Single-Step Formation of Metal Oxide Nanostructures Wrapped in Mesoporous Silica and Silica–Niobia Catalysts for the Condensation of Furfural with Acetone
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. General Synthesis of Metal Oxide Nanostructures Wrapped in Mesoporous Silica
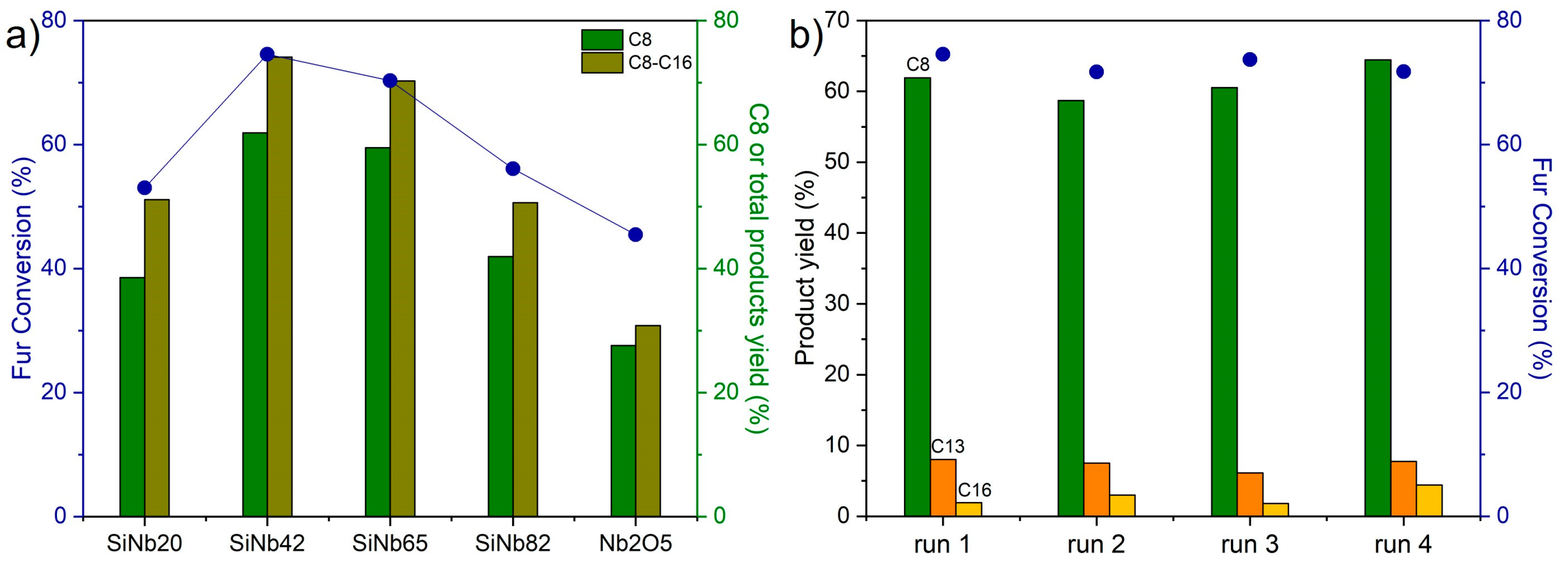
3.2. Surface and Catalytic Properties of Silica–Niobia Structures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Lee, D.W.; Yoo, B.R. Advanced metal oxide (supported) catalysts: Synthesis and applications. J. Ind. Eng. Chem. 2014, 20, 3947–3959. [Google Scholar] [CrossRef]
- Védrine, J.C. Importance, features and uses of metal oxide catalysts in heterogeneous catalysis. Chin. J. Catal. 2019, 40, 1627–1636. [Google Scholar] [CrossRef]
- Xie, C.; Yan, D.; Li, H.; Du, S.; Chen, W.; Wang, Y.; Zou, Y.; Chen, R.; Wang, S. Defect Chemistry in Heterogeneous Catalysis: Recognition, Understanding, and Utilization. ACS Catal. 2020, 10, 11082–11098. [Google Scholar] [CrossRef]
- Wang, G.; Yang, Y.; Han, D.; Li, Y. Oxygen defective metal oxides for energy conversion and storage. Nano Today 2017, 13, 23–39. [Google Scholar] [CrossRef]
- Jia, J.; Qian, C.; Dong, Y.; Li, Y.F.; Wang, H.; Ghoussoub, M.; Butler, K.T.; Walsh, A.; Ozin, G.A. Heterogeneous catalytic hydrogenation of CO2 by metal oxides: Defect engineering—Perfecting imperfection. Chem. Soc. Rev. 2017, 46, 4631–4644. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Xiao, C.; Kou, Y. Transition metal nanoparticle catalysis in green solvents. Coord. Chem. Rev. 2010, 254, 1179–1218. [Google Scholar] [CrossRef]
- Hansen, T.W.; DeLaRiva, A.T.; Challa, S.R.; Datye, A.K. Sintering of Catalytic Nanoparticles: Particle Migration or Ostwald Ripening? Acc. Chem. Res. 2013, 46, 1720–1730. [Google Scholar] [CrossRef]
- Lwin, S.; Wachs, I.E. Olefin Metathesis by Supported Metal Oxide Catalysts. ACS Catal. 2014, 4, 2505–2520. [Google Scholar] [CrossRef]
- Nava, R.; Pawelec, B.; Castaño, P.; Álvarez-Galván, M.C.; Loricera, C.V.; Fierro, J.L.G. Upgrading of bio-liquids on different mesoporous silica-supported CoMo catalysts. Appl. Catal. B 2009, 92, 154–167. [Google Scholar] [CrossRef]
- Esposito, S. “Traditional” Sol-Gel Chemistry as a Powerful Tool for the Preparation of Supported Metal and Metal Oxide Catalysts. Materials 2019, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- McCarty, J.G.; Gusman, M.; Lowe, D.M.; Hildenbrand, D.L.; Lau, K.N. Stability of supported metal and supported metal oxide combustion catalysts. Catal. Today 1999, 47, 5–17. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Q.; Zhang, T.; Yin, Y. Core–Satellite Nanocomposite Catalysts Protected by a Porous Silica Shell: Controllable Reactivity, High Stability, and Magnetic Recyclability. Angew. Chem. Int. Ed. 2008, 47, 8924–8928. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cui, X.; Li, S.; Cen, Y.; Deng, T.; Wang, J.; Olsbye, U.; Fan, W. Developing a general method for encapsulation of metal oxide nanoparticles in mesoporous silica shell by unraveling its formation mechanism. Microporous Mesoporous Mater. 2020, 305, 110381. [Google Scholar] [CrossRef]
- Forman, A.J.; Park, J.-N.; Tang, W.; Hu, Y.-S.; Stucky, G.D.; McFarland, E.W. Silica-Encapsulated Pd Nanoparticles as a Regenerable and Sintering-Resistant Catalyst. ChemCatChem 2010, 2, 1318–1324. [Google Scholar] [CrossRef]
- Fang, R.; Tian, P.; Yang, X.; Luque, R.; Li, Y. Encapsulation of ultrafine metal-oxide nanoparticles within mesopores for biomass-derived catalytic applications. Chem. Sci. 2018, 9, 1854–1859. [Google Scholar] [CrossRef]
- Deshmukh, R.; Niederberger, M. Mechanistic Aspects in the Formation, Growth and Surface Functionalization of Metal Oxide Nanoparticles in Organic Solvents. Chem. Eur. J. 2017, 23, 8542–8570. [Google Scholar] [CrossRef]
- Mutin, P.H.; Vioux, A. Recent advances in the synthesis of inorganic materials via non-hydrolytic condensation and related low-temperature routes. J. Mater. Chem. A 2013, 1, 11504–11512. [Google Scholar] [CrossRef]
- Skrodczky, K.; Antunes, M.M.; Han, X.; Santangelo, S.; Scholz, G.; Valente, A.A.; Pinna, N.; Russo, P.A. Niobium pentoxide nanomaterials with distorted structures as efficient acid catalysts. Commun. Chem. 2019, 2, 129. [Google Scholar] [CrossRef]
- Beach, E.R.; Shqau, K.; Brown, S.E.; Rozeveld, S.J.; Morris, P.A. Solvothermal synthesis of crystalline nickel oxide nanoparticles. Mater. Chem. Phys. 2009, 115, 371–377. [Google Scholar] [CrossRef]
- Debecker, D.P.; Bouchmella, K.; Poleunis, C.; Eloy, P.; Bertrand, P.; Gaigneaux, E.M.; Mutin, P.H. Design of SiO2−Al2O3−MoO3 Metathesis Catalysts by Nonhydrolytic Sol−Gel. Chem. Mater. 2009, 21, 2817–2824. [Google Scholar] [CrossRef]
- Bouchmella, K.; Hubert Mutin, P.; Stoyanova, M.; Poleunis, C.; Eloy, P.; Rodemerck, U.; Gaigneaux, E.M.; Debecker, D.P. Olefin metathesis with mesoporous rhenium–silicium–aluminum mixed oxides obtained via a one-step non-hydrolytic sol–gel route. J. Catal. 2013, 301, 233–241. [Google Scholar] [CrossRef]
- Dochain, D.D.; Stýskalík, A.; Debecker, D.P. Ag- and Cu-Promoted Mesoporous Ta-SiO2 Catalysts Prepared by Non-Hydrolytic Sol-Gel for the Conversion of Ethanol to Butadiene. Catalysts 2019, 9, 920. [Google Scholar] [CrossRef]
- Smeets, V.; Ben Mustapha, L.; Schnee, J.; Gaigneaux, E.M.; Debecker, D.P. Mesoporous SiO2-TiO2 epoxidation catalysts: Tuning surface polarity to improve performance in the presence of water. Mol. Catal. 2018, 452, 123–128. [Google Scholar] [CrossRef]
- Mathew, A.K.; Abraham, A.; Mallapureddy, K.K.; Sukumaran, R.K. Waste Biorefinery; Bhaskar, T., Pandey, A., Mohan, S.V., Lee, D.-J., Khanal, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 267–297. [Google Scholar]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Truong, C.C.; Verma, V.K.; Mishra, P.; Suh, Y.-W.; Mishra, D.K. Biomass, Biofuels, Biochemicals; Li, H., Saravanamurugan, S., Pandey, A., Elumalai, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 673–720. [Google Scholar]
- Wegenhart, B.L.; Yang, L.; Kwan, S.C.; Harris, R.; Kenttämaa, H.I.; Abu-Omar, M.M. From Furfural to Fuel: Synthesis of Furoins by Organocatalysis and their Hydrodeoxygenation by Cascade Catalysis. ChemSusChem 2014, 7, 2742–2747. [Google Scholar] [CrossRef]
- Bohre, A.; Dutta, S.; Saha, B.; Abu-Omar, M.M. Upgrading Furfurals to Drop-in Biofuels: An Overview. ACS Sustain. Chem. Eng. 2015, 3, 1263–1277. [Google Scholar] [CrossRef]
- Zang, H.; Wang, K.; Zhang, M.; Xie, R.; Wang, L.; Chen, E.Y.X. Catalytic coupling of biomass-derived aldehydes into intermediates for biofuels and materials. Catal. Sci. Technol. 2018, 8, 1777–1798. [Google Scholar] [CrossRef]
- Huber, G.W.; Chheda, J.N.; Barrett, C.J.; Dumesic, J.A. Production of Liquid Alkanes by Aqueous-Phase Processing of Biomass-Derived Carbohydrates. Science 2005, 308, 1446–1450. [Google Scholar] [CrossRef]
- Zitouni, A.; Bachir, R.; Bendedouche, W.; Bedrane, S. Production of bio-jet fuel range hydrocarbons from catalytic HDO of biobased difurfurilydene acetone over Ni/SiO2-ZrO2 catalysts. Fuel 2021, 297, 120783. [Google Scholar] [CrossRef]
- Jaroniec, M.; Kruk, M.; Olivier, J.P. Standard Nitrogen Adsorption Data for Characterization of Nanoporous Silicas. Langmuir 1999, 15, 5410–5413. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of Integrated Molar Extinction Coefficients for Infrared Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Koziej, D.; Rossell, M.D.; Ludi, B.; Hintennach, A.; Novák, P.; Grunwaldt, J.-D.; Niederberger, M. Interplay Between Size and Crystal Structure of Molybdenum Dioxide Nanoparticles—Synthesis, Growth Mechanism, and Electrochemical Performance. Small 2011, 7, 377–387. [Google Scholar] [CrossRef]
- Busca, G. Spectroscopic characterization of the acid properties of metal oxide catalysts. Catal. Today 1998, 41, 191–206. [Google Scholar] [CrossRef]
- Tamura, M.; Shimizu, K.-i.; Satsuma, A. Comprehensive IR study on acid/base properties of metal oxides. Appl. Catal. A 2012, 433–434, 135–145. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Kelbichová, V.; Vitvarová, D.; Kubů, M.; Kubička, D. Aldol condensation of furfural and acetone on zeolites. Catal. Today 2014, 227, 154–162. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Kubička, D.; Čejka, J. Toward understanding of the role of Lewis acidity in aldol condensation of acetone and furfural using MOF and zeolite catalysts. Catal. Today 2015, 243, 158–162. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Bulánek, R.; Frolich, K.; Čejka, J.; Kubička, D. Aldol condensation of furfural with acetone over ion-exchanged and impregnated potassium BEA zeolites. J. Mol. Catal. A 2016, 424, 358–368. [Google Scholar] [CrossRef]
- Balaga, R.; Yan, P.; Ramineni, K.; Du, H.; Xia, Z.; Marri, M.R.; Zhang, Z.C. The Role and Performance of Isolated Zirconia Sites on Mesoporous Silica for Aldol Condensation of Furfural with Acetone. Appl. Catal. A Gen. 2022, 648, 118901. [Google Scholar] [CrossRef]
- Gandhi, P.; Saha, B.; Vedachalam, S.; Dalai, A.K. Renewable Fuel Intermediates from Furfural over Copper-Loaded Mesoporous Aldol Condensation Catalysts. Sustain. Energy Fuels 2023, 7, 4260–4272. [Google Scholar] [CrossRef]
- Arumugam, M.; Kikhtyanin, O.; Osatiashtiani, A.; Kyselová, V.; Fila, V.; Paterova, I.; Wong, K.L.; Kubička, D. Potassium-Modified Bifunctional MgAl-SBA-15 for Aldol Condensation of Furfural and Acetone. Sustain. Energy Fuels 2023, 7, 3047–3059. [Google Scholar] [CrossRef]
- Kondratowicz, T.; Slang, S.; Dubnová, L.; Kikhtyanin, O.; Belina, P.; Capek, L. Controlled Silica Core Removal from SiO2@MgAl Core-Shell System as a Tool to Prepare Well-Oriented and Highly Active Catalysts. Appl. Clay Sci. 2022, 216, 106365. [Google Scholar] [CrossRef]
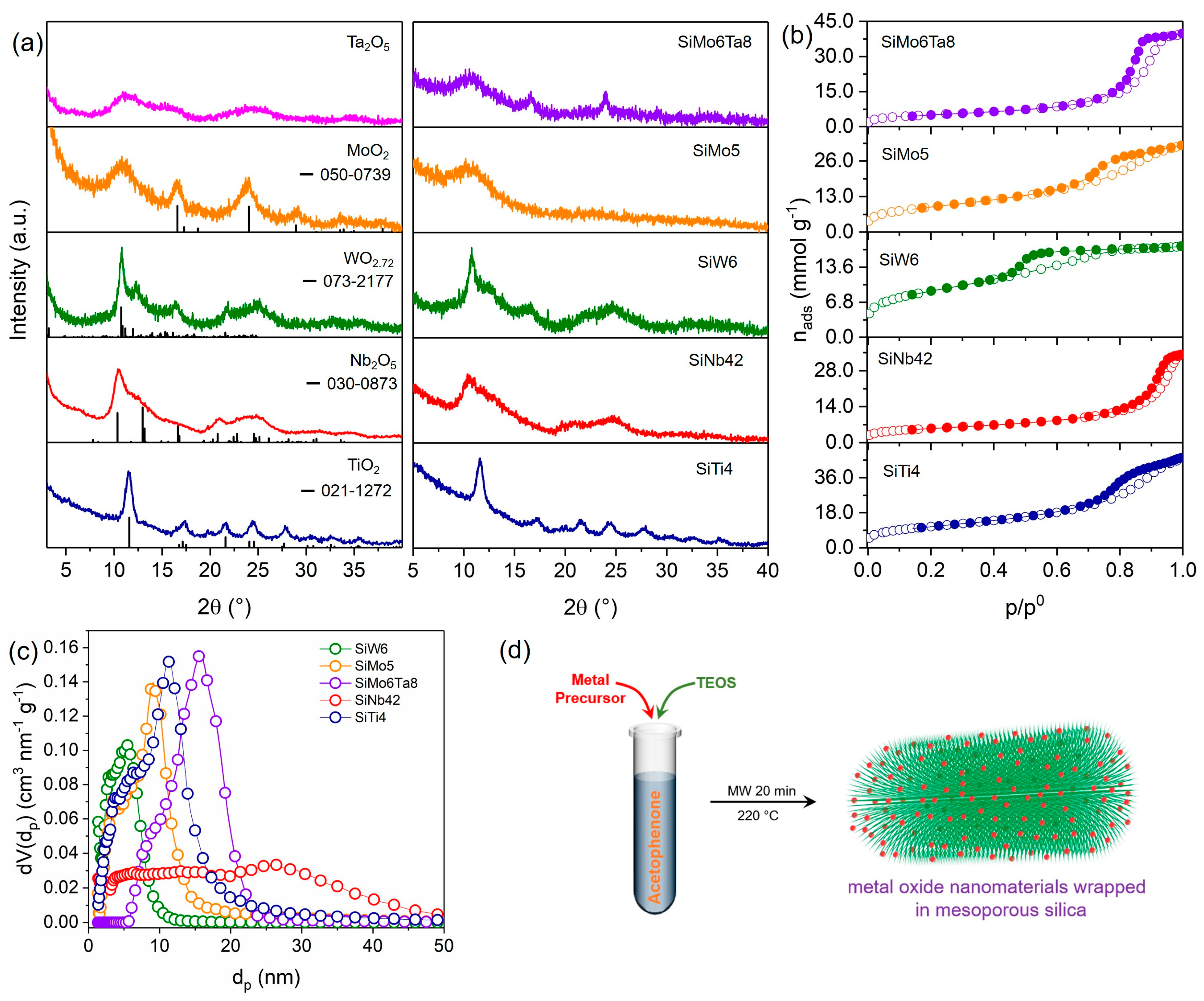
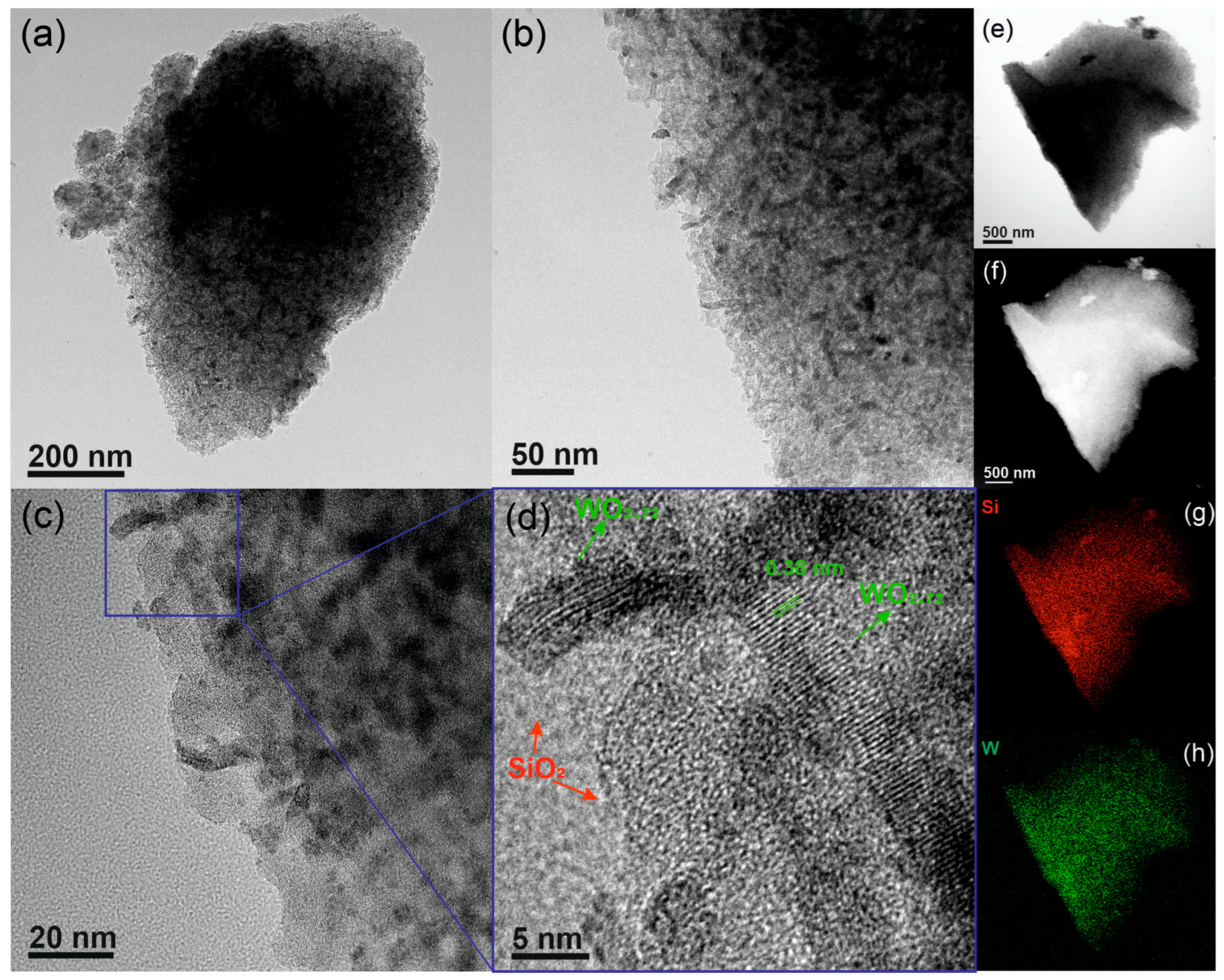
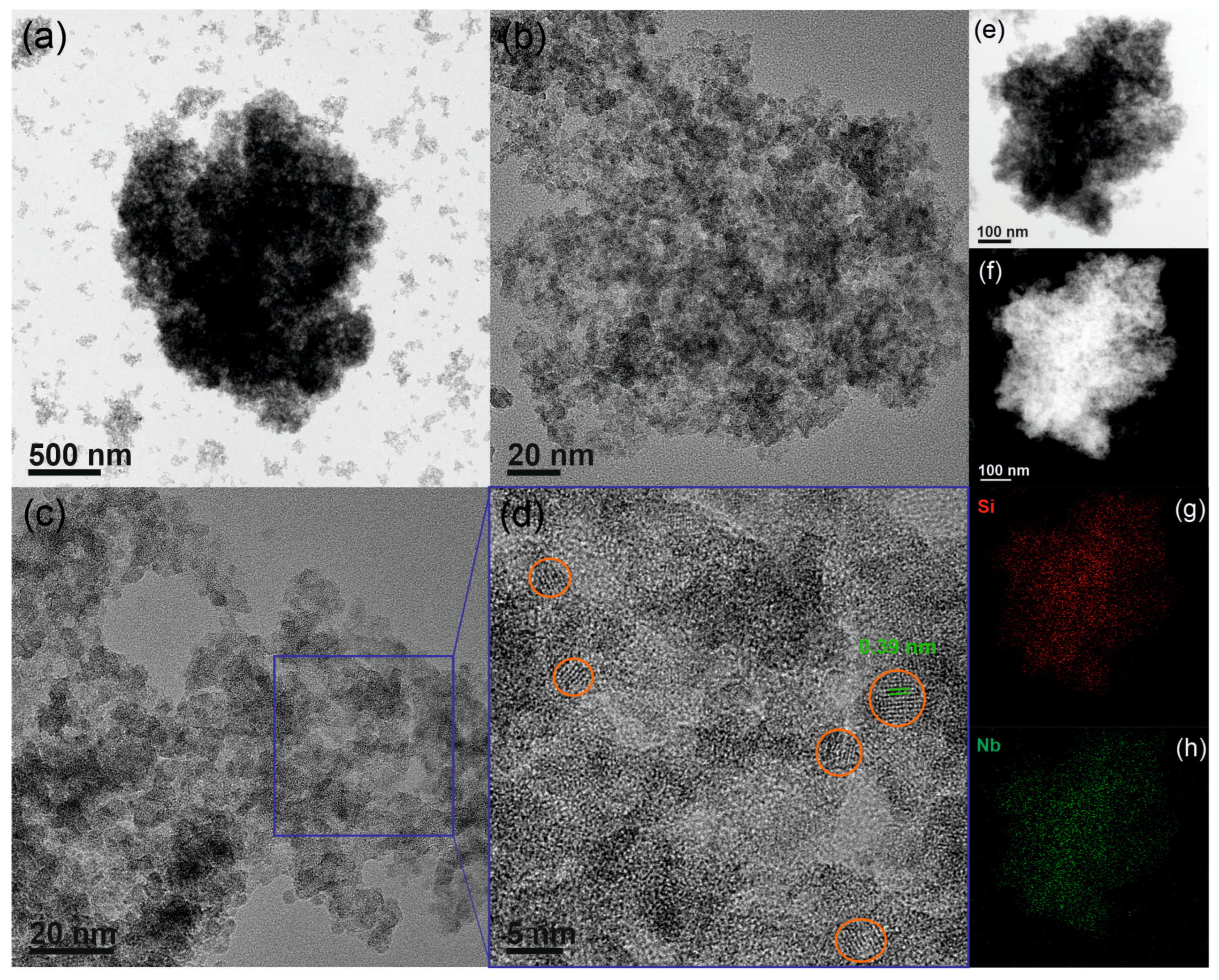
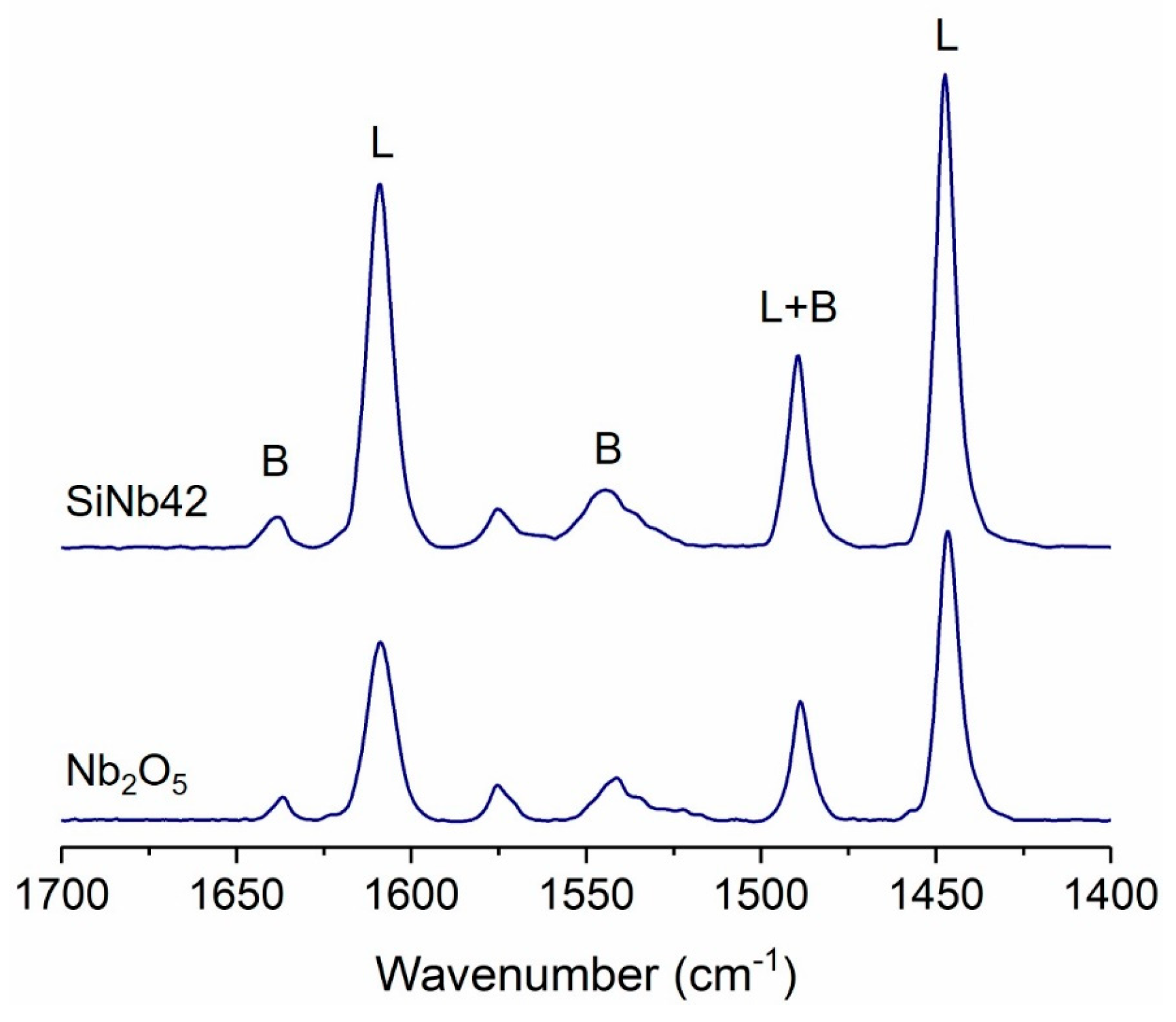
| Sample | SBET (m2 g−1) 1 | Vmp (cm3 g−1) 2 | dp (nm) 3 |
|---|---|---|---|
| SiO2 | 1196 | 1.40 | 9.1 |
| SiTi4 | 895 | 1.27 | 11.3 |
| SiW6 | 724 | 0.58 | 5.5 |
| SiMo5 | 755 | 0.94 | 9.1 |
| SiMo6Ta8 | 413 | 1.26 | 15.5 |
| Sample | SBET (m2 g−1) 1 | Vmp (cm3 g−1) 2 | dp (nm) 3 | AST (μmol g−1) 4 | ASL (μmol g−1) 5 | ASB (μmol g−1) 6 |
|---|---|---|---|---|---|---|
| SiNb7 | 913 | 1.02 | 7.3 | 138 | 88 | 50 |
| SiNb20 | 682 | 1.40 | 18.6 | 195 | 137 | 58 |
| SiNb42 | 449 | 0.79 | 26.4 | 267 | 198 | 69 |
| SiNb65 | 296 | 0.42 | 27.2 | 252 | 190 | 62 |
| SiNb82 | 224 | 0.25 | 17.3 | 156 | 123 | 33 |
| Nb2O5 | 161 | - | - | 150 | 120 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrodczky, K.; Antunes, M.M.; Zhu, Q.; Valente, A.A.; Pinna, N.; Russo, P.A. Single-Step Formation of Metal Oxide Nanostructures Wrapped in Mesoporous Silica and Silica–Niobia Catalysts for the Condensation of Furfural with Acetone. Nanomaterials 2023, 13, 3046. https://doi.org/10.3390/nano13233046
Skrodczky K, Antunes MM, Zhu Q, Valente AA, Pinna N, Russo PA. Single-Step Formation of Metal Oxide Nanostructures Wrapped in Mesoporous Silica and Silica–Niobia Catalysts for the Condensation of Furfural with Acetone. Nanomaterials. 2023; 13(23):3046. https://doi.org/10.3390/nano13233046
Chicago/Turabian StyleSkrodczky, Kai, Margarida M. Antunes, Qingjun Zhu, Anabela A. Valente, Nicola Pinna, and Patrícia A. Russo. 2023. "Single-Step Formation of Metal Oxide Nanostructures Wrapped in Mesoporous Silica and Silica–Niobia Catalysts for the Condensation of Furfural with Acetone" Nanomaterials 13, no. 23: 3046. https://doi.org/10.3390/nano13233046
APA StyleSkrodczky, K., Antunes, M. M., Zhu, Q., Valente, A. A., Pinna, N., & Russo, P. A. (2023). Single-Step Formation of Metal Oxide Nanostructures Wrapped in Mesoporous Silica and Silica–Niobia Catalysts for the Condensation of Furfural with Acetone. Nanomaterials, 13(23), 3046. https://doi.org/10.3390/nano13233046







