Monitoring Tools and Strategies for Effective Electrokinetic Nanoparticle Treatment
Abstract
:1. Background
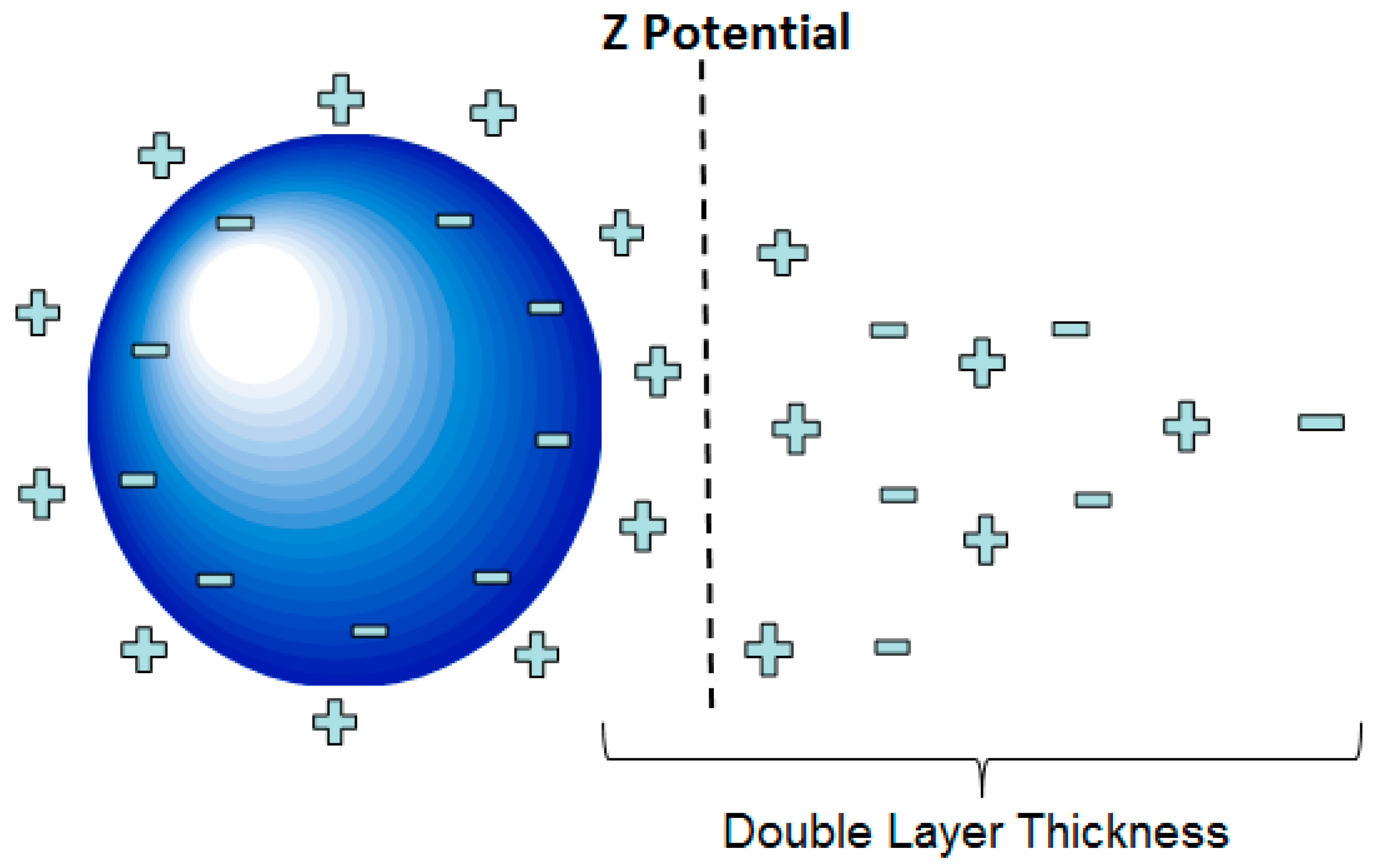
1.1. Theory of Electrokinetic Nanoparticle Treatment
1.2. Particle Destabilization Mechanisms
1.3. Turbidity of a Suspension
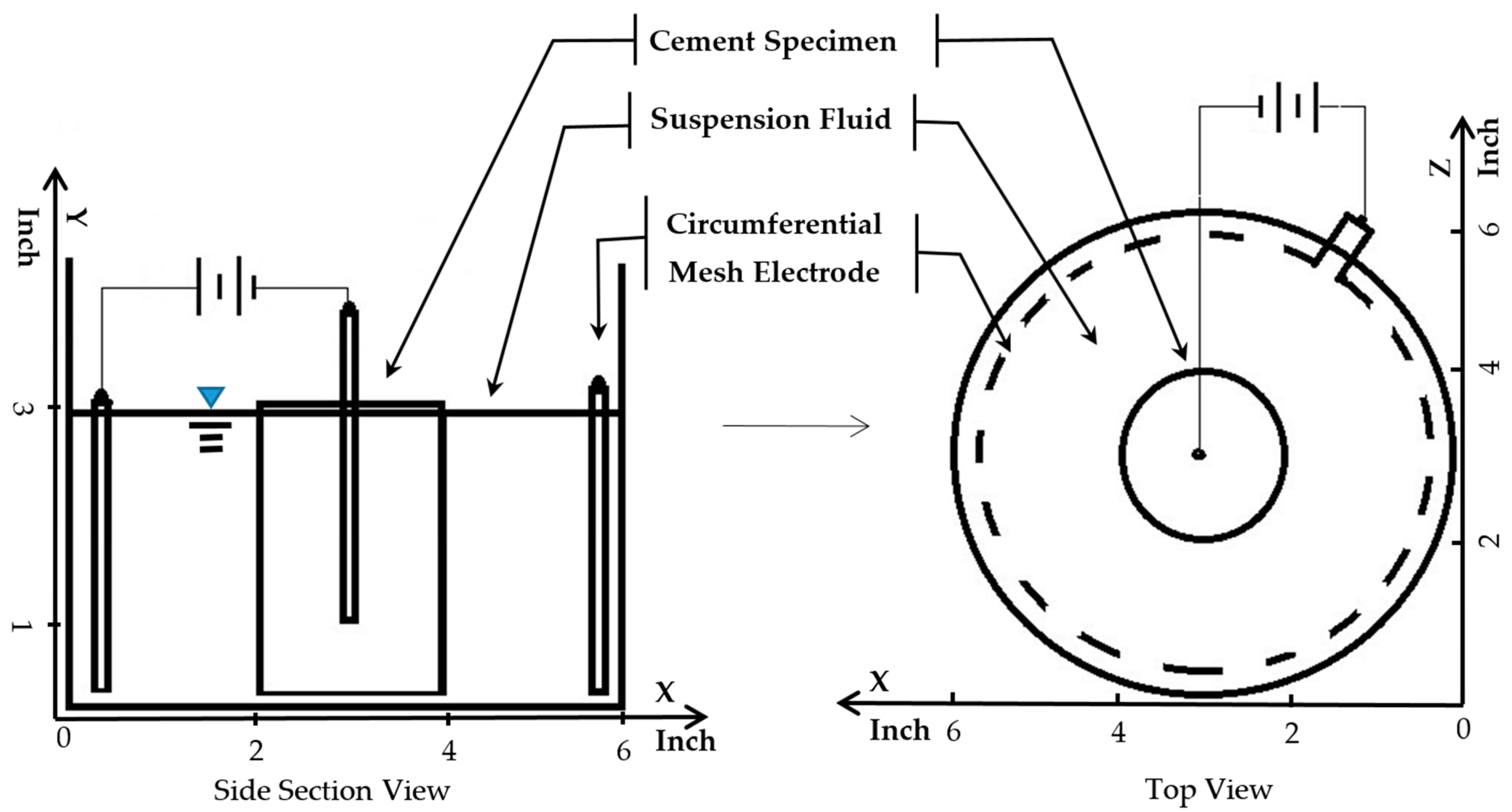
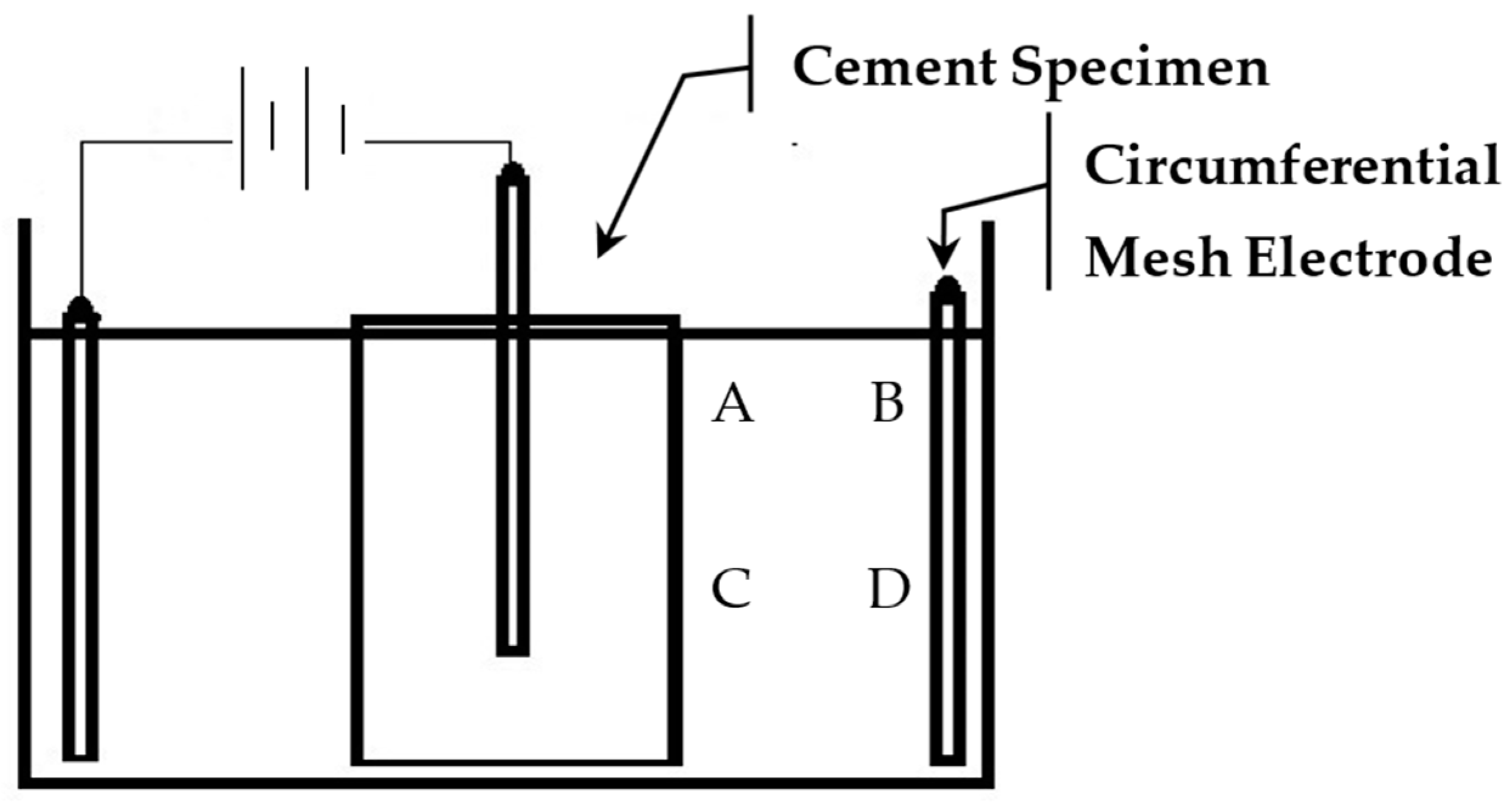
2. Methodology and Experiment Setup
2.1. Batching and Curing
2.2. pH and Turbidity Measurement
3. Results and Discussion
3.1. Treatment Approaches Examination
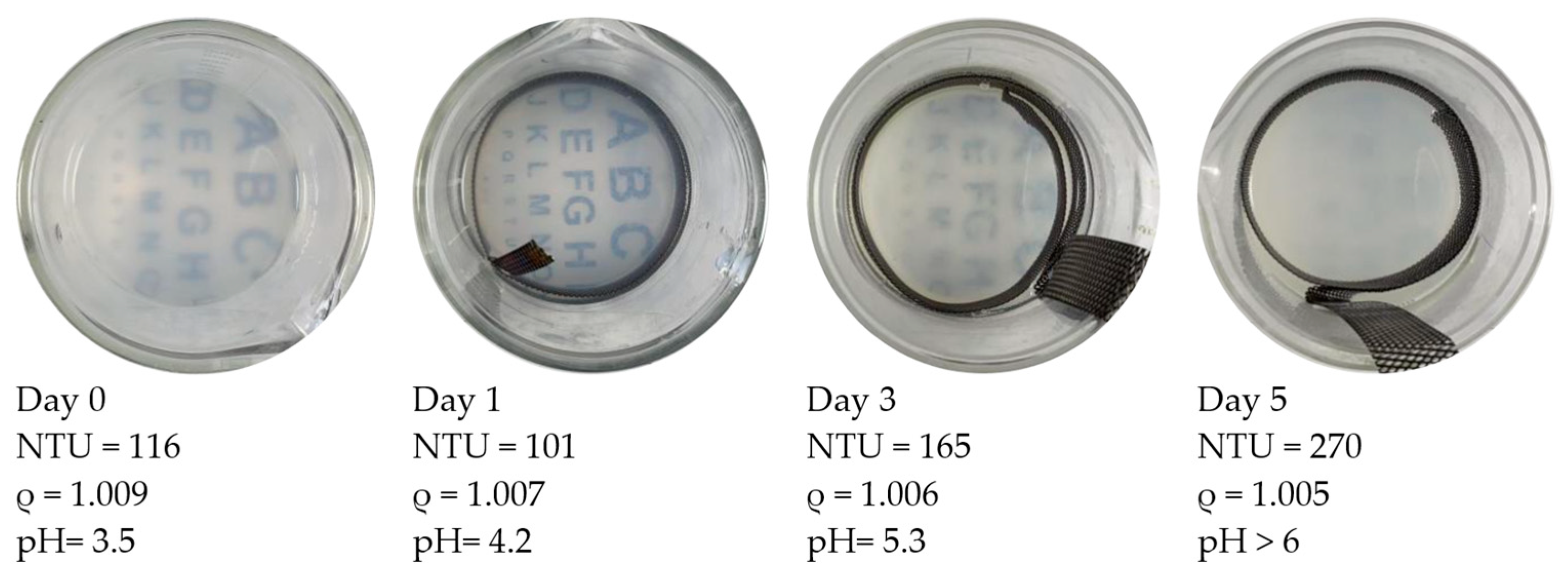
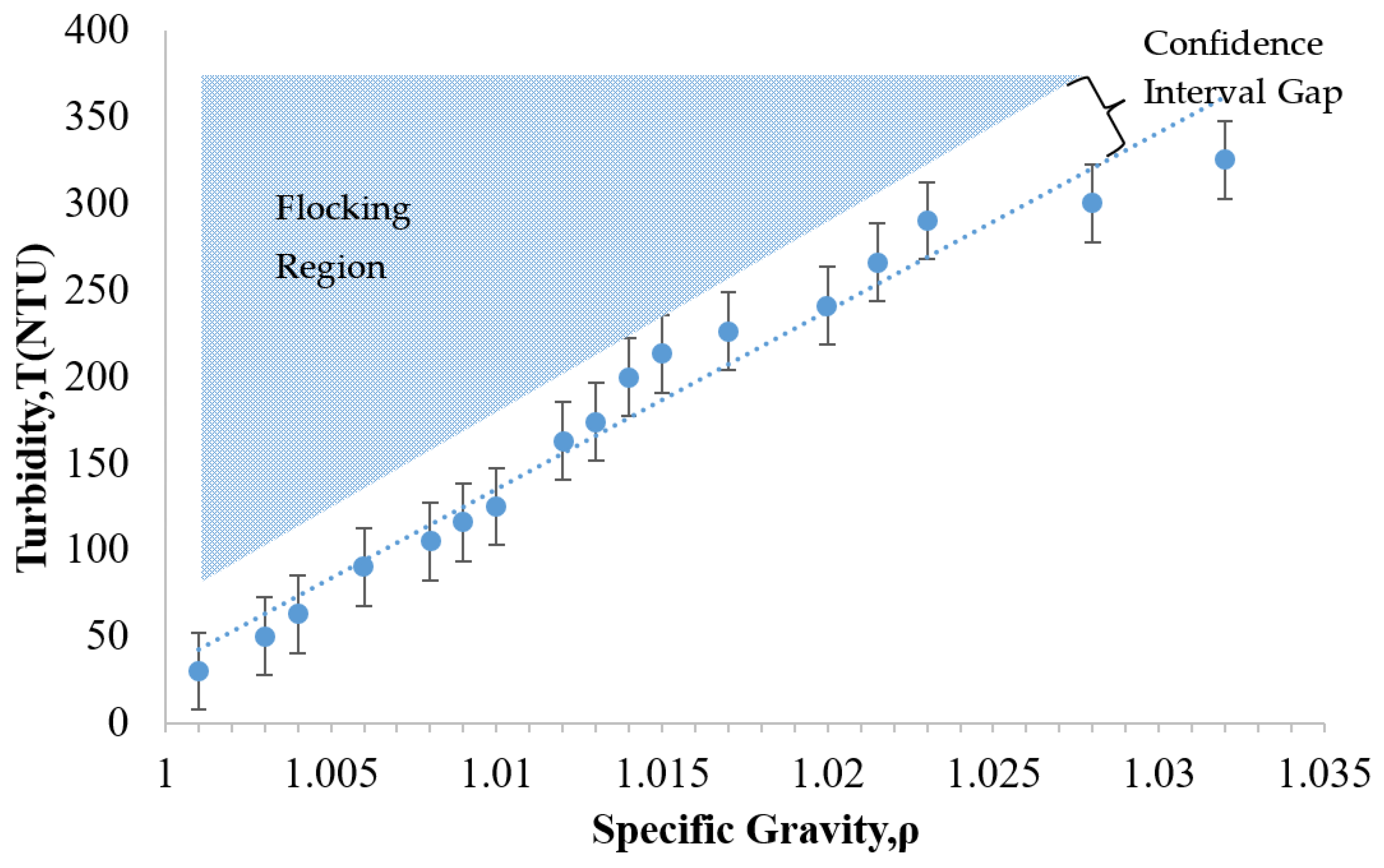
3.2. Flocking Behavior Observation
3.3. pH Control Approaches

3.4. Specific Gravity Monitoring and Comparison
3.5. Flocking Behavior Plot of NALCO 1056 Particles
3.6. Flocking Behavior Plot of Grace CL Particles
4. Conclusions and Discussion
- (1)
- To achieve an efficient treatment and avoid particle loss due to flocking or coagulation, pH adjustments appear to be necessary to support the stability and efficiency of a given EN treatment.
- (2)
- The effective and efficient treatments obtained in this work exhibited successful particle transport into cement pores, which was identified by declining specific gravities and turbidities while the treatment particles remained in stable suspension.
- (3)
- This work confirmed that periodically adjusting the pH of a particle suspension back to the starting level (during a long-term treatment period) may prevent treatment suspension instability by delaying the pH rise that can cause flocking and suspension collapse.
- (4)
- While visual inspection is a convenient way for assessing particle transport progress, it is recommended that utilizing turbidity measurements could more definitively identify important particle suspension changes that can confirm acceptable treatment progress.
- (5)
- Identifying the flocking region of a given particle suspension may provide a convenient benchmark for assessing the risk of particle loss during a given treatment.
- (6)
- The relationship between the specific gravity and the turbidity was approximately linear for the Grace CL (silica) particles and thus similar to the NALCO 1056 (alumina-coated silica) particles.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cardenas, H.; Kupwade-Patil, K.; Eklund, S. Corrosion mitigation in mature reinforced concrete using nanoscale pozzolan deposition. J. Mater. Civ. Eng. 2011, 23, 752–760. [Google Scholar] [CrossRef]
- Cardenas, H.; Kupwade-Patil, K.; Eklund, S. Recovery from sulfate attack in concrete via electrokinetic nanoparticle treatment. J. Mater. Civ. Eng. 2011, 23, 1103–1112. [Google Scholar] [CrossRef]
- Cardenas, H.E.; Struble, L.J. Electrokinetic nanoparticle treatment of hardened cement paste for reduction of permeability. J. Mater. Civ. Eng. 2006, 18, 554–560. [Google Scholar] [CrossRef]
- Kupwade-Patil, K.; Cardenas, H.E.; Gordon, K.; Lee, L.S. Corrosion mitigation in reinforced concrete beams via nanoparticle treatment. ACI Mater. J. 2012, 109, 617–626. [Google Scholar]
- Laster, B.H.; Thomlinson, W.C.; Fairchild, R.G. Photon activation of iododeoxyuridine: Biological efficacy of Auger electrons. Radiat. Res. 1993, 133, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Roeske, J.C.; Nunez, L.; Hoggarth, M.; Labay, E.; Weichselbaum, R.R. Characterization of the theoretical radiation dose enhancement from nanoparticles. Technol. Cancer Res. Treat. 2007, 6, 395–401. [Google Scholar] [CrossRef]
- Pohanka, M. Copper and copper nanoparticles toxicity and their impact on basic functions in the body. Bratisl. Lek. Listy 2019, 120, 397–409. [Google Scholar] [CrossRef]
- Pavlin, M.; Bregar, V.B. Stability of nanoparticle suspensions in different biologically relevant media. Dig. J. Nanomater. Biostruct. (DJNB) 2012, 7, 1389–1400. [Google Scholar]
- Ji, Z.; Jin, X.; George, S.; Xia, T.; Meng, H.; Wang, X.; Suarez, E.; Zhang, H.; Hoek, E.M.V.; Godwin, H.; et al. Dispersion and stability optimization of TiO2 nanoparticles in cell culture media. Environ. Sci. Technol. 2010, 44, 7309–7314. [Google Scholar] [CrossRef]
- Derjaguin, B.; Landau, L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Prog. Surf. Sci. 1993, 43, 30–59. [Google Scholar] [CrossRef]
- Verwey, E.J.W. Theory of the stability of lyophobic colloids. J. Phys. Chem. 1947, 51, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.; Zhuge, Y.; Beecham, S. The relationship between porosity and strength for porous concrete. Constr. Build. Mater. 2011, 25, 4294–4298. [Google Scholar] [CrossRef]
- Ben-Moshe, T.; Dror, I.; Berkowitz, B. Transport of metal oxide nanoparticles in saturated porous media. Chemosphere 2010, 81, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Lecoanet, H.F.; Bottero, J.Y.; Wiesner, M.R. Laboratory assessment of the mobility of nanomaterials in porous media. Environ. Sci. Technol. 2004, 38, 5164–5169. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, H.E.; Zhong, H. Electrokinetic nanoparticle treatment success factors. Mater. Sci. Appl. 2020, 11, 767–786. [Google Scholar] [CrossRef]
- Zhong, H.; Cardenas, H.E. Effective Transport and Efficiency Parameters for Electrokinetic Nanopozzolan Treatment of Hardened Cement Paste. ACI Mater. J. 2022, 119, 139–149. [Google Scholar]
- Jenkins, S.; Kirk, S.R.; Persson, M.; Carlen, J.; Abbas, Z. Molecular dynamics simulation of nanocolloidal amorphous silica particles: Part I. J. Chem. Phys. 2007, 127, 224711. [Google Scholar] [CrossRef]
- Zhou, D.; Abdel-Fattah, A.I.; Keller, A.A. Clay particles destabilize engineered nanoparticles in aqueous environments. Environ. Sci. Technol. 2012, 46, 7520–7526. [Google Scholar] [CrossRef]
- Jung, Y.; Son, Y.H.; Lee, J.K.; Phuoc, T.X.; Soong, Y.; Chyu, M.K. Rheological behavior of clay–nanoparticle hybrid-added bentonite suspensions: Specific role of hybrid additives on the gelation of clay-based fluids. ACS Appl. Mater. Interfaces 2011, 3, 3515–3522. [Google Scholar] [CrossRef]
- Jenkins, P.; Snowden, M. Depletion flocculation in colloidal dispersions. Adv. Colloid Interface Sci. 1996, 68, 57–96. [Google Scholar] [CrossRef]
- Gregory, J.; Barany, S. Adsorption and flocculation by polymers and polymer mixtures. Adv. Colloid Interface Sci. 2011, 169, 1–12. [Google Scholar] [CrossRef]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle aggregation: Challenges to understanding transport and reactivity in the environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef]
- Sadar, M. Turbidity Standards Technical Information Series—Booklet No. 12; Hach Co., Ltd.: Loveland, CO, USA, 1998. [Google Scholar]
- Foster, I.D.L.; Millington, R.; Grew, R.G. The impact of particle size controls on stream turbidity measurement; some implications for suspended sediment yield estimation. Eros. Sediment Transp. Monit. Programmes River Basins 1992, 51–62. [Google Scholar]
- Webb, B.W. Erosion and Sedimentation; IAHS Pub: Wallingford, UK, 1987; Volume 171, pp. 79–90. [Google Scholar]
- Yang, K.C.; Hogg, R. Estimation of particle size distributions from turbidimetric measurements. Anal. Chem. 1979, 51, 758–763. [Google Scholar] [CrossRef]
- Bhargava, D.S.; Mariam, D.W. Effects of suspended particle size and concentration on reflectance measurements. Photogramm. Eng. Remote Sens. 1991, 57, 519–529. [Google Scholar]
- Bunt, J.A.C.; Larcombe, P.; Jago, C.F. Quantifying the response of optical backscatter devices and transmissometers to variations in suspended particulate matter. Cont. Shelf Res. 1999, 19, 1199–1220. [Google Scholar] [CrossRef]
- ISO 7027-2:2019; Water Quality—Determination of Turbidity—Part 2: Semi-Quantitative Methods for the Assessment of Transparency of Waters. ISO: Geneva, Switzerland, 2019.
- Bartels, P.; Hirsch, P.E.; Svanbäck, R.; Eklöv, P. Water transparency drives intra-population divergence in Eurasian perch (Perca fluviatilis). PLoS ONE 2012, 7, e43641. [Google Scholar] [CrossRef] [PubMed]
- Nellis, M.D.; Harrington, J.A., Jr.; Wu, J. Remote sensing of temporal and spatial variations in pool size, suspended sediment, turbidity, and Secchi depth in Tuttle Creek Reservoir, Kansas: 1993. Geomorphology 1998, 21, 281–293. [Google Scholar] [CrossRef]
- Neukermans, G.; Ruddick, K.; Loisel, H.; Roose, P. Optimization and quality control of suspended particulate matter concentration measurement using turbidity measurements. Limnol. Oceanogr. Methods 2012, 10, 1011–1023. [Google Scholar] [CrossRef]
- Guillén, J.; Palanques, A.; Puig, P.; Durrieu De Madron, X.; Nyffeler, F. Field calibration of optical sensors for measuring suspended sediment concentration in the western Mediterranean. Sci. Mar. 2000, 64, 427–435. [Google Scholar] [CrossRef]
- Vangriesheim, A.; Gouillou, J.P.; Prieur, L. A deep-ocean nephelometer to detect bottom and intermediate nepheloid layers. Deep. Sea Res. Part A Oceanogr. Res. Pap. 1992, 39, 1403–1416. [Google Scholar] [CrossRef]
- Center for Pavement Technology. Cementitious Materials. CPTech Center. Available online: https://cptechcenter.org/cementitious-materials/#:~:text=Ordinary%20Portland%20Cement,-Gypsum%20(used%20with&text=(2%20cm)%20hard%20spheres%20known,85%2C%20and%20AASHTO%20M%20240 (accessed on 1 September 2023).
- ASTM C305-20; Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency. ASTM International: West Conshohocken, PA, USA, 2020.
- Neville, A.M. Properties of Concrete; Longman: London, UK, 1995; Volume 4. [Google Scholar]
- Ma, X.; Zeng, G.; Zahng, C.; Wang, Z.; Yu, J.; Li, J.; Huang, G.; Liu, H. Characteristics of BPA removal from water by PACl-Al13 in coagulation process. J. Colloid Interface Sci. 2009, 337, 408–413. [Google Scholar]
- Wheeler, A.J.; Ganji, A.R.; Krishnan, V.V.; Thurow, B.S. Introduction to Engineering Experimentation; Pearson: London, UK, 2010; Volume 480. [Google Scholar]


| Component | CaCO3 | SiO2 | Al2O3 | Fe2O3 | CaO | SO3 | Na2O | K2O |
|---|---|---|---|---|---|---|---|---|
| Amount (mass %) | 2.41 | 20.15 | 4.62 | 4.03 | 63.61 | 3.20 | 0.16 | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, H.; Cardenas, H.E. Monitoring Tools and Strategies for Effective Electrokinetic Nanoparticle Treatment. Nanomaterials 2023, 13, 3045. https://doi.org/10.3390/nano13233045
Zhong H, Cardenas HE. Monitoring Tools and Strategies for Effective Electrokinetic Nanoparticle Treatment. Nanomaterials. 2023; 13(23):3045. https://doi.org/10.3390/nano13233045
Chicago/Turabian StyleZhong, Huayuan, and Henry E. Cardenas. 2023. "Monitoring Tools and Strategies for Effective Electrokinetic Nanoparticle Treatment" Nanomaterials 13, no. 23: 3045. https://doi.org/10.3390/nano13233045
APA StyleZhong, H., & Cardenas, H. E. (2023). Monitoring Tools and Strategies for Effective Electrokinetic Nanoparticle Treatment. Nanomaterials, 13(23), 3045. https://doi.org/10.3390/nano13233045





