Recent Development and Applications of Stretchable SERS Substrates
Abstract
:1. Introduction
2. A Roadmap to Stretchable SERS Substrates
2.1. Fundamentals of the SERS Mechanism: A Brief Overview
2.2. Traditional Rigid SERS Substrate
2.3. Flexible SERS Substrate
2.4. Stretchable SERS Substrate
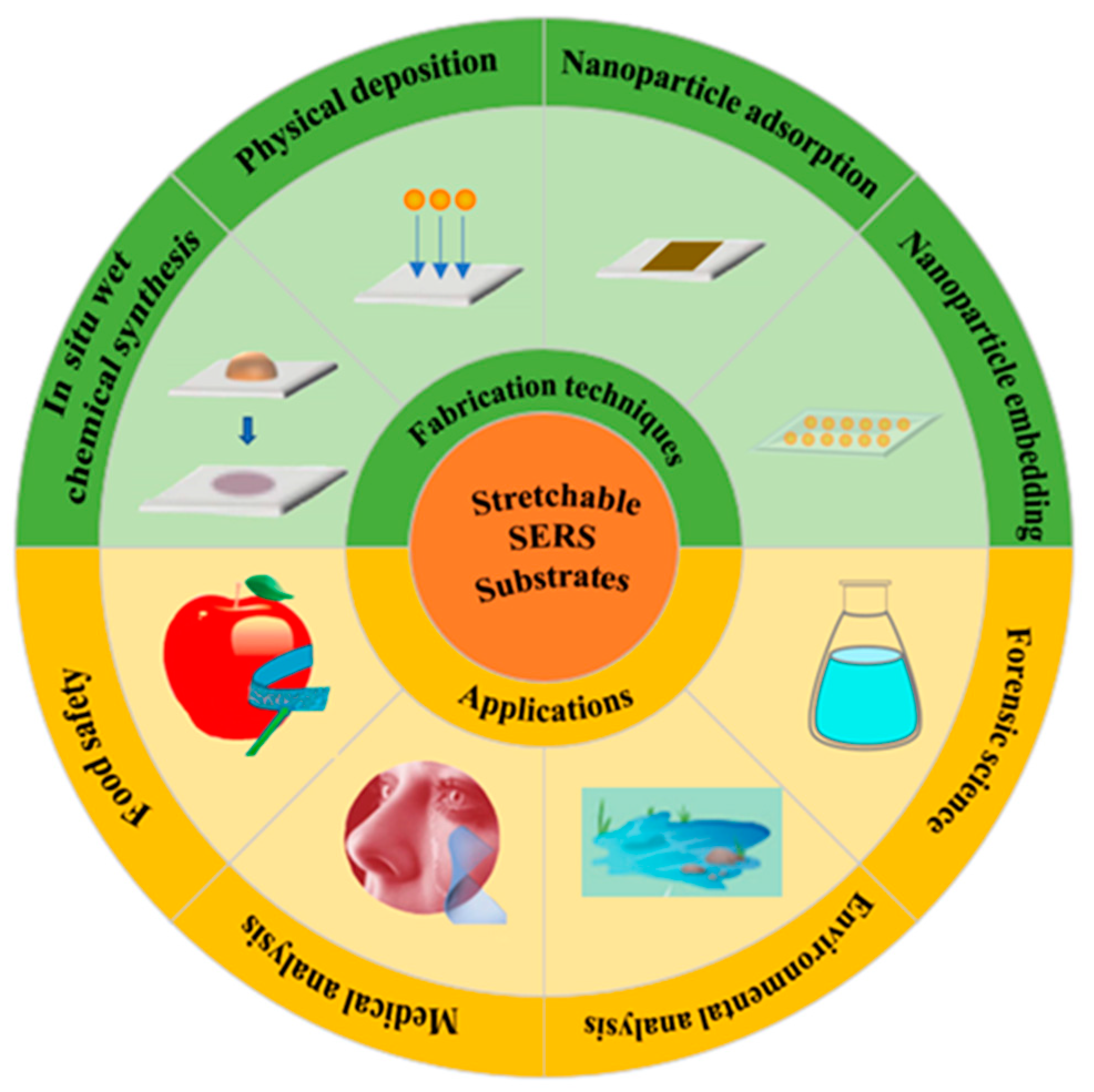
3. Fabrication of Stretchable SERS Substrates
3.1. Stretchable Supporting Materials
| Stretchable Substrate | Physical Property | Advantage | Disadvantage | Ref. | ||
|---|---|---|---|---|---|---|
| Polymer elastomer | PDMS | 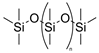 | ~150% elongation | Easy to obtain, low cost, good flexibility, and optical transparency (~100%) | Not resistant to high temperature | [53,54,55,56] |
| PCL | 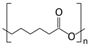 | ~650% elongation | Good transparency (~90%) and temperature stability (9.62%) | Insufficient mechanical strength | [57] | |
| PMMA | 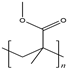 | ~3160 MPa elastic modulus | Excellent optical transparency (~92%) and good flexibility | Low surface hardness, easy to scratch | [58,59] | |
| PVA | 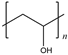 | 4400~5400 MPa elastic modulus | Ultrathin, flexible, stretchable, adhesive, and bio-integrable | Poor water resistance | [47] | |
| PC | 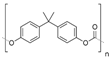 | ~2200 MPa elastic modulus | High tensile, flexural, and compressive strengths | Not resistant to strong alkali | [60] | |
| PVP | 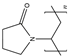 | - | Biocompatible, highly plastic, and adhesive | - | [61] | |
| Textiles | - | ~30% elongation | Washable and reusable | Not resistant to high temperature | [50] | |
| Silicone rubber | - | 200~900 MPa elastic modulus | Reversible gap adjustment | Low tensile strength | [19] | |
| Electrical tape | - | ~150% elongation | Paste and peel off and cost-effective | Non-biodegradable | [51] | |
| Hydrogels | Poly (acrylic acid) | 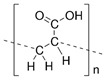 | 1~100 kPa elastic modulus | Stimulus responsiveness, large volume change, and biocompatibility | - | [52] |
3.2. In Situ Wet Chemical Synthesis of Plasmonic Nanostructures

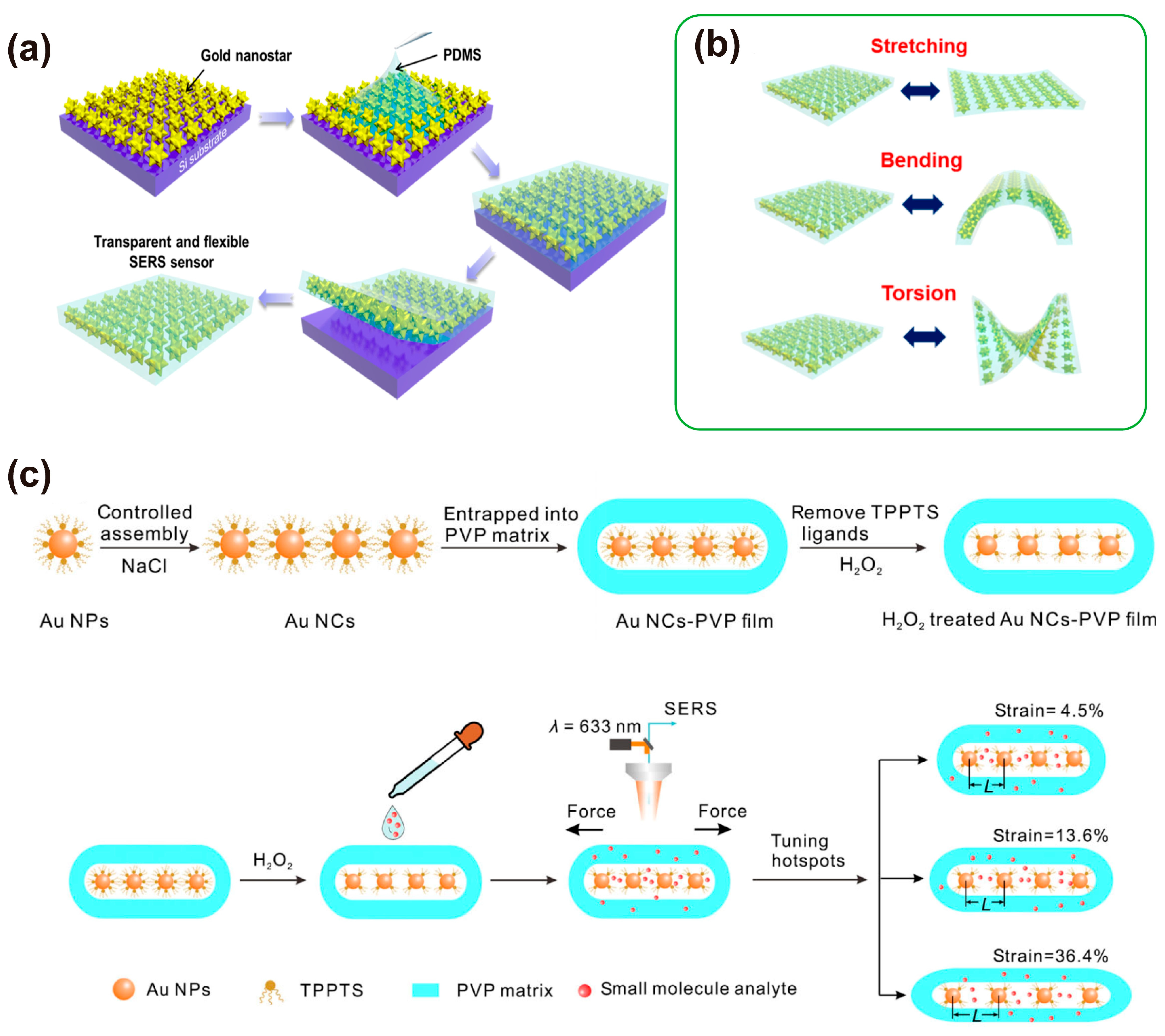
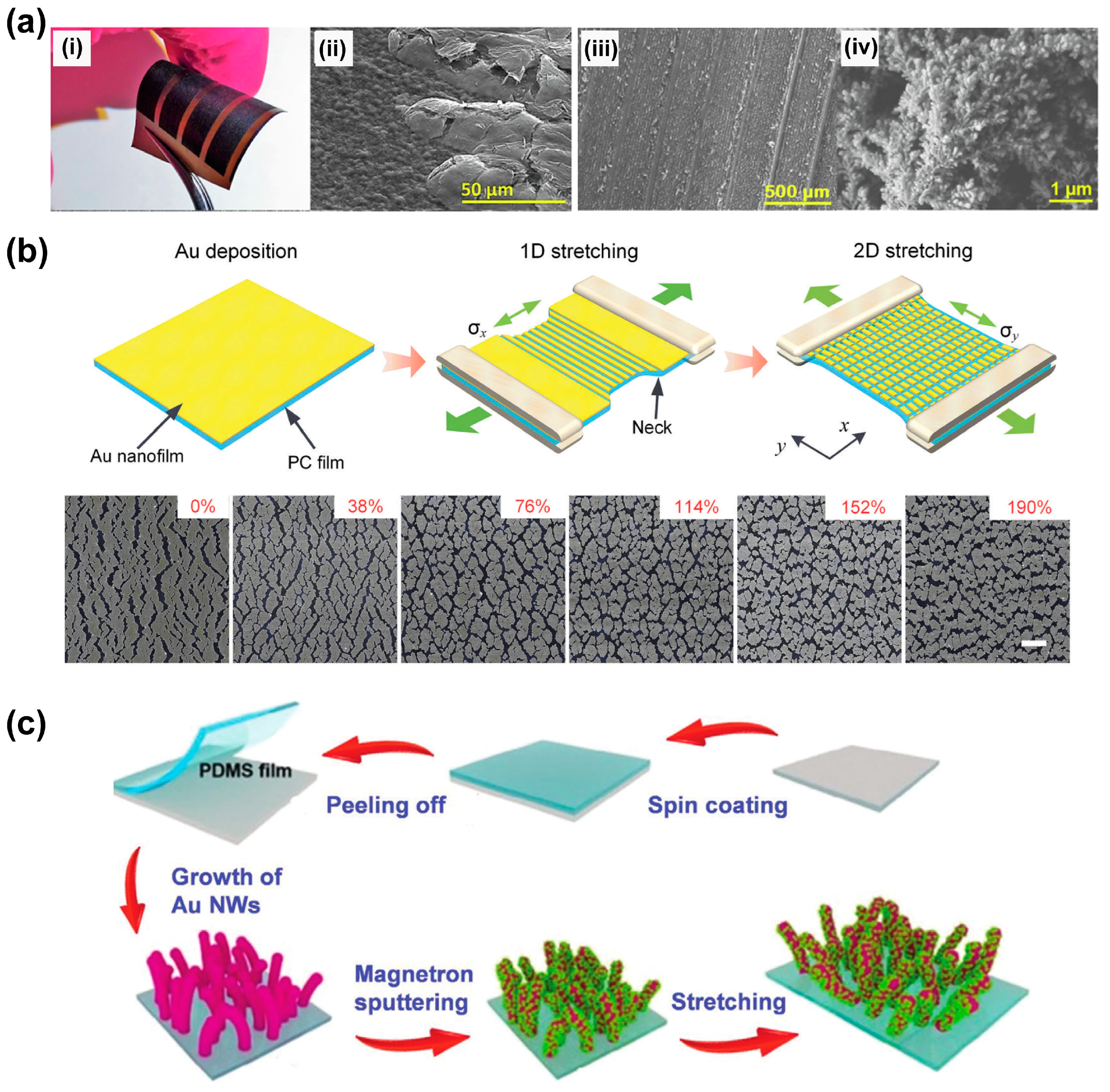
3.3. Physical Deposition Method
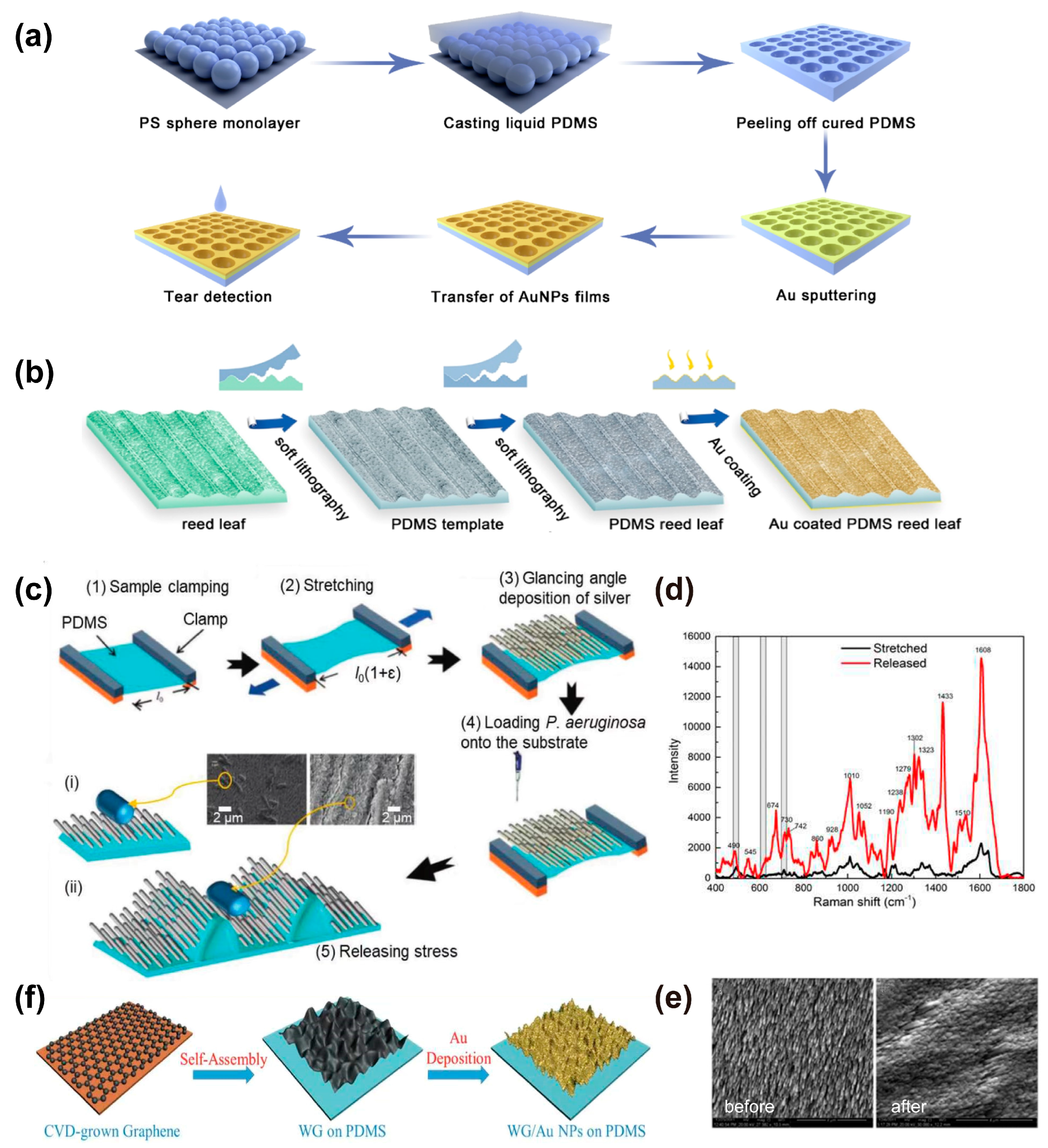
3.4. Physical Adsorption Method
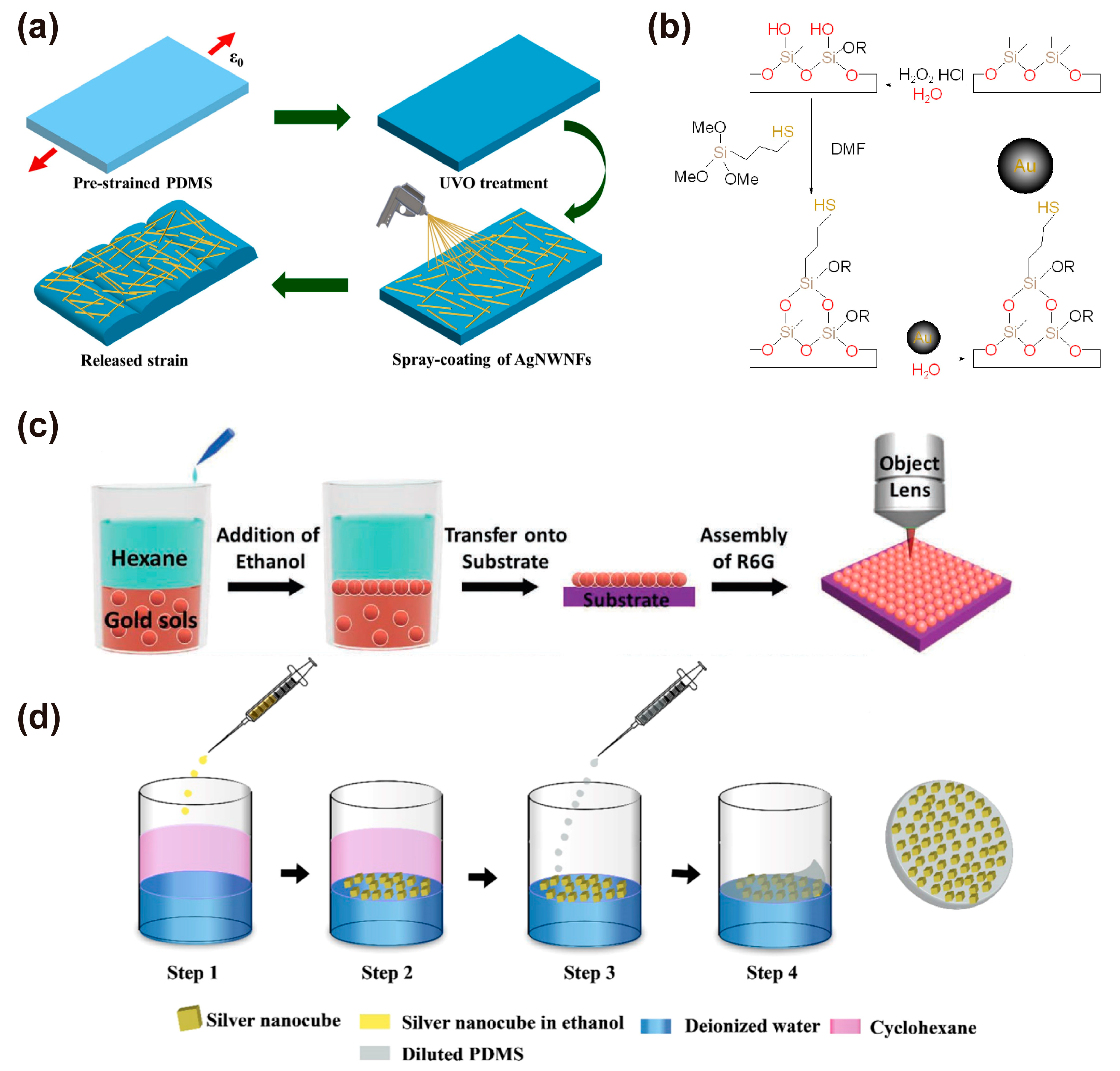
3.5. Embedding of Nanomaterials
3.6. Other Methods
4. Applications of Stretchable SERS Substrates
4.1. Environmental Analyses
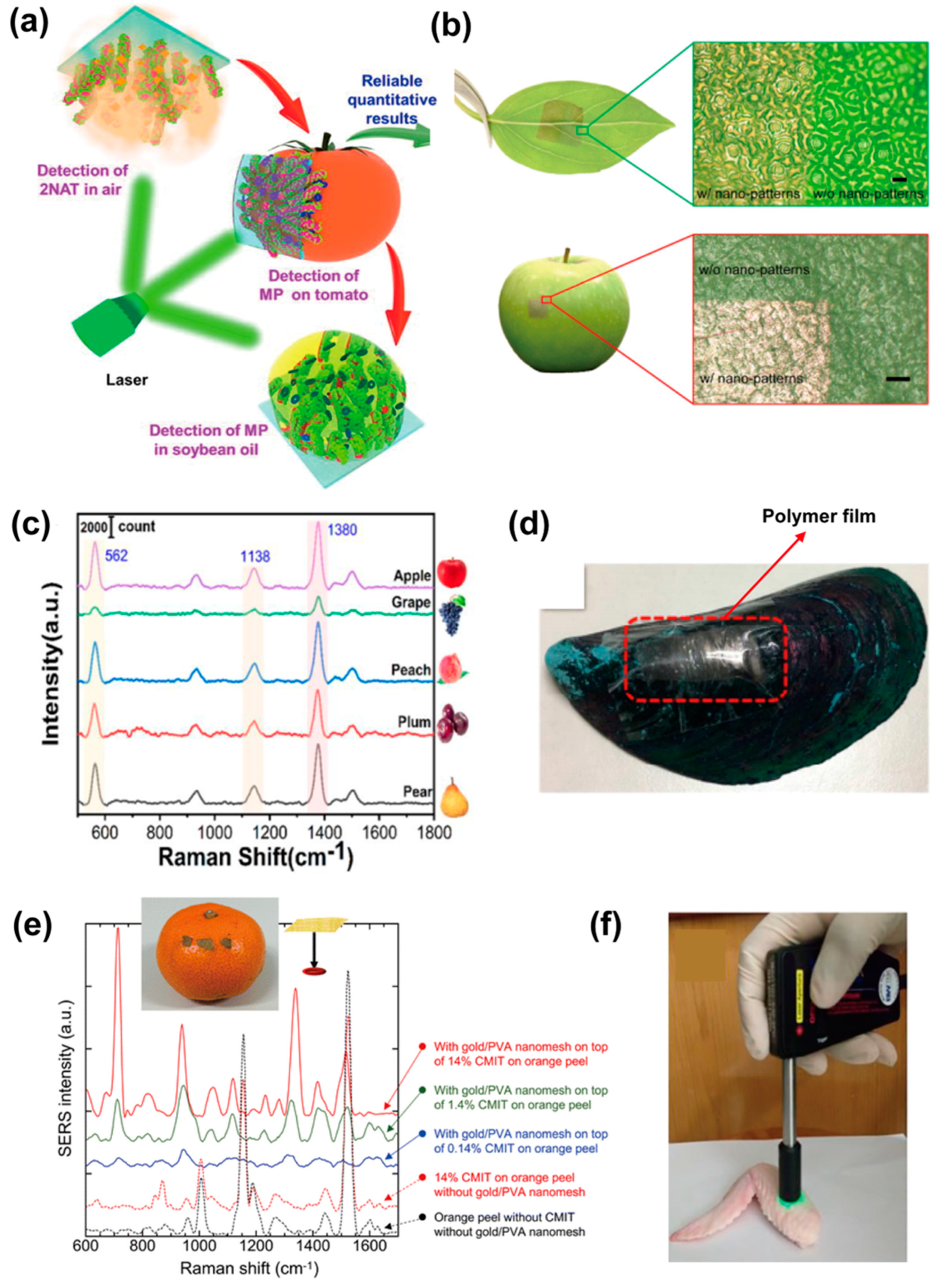
4.2. Food Safety Monitoring
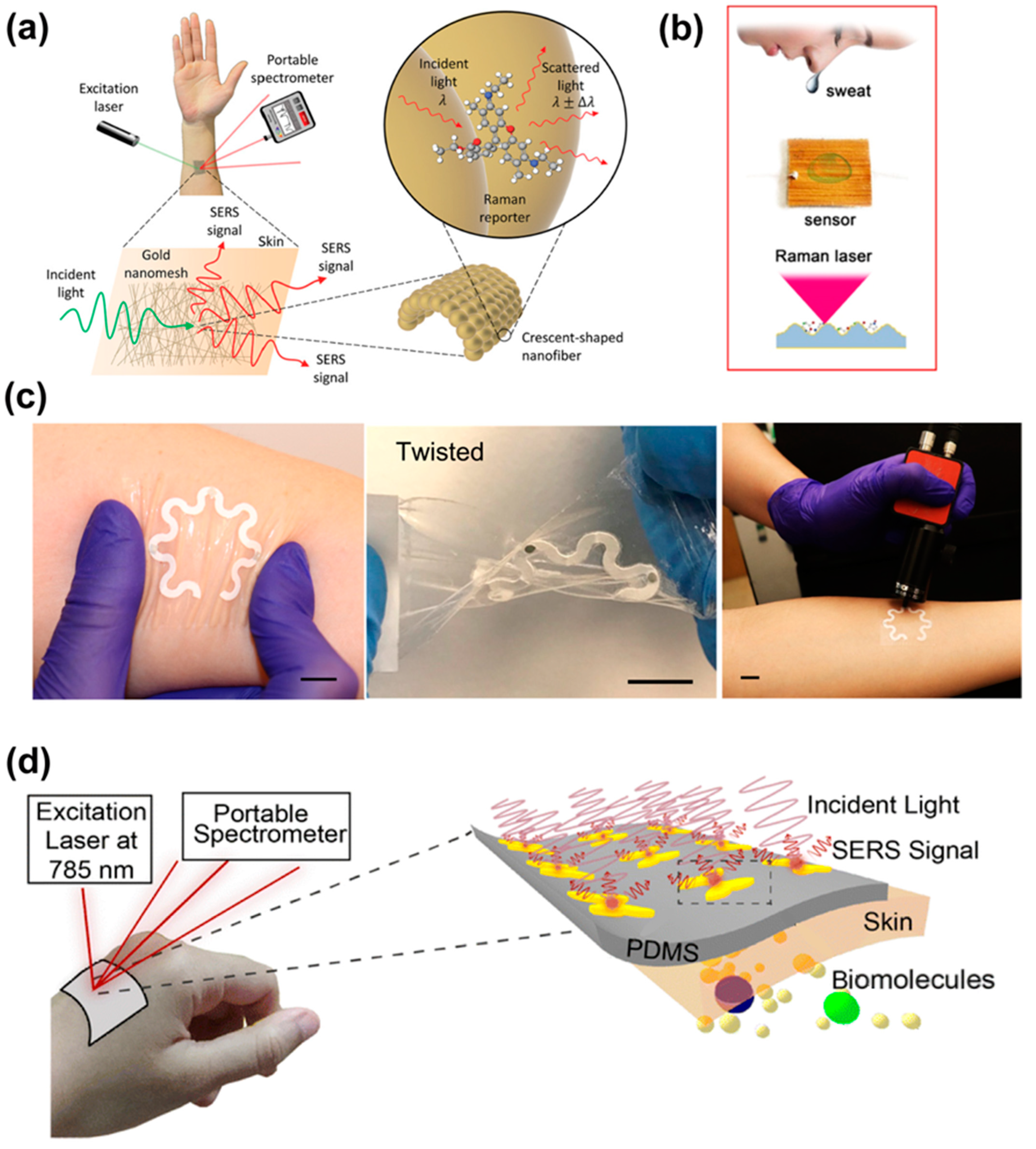
4.3. Biomedical Applications
4.4. Other Applications
5. Conclusions and Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Jeanmaire, D.; Van Duyne, R.P. Surface Raman Spectroelectrochemistry Part I. Heterocyclic, Aromatic, and Aliphatic Amines Adsorbed on the Anodized Silver Electrode. J. Electroanal. Chem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Kwart, H.; George, T.J. Anomalously Intense Raman Spectra of Pyridine at a Silver Electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar]
- Song, D.; Yang, R.; Long, F.; Zhu, A. ScienceDirect Applications of Magnetic Nanoparticles in Surface-Enhanced Raman Scattering (SERS) Detection of Environmental Pollutants. J. Environ. Sci. 2018, 80, 14–34. [Google Scholar] [CrossRef]
- Luo, W.; Chen, M.; Hao, N.; Huang, X.; Zhao, X.; Zhu, Y.; Yang, H.; Chen, X. In Situ Synthesis of Gold Nanoparticles on Pseudo- Paper Films as Flexible SERS Substrate for Sensitive Detection of Surface Organic Residues. Talanta 2018, 197, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Pu, H.; Sun, D. Trends in Food Science & Technology Multifunctional Cellulose Based Substrates for SERS Smart Sensing: Principles, Applications and Emerging Trends for Food Safety Detection. Trends Food Sci. Technol. 2021, 110, 304–320. [Google Scholar] [CrossRef]
- Saviñon-flores, F.; Erika, M.; Lopez-Castanos, M.; Carabarin-lima, A.; Karen, A.L.; Gonz, M.A.; Alia, M. A Review on SERS-Based Detection of Human Virus Infections: Influenza and Coronavirus. Biosensors 2021, 11, 66. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Rahmanian, V.; Kalashgrani, M.Y.; Gholami, A.; Omidifar, N.; Chiang, W.H. Highly Sensitive Flexible SERS-Based Sensing Platform for Detection of COVID-19. Biosensors 2022, 12, 466. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, X.; Li, S.; Ding, F.; Li, N.; Meng, S.; Li, R.; Qi, J.; Liu, Q.; Liu, G.L. Plasmonic Nano-Arrays for Ultrasensitive Bio-Sensing. Nanophotonics 2018, 7, 1517–1531. [Google Scholar]
- Huang, Z.; Zhang, A.; Zhang, Q.; Cui, D. Nanomaterial-Based SERS Sensing Technology for Biomedical Application. J. Mater. Chem. B 2019, 7, 3755–3774. [Google Scholar]
- Peng, W.; Wu, H. Flexible and Stretchable Photonic Sensors Based on Modulation of Light Transmission. Adv. Opt. Mater. 2019, 7, 1900329. [Google Scholar] [CrossRef]
- Sun, J.; Gong, L.; Wang, W.; Gong, Z.; Wang, D.; Fan, M. Surface-Enhanced Raman Spectroscopy for on-Site Analysis: A Review of Recent Developments. Luminescence 2020, 35, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.A.; Dina, N.E.; Cheng, H.; Valev, V.K.; Zhang, L. Surface-Enhanced Raman Spectroscopy for Bioanalysis and Diagnosis. Nanoscale 2021, 13, 11593–11634. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Ju, W.; Yang, H.Y.; Li, Z. Dimensional Design for Surface-Enhanced Raman Spectroscopy. ACS Mater. Au 2022, 2, 552–575. [Google Scholar] [CrossRef] [PubMed]
- Schatz, G.C.; Van Duyne, R.P. Surface-Enhanced Vibrational Spectroscopy Electromagnetic Mechanism of Surface-Enhanced Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Liu, H.; He, Y.; Cao, K. Flexible Surface-Enhanced Raman Scattering Substrates: A Review on Constructions, Applications, and Challenges. Adv. Mater. Interfaces 2021, 8, 2100982. [Google Scholar] [CrossRef]
- Song, L.; Chen, J.; Bin Xu, B.; Huang, Y. Flexible Plasmonic Biosensors for Healthcare Monitoring: Progress and Prospects. ACS Nano 2021, 15, 18822–18847. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.X.; Duan, G.; Xu, W.; Xu, C.; Jiang, J.; Yang, Z.; Wu, Y.; Pi, F. Flexible Surface-Enhanced Raman Scatting Substrates: Recent Advances in Their Principles, Design Strategies, Diversified Material Selections and Applications. Crit. Rev. Food Sci. Nutr. 2022, 10, 1–45. [Google Scholar] [CrossRef]
- Kang, H.; Heo, C.J.; Jeon, H.C.; Lee, S.Y.; Yang, S.M. Durable Plasmonic Cap Arrays on Flexible Substrate with Real-Time Optical Tunability for High-Fidelity SERS Devices. ACS Appl. Mater. Interfaces 2013, 5, 4569–4574. [Google Scholar] [CrossRef]
- Alexander, K.D.; Skinner, K.; Zhang, S.; Wei, H.; Lopez, R. Tunable SERS in Gold Nanorod Dimers through Strain Control on an Elastomeric Substrate. Nano Lett. 2010, 10, 4488–4493. [Google Scholar] [CrossRef] [PubMed]
- Raman, C.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Ge, K.; Hu, Y.; Li, G. Recent Progress on Solid Substrates for Surface-Enhanced Raman Spectroscopy Analysis. Biosensors 2022, 12, 194. [Google Scholar]
- Liu, H.; Yang, L.; Liu, J. Trends in Analytical Chemistry Three-Dimensional SERS Hot Spots for Chemical Sensing: Towards Developing a Practical Analyzer. Trends Anal. Chem. 2016, 80, 364–372. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman Spectra of Pyridine Adsorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Xie, L.; Zeng, H.; Zhu, J.; Zhang, Z.; Sun, H.; Xia, W.; Du, Y. State of the Art in Flexible SERS Sensors toward Label-Free and Onsite Detection: From Design to Applications. Nano Res. 2022, 15, 4374–4394. [Google Scholar] [CrossRef]
- Hemal, A.K.; Menon, M.; Vattikuti, R.; Vattikuti, P. Single Molecule Detection Using Surface-Enhanced Raman Scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667. [Google Scholar]
- Pandey, P.; Vongphachanh, S.; Yoon, J.; Kim, B.; Choi, C.J.; Sohn, J.I.; Hong, W.K. Silver Nanowire-Network-Film-Coated Soft Substrates with Wrinkled Surfaces for Use as Stretchable Surface Enhanced Raman Scattering Sensors. J. Alloys Compd. 2021, 859, 157862. [Google Scholar] [CrossRef]
- Pang, S.; Yang, T.; He, L. Review of Surface Enhanced Raman Spectroscopic (SERS) Detection of Synthetic Chemical Pesticides. TrAC–Trends Anal. Chem. 2016, 85, 73–82. [Google Scholar] [CrossRef]
- Horimoto, N.; Ishikawa, N.; Nakajima, A. Preparation of a SERS Substrate Using Vacuum-Synthesized Silver Nanoparticles. Chem. Phys. Lett. 2005, 413, 78–83. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Jiang, X.X.; Su, S.; Wei, X.P.; Lee, S.T.; He, Y. Silicon-Based Reproducible and Active Surface-Enhanced Raman Scattering Substrates for Sensitive, Specific, and Multiplex DNA Detection. Appl. Phys. Lett. 2012, 100, 203104. [Google Scholar] [CrossRef]
- Martín, A.; Wang, J.J.; Iacopino, D. Flexible SERS Active Substrates from Ordered Vertical Au Nanorod Arrays. RSC Adv. 2014, 4, 20038–20043. [Google Scholar] [CrossRef]
- Farrell, M.E.; Singamaneni, S.; Pellegrino, P.M. Flexible SERS-Based Substrates: Challenges and Opportunities toward an Army Relevant Universal Sensing Platform. Smart Biomed. Physiol. Sens. Technol. XII 2015, 9487, 45–60. [Google Scholar] [CrossRef]
- Zeng, P.; Ma, D.; Zheng, M.; Chen, L.; Liang, H.; Shu, Z.; Fu, Y.; Pan, M.; Zhao, Q.; Duan, H. Flexible Plasmonic Nanoparticle-on-a-Mirror Metasurface-Enabled Substrates for High-Quality Surface-Enhanced Raman Spectroscopy Detection. Colloid Interface Sci. Commun. 2023, 55, 100728. [Google Scholar] [CrossRef]
- Li, D.; Zhu, Z.; Sun, D. Visualization of the in Situ Distribution of Contents and Hydrogen Bonding States of Cellular Level Water in Apple Tissues by Confocal Raman Microscopy. Analyst 2019, 145, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hu, X.; Fu, F.; Liu, L.; Liu, X. DNA-Induced Assembly of Silver Nanoparticle Decorated Cellulose Nanofiber: A Flexible Surface-Enhanced Raman Spectroscopy Substrate for the Selective Charge Molecular Detection and Wipe Test of Pesticide Residues in Fruits. ACS Sustain. Chem. Eng. 2021, 9, 5217–5229. [Google Scholar] [CrossRef]
- Xu, J.; Shang, S.; Gao, W.; Zeng, P.; Jiang, S. Ag@ZIF-67 Decorated Cotton Fabric as Flexible, Stable and Sensitive SERS Substrate for Label-Free Detection of Phenol-Soluble Modulin. Cellulose 2021, 28, 7389–7404. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, W.; El-sayed, M.A. On the Universal Scaling Behavior of the Distance Decay of Plasmon Coupling in Metal Nanoparticle Pairs: A Plasmon Ruler Equation. Nano Lett. 2007, 7, 2080–2088. [Google Scholar] [CrossRef]
- Sönnichsen, C.; Reinhard, B.M.; Liphardt, J.; Alivisatos, A.P. A Molecular Ruler Based on Plasmon Coupling of Single Gold and Silver Nanoparticles. Nat. Biotechnol. 2005, 23, 741–745. [Google Scholar] [CrossRef]
- Tong, Q.; Wang, W.; Fan, Y.; Dong, L. Trends in Analytical Chemistry Recent Progressive Preparations and Applications of Silver-Based SERS Substrates. Trends Anal. Chem. 2018, 106, 246–258. [Google Scholar] [CrossRef]
- Shiohara, A.; Wang, Y.; Liz-marzán, L.M. Recent Approaches toward Creation of Hot Spots for SERS Detection. J. Photochem. Photobiol. C Photochem. Rev. 2014, 21, 2–25. [Google Scholar] [CrossRef]
- Chen, W.; Gui, X.; Zheng, Y.; Liang, B.; Lin, Z.; Zhao, C.; Chen, H.; Chen, Z.; Li, X.; Tang, Z. Synergistic Effects of Wrinkled Graphene and Plasmonics in Stretchable Hybrid Platform for Surface-Enhanced Raman Spectroscopy. Adv. Opt. Mater. 2017, 5, 1600715. [Google Scholar] [CrossRef]
- Won, S.; Kim, D.; Kim, J.; You, J.; Min, H. Flexible Nanocellulose-Based SERS Substrates for Fast Analysis of Hazardous Materials by Spiral Scanning. J. Hazard. Mater. 2021, 414, 125160. [Google Scholar] [CrossRef]
- Xu, W.; Ling, X.; Xiao, J.; Dresselhaus, M.S.; Kong, J.; Xu, H.; Liu, Z.; Zhang, J. Surface Enhanced Raman Spectroscopy on a Flat Graphene Surface. Proc. Natl. Acad. Sci. USA 2012, 109, 9281–9286. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Y.; Kannan, P.; Zhang, L.; Lin, Z.; Zhang, J.; Chen, T.; Guo, L. Detection of Pesticide Residues in Fruits and Vegetables Flexible and Adhesive SERS Active Tape for Rapid Detection of Pesticide Residues in Fruits and Vegetables. Anal. Chem. 2016, 88, 2149–2155. [Google Scholar] [CrossRef]
- Zhang, D.; Pu, H.; Huang, L.; Sun, D. Advances in Flexible Surface-Enhanced Raman Scattering (SERS) Substrates for Nondestructive Food Detection: Fundamentals and Recent Applications. Trends Food Sci. Technol. 2021, 109, 690–701. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y.; Tian, Y.; Gu, C.; Jiang, T. Contrastive Study of in Situ Sensing and Swabbing Detection Based on SERS-Active Gold Nanobush-PDMS Hybrid Film. J. Agric. Food Chem. 2021, 69, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Wang, M.; Jiang, S.; Zhang, L.; Yang, Z.; Li, L. Reliable Molecular Trace-Detection Based on Flexible SERS Substrate of Graphene/Ag-Nanoflowers/PMM. Sens. Actuators B Chem. 2017, 249, 439–450. [Google Scholar] [CrossRef]
- Kitahama, Y.; Pancorbo, P.M.; Segawa, H.; Marumi, M.; Xiao, T.; Hiramatsu, K.; Yang, W.; Goda, K. Place & Play SERS: Sample Collection and Preparation-Free Surface-Enhanced Raman Spectroscopy. Anal. Methods 2023, 15, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Qiao, Y.; Wei, M.; Gao, L.; Wang, L.; Yan, Y.; Li, H. High-Sensitive Imprinted Membranes Based on Surface-Enhanced Raman Scattering for Selective Detection of Antibiotics in Water. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2019, 222, 117116. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Zhang, J.R.; Han, D.D.; Zhang, Y.L.; Sun, H.B. Versatile Electronic Skins with Biomimetic Micronanostructures Fabricated Using Natural Reed Leaves as Templates. ACS Appl. Mater. Interfaces 2019, 11, 38084–38091. [Google Scholar] [CrossRef]
- Garg, A.; Nam, W.; Zhou, W. Reusable Surface-Enhanced Raman Spectroscopy Membranes and Textiles via Template-Assisted Self-Assembly and Micro/Nanoimprinting. ACS Appl. Mater. Interfaces 2020, 12, 56290–56299. [Google Scholar] [CrossRef]
- Dai, X.; Xue, D.; Liu, X.; Gu, C. Analytical Methods An Adhesive SERS Substrate Based on a Stretched Silver Nanowire-Tape for the in Situ Multicomponent Analysis of Pesticide Residues. Anal. Methods 2023, 15, 1261–1273. [Google Scholar] [CrossRef]
- Hamajima, S.; Mitomo, H.; Tani, T.; Matsuo, Y.; Niikura, K.; Naya, M.; Ijiro, K. Nanoscale Uniformity in the Active Tuning of a Plasmonic Array by Polymer Gel Volume Change. Nanoscale Adv. 2019, 1, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Yang, K.; Zhang, X.; Zhou, Z.; Xu, Y.; Xie, A.; Xue, C. Stretchable and Flexible Micro-Nano Substrates for SERS Detection of Organic Dyes. ACS Omega 2023, 8, 14541–14548. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Park, C.; Chung, K.; Lim, J.W.; Marques Mota, F.; Jeong, U.; Kim, D.H. Viable Stretchable Plasmonics Based on Unidirectional Nanoprisms. Nanoscale 2018, 10, 4105–4112. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, H.; Chen, H.; Zhang, W.; Chen, J. Stretchable Plasmonic Substrate with Tunable Resonances for Surface-Enhanced Raman Spectroscopy. J. Opt. 2015, 17, 114015. [Google Scholar] [CrossRef]
- Haque Chowdhury, M.A.; Tasnim, N.; Hossain, M.; Habib, A. Flexible, Stretchable, and Single-Molecule-Sensitive SERS-Active Sensor for Wearable Biosensing Applications. RSC Adv. 2023, 13, 20787–20798. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Z.; Tan, C.F.; Kang, N.; Chen, L.; Ren, L.; Thian, E.S.; Ho, G.W.; Ji, R.; Hong, M. Uniaxially Stretched Flexible Surface Plasmon Resonance Film for Versatile Surface Enhanced Raman Scattering Diagnostics. ACS Appl. Mater. Interfaces 2017, 9, 26341–26349. [Google Scholar] [CrossRef]
- Zhong, L.B.; Yin, J.; Zheng, Y.M.; Liu, Q.; Cheng, X.X.; Luo, F.H. Self-Assembly of Au Nanoparticles on PMMA Template as Flexible, Transparent, and Highly Active SERS Substrates. Anal. Chem. 2014, 86, 6262–6267. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, J.; Zhang, C.; Chen, C.; Xu, S.; Li, C.; Li, Z. Flexible and Stretchable SERS Substrate Based on a Pyramidal PMMA Structure Hybridized with Graphene Oxide Assivated AgNPs. Appl. Surf. Sci. 2018, 455, 1171–1178. [Google Scholar] [CrossRef]
- Li, K.; Zhang, N.; Zhang, T.; Wang, Z.; Chen, M.; Wu, T.; Ma, S.; Zhang, M.; Zhang, J.; Dinish, U.S.; et al. Formation of Ultra-Flexible, Conformal, and Nano-Patterned Photonic Surfaces: Via Polymer Cold-Drawing. J. Mater. Chem. C 2018, 6, 4649–4657. [Google Scholar] [CrossRef]
- Yan, X.; Chen, Q.; Song, Q.; Huo, Z.; Zhang, N.; Ma, M. Continuous Mechanical Tuning of Plasmonic Nanoassemblies for Tunable and Selective SERS Platforms. Nano Res. 2021, 14, 275–284. [Google Scholar] [CrossRef]
- Li, Z.; Huang, X.; Lu, G. Recent Developments of Flexible and Transparent SERS Substrates. J. Mater. Chem. C 2020, 8, 3956–3969. [Google Scholar] [CrossRef]
- Ying, B.; Liu, X. A Highly-Transparent Nanocellulose-Paper-Based Microfluidic Device. In Proceedings of the International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS), Kaohsiung, Taiwan, 11–15 November 2018. [Google Scholar]
- Wu, W.; Bian, Z.; Wang, W.; Wang, W.; Zhu, J. PDMS Gold Nanoparticle Composite Film-Based Silver Enhanced Colorimetric Detection of Cardiac Troponin I. Sens. Actuators B. Chem. 2010, 147, 298–303. [Google Scholar] [CrossRef]
- Fortuni, B.; Inose, T.; Uezono, S.; Toyouchi, S.; Umemoto, K.; Sekine, S.; Fujita, Y.; Ricci, M.; Lu, G.; Masuhara, A.; et al. In situ Synthesis of Au-Shelled Ag Nanoparticles on PDMS for Flexible, Long-Life, and Broad Spectrum-Sensitive SERS Substrates. Chem. Commun. 2017, 53, 11298–11301. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, U.; Caputo, R.; Kurylyak, Y.; Klein, G.; Chekini, M.; Umeton, C.; Bürgi, T. Growing Gold Nanoparticles on a Flexible Substrate to Enable Simple Mechanical Control of Their Plasmonic Coupling. J. Mater. Chem. C 2014, 2, 7927–7933. [Google Scholar] [CrossRef]
- Zhao, L.; Gu, W.; Zhang, C.; Shi, X.; Xian, Y. In Situ Regulation Nanoarchitecture of Au Nanoparticles/Reduced Graphene Oxide Colloid for Sensitive and Selective SERS Detection of Lead Ions. J. Colloid Interface Sci. 2016, 465, 279–285. [Google Scholar] [CrossRef]
- Zhang, L. Self-Assembly Ag Nanoparticle Monolayer Film as SERS Substrate for Pesticide Detection. Appl. Surf. Sci. 2013, 270, 292–294. [Google Scholar] [CrossRef]
- Leem, J.; Wang, M.C.; Kang, P.; Nam, S. Mechanically Self-Assembled, Three-Dimensional Graphene-Gold Hybrid Nanostructures for Advanced Nanoplasmonic Sensors. Nano Lett. 2015, 15, 7684–7690. [Google Scholar] [CrossRef]
- Wang, M.C.; Chun, S.; Han, R.S.; Ashraf, A.; Kang, P.; Nam, S. Heterogeneous, Three-Dimensional Texturing of Graphene. Nano Lett. 2015, 15, 1829–1835. [Google Scholar] [CrossRef]
- Li, T.; Xu, Y.; Feng, Y.; Zhang, C.; Yang, C. Self-Assembly of the Stretchable AuNPs@MoS2@GF Substrate for the SERS Application. Appl. Surf. Sci. 2017, 423, 1072–1079. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, M.; Zhou, F.; Liu, D.; Liu, G.; Duan, G.; Cai, W.; Li, Y. Physical Deposition Improved SERS Stability of Morphology Controlled Periodic Micro/Nanostructured Arrays Based on Colloidal Templates. Small 2015, 11, 844–853. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.; Cui, B.; Bravo-Vasquez, J.P.; Veres, T.; Fenniri, H. Nanoimprinted SERS-Active Substrates with Tunable Surface Plasmon Resonances. J. Phys. Chem. C 2007, 111, 6720–6723. [Google Scholar] [CrossRef]
- Durmanov, N.N.; Guliev, R.R.; Eremenko, A.V.; Boginskaya, I.A.; Ryzhikov, I.A.; Trifonova, E.A.; Putlyaev, E.V.; Mukhin, A.N.; Kalnov, S.L.; Balandina, M.V.; et al. Non-Labeled Selective Virus Detection with Novel SERS-Active Porous Silver Nanofilms Fabricated by Electron Beam Physical Vapor Deposition. Sens. Actuators B Chem. 2018, 257, 37–47. [Google Scholar] [CrossRef]
- Yao, X.; Jiang, S.; Luo, S.; Liu, B.; Huang, T.; Hu, S.; Zhu, J.; Wang, X.; Ren, B. Uniform Periodic Bowtie SERS Substrate with Narrow Nanogaps Obtained by Monitored Pulsed Electrodeposition. ACS Appl. Mater. Interfaces 2020, 12, 36505–36512. [Google Scholar] [CrossRef]
- Kahraman, M.; Daggumati, P.; Kurtulus, O.; Seker, E.; Wachsmann-Hogiu, S. Fabrication and Characterization of Flexible and Tunable Plasmonic Nanostructures. Sci. Rep. 2013, 3, 3396. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, Y.; Chen, M.; Xiao, X.; Zhang, T.; Wang, J.; Jiang, K.; Fan, S.; Li, Q. Highly Sensitive, Uniform, and Reproducible Surface-Enhanced Raman Spectroscopy Substrate with Nanometer-Scale Quasi-Periodic Nanostructures. ACS Appl. Mater. Interfaces 2017, 9, 32369–32376. [Google Scholar] [CrossRef]
- Celik, M.; Buyukserin, F. The Use of Anodized Alumina Molds for the Fabrication of Polymer Nanopillar Arrays as SERS Substrates with Tunable Properties. Vib. Spectrosc. 2019, 104, 102965. [Google Scholar] [CrossRef]
- Lee, S.; Ongko, A.; Kim, H.Y.; Yim, S. Sub-100nm Gold Nanohole-Enhanced Raman Scattering on Flexible PDMS Sheets. Nanotechnology 2016, 27, 315301. [Google Scholar] [CrossRef]
- Li, J.; Cui, X.; Yang, X.; Qiu, Y.; Li, Y.; Cao, H.; Wang, D.; He, W.; Feng, Y.; Yang, Z. Quantification of Uric Acid Concentration in Tears by Using PDMS Inverse Opal Structure Surface-Enhanced Raman Scattering Substrates: Application in Hyperuricemia. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2022, 278, 121326. [Google Scholar] [CrossRef]
- Kumar, P.; Khosla, R.; Soni, M.; Deva, D.; Sharma, S.K. A Highly Sensitive, Flexible SERS Sensor for Malachite Green Detection Based on Ag Decorated Microstructured PDMS Substrate Fabricated from Taro Leaf as Template. Sens. Actuators B Chem. 2017, 246, 477–486. [Google Scholar] [CrossRef]
- Qi, J.; Li, Y.; Yang, M.; Wu, Q.; Chen, Z.; Wang, W.; Lu, W.; Yu, X.; Xu, J.; Sun, Q. Large-Area High-Performance SERS Substrates with Deep Controllable Sub-10-Nm Gap Structure Fabricated by Depositing Au Film on the Cicada Wing. Nanoscale Res. Lett. 2013, 8, 437. [Google Scholar] [CrossRef]
- Yuan, W.; Wu, Y.; Zhang, Z.; Shi, G.; Han, W.; Li, K.; Gu, J.; Chen, C.; Ge, J.; Zhou, W.; et al. Optimization of Surface Enhanced Raman Scattering Performance Based on Ag Nanoparticle-Modified Vanadium-Titanium Nanorods with Tunable Nanogaps. Opt. Express 2022, 30, 38613. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lodhi, D.K.; Goel, P.; Neeti; Mishra, P.; Singh, J.P. Facile Method for Fabrication of Buckled PDMS Silver Nanorod Arrays as Active 3D SERS Cage for Bacterial Sensing. Chem. Commun. 2015, 51, 12411–12414. [Google Scholar] [CrossRef] [PubMed]
- Gabardo, C.M.; Yang, J.; Smith, N.J.; Adams-McGavin, R.C.; Soleymani, L. Programmable Wrinkling of Self-Assembled Nanoparticle Films on Shape Memory Polymers. ACS Nano 2016, 10, 8829–8836. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Chu, H.; Abell, J.; Tripp, A.; Zhao, Y. Flexible and Mechanical Strain Resistant Large Area SERS Active Substrates. Nanoscale 2012, 4, 3410–3414. [Google Scholar] [CrossRef]
- Ling, X.; Xie, L.; Fang, Y.; Xu, H.; Zhang, H.; Kong, J.; Dresselhaus, M.S.; Zhang, J.; Liu, Z. Can Graphene Be Used as a Substrate for Raman Enhancement? Nano Lett. 2010, 10, 553–561. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, T.H.; El-Said, W.A.; Lee, J.H.; Yang, L.; Conley, B.; Choi, J.-W.; Lee, K.-B. In Situ Detection of Neurotransmitters from Stem Cell-Derived Neural Interface at the Single-Cell Level via Graphene-Hybrid SERS Nanobiosensing. Nano Lett. 2020, 20, 7670–7679. [Google Scholar] [CrossRef]
- Zhang, W.; Man, P.; Wang, M.; Shi, Y.; Xu, Y.; Li, Z.; Yang, C.; Ma, B. Roles of Graphene Nanogap for the AgNFs Electrodeposition on the Woven Cu Net as Flexible Substrate and Its Application in SERS. Carbon 2018, 133, 300–305. [Google Scholar] [CrossRef]
- Aparicio-Martínez, E.; Estrada-Moreno, I.; Dominguez, R. Fabrication of Flexible Composite of Laser Reduced Graphene@Ag Dendrites as Active Material for Surface Enhanced Raman Spectroscopy. Mater. Lett. 2020, 277, 128380. [Google Scholar] [CrossRef]
- Xu, K.; Zhou, R.; Takei, K.; Hong, M. Toward Flexible Surface-Enhanced Raman Scattering (SERS) Sensors for Point-of-Care Diagnostics. Adv. Sci. 2019, 6, 1900925. [Google Scholar] [CrossRef]
- Xian, L.; You, R.; Lu, D.; Wu, C.; Feng, S.; Lu, Y. Surface-Modified Paper-Based SERS Substrates for Direct- Droplet Quantitative Determination of Trace Substances. Cellulose 2019, 6, 1483–1495. [Google Scholar] [CrossRef]
- Mir-simon, B.; Morla-folch, J.; Gisbert-quilis, P.; Chen, H.; Schatz, G.C.; Ratner, M.A.; Langer, J.; Novikov, S.M.; Liz-marzán, L.M.; Wen, J.; et al. Mechanical Control of the Plasmon Coupling with Au Nanoparticle Arrays Fixed on the Elastomeric Film via Chemical Bond. Jpn. J. Appl. Phys. 2017, 56, 035201. [Google Scholar]
- Yan, S.; An, R.; Zou, Y.; Yang, N.; Zhang, Y. Fabrication of Polymer Colloidal/Au Composite Nanofilms for Stable and Reusable SERS-Active Substrates with Highly-Dense Hotspots. Sens. Actuators B Chem. 2020, 302, 127107. [Google Scholar] [CrossRef]
- Si, S.; Liang, W.; Sun, Y.; Huang, J.; Ma, W. Facile Fabrication of High-Density Sub-1-Nm Gaps from Au Nanoparticle Monolayers as Reproducible SERS Substrates. Adv. Funct. Mater. 2016, 26, 8137–8145. [Google Scholar] [CrossRef]
- Li, L.; Chin, W.S. Rapid Fabrication of a Flexible and Transparent Ag Nanocubes@PDMS Film as a SERS Substrate with High Performance. ACS Appl. Mater. Interfaces 2020, 12, 37538–37548. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.; Ko, H. Transparent and Flexible Surface-Enhanced Raman Scattering (SERS) Sensors Based on Gold Nanostar Arrays Embedded in Silicon Rubber Film. ACS Appl. Mater. Interfaces 2017, 9, 44088–44095. [Google Scholar] [CrossRef]
- Lu, G.; Li, H.; Zhang, H. Gold-Nanoparticle-Embedded Polydimethylsiloxane Elastomers for Highly Sensitive Raman Detection. Small 2012, 8, 1336–1340. [Google Scholar] [CrossRef]
- Vinod, M.; Gopchandran, K.G. Au, Ag and Au: Ag Colloidal Nanoparticles Synthesized by Pulsed Laser Ablation as SERS Substrates. Prog. Nat. Sci. Mater. Int. 2014, 24, 569–578. [Google Scholar] [CrossRef]
- Aleknavičienė, I.; Pabrėža, E.; Talaikis, M.; Jankunec, M.; Račiukaitis, G. Low-Cost SERS Substrate Featuring Laser-Ablated Amorphous Nanostructure. Appl. Surf. Sci. 2022, 571, 151248. [Google Scholar] [CrossRef]
- Cao, W.; Jiang, L.; Hu, J.; Wang, A.; Li, X.; Lu, Y. Optical Field Enhancement in Au Nanoparticle-Decorated Nanorod Arrays Prepared by Femtosecond Laser and Their Tunable Surface-Enhanced Raman Scattering Applications. ACS Appl. Mater. Interfaces 2018, 10, 1297–1305. [Google Scholar] [CrossRef]
- Ma, Y.; Du, Y.; Chen, Y.; Gu, C.; Jiang, T.; Wei, G.; Zhou, J. Intrinsic Raman Signal of Polymer Matrix Induced Quantitative Multiphase SERS Analysis Based on Stretched PDMS Film with Anchored Ag Nanoparticles/Au Nanowires. Chem. Eng. J. 2020, 381, 122710. [Google Scholar] [CrossRef]
- Chang, Y.; Lai, I.; Lu, L.; Chang, S.; Sun, A.Y.; Wan, D.; Chen, H. Wafer-Scale Nanocracks Enable Single-Molecule Detection and on-Site Analysis. Biosens. Bioelectron. 2022, 200, 113920. [Google Scholar] [CrossRef] [PubMed]
- Pomfret, M.B.; Pietron, J.J.; Owrutsky, J.C. Measurement of Benzenethiol Adsorption to Nanostructured Pt, Pd, and PtPd Films Using Raman Spectroelectrochemistry. Langmuir 2010, 26, 6809–6817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lai, K.; Zhou, J.; Wang, X.; Rasco, B.A.; Huang, Y. A Novel Approach to Determine Leucomalachite Green and Malachite Green in Fish Fillets with Surface-Enhanced Raman Spectroscopy (SERS) and Multivariate Analyses. J. Raman Spectrosc. 2012, 43, 1208–1213. [Google Scholar] [CrossRef]
- Mandic-Rajcevic, S.; Rubino, F.M.; Ariano, E.; Cottica, D.; Negri, S.; Colosio, C. Exposure Duration and Absorbed Dose Assessment in Pesticide-Exposed Agricultural Workers: Implications for Risk Assessment and Modeling. Int. J. Hyg. Environ. Health 2019, 222, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Memon, Q.U.A.; Wagan, S.A.; Chunyu, D.; Shuangxi, X.; Jingdong, L.; Damalas, C.A. Health Problems from Pesticide Exposure and Personal Protective Measures among Women Cotton Workers in Southern Pakistan. Sci. Total Environ. 2019, 685, 659–666. [Google Scholar] [CrossRef]
- Li, D.; Duan, H.; Ma, Y.; Deng, W. Headspace-Sampling Paper-Based Analytical Device for Colorimetric/Surface-Enhanced Raman Scattering Dual Sensing of Sulfur Dioxide in Wine. Anal. Chem. 2018, 90, 5719–5727. [Google Scholar] [CrossRef]
- Luong, H.N.; Nguyen, N.M.; Nguyen, L.N.T.; Tran, C.K.; Nguyen, T.T.; Duy, L.T.; Nguyen, N.P.; Huynh, T.M.H.; Tran, T.T.; Phan, B.T.; et al. Detection of Carbendazim by Utilizing Multi-Shaped Ag NPs Decorated ZnO NRs on Patterned Stretchable Substrate through Surface-Enhanced Raman Scattering Effect. Sens. Actuators A. Phys. 2022, 346, 113816. [Google Scholar] [CrossRef]
- Liang, L.; Zhao, X.; Wen, J.; Liu, J.; Zhang, F.; Guo, X.; Zhang, K.; Wang, A.; Gao, R.; Wang, Y.; et al. Flexible SERS Substrate with a Ag-SiO2Cosputtered Film for the Rapid and Convenient Detection of Thiram. Langmuir 2022, 38, 13753–13762. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, H.; Zhao, X.R.; Lv, K.; Zhu, T.; Xia, Y.; Yang, C.; Bai, C. Three-Dimensional SERS Sensor Based on the Sandwiched G@AgNPs@G/PDMS Film. Talanta 2021, 233, 122481. [Google Scholar] [CrossRef]
- Riswana Barveen, N.; Wang, T.J.; Chang, Y.H. Photochemical Synthesis of Au Nanostars on PMMA Films by Ethanol Action as Flexible SERS Substrates for In-Situ Detection of Antibiotics on Curved Surfaces. Chem. Eng. J. 2022, 431, 134240. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, Z.; Zhao, B.; Hou, R.; Kinchla, A.; Clark, J.M.; He, L. Real-Time and in Situ Monitoring of Pesticide Penetration in Edible Leaves by Surface-Enhanced Raman Scattering Mapping. Anal. Chem. 2016, 88, 5243–5250. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Barcelo, S.J.; Li, Z. SERS-Based Pesticide Detection by Using Nanofinger Sensors. Nanotechnology 2015, 26, 15502. [Google Scholar] [CrossRef] [PubMed]
- Walton, K.; Walker, R.; Van De Sandt, J.J.M.; Castell, J.V. The Application of In Vitro Data in the Derivation of the Acceptable Daily Intake of Food Additives. Food Chem. Toxicol. 1999, 37, 1175–1197. [Google Scholar] [CrossRef]
- Tang, Z.; Wu, J.; Liu, X.; Su, Q.; Yin, X.; Huang, Z.; Lin, X.; Lin, W.; Yi, G. Au-Nanoparticle-Array/Aligned-Ag-Nanowire-Based Flexible Dual Plasmonic Substrate for Sensitive Surface-Enhanced Raman Scattering Detection. Part. Part. Syst. Charact. 2021, 38, 2100160. [Google Scholar] [CrossRef]
- Yue, S.; Sun, X.T.; Wang, Y.; Zhang, W.S.; Xu, Z.R. Microparticles with Size/Charge Selectivity and PH Response for SERS Monitoring of 6-Thioguanine in Blood Serum. Sens. Actuators B Chem. 2018, 273, 1539–1547. [Google Scholar] [CrossRef]
- Liu, L.; Martinez Pancorbo, P.; Xiao, T.H.; Noguchi, S.; Marumi, M.; Segawa, H.; Karhadkar, S.; Gala de Pablo, J.; Hiramatsu, K.; Kitahama, Y.; et al. Highly Scalable, Wearable Surface-Enhanced Raman Spectroscopy. Adv. Opt. Mater. 2022, 10, 2200054. [Google Scholar] [CrossRef]
- Yuan, Q.; Fang, H.; Wu, X.; Wu, J.; Luo, X.; Peng, R.; Xu, S.; Yan, S. Self-Adhesive, Biocompatible, Wearable Microfluidics with Erasable Liquid Metal Plasmonic Hotspots for Glucose Detection in Sweat. ACS Appl. Mater. Interfaces 2023, 1944–8244. [Google Scholar] [CrossRef]
- Mogera, U.; Guo, H.; Namkoong, M.; Rahman, M.S.; Nguyen, T.; Tian, L. Wearable Plasmonic Paper-Based Microfluidics for Continuous Sweat Analysis. Sci. Adv. 2022, 8, eabn1736. [Google Scholar] [CrossRef]
- Wang, K.S.; Tseng, Z.L.; Liu, C.Y.; Kuan, T.Y.; Jeng, R.J.; Yang, M.C.; Wang, Y.L.; Liu, T.Y. Novel Strategy for Flexible and Super-Hydrophobic SERS Substrate Fabricated by Deposited Gold Nanoislands on Organic Semiconductor Nanostructures for Bio-Detection. Surf. Coat. Technol. 2022, 435, 128251. [Google Scholar] [CrossRef]
- Bumbrah, G.S.; Sharma, R.M. Raman Spectroscopy—Basic Principle, Instrumentation and Selected Applications for the Characterization of Drugs of Abuse. Egypt. J. Forensic Sci. 2016, 6, 209–215. [Google Scholar] [CrossRef]
- De Oliveira Penido, C.A.F.; Pacheco, M.T.T.; Lednev, I.K.; Silveira, L. Raman Spectroscopy in Forensic Analysis: Identification of Cocaine and Other Illegal Drugs of Abuse. J. Raman Spectrosc. 2016, 47, 28–38. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, Y.; Zhang, L.; Xu, X.; Li, B.; Zhao, X.; Yan, X.; Wang, C.; Sun, P.; Liu, X.; et al. Flexible and Wearable Glove-Based SERS Sensor for Rapid Sampling and Sensitive Detection of Controlled Drugs. Sens. Actuators B Chem. 2023, 386, 133738. [Google Scholar] [CrossRef]
- Maddipatla, D.; Janabi, F.; Narakathu, B.B.; Ali, S.; Turkani, V.S.; Bazuin, B.J.; Fleming, P.D.; Atashbar, M.Z. Sensing and Bio-Sensing Research Development of a Novel Wrinkle-Structure Based SERS Substrate for Drug Detection Applications Ag-NP Ink. Sens. Bio-Sens. Res. 2019, 24, 100281. [Google Scholar] [CrossRef]
- Mitomo, H.; Horie, K.; Matsuo, Y.; Niikura, K.; Tani, T.; Naya, M.; Ijiro, K. Active Gap SERS for the Sensitive Detection of Biomacromolecules with Plasmonic Nanostructures on Hydrogels. Adv. Opt. Mater. 2016, 4, 259–263. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, R.; Zhang, T.; Yan, S.; Song, Y.; Liu, X.; Wang, J. Recent Development and Applications of Stretchable SERS Substrates. Nanomaterials 2023, 13, 2968. https://doi.org/10.3390/nano13222968
Peng R, Zhang T, Yan S, Song Y, Liu X, Wang J. Recent Development and Applications of Stretchable SERS Substrates. Nanomaterials. 2023; 13(22):2968. https://doi.org/10.3390/nano13222968
Chicago/Turabian StylePeng, Ran, Tingting Zhang, Sheng Yan, Yongxin Song, Xinyu Liu, and Junsheng Wang. 2023. "Recent Development and Applications of Stretchable SERS Substrates" Nanomaterials 13, no. 22: 2968. https://doi.org/10.3390/nano13222968
APA StylePeng, R., Zhang, T., Yan, S., Song, Y., Liu, X., & Wang, J. (2023). Recent Development and Applications of Stretchable SERS Substrates. Nanomaterials, 13(22), 2968. https://doi.org/10.3390/nano13222968










