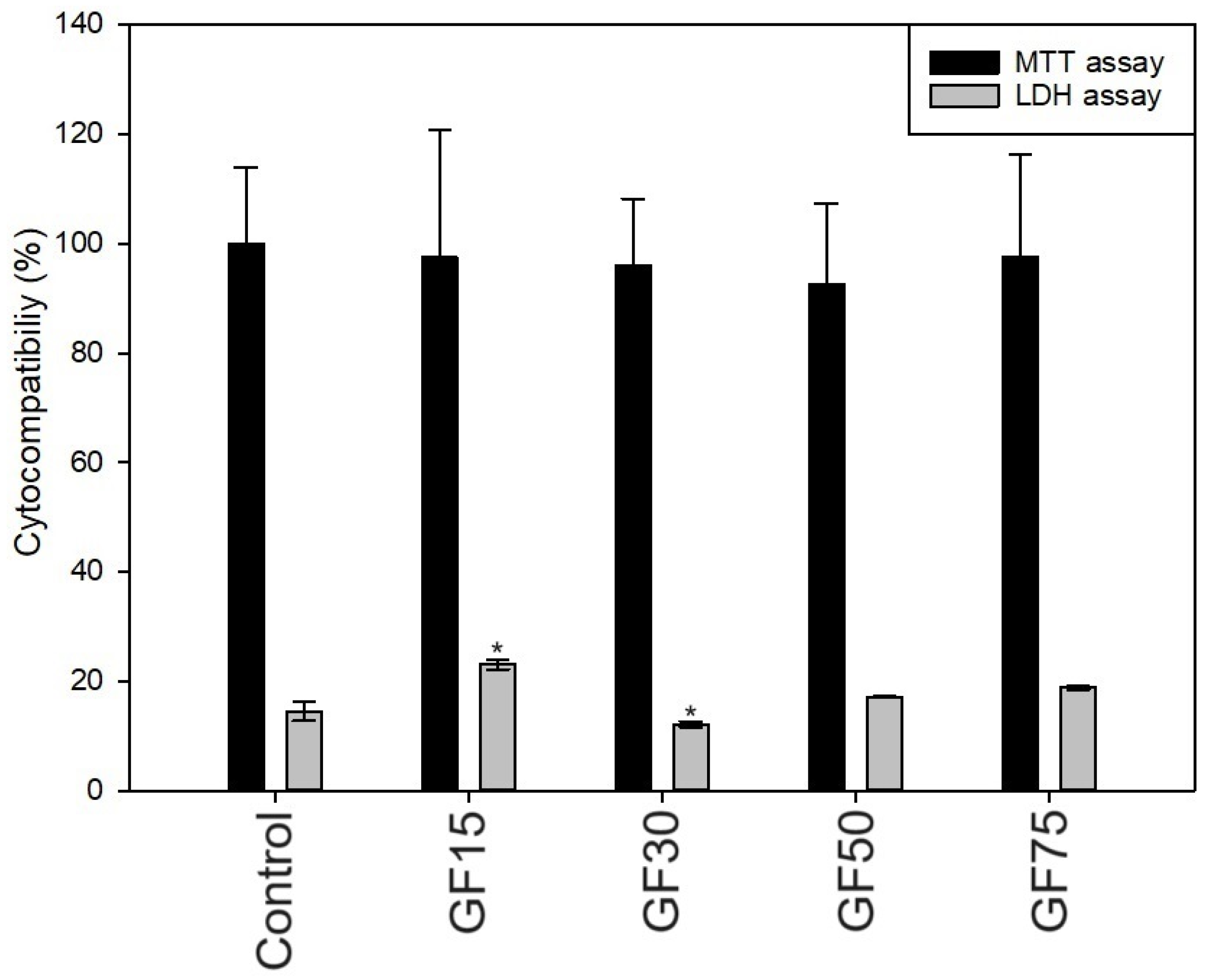
2.3.2. Cytocompatibility
2.4. Film Preparation and Characterization
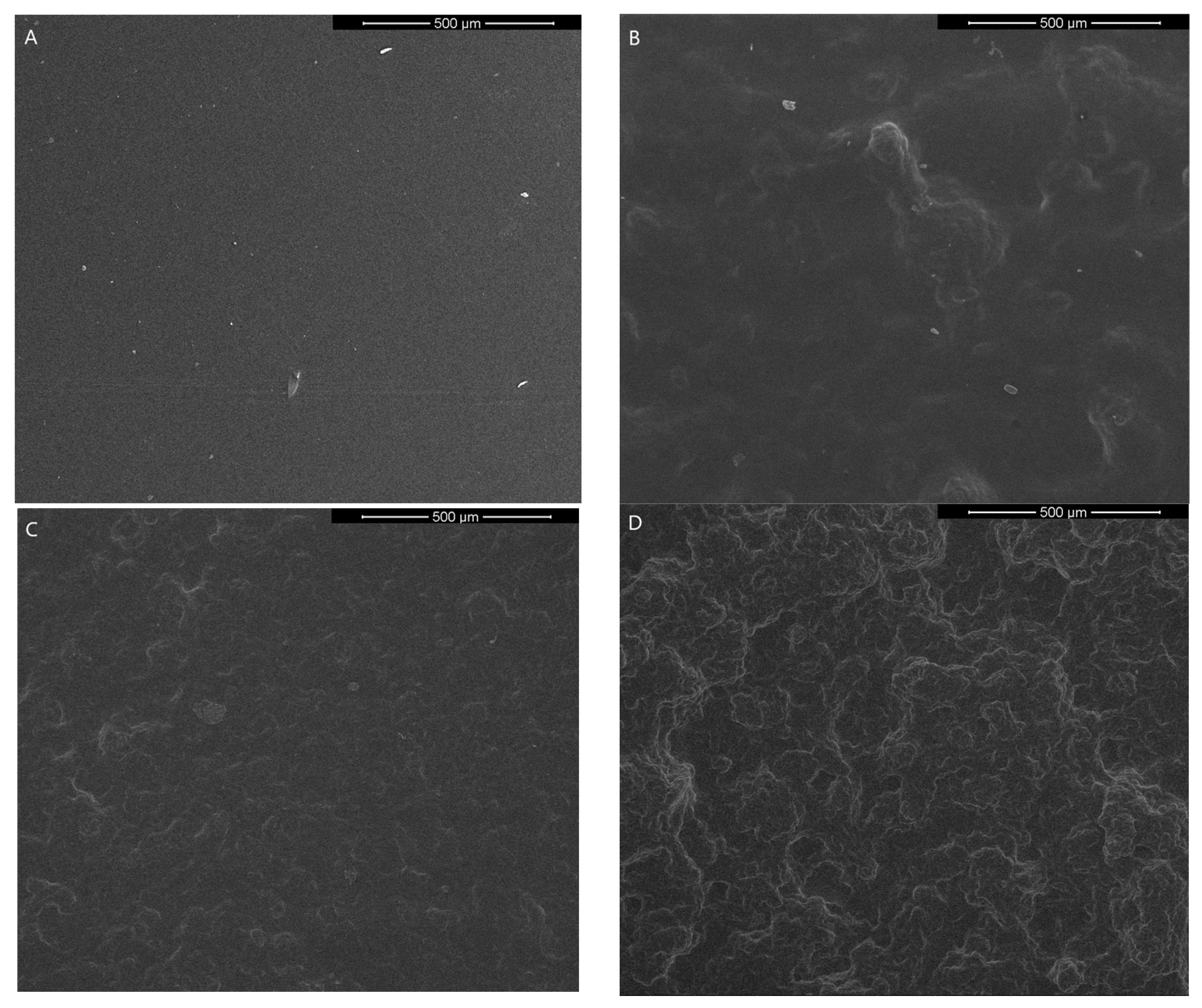
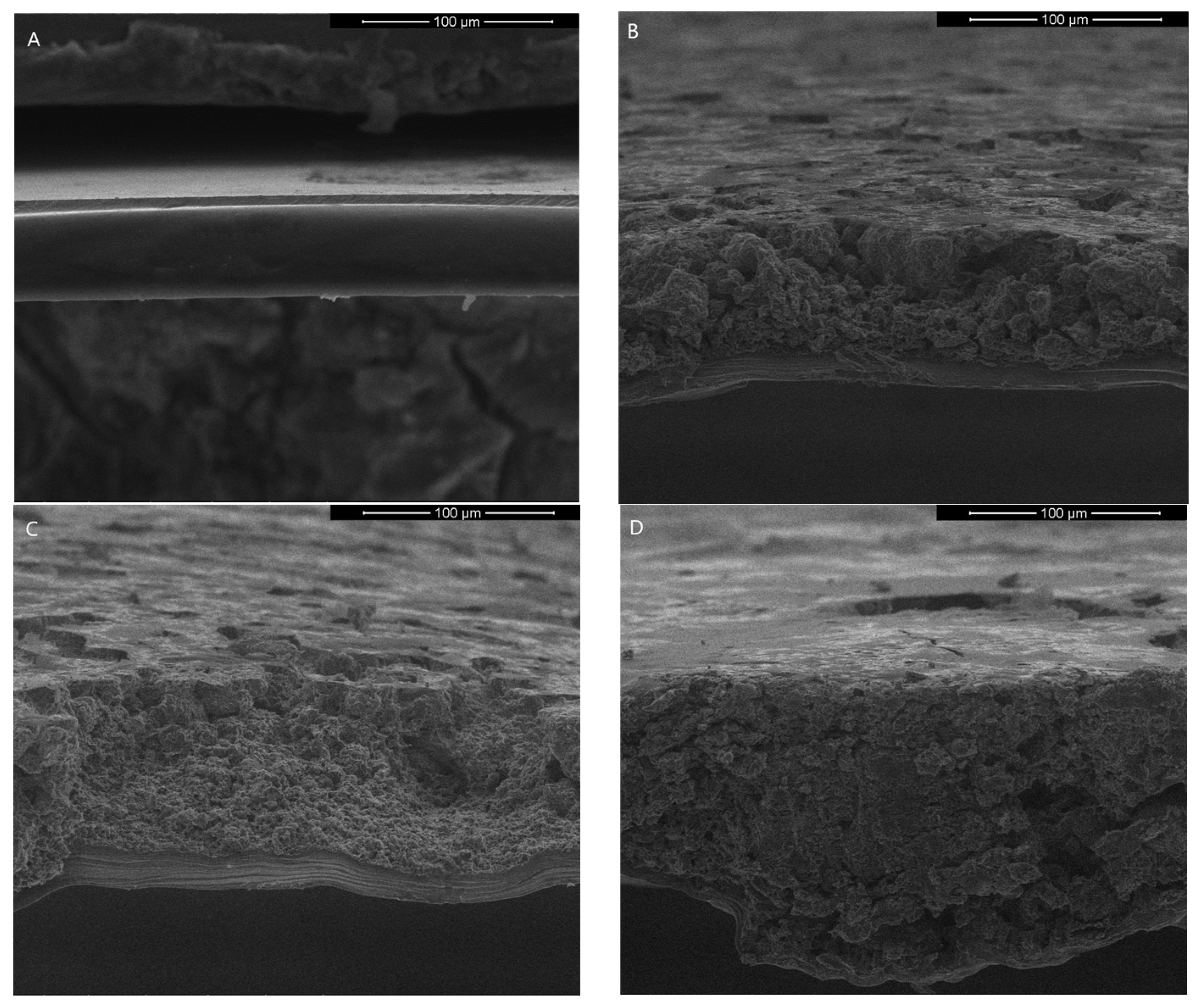
2.4.1. Scanning Electron Microscope (SEM)
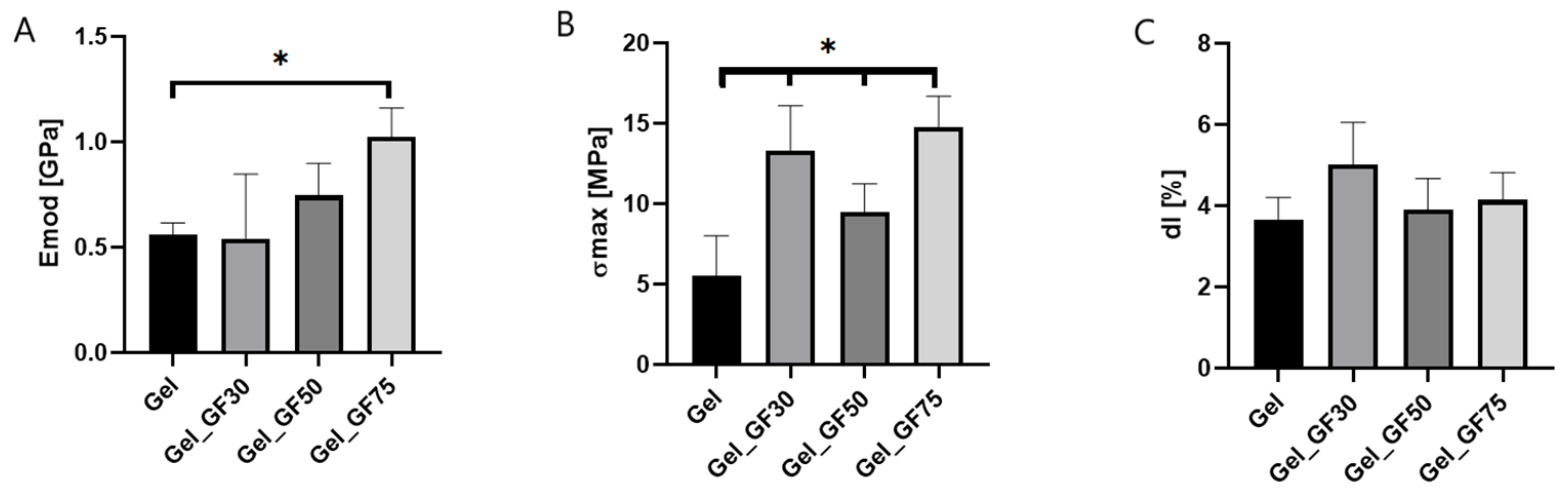
2.4.2. Mechanical Properties
2.4.3. Antioxidant Activity
2.4.4. Roughness of Surface
2.4.5. Surface Free Energy
2.4.6. Water Vapor Permeation Rate (WVPR)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Graphene Nanoparticle Characterization
3.2. In Vitro Biocompatibility
3.2.1. Hemocompatibility
3.2.2. Cytocompatibility
3.3. Film Characterization
3.3.1. Scanning Electron Microscope (SEM)
3.3.2. Mechanical Properties
3.3.3. Antioxidant Activity
3.3.4. Roughness of Surface
3.3.5. Surface Free Energy
3.3.6. Water Vapor Permeation Rate (WVPR)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug Delivery Systems and Materials for Wound Healing Applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable Polymers and Green-Based Antimicrobial Packaging Materials: A Mini-Review. Adv. Ind. Eng. Polym. Res. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current Development of Biodegradable Polymeric Materials for Biomedical Applications. Drug. Des. Devel. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed]
- Shariati, A.; Hosseini, S.M.; Chegini, Z.; Seifalian, A.; Arabestani, M.R. Graphene-Based Materials for Inhibition of Wound Infection and Accelerating Wound Healing. Biomed. Pharmacother. 2023, 158, 114184. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Guan, J. Nanoparticle-Based Drug Delivery Systems for Cancer Therapy. Smart. Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef]
- Kassaee, M.Z.; Motamedi, E.; Majdi, M. Magnetic Fe3O4-Graphene Oxide/Polystyrene: Fabrication and Characterization of a Promising Nanocomposite. Chem. Eng. J. 2011, 172, 540–549. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepańska, B.; Michalska-Sionkowska, M.; Binkowski, P.; Lukaszewicz, J.P.; Kamedulski, P. 3D-Structured and Blood-Contact-Safe Graphene Materials. Int. J. Mol. Sci. 2023, 24, 3576. [Google Scholar] [CrossRef]
- Chen, C.; Xi, J.; Zhou, E.; Peng, L.; Chen, Z.; Gao, C. Porous Graphene Microflowers for High-Performance Microwave Absorption. Nanomicro. Lett. 2018, 10, 26. [Google Scholar] [CrossRef]
- Vo, T.T.N.; Lim, S.T.; Kim, J.H.; Shim, G.H.; Kim, K.M.; Kweon, B.; Kim, M.; Lee, C.Y.; Ahn, H.S. Nanostructured Micro/Mesoporous Graphene: Removal Performance of Volatile Organic Compounds. RSC Adv. 2022, 12, 14570–14577. [Google Scholar] [CrossRef]
- Andreazza, R.; Morales, A.; Pieniz, S.; Labidi, J. Gelatin-Based Hydrogels: Potential Biomaterials for Remediation. Polymers 2023, 15, 1026. [Google Scholar] [CrossRef]
- Ahmady, A.; Abu Samah, N.H. A Review: Gelatine as a Bioadhesive Material for Medical and Pharmaceutical Applications. Int. J. Pharm. 2021, 608, 121037. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Li, Y.-C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-Based Materials for Tissue Engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E.; Akhavan, A. Size-Dependent Genotoxicity of Graphene Nanoplatelets in Human Stem Cells. Biomaterials 2012, 33, 8017–8025. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, W.; Sun, L.; Aifantis, K.E.; Yu, B.; Fan, Y.; Feng, Q.; Cui, F.; Watari, F. Effects of Physicochemical Properties of Nanomaterials on Their Toxicity. J. Biomed. Mater. Res. A 2015, 103, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Shvedova, A.; Castranova, V.; Kisin, E.; Schwegler-Berry, D.; Murray, A.; Gandelsman, V.; Maynard, A.; Baron, P. Exposure to Carbon Nanotube Material: Assessment of Nanotube Cytotoxicity Using Human Keratinocyte Cells. J. Toxicol. Environ. Health A 2003, 66, 1909–1926. [Google Scholar] [CrossRef] [PubMed]
- Govindaraj, P.; Mirabedini, A.; Jin, X.; Antiohos, D.; Salim, N.; Aitchison, P.; Parker, J.; Fuss, F.K.; Hameed, N. Health and Safety Perspectives of Graphene in Wearables and Hybrid Materials. J. Mater. Sci. Technol. 2023, 155, 10–32. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol 2018, 6, 99. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization (ISO): Geneve, Switzerland, 2009.
- Kaczmarek-Szczepańska, B.; Zasada, L.; Grabska-Zielińska, S. The Physicochemical, Antioxidant, and Color Properties of Thin Films Based on Chitosan Modified by Different Phenolic Acids. Coatings 2022, 12, 126. [Google Scholar] [CrossRef]
- Kamedulski, P.; Skorupska, M.; Binkowski, P.; Arendarska, W.; Ilnicka, A.; Lukaszewicz, J.P. High Surface Area Micro-Mesoporous Graphene for Electrochemical Applications. Sci. Rep. 2021, 11, 22054. [Google Scholar] [CrossRef]
- Kamedulski, P.; Truszkowski, S.; Lukaszewicz, J.P. Highly Effective Methods of Obtaining N-Doped Graphene by Gamma Irradiation. Materials 2020, 13, 4975. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Le, H.D.; Nguyen, V.C.; Tam Ngo, T.T.; Le, D.Q.; Nguyen, X.N.; Phan, N.M. Synthesis of Multi-Layer Graphene Films on Copper Tape by Atmospheric Pressure Chemical Vapor Deposition Method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 035012. [Google Scholar] [CrossRef]
- Fedel, M. Hemocompatibility of Carbon Nanostructures. C—J. Carbon Res. 2020, 6, 12. [Google Scholar] [CrossRef]
- Kenry Understanding the Hemotoxicity of Graphene Nanomaterials through Their Interactions with Blood Proteins and Cells. J. Mater. Res. 2018, 33, 44–57. [CrossRef]
- Sasidharan, A.; Panchakarla, L.S.; Sadanandan, A.R.; Ashokan, A.; Chandran, P.; Girish, C.M.; Menon, D.; Nair, S.V.; Rao, C.N.R.; Koyakutty, M. Hemocompatibility and Macrophage Response of Pristine and Functionalized Graphene. Small 2012, 8, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Aggarwal, P.; Hall, J.B.; McNeil, S.E. Preclinical Studies To Understand Nanoparticle Interaction with the Immune System and Its Potential Effects on Nanoparticle Biodistribution. Mol. Pharm. 2008, 5, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Carey, T.; Alhourani, A.; Tian, R.; Seyedin, S.; Arbab, A.; Maughan, J.; Šiller, L.; Horvath, D.; Kelly, A.; Kaur, H.; et al. Cyclic Production of Biocompatible Few-Layer Graphene Ink with in-Line Shear-Mixing for Inkjet-Printed Electrodes and Li-Ion Energy Storage. NPJ 2D Mater. Appl. 2022, 6, 3. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, S.-T.; Liu, J.-H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In Vitro Toxicity Evaluation of Graphene Oxide on A549 Cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef]
- Guo, C.; Lu, R.; Wang, X.; Chen, S. Antibacterial Activity, Bio-Compatibility and Osteogenic Differentiation of Graphene Oxide Coating on 3D-Network Poly-Ether-Ether-Ketone for Orthopaedic Implants. J. Mater. Sci. Mater. Med. 2021, 32, 135. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2010, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Ricci, R.; Leite, N.C.S.; da-Silva, N.S.; Pacheco-Soares, C.; Canevari, R.A.; Marciano, F.R.; Webster, T.J.; Lobo, A.O. Graphene Oxide Nanoribbons as Nanomaterial for Bone Regeneration: Effects on Cytotoxicity, Gene Expression and Bactericidal Effect. Mater. Sci. Eng. C 2017, 78, 341–348. [Google Scholar] [CrossRef]
- Mayer, A.; Vadon, M.; Rinner, B.; Novak, A.; Wintersteiger, R.; Fröhlich, E. The Role of Nanoparticle Size in Hemocompatibility. Toxicology 2009, 258, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Napierska, D.; Thomassen, L.C.J.; Rabolli, V.; Lison, D.; Gonzalez, L.; Kirsch-Volders, M.; Martens, J.A.; Hoet, P.H. Size-Dependent Cytotoxicity of Monodisperse Silica Nanoparticles in Human Endothelial Cells. Small 2009, 5, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.-H.; Lin, Y.-S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of Graphene Oxide and Graphene in Human Erythrocytes and Skin Fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef]
- Baali, N.; Khecha, A.; Bensouici, A.; Speranza, G.; Hamdouni, N. Assessment of Antioxidant Activity of Pure Graphene Oxide (GO) and ZnO-Decorated Reduced Graphene Oxide (RGO) Using DPPH Radical and H2O2 Scavenging Assays. C—J. Carbon Res. 2019, 5, 75. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Hahnel, S.; Cazzaniga, G.; Ottobelli, M.; Braga, R.R.; Rodrigues, M.C.; Brambilla, E. Streptococcus Mutans Adherence and Biofilm Formation on Experimental Composites Containing Dicalcium Phosphate Dihydrate Nanoparticles. J. Mater. Sci. Mater. Med. 2017, 28, 108. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Abidi, N.; Cabrales, L. Wettability and Surface Free Energy of Graphene Films. Langmuir 2009, 25, 11078–11081. [Google Scholar] [CrossRef]
- Avena-Bustillos, R.J.; Olsen, C.W.; Olson, D.A.; Chiou, B.; Yee, E.; Bechtel, P.J.; McHugh, T.H. Water Vapor Permeability of Mammalian and Fish Gelatin Films. J. Food Sci. 2006, 71, E202–E207. [Google Scholar] [CrossRef]
- Takenaka, R.; Moriyama, N.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Permeation Properties of Water Vapor through Graphene Oxide/Polymer Substrate Composite Membranes. Membranes 2023, 13, 533. [Google Scholar] [CrossRef]

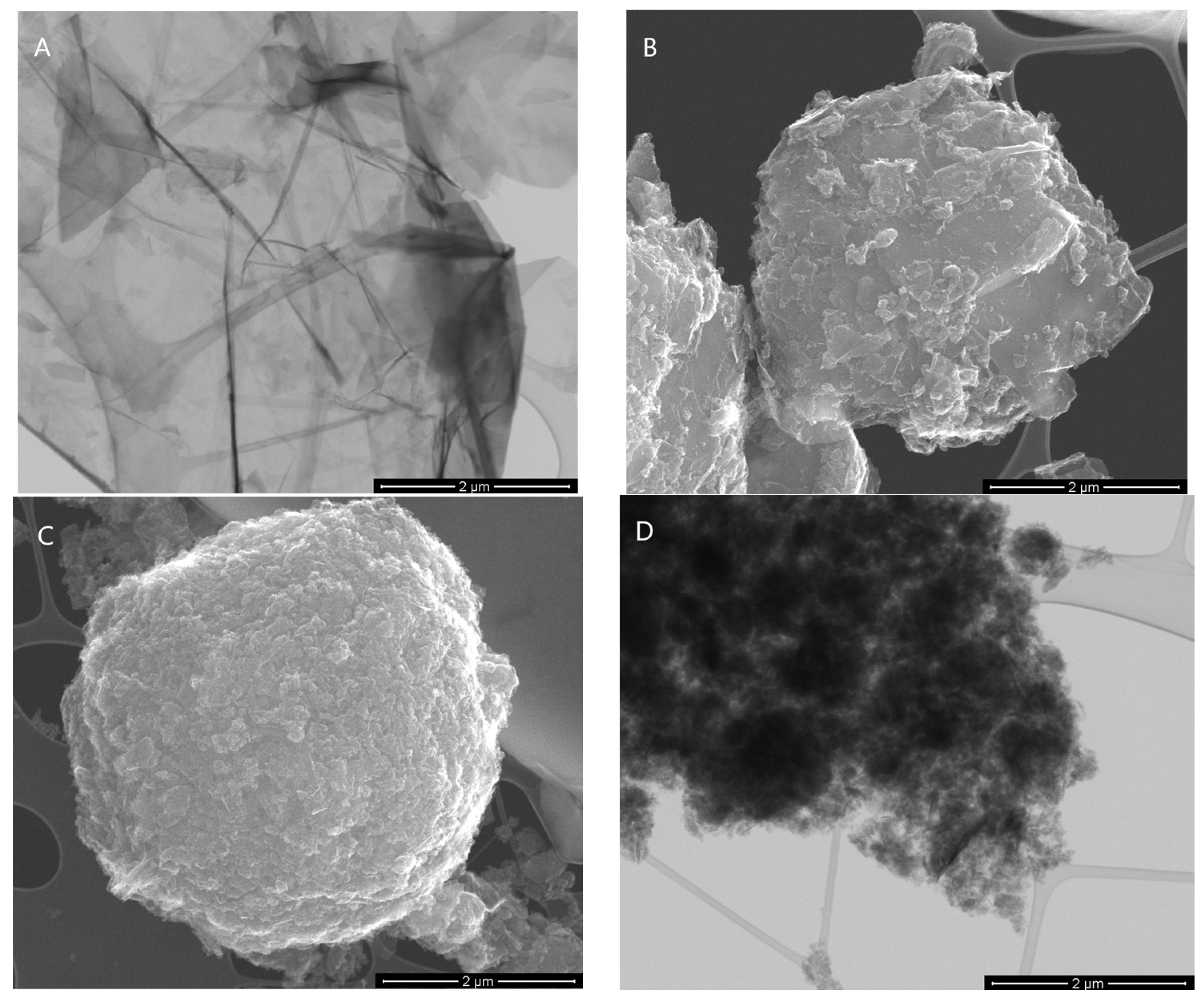

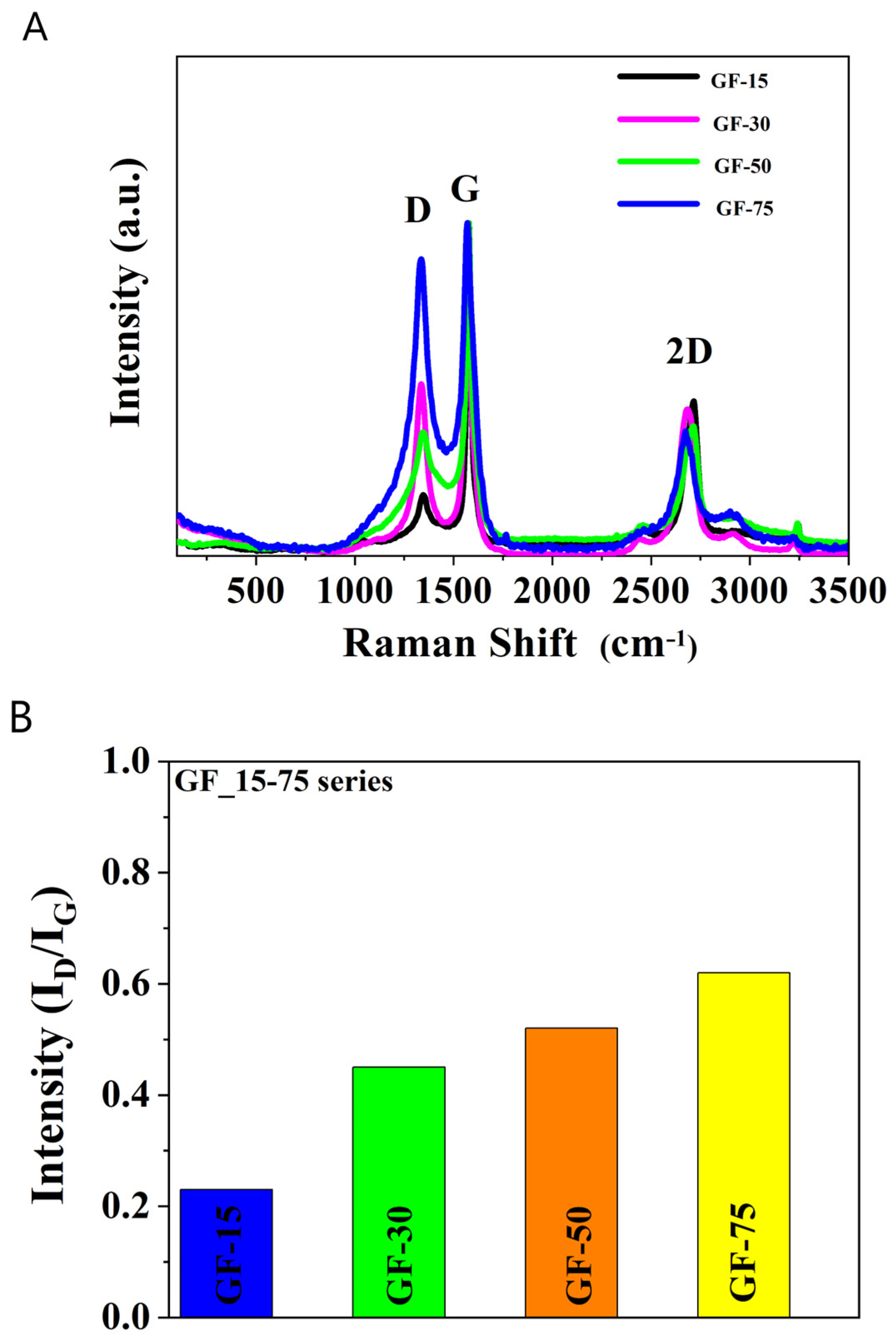


| Sample | Elemental Content (wt%) | SBET | Vt | Vmi | Vme | Vme/Vt | |||
|---|---|---|---|---|---|---|---|---|---|
| N | C | H | Rest | (m2/g) | (cm3/g) | (cm3/g) | (cm3/g) | (%) | |
| GF-15 | 0.6 | 90.8 | 0.9 | 7.7 | 145 | 0.256 | 0.178 | 0.078 | 44 |
| GF-30 | 0.3 | 98.0 | 0.5 | 1.2 | 326 | 0.416 | 0.156 | 0.260 | 63 |
| GF-50 | 0.5 | 91.7 | 0.6 | 7.2 | 431 | 0.678 | 0.139 | 0.539 | 79 |
| GF-75 | 0.7 | 89.3 | 0.9 | 9.1 | 750 | 0.999 | 0.127 | 0.873 | 87 |
| Specimen | RSA [%] |
|---|---|
| Gel | 2.67 ± 0.02 |
| Gel_GF30 | 35.70 ± 0.06 * |
| Gel_GF50 | 42.42 ± 0.11 * |
| Gel_GF75 | 46.14 ± 0.05 * |
| Specimen | Ra [nm] | Rq [nm] |
|---|---|---|
| Gel | 1.75 ± 0.14 | 2.22 ± 0.21 |
| Gel_GF30 | 1.83 ± 0.17 | 2.27 ± 0.13 |
| Gel_GF50 | 2.22 ± 0.07 * | 2.80 ± 0.03 * |
| Gel_GF75 | 2.47 ± 0.19 * | 3.25 ± 0.16 * |
| Specimen | ΘW [o] | ΘD [o] | IFT(s) [mJ/m2] | IFT(s,D) [mJ/m2] | IFT(s,P) [mJ/m2] |
|---|---|---|---|---|---|
| Gel | 78 ± 1.12 | 21.15 ± 0.95 | 42.11 ± 0.11 | 35.40 ± 0.31 | 7.20 ± 0.19 |
| Gel_GF30 | 64.43 ± 1.40 * | 41.83 ± 0.23 * | 40.51 ± 0.20 * | 31.93 ± 0.08 * | 8.58 ± 0.12 * |
| Gel_GF50 | 63.45 ± 0.60 * | 47.33 ± 1.16 * | 38.48 ± 0.38 * | 28.75 ± 0.26 * | 9.73 ± 0.12 * |
| Gel+GF75 | 54.67 ± 2.28 * | 62.70 ± 1.30 * | 36.88 ± 0.75 * | 23.88 ± 0.47 * | 13.00 ± 0.28 * |
| Specimen | WVPR [mg/cm2/h] |
|---|---|
| Gel | 0.114 ± 0.021 |
| Gel_GF30 | 0.108 ± 0.026 |
| Gel_GF50 | 0.102 ± 0.030 |
| Gel+GF75 | 0.072 ± 0.01 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamedulski, P.; Wekwejt, M.; Zasada, L.; Ronowska, A.; Michno, A.; Chmielniak, D.; Binkowski, P.; Łukaszewicz, J.P.; Kaczmarek-Szczepańska, B. Evaluating Gelatin-Based Films with Graphene Nanoparticles for Wound Healing Applications. Nanomaterials 2023, 13, 3068. https://doi.org/10.3390/nano13233068
Kamedulski P, Wekwejt M, Zasada L, Ronowska A, Michno A, Chmielniak D, Binkowski P, Łukaszewicz JP, Kaczmarek-Szczepańska B. Evaluating Gelatin-Based Films with Graphene Nanoparticles for Wound Healing Applications. Nanomaterials. 2023; 13(23):3068. https://doi.org/10.3390/nano13233068
Chicago/Turabian StyleKamedulski, Piotr, Marcin Wekwejt, Lidia Zasada, Anna Ronowska, Anna Michno, Dorota Chmielniak, Paweł Binkowski, Jerzy P. Łukaszewicz, and Beata Kaczmarek-Szczepańska. 2023. "Evaluating Gelatin-Based Films with Graphene Nanoparticles for Wound Healing Applications" Nanomaterials 13, no. 23: 3068. https://doi.org/10.3390/nano13233068
APA StyleKamedulski, P., Wekwejt, M., Zasada, L., Ronowska, A., Michno, A., Chmielniak, D., Binkowski, P., Łukaszewicz, J. P., & Kaczmarek-Szczepańska, B. (2023). Evaluating Gelatin-Based Films with Graphene Nanoparticles for Wound Healing Applications. Nanomaterials, 13(23), 3068. https://doi.org/10.3390/nano13233068










