Design, Optimization, and Application of a 3D-Printed Polymer Sample Introduction System for the ICP-MS Analysis of Nanoparticles and Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Samples and Materials
2.3. Software
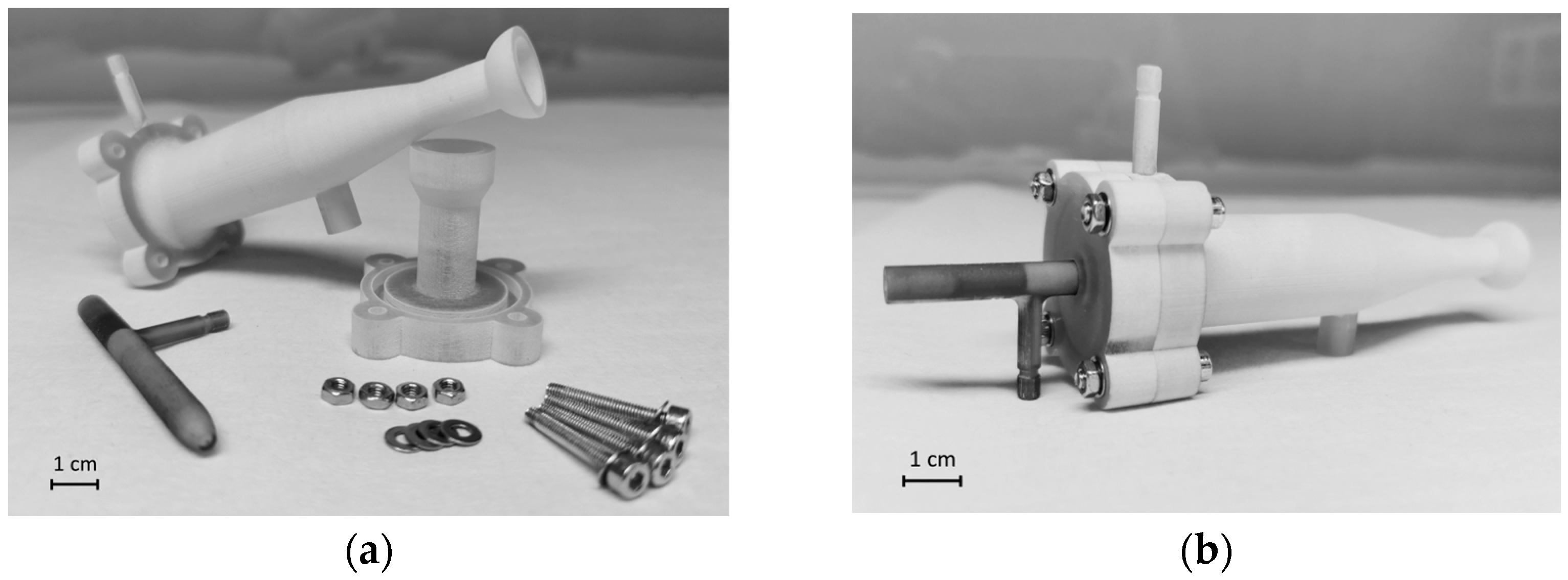
2.4. 3D Printing
3. Results and Discussions
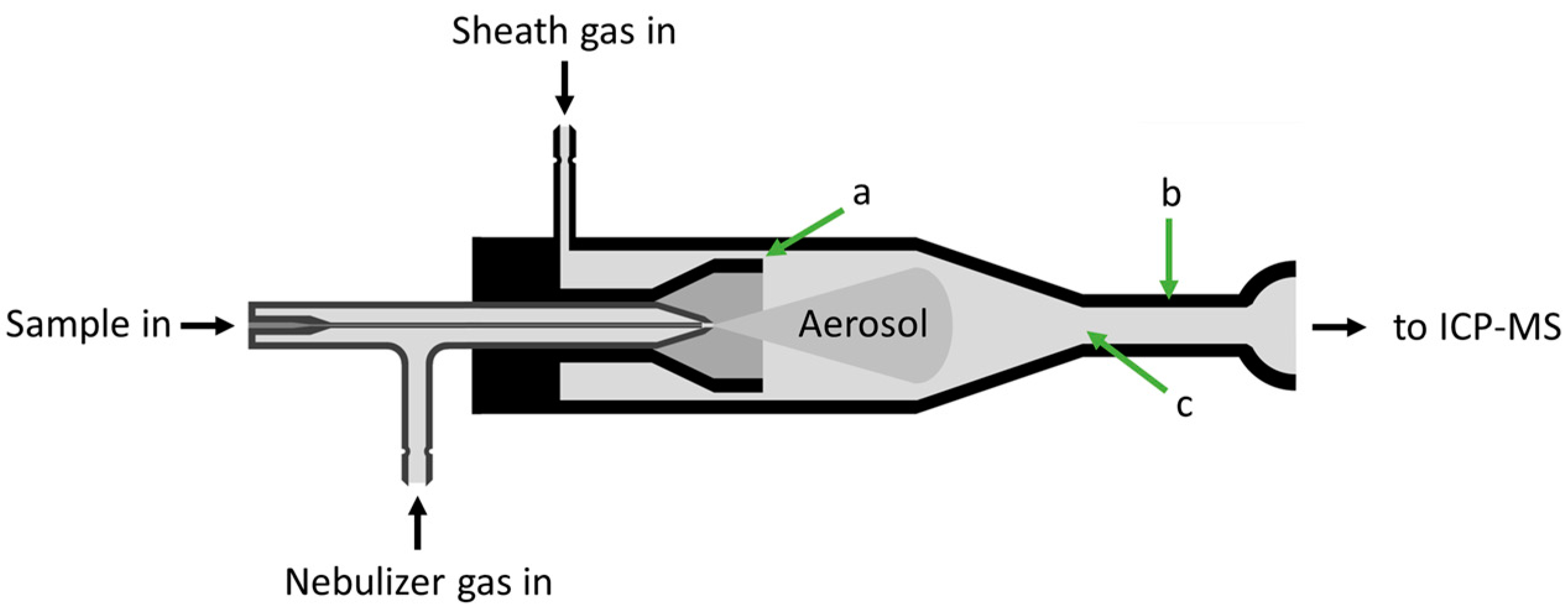
3.1. Development and Fabrication of the SIS
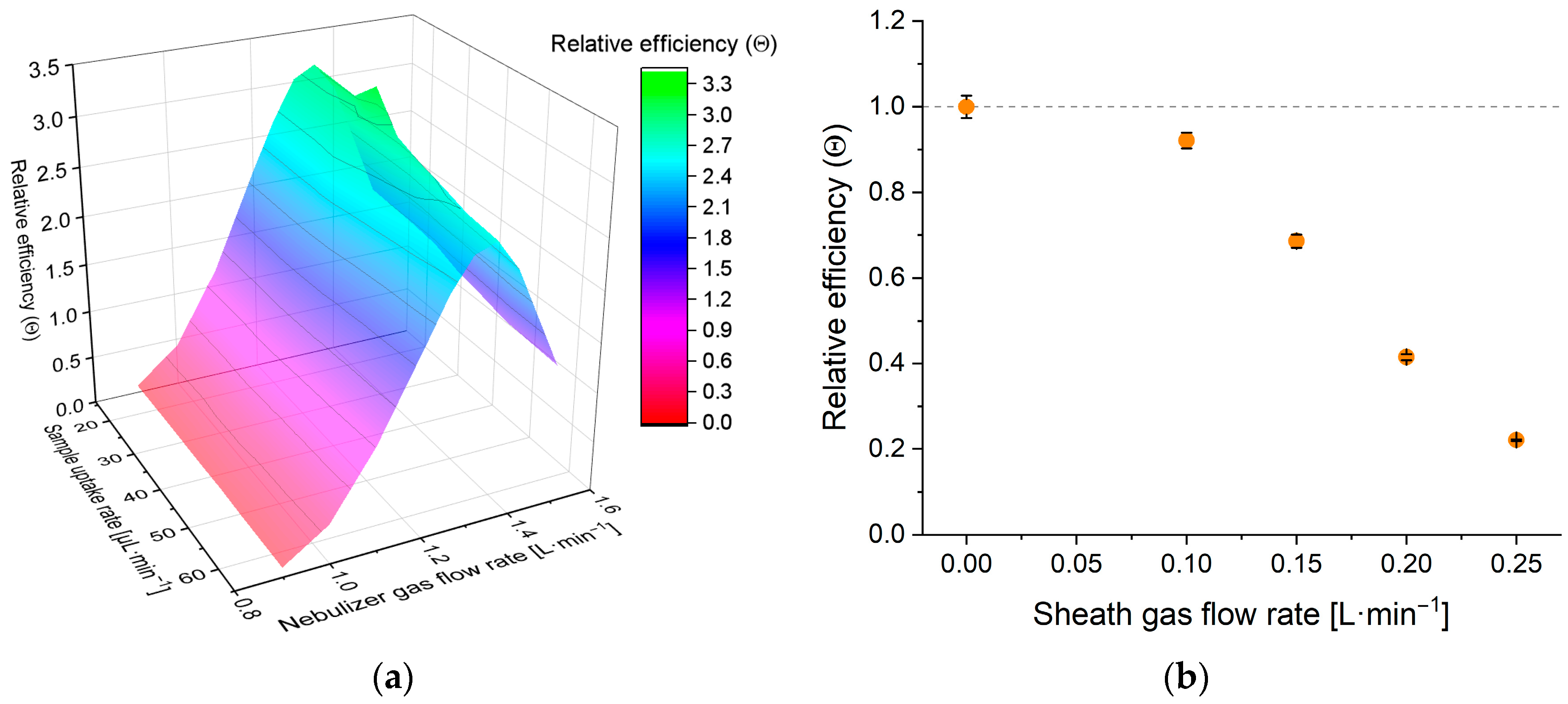
3.2. Performance Assessment
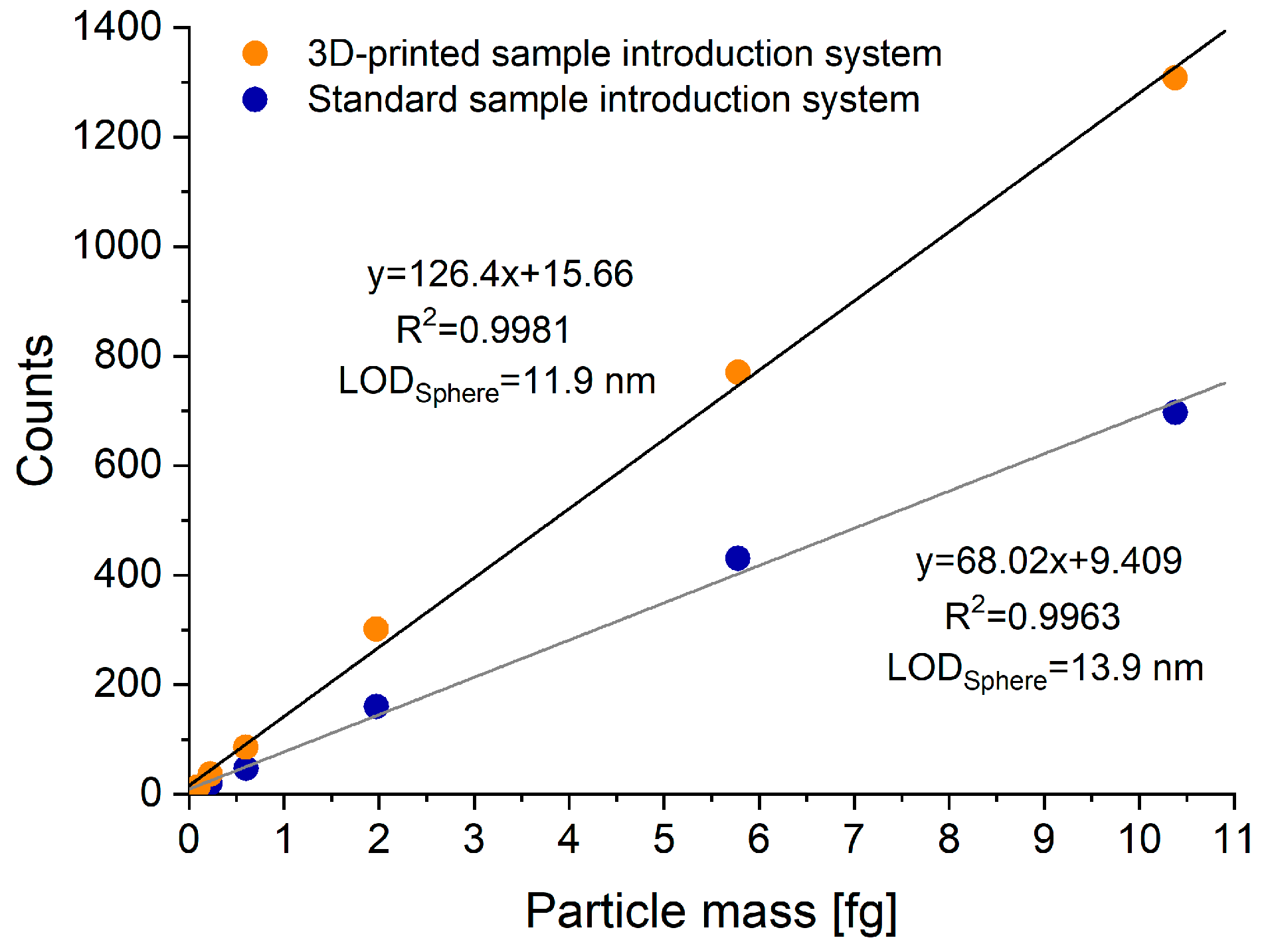
3.2.1. Performance with True Solutions
3.2.2. Performance with Dispersions
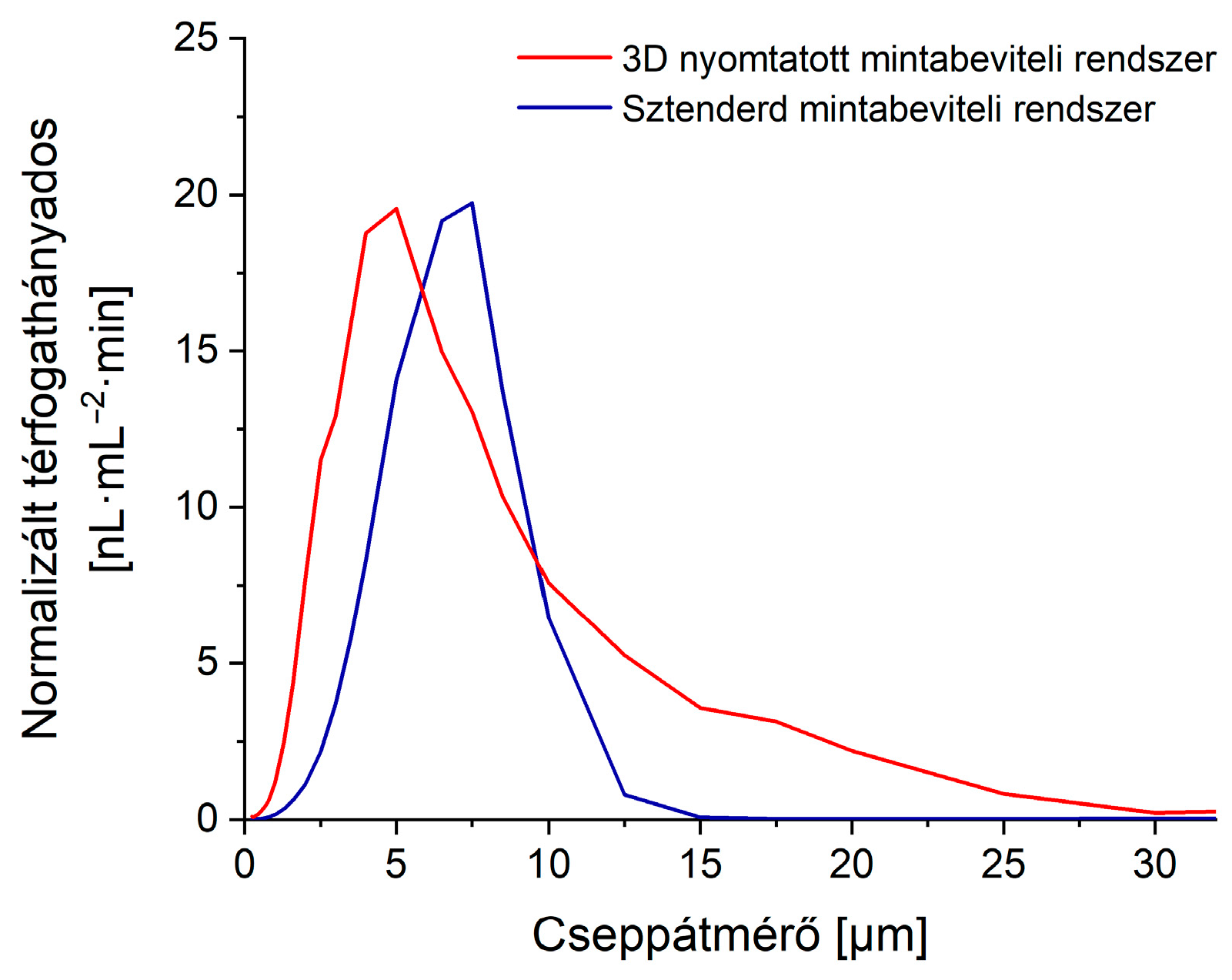
3.3. Comparison with the Standard Sample Introduction System
3.3.1. Aerosol Characteristics
3.3.2. Relative Particle Detection Efficiencies
3.4. Applications in Single Cell ICP-MS Analysis
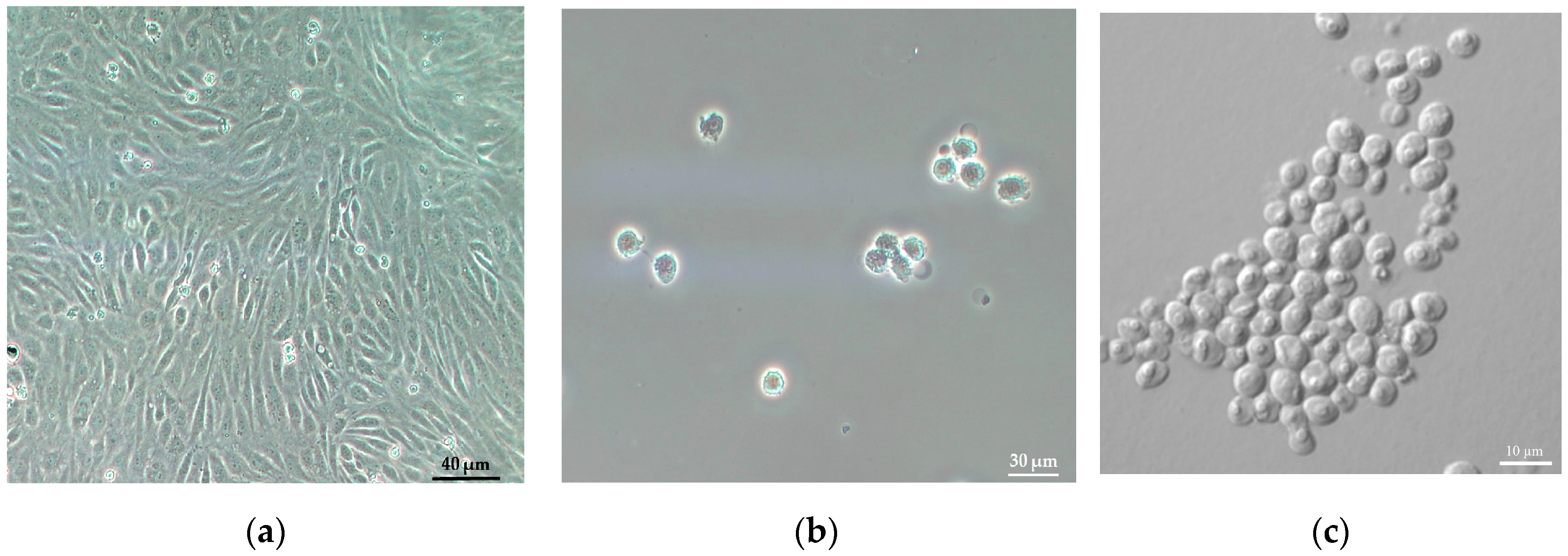
3.4.1. Monitoring the Effects of Nickel Ions on the Proliferation of Chlorella Algal Cells
3.4.2. Counting of Human Endothelial Cells
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vanhaecke, F.; Degryse, P. Isotopic Analysis: Fundamentals and Applications Using ICP-MS, 1st ed.; Wiley-VCH: Weinheim, Germany, 2012; pp. 31–517. [Google Scholar]
- Degueldre, C.; Favarger, P.Y. Colloid analysis by single particle inductively coupled plasma-mass spectroscopy: A feasibility study. Colloids Surf. A Physicochem. Eng. 2003, 217, 137–142. [Google Scholar] [CrossRef]
- Galbács, G.; Kéri, A.; Kohut, A.; Veres, M.; Geretovszky, Z. Nanoparticles in analytical laser and plasma spectroscopy—A review of recent developments in methodology and applications. J. Anal. At. Spectrom. 2021, 36, 1826–1872. [Google Scholar] [CrossRef]
- Montano, M.D.; Olesik, J.W.; Barber, A.G.; Challis, K.; Ranville, J.F. Single particle ICP-MS: Advances toward routine analysis of nanomaterials. Anal. Bioanal. Chem. 2016, 408, 5053–5074. [Google Scholar] [CrossRef] [PubMed]
- Kálomista, I.; Kéri, A.; Ungor, D.; Csapó, E.; Dékány, I.; Prohaska, T.; Galbács, G. Dimensional characterization of gold nanorods by combining millisecond and microsecond temporal resolution single particle ICP-MS measurements. J. Anal. At. Spectrom. 2017, 32, 2455–2462. [Google Scholar] [CrossRef]
- Kéri, A.; Kálomista, I.; Ungor, D.; Bélteki, Á.; Csapó, E.; Dékány, I.; Prohaska, T.; Galbács, G. Determination of the structure and composition of Au-Ag bimetallic spherical nanoparticles using single particle ICP-MS measurements performed with normal and high temporal resolution. Talanta 2018, 179, 193–199. [Google Scholar] [CrossRef]
- Kéri, A.; Sápi, A.; Ungor, D.; Sebők, D.; Csapó, E.; Kónya, Z.; Galbács, G. Porosity determination of nano- and sub-micron particles by single particle inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2020, 35, 1139–1147. [Google Scholar] [CrossRef]
- Sápi, A.; Kéri, A.; Kálomista, I.; Dobó, D.G.; Szamosvölgyi, Á.; Juhász, K.L.; Kukovecz, Á.; Kónya, Z.; Galbács, G. Determination of the platinum concentration of a Pt/silica nanocomposite decorated with ultra small Pt nanoparticles using single particle inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2017, 32, 996–1003. [Google Scholar] [CrossRef]
- Nomizu, T.; Kaneco, S.; Tanaka, T.; Ito, D.; Kawaguchi, H. Determination of calcium content in individual biological cells by inductively coupled plasma atomic emission spectrometry. Anal. Chem. 1994, 66, 3000–3004. [Google Scholar] [CrossRef]
- Miyashita, S.; Fujii, S.; Shigeta, K.; Inagaki, K. Chapter 5: Single cell analysis by using ICP-MS. In Metallomics; Ogra, Y., Hirata, T., Eds.; Springer: New York, NY, USA, 2017; pp. 107–124. [Google Scholar]
- Santos da Silva, A.B.; Arruda, M.A.Z. Single-cell ICP-MS to address the role of trace elements at a cellular level. J. Trace Elem. Med. Biol. 2023, 75, 127086. [Google Scholar]
- Corte-Rodríguez, M.; Álvarez-Fernández, R.; García-Cancela, P.; Montes-Bayón, M.; Bettmer, J. Single cell ICP-MS using online sample introduction systems: Current developments and remaining challenges. Trends Anal. Chem. 2020, 132, 116042. [Google Scholar] [CrossRef]
- Ho, K.S.; Chan, W.T. Time-resolved ICP-MS measurement for single-cell analysis and on-line cytometry. J. Anal. At. Spectrom. 2010, 25, 1114–1122. [Google Scholar] [CrossRef]
- Sneddon, J. Analytical Spectroscopy Library Volume 4: Sample Introduction in Atomic Spectroscopy, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 1–101. [Google Scholar]
- Browner, R.F.; Boorn, A.W. Sample Introduction: The Achilles’ heel of atomic spectroscopy? Anal. Chem. 1984, 56, 786–798. [Google Scholar] [CrossRef]
- Thomas, R. Practical Guide to ICP-MS: A Tutorial for Beginners, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 1–376. [Google Scholar]
- Miyashita, S.; Groombridge, A.S.; Fujii, S.; Minoda, A.; Takatsu, A.; Hioki, A.; Chiba, K.; Inagaki, K. Highly efficient single-cell analysis of microbial cells by time-resolved inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2014, 29, 1598–1606. [Google Scholar] [CrossRef]
- Tharaud, M.; Louvat, P.; Benetti, M.F. Detection of nanoparticles by single-particle ICP-MS with complete transport efficiency through direct nebulization at few-microlitres-per-minute uptake rates. Anal. Bioanal. Chem. 2021, 413, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Sharp, B.L. Pneumatic nebulisers and spray chambers for inductively coupled plasma spectrometry. A review. J. Anal. At. Spectrom. 1988, 3, 613–652. [Google Scholar] [CrossRef]
- Todolí, J.L.; Mermet, J.M. Evaluation of a direct injection high-efficiency nebulizer (DIHEN) by comparison with a high-efficiency nebulizer (HEN) coupled to a cyclonic spray chamber as a liquid sample introduction system for ICP-AES. J. Anal. At. Spectrom. 2001, 16, 514–520. [Google Scholar] [CrossRef]
- Todolí, J.L.; Mermet, J.M. Sample introduction systems for the analysis of liquid microsamples by ICP-AES and ICP-MS. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 239–283. [Google Scholar] [CrossRef]
- Lin, F.; Miyashita, S.; Inagaki, K.; Liu, Y.H.; Hsu, I. H Evaluation of three different sample introduction systems for single-particle inductively coupled plasma mass spectrometry (spICP-MS) applications. J. Anal. At. Spectrom. 2019, 34, 401–406. [Google Scholar] [CrossRef]
- Bings, N.H.; Orlandini von Niessen, J.O.; Schaper, J.N. Liquid sample introduction in inductively coupled plasma atomic emission and mass spectrometry—Critical review. Spectrochim. Acta Part B At. Spectrosc. 2014, 100, 14–37. [Google Scholar] [CrossRef]
- Inagaki, K.; Fujii, S.; Takatsu, A.; Chiba, K. High performance concentric nebulizer for low-flow rate liquid sample introduction to ICP-MS. J. Anal. At. Spectrom. 2011, 26, 623–630. [Google Scholar] [CrossRef]
- Khedkar, N.K.; Sonawane, C.; Chandras, A.; Kulkarni, A.; Sawant, R. Experimental and numerical investigation of Cross-Flow nebulizer for developing optimized suction pressure. Mater. Today Proc. 2022, 59, 617–622. [Google Scholar] [CrossRef]
- Rösch, M.; Cziczo, D.J. Aqueous particle generation with a 3D printed nebulizer. Atmos. Meas. Tech. 2020, 13, 6807–6812. [Google Scholar] [CrossRef]
- Thompson, D.F. Rapid production of cyclonic spray chambers for inductively coupled plasma applications using low cost 3D printer technology. J. Anal. At. Spectrom. 2014, 29, 2262–2266. [Google Scholar] [CrossRef]
- Geertsen, V.; Barruet, E.; Taché, O. 3D printing for cyclonic spray chambers in ICP spectrometry. J. Anal. At. Spectrom. 2015, 30, 1369–1376. [Google Scholar] [CrossRef][Green Version]
- Carcia-Montoto, V.; Mallet, S.; Arnaudguilhem, C.; Christensen, J.H.; Bouyssiere, B. 3D-printed total consumption microflow nebuliser development for trace element analysis in organic matrices via inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2020, 35, 1552–1557. [Google Scholar] [CrossRef]
- Aladese, A.D.; Jeong, H.H. Recent developments in 3D printing of droplet-based microfluidics. Biochip J. 2021, 15, 313–333. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Floch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef]
- Kajner, G. Development and Application of a 3D-Printed Sample Introduction System Suitable for the Analysis of Organic and Inorganic Particle Dispersions by ICP-MS. Master’s Thesis, University of Szeged, Szeged, Hungary, 7 January 2023. [Google Scholar]
- Kajner, G.; Bélteki, Á.; Cseh, M.; Vajda, B.; Geretovszky, Z.; Papp, E.; Braun, M.; Galbács, G. Single particle ICP-MS analysis using 3D-printed nebulizers and spray chambers. In Proceedings of the 2023 European Winter Conference on Plasma Spectrochemistry, Ljubljana, Slovenia, 29 January 2023. [Google Scholar]
- Mészáros, M.; Phan, T.H.M.; Vigh, J.P.; Porkoláb, G.; Kocsis, A.; Páli, E.K.; Polgár, T.F.; Walter, F.R.; Bolognin, S.; Schwamborn, J.C.; et al. Targeting human endothelial cells with glutathione and alanine increases the crossing of a polypeptide nanocarrier through a blood-brain barrier model and entry to human brain organoids. Cells 2023, 12, 503. [Google Scholar] [CrossRef]
- Lee, S.; Bi, X.; Reed, R.B.; Ranville, J.F.; Herckes, P.; Westerhoff, P. Nanoparticle size detection limits by single particle ICP-MS for 40 elements. Envorion. Sci. Technol. 2014, 48, 10291–10300. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sust. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Qi, Z.; Liu, K.J. The interaction of zinc and the blood-brain barrier under physiological and ischemic conditions. Toxicol. Appl. Pharmacol. 2019, 364, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Yokel, R.A. Manganese flux across the blood–brain barrier. Neuromol. Med. 2009, 11, 297–310. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kajner, G.; Bélteki, Á.; Cseh, M.; Geretovszky, Z.; Ajtai, T.; Barna, L.; Deli, M.A.; Pap, B.; Maróti, G.; Galbács, G. Design, Optimization, and Application of a 3D-Printed Polymer Sample Introduction System for the ICP-MS Analysis of Nanoparticles and Cells. Nanomaterials 2023, 13, 3018. https://doi.org/10.3390/nano13233018
Kajner G, Bélteki Á, Cseh M, Geretovszky Z, Ajtai T, Barna L, Deli MA, Pap B, Maróti G, Galbács G. Design, Optimization, and Application of a 3D-Printed Polymer Sample Introduction System for the ICP-MS Analysis of Nanoparticles and Cells. Nanomaterials. 2023; 13(23):3018. https://doi.org/10.3390/nano13233018
Chicago/Turabian StyleKajner, Gyula, Ádám Bélteki, Martin Cseh, Zsolt Geretovszky, Tibor Ajtai, Lilla Barna, Mária A. Deli, Bernadett Pap, Gergely Maróti, and Gábor Galbács. 2023. "Design, Optimization, and Application of a 3D-Printed Polymer Sample Introduction System for the ICP-MS Analysis of Nanoparticles and Cells" Nanomaterials 13, no. 23: 3018. https://doi.org/10.3390/nano13233018
APA StyleKajner, G., Bélteki, Á., Cseh, M., Geretovszky, Z., Ajtai, T., Barna, L., Deli, M. A., Pap, B., Maróti, G., & Galbács, G. (2023). Design, Optimization, and Application of a 3D-Printed Polymer Sample Introduction System for the ICP-MS Analysis of Nanoparticles and Cells. Nanomaterials, 13(23), 3018. https://doi.org/10.3390/nano13233018






