A Comprehensive Review on Electrocatalytic Applications of 2D Metallenes
Abstract
:1. Introduction
2. Three-Dimensional and 2D Metals as Electrocatalysts
3. Fundamentals of 2D Metals
3.1. Understanding 2D Materials and Their Properties
3.2. Synthesis Techniques for 2D Metals
3.2.1. Exfoliation
3.2.2. Surfactant-Directed Synthesis
3.2.3. Vapor Deposition Techniques
3.2.4. Advantages and Disadvantages of Preparation Methods of Metallenes
4. Applications of Metallenes in Heterogeneous Catalysis
4.1. Oxygen Evolution Reaction
4.2. Oxygen Reduction Reaction
4.3. Fuel Oxidation Reaction
4.4. Carbon Dioxide Reduction Reaction
5. Electrocatalytic Organic Synthesis
5.1. C-C and C-X Bond Formation
5.2. Functional Group Transformations
5.3. Green Chemistry Principles
6. Outstanding Properties of Electrocatalytic Activity in 2D Metallene
6.1. High Surface Area and Active Sites
6.2. Electronic Structure and Bandgap Engineering
6.3. Catalytic Synergy of Heteroatoms
6.4. Reaction Mechanism and Kinetics
6.5. Stability and Resistance to Poisoning
6.6. Quantum Effects and Size-Dependent Properties
7. Future Challenges and Opportunities
7.1. Toward Enhanced Application of Metallene Electrocatalysts
7.1.1. Stability and Durability
7.1.2. Scalability and Synthesis
7.1.3. Multifunctionality
7.2. Guiding Catalyst Design for Future Research Directions
7.2.1. Rational Design Principles
7.2.2. Multidisciplinary Approaches
7.2.3. Environmental and Economic Considerations
8. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Two-dimensional | 2D |
| Metal–organic frameworks | MOFs |
| Chemical vapor deposition | CVD |
| Ultra-high-vacuum chemical vapor deposition | UHV-CVD |
| Three-dimensional | 3D |
| Oxygen Evolution Reaction | OER |
| Oxygen Reduction Reaction | ORR |
| Carbon Dioxide Reduction Reaction | CO2 RR |
| Fuel Oxidation Reaction | FOR |
| Proton exchange membrane | PEM |
| Methanol oxidation reaction | MOR |
| Formic acid oxidation reaction | FAOR |
| Transition metal dichalcogenides | TMDs |
| Localized surface plasmon resonance | LSPR |
| Faradaic efficiency | FE |
| Direct Formic Acid Fuel Cells | DFAFCs |
| Molecular beam epitaxy | MBE |
| X-ray absorption fine structure spectroscopy | XAFS |
| Scanning transmission electron microscopy | STEM |
| Density Functional Theory | DFT |
| Cyclic voltammogram | CV |
| X-ray Photoelectron Spectroscopy | XPS |
| Underpotential deposition | UPD |
| Turnover frequency | TOF |
| Brunauer–Emmett–Teller | BET |
| Hydrogen evolution reaction | HER |
| Formic acid oxidation reaction | FAOR |
| Mass activity | MA |
| Ionic liquid | IL |
References
- Almomani, F.; Al-Rababah, A.; Tawalbeh, M.; Al-Othman, A. A comprehensive review of hydrogen generation by water splitting using 2D nanomaterials: Photo vs. electro-catalysis. Fuel 2023, 332, 125905. [Google Scholar] [CrossRef]
- Yusuf, M.; Farooqi, A.S.; Keong, L.K.; Hellgardt, K.; Abdullah, B. Contemporary trends in composite Ni-based catalysts for CO2 reforming of methane. Chem. Eng. Sci. 2021, 229, 116072. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, M.; Long, A.; Xue, Y.; Liao, H.; Wei, C.; Xu, Z.J. Heterostructured electrocatalysts for hydrogen evolution reaction under alkaline conditions. Nano Micro Lett. 2018, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Rosdin, R.D.B.; Yusuf, M.; Abdullah, B. Dry reforming of methane over Ni-based catalysts: Effect of ZrO2 and MgO addition as support. Mater. Lett. X 2021, 12, 100095. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Advancing the electrochemistry of the hydrogen-evolution reaction through combining experiment and theory. Angew. Chem. Int. Ed. 2015, 54, 52–65. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Yusuf, M.; Bazli, L.; Alam, M.A.; Masood, F.; Keong, L.K.; Noor, A.; Hellgardt, K.; Abdullah, B. Hydrogen production via natural gas reforming: A comparative study between DRM, SRM and BRM Techniques. In Proceedings of the 2021 Third International Sustainability and Resilience Conference: Climate Change, Online, 15–16 November 2021; pp. 155–158. [Google Scholar]
- Chen, Z.; Duan, X.; Wei, W.; Wang, S.; Ni, B.-J. Recent advances in transition metal-based electrocatalysts for alkaline hydrogen evolution. J. Mater. Chem. A 2019, 7, 14971–15005. [Google Scholar] [CrossRef]
- Xie, M.; Tang, S.; Zhang, B.; Yu, G. Metallene-related materials for electrocatalysis and energy conversion. Mater. Horiz. 2023, 10, 407–431. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-E.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Berkani, M.; Smaali, A.; Almomani, F. Vasseghian, Recent advances in MXene-based nanomaterials for desalination at water interfaces. Environ. Res. 2022, 203, 111845. [Google Scholar] [CrossRef]
- Jiang, B.; Guo, Y.; Sun, F.; Wang, S.; Kang, Y.; Xu, X.; Zhao, J.; You, J.; Eguchi, M.; Yamauchi, Y. Nanoarchitectonics of metallene materials for electrocatalysis. ACS Nano 2023, 17, 13017–13043. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Cheng, H.; Liu, J.; Shao, M.; Wei, M.; Evans, D.G.; Zhang, H.; Duan, X. Confined synthesis of 2D nanostructured materials toward electrocatalysis. Adv. Energy Mater. 2020, 10, 1900486. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Lin, Y.; Wang, X.; Li, J.; Li, Y.; Gao, L.; Zhang, L.; Chao, D.; Xiao, X. Electronic modulation of non-van der Waals 2D electrocatalysts for efficient energy conversion. Adv. Mater. 2021, 33, 2008422. [Google Scholar] [CrossRef]
- Yuan, L.-P.; Tang, T.; Hu, J.-S.; Wan, L.-J. Confinement strategies for precise synthesis of efficient electrocatalysts from the macroscopic to the atomic level. Acc. Mater. Res. 2021, 2, 907–919. [Google Scholar] [CrossRef]
- Tang, L.; Meng, X.; Deng, D.; Bao, X. Confinement catalysis with 2D materials for energy conversion. Adv. Mater. 2019, 31, 1901996. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Zhang, Y.; Zhang, H.; Wu, H.; Shao, J.; Shao, J.; Liu, K.; Chen, S.; Zhou, C.; Cheng, X. Centimeter-Scale Two-Dimensional Metallenes for High-Efficiency Electrocatalysis and Sensing. ACS Mater. Lett. 2023, 5, 397–405. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, H.; Liu, J.; Zhang, D.; Wu, X.; Nie, N.; Wu, D.; Xu, W.; Lai, J.; Wang, L. The PdHx metallene with vacancies for synergistically enhancing electrocatalytic N2 fixation. Chem. Eng. J. 2022, 450, 137951. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Li, T.; Wang, Y.; Li, H.; Cabot, A. PdMoSb trimetallene as high-performance alcohol oxidation electrocatalyst. Nano Res. 2023, 16, 2041–2048. [Google Scholar] [CrossRef]
- Wang, H.F.; Tang, C.; Zhao, C.X.; Huang, J.Q.; Zhang, Q. Emerging graphene derivatives and analogues for efficient energy electrocatalysis. Adv. Funct. Mater. 2022, 32, 2204755. [Google Scholar] [CrossRef]
- Al-Othman, A.; Hassan, M.F.; Tawalbeh, M.; Ka’ki, A. Proton conductivity studies in zirconium phosphate/MXenes in PEM fuel cells. In Proceedings of the 2022 Advances in Science and Engineering Technology International Conferences (ASET), Dubai, United Arab Emirates, 21–24 February 2022; pp. 1–5. [Google Scholar]
- Javed, R.M.N.; Al-Othman, A.; Tawalbeh, M.; Olabi, A.G. Recent developments in graphene and graphene oxide materials for polymer electrolyte membrane fuel cells applications. Renew. Sustain. Energy Rev. 2022, 168, 112836. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, G.; Li, J.; Li, Y.; Wu, X. 0D/2D Z-scheme heterojunctions of bismuth tantalate quantum dots/ultrathin g-C3N4 nanosheets for highly efficient visible light photocatalytic degradation of antibiotics. ACS Appl. Mater. Interfaces 2017, 9, 43704–43715. [Google Scholar] [CrossRef] [PubMed]
- Chia, X.; Pumera, M. Characteristics and performance of two-dimensional materials for electrocatalysis. Nat. Catal. 2018, 1, 909–921. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, J.; Meng, X.; Yu, L.; Deng, D.; Bao, X. Catalysis with two-dimensional materials confining single atoms: Concept, design, and applications. Chem. Rev. 2018, 119, 1806–1854. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, X.; Ren, X.; Ma, Y.; Shi, X.; Tian, Z.; Asiri, A.M.; Chen, L.; Tang, B.; Sun, X. Electrochemical ammonia synthesis via nitrogen reduction reaction on a MoS2 catalyst: Theoretical and experimental studies. Adv. Mater. 2018, 30, 1800191. [Google Scholar] [CrossRef]
- Jin, C.; Regan, E.C.; Yan, A.; Utama, M.I.B.; Wang, D.; Zhao, S.; Qin, Y.; Yang, S.; Zheng, Z.; Shi, S. Observation of moiré excitons in WSe2/WS2 heterostructure superlattices. Nature 2019, 567, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Y.; Li, J.; Zhang, G. Boosting interfacial charge separation of Ba5Nb4O15/g-C3N4 photocatalysts by 2D/2D nanojunction towards efficient visible-light driven H2 generation. Appl. Catal. B Environ. 2020, 263, 117730. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Q.; O’Hare, D.; Sun, L. Preparation of two dimensional layered double hydroxide nanosheets and their applications. Chem. Soc. Rev. 2017, 46, 5950–5974. [Google Scholar] [CrossRef]
- Vasseghian, Y.; Dragoi, E.-N.; Almomani, F. A comprehensive review on MXenes as new nanomaterials for degradation of hazardous pollutants: Deployment as heterogeneous sonocatalysis. Chemosphere 2022, 287, 132387. [Google Scholar] [CrossRef]
- Jing, H.; Zhu, P.; Zheng, X.; Zhang, Z.; Wang, D.; Li, Y. Theory-oriented screening and discovery of advanced energy transformation materials in electrocatalysis. Adv. Powder Mater. 2022, 1, 100013. [Google Scholar] [CrossRef]
- Liu, Y.; Dinh, K.N.; Dai, Z.; Yan, Q. Metallenes: Recent advances and opportunities in energy storage and conversion applications. ACS Mater. Lett. 2020, 2, 1148–1172. [Google Scholar] [CrossRef]
- Prabhu, P.; Lee, J.-M. Metallenes as functional materials in electrocatalysis. Chem. Soc. Rev. 2021, 50, 6700–6719. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Baek, J.-B. Recent advances in noble metal (Pt, Ru, and Ir)-based electrocatalysts for efficient hydrogen evolution reaction. ACS Omega 2019, 5, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lin, Y.; Liu, S.; Li, J.; Bu, L.; Chen, J.; Xiao, X.; Choi, J.-H.; Gao, L.; Lee, J.-M. Confined growth of pyridinic N–Mo2 C sites on MXenes for hydrogen evolution. J. Mater. Chem. A 2020, 8, 7109–7116. [Google Scholar] [CrossRef]
- Fu, G.; Lee, J.-M. Ternary metal sulfides for electrocatalytic energy conversion. J. Mater. Chem. A 2019, 7, 9386–9405. [Google Scholar] [CrossRef]
- Voiry, D.; Shin, H.S.; Loh, K.P.; Chhowalla, M. Low-dimensional catalysts for hydrogen evolution and CO2 reduction. Nat. Rev. Chem. 2018, 2, 0105. [Google Scholar] [CrossRef]
- Deng, D.; Novoselov, K.; Fu, Q.; Zheng, N.; Tian, Z.; Bao, X. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 2016, 11, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhao, Z.; Zhang, Y.; Sun, Y.; Xing, Y.; Lv, F.; Yang, Y.; Zhang, X.; Hwang, S.; Qin, Y. PdMo bimetallene for oxygen reduction catalysis. Nature 2019, 574, 81–85. [Google Scholar] [CrossRef]
- Fan, J.; Wu, J.; Cui, X.; Gu, L.; Zhang, Q.; Meng, F.; Lei, B.-H.; Singh, D.J.; Zheng, W. Hydrogen stabilized RhPdH 2D bimetallene nanosheets for efficient alkaline hydrogen evolution. J. Am. Chem. Soc. 2020, 142, 3645–3651. [Google Scholar] [CrossRef]
- Dang, R.; Song, L.; Dong, W.; Li, C.; Zhang, X.; Wang, G.; Chen, X. Synthesis and self-assembly of large-area Cu nanosheets and their application as an aqueous conductive ink on flexible electronics. ACS Appl. Mater. Interfaces 2014, 6, 622–629. [Google Scholar] [CrossRef]
- Basyooni, M.A.; Zaki, S.E.; Alfryyan, N.; Tihtih, M.; Eker, Y.R.; Attia, G.F.; Yılmaz, M.; Ateş, Ş.; Shaban, M. Nanostructured MoS2 and WS2 Photoresponses under Gas Stimuli. Nanomaterials 2022, 12, 3585. [Google Scholar] [CrossRef]
- Basyooni, M.A.; Zaki, S.E.; Shaban, M.; Eker, Y.R.; Yilmaz, M. Efficient MoWO3/VO2/MoS2/Si UV Schottky photodetectors; MoS2 optimization and monoclinic VO2 surface modifications. Sci. Rep. 2020, 10, 15926. [Google Scholar] [CrossRef]
- Duan, H.; Yan, N.; Yu, R.; Chang, C.-R.; Zhou, G.; Hu, H.-S.; Rong, H.; Niu, Z.; Mao, J.; Asakura, H. Ultrathin rhodium nanosheets. Nat. Commun. 2014, 5, 3093. [Google Scholar] [CrossRef]
- Cao, C.; Ma, D.D.; Gu, J.F.; Xie, X.; Zeng, G.; Li, X.; Han, S.G.; Zhu, Q.L.; Wu, X.T.; Xu, Q. Metal–organic layers leading to atomically thin bismuthene for efficient carbon dioxide electroreduction to liquid fuel. Angew. Chem. Int. Ed. 2020, 59, 15014–15020. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Lin, Y.; Jiao, X.; Sun, Y.; Luo, Q.; Zhang, W.; Li, D.; Yang, J.; Xie, Y. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature 2016, 529, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, S.; Chen, Z.; Wang, Y.; Gao, H.; Gómez-Herrero, J.; Ares, P.; Zamora, F.; Zhu, Z.; Zeng, H. Recent progress in 2D group-VA semiconductors: From theory to experiment. Chem. Soc. Rev. 2018, 47, 982–1021. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Chai, J.; Lu, S.; Mu, X.; Liu, R.; Wang, Q.; Chen, F.; Li, Y.; Wang, J.; Wang, B. Solution-phase vertical growth of aligned NiCo2O4 nanosheet arrays on Au nanosheets with weakened oxygen–hydrogen bonds for photocatalytic oxygen evolution. Nanoscale 2020, 12, 6195–6203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Deng, Q.; Bachmatiuk, A.; Sandeep, G.; Popov, A.; Eckert, J.; Rümmeli, M.H. Free-standing single-atom-thick iron membranes suspended in graphene pores. Science 2014, 343, 1228–1232. [Google Scholar] [CrossRef]
- Ma, Y.; Li, B.; Yang, S. Ultrathin two-dimensional metallic nanomaterials. Mater. Chem. Front. 2018, 2, 456–467. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Y.; Lu, P.; Wei, C.; Zhu, C.; Yao, H.; Xu, F.; Shi, J. Cryogenic Exfoliation of Non-layered Magnesium into Two-Dimensional Crystals. Angew. Chem. Int. Ed. 2019, 58, 8814–8818. [Google Scholar] [CrossRef]
- Lu, W.; Birmingham, B.; Voronine, D.V.; Stolpman, D.; Ambardar, S.; Erdogan, D.A.; Ozensoy, E.; Zhang, Z.; Solouki, T. From aluminum foil to two-dimensional nanocrystals using ultrasonic exfoliation. J. Phys. Chem. C 2021, 125, 7746–7755. [Google Scholar] [CrossRef]
- Yadav, T.P.; Woellner, C.F.; Sharifi, T.; Sinha, S.K.; Qu, L.-L.; Apte, A.; Mukhopadhyay, N.; Srivastava, O.; Vajtai, R.; Galvao, D.S. Extraction of two-dimensional aluminum alloys from decagonal quasicrystals. ACS Nano 2020, 14, 7435–7443. [Google Scholar] [CrossRef]
- Funatsu, A.; Tateishi, H.; Hatakeyama, K.; Fukunaga, Y.; Taniguchi, T.; Koinuma, M.; Matsuura, H.; Matsumoto, Y. Synthesis of monolayer platinum nanosheets. Chem. Commun. 2014, 50, 8503–8506. [Google Scholar] [CrossRef]
- Yin, J.; Yin, Z.; Jin, J.; Sun, M.; Huang, B.; Lin, H.; Ma, Z.; Muzzio, M.; Shen, M.; Yu, C. A new hexagonal cobalt nanosheet catalyst for selective CO2 conversion to ethanal. J. Am. Chem. Soc. 2021, 143, 15335–15343. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Zhao, P.; Xue, X.; Chen, R.; Yang, S.; Ma, J.; Liu, J. Liquid-phase exfoliated ultrathin Bi nanosheets: Uncovering the origins of enhanced electrocatalytic CO2 reduction on two-dimensional metal nanostructure. Nano Energy 2018, 53, 808–816. [Google Scholar] [CrossRef]
- Ah, C.S.; Yun, Y.J.; Park, H.J.; Kim, W.-J.; Ha, D.H.; Yun, W.S. Size-controlled synthesis of machinable single crystalline gold nanoplates. Chem. Mater. 2005, 17, 5558–5561. [Google Scholar] [CrossRef]
- Xu, D.; Liu, X.; Lv, H.; Liu, Y.; Zhao, S.; Han, M.; Bao, J.; He, J.; Liu, B. Ultrathin palladium nanosheets with selectively controlled surface facets. Chem. Sci. 2018, 9, 4451–4455. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Kang, X.; Xu, C.; Zhou, J.; Deng, J.; Li, Y.; Cheng, S. 2D PdAg alloy nanodendrites for enhanced ethanol electroxidation. Adv. Mater. 2018, 30, 1706962. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Fan, X.; Ke, X.; Xu, J.; Zhao, X.; Jia, L.; Pan, B.; Han, N.; Li, L.; Liu, X. Two-dimensional palladium–copper alloy nanodendrites for highly stable and selective electrochemical formate production. Nano Lett. 2021, 21, 4092–4098. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-S.; Chen, Y.-Z.; Medina, H.; Su, T.-Y.; Chou, T.-S.; Chen, Y.-H.; Chueh, Y.-L.; Liang, J.-H. Direct formation of large-scale multi-layered germanene on Si substrate. Phys. Chem. Chem. Phys. 2015, 17, 21389–21393. [Google Scholar] [CrossRef]
- Kuriakose, S.; Jain, S.K.; Tawfik, S.A.; Spencer, M.J.; Murdoch, B.J.; Singh, M.; Rahman, F.; Mayes, E.L.; Taha, M.Y.; Ako, R.T. Monocrystalline antimonene nanosheets via physical vapor deposition. Adv. Mater. Interfaces 2020, 7, 2001678. [Google Scholar] [CrossRef]
- Wu, X.; Shao, Y.; Liu, H.; Feng, Z.; Wang, Y.L.; Sun, J.T.; Liu, C.; Wang, J.O.; Liu, Z.L.; Zhu, S.Y. Epitaxial growth and air-stability of monolayer antimonene on PdTe2. Adv. Mater. 2017, 29, 1605407. [Google Scholar] [CrossRef]
- Niu, T.; Meng, Q.; Zhou, D.; Si, N.; Zhai, S.; Hao, X.; Zhou, M.; Fuchs, H. Large-scale synthesis of strain-tunable semiconducting antimonene on copper oxide. Adv. Mater. 2020, 32, 1906873. [Google Scholar] [CrossRef] [PubMed]
- Fortin-Deschênes, M.; Waller, O.; Mentes, T.; Locatelli, A.; Mukherjee, S.; Genuzio, F.; Levesque, P.; Hébert, A.; Martel, R.; Moutanabbir, O. Synthesis of antimonene on germanium. Nano Lett. 2017, 17, 4970–4975. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.S.; Na, S.R.; Jung, D.; March, S.D.; Kim, J.-S.; Trivedi, T.; Li, W.; Tao, L.; Lee, M.L.; Liechti, K.M. Large-area dry transfer of single-crystalline epitaxial bismuth thin films. Nano Lett. 2016, 16, 6931–6938. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Kim, J.; Guo, Y.; Wu, K.C.; Alshehri, S.M.; Ahamad, T.; Alhokbany, N.; Henzie, J.; Yamachi, Y. Efficient oxygen evolution on mesoporous IrO x nanosheets. Catal. Sci. Technol. 2019, 9, 3697–3702. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Song, J.; Dou, S.; Li, X.; Wang, J.; Wang, X. Strategies to break the scaling relation toward enhanced oxygen electrocatalysis. Matter 2019, 1, 1494–1518. [Google Scholar] [CrossRef]
- Zu, L.; Qian, X.; Zhao, S.; Liang, Q.; Chen, Y.E.; Liu, M.; Su, B.-J.; Wu, K.-H.; Qu, L.; Duan, L. Self-assembly of Ir-based nanosheets with ordered interlayer space for enhanced electrocatalytic water oxidation. J. Am. Chem. Soc. 2022, 144, 2208–2217. [Google Scholar] [CrossRef]
- Wu, G.; Zheng, X.; Cui, P.; Jiang, H.; Wang, X.; Qu, Y.; Chen, W.; Lin, Y.; Li, H.; Han, X. A general synthesis approach for amorphous noble metal nanosheets. Nat. Commun. 2019, 10, 4855. [Google Scholar] [CrossRef]
- Li, L.; Bu, L.; Huang, B.; Wang, P.; Shen, C.; Bai, S.; Chan, T.S.; Shao, Q.; Hu, Z.; Huang, X. Compensating electronic effect enables fast site-to-site electron transfer over ultrathin RuMn nanosheet branches toward highly electroactive and stable water splitting. Adv. Mater. 2021, 33, 2105308. [Google Scholar] [CrossRef]
- Yao, Q.; Huang, B.; Zhang, N.; Sun, M.; Shao, Q.; Huang, X. Channel-rich RuCu nanosheets for pH-universal overall water splitting electrocatalysis. Angew. Chem. 2019, 131, 14121–14126. [Google Scholar] [CrossRef]
- Wu, D.; Kusada, K.; Yoshioka, S.; Yamamoto, T.; Toriyama, T.; Matsumura, S.; Chen, Y.; Seo, O.; Kim, J.; Song, C. Efficient overall water splitting in acid with anisotropic metal nanosheets. Nat. Commun. 2021, 12, 1145. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.; Liu, S.; Yin, S.; Yu, H.; Wang, Z.; Li, X.; Wang, L.; Wang, H. Facile construction of IrRh nanosheet assemblies as efficient and robust bifunctional electrocatalysts for overall water splitting. ACS Sustain. Chem. Eng. 2019, 7, 15747–15754. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, Q.; Wang, X. Cr-doped Pd metallene nanoribbon superstructures for the oxygen reduction reaction and formic acid oxidation. Chem. Commun. 2023, 59, 2473–2476. [Google Scholar] [CrossRef] [PubMed]
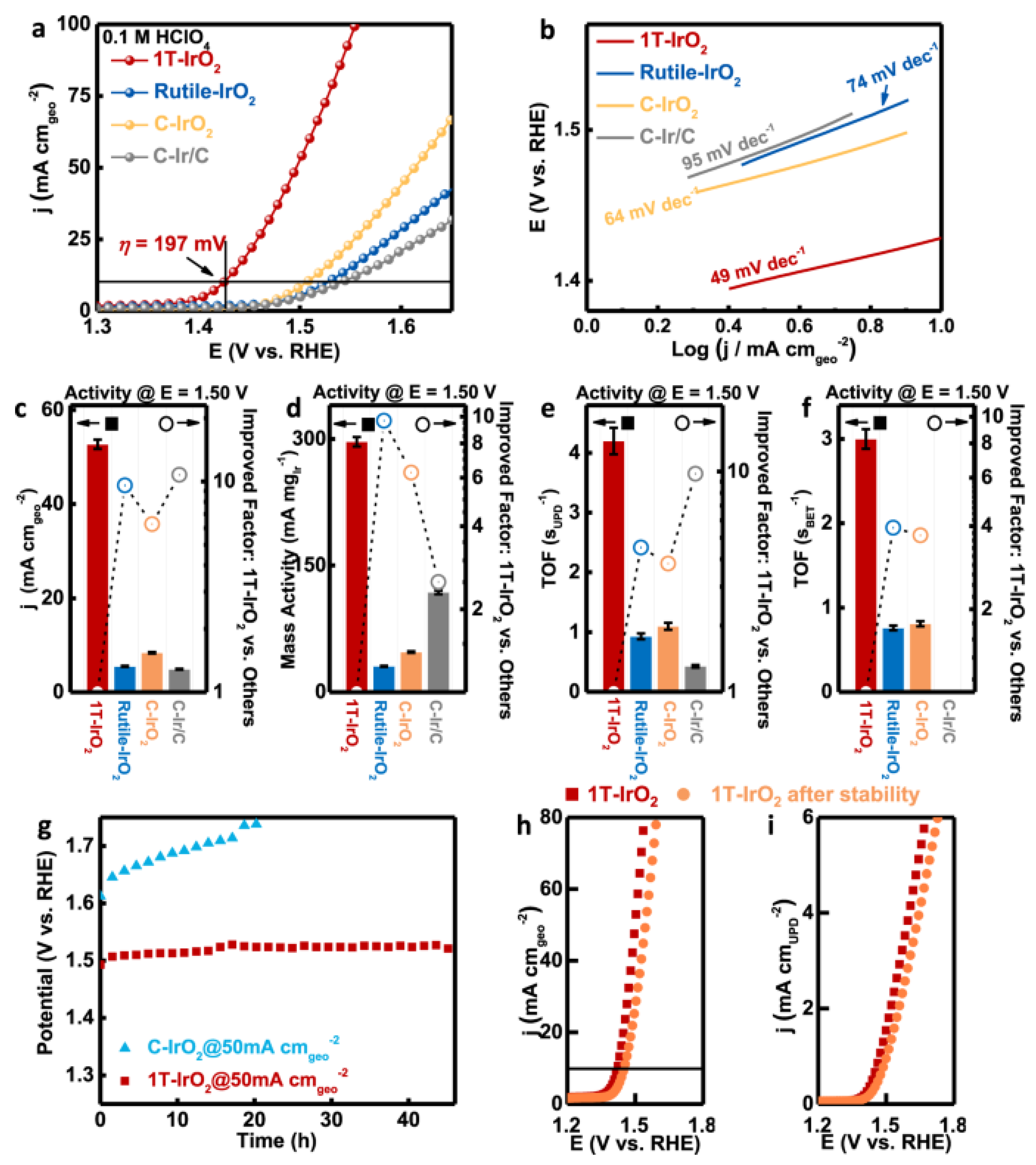
- Dang, Q.; Lin, H.; Fan, Z.; Ma, L.; Shao, Q.; Ji, Y.; Zheng, F.; Geng, S.; Yang, S.-Z.; Kong, N. Iridium metallene oxide for acidic oxygen evolution catalysis. Nat. Commun. 2021, 12, 6007. [Google Scholar] [CrossRef]
- Ooka, H.; Huang, J.; Exner, K.S. The sabatier principle in electrocatalysis: Basics, limitations, and extensions. Front. Energy Res. 2021, 9, 654460. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, T.; Wang, Z.; Xu, Y.; Li, X.; Wang, L.; Wang, H. Defect-rich porous palladium metallene for enhanced alkaline oxygen reduction electrocatalysis. Angew. Chem. 2021, 133, 12134–12138. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jonsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Montemore, M.M.; van Spronsen, M.A.; Madix, R.J.; Friend, C.M. O2 activation by metal surfaces: Implications for bonding and reactivity on heterogeneous catalysts. Chem. Rev. 2017, 118, 2816–2862. [Google Scholar] [CrossRef]
- Takimoto, D.; Toma, S.; Suda, Y.; Shirokura, T.; Tokura, Y.; Fukuda, K.; Matsumoto, M.; Imai, H.; Sugimoto, W. Platinum nanosheets synthesized via topotactic reduction of single-layer platinum oxide nanosheets for electrocatalysis. Nat. Commun. 2023, 14, 19. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, B.; Yang, C.; Shao, Q.; Huang, X. Platinum porous nanosheets with high surface distortion and Pt utilization for enhanced oxygen reduction catalysis. Adv. Funct. Mater. 2019, 29, 1904429. [Google Scholar] [CrossRef]
- Chen, W.; Gao, W.; Tu, P.; Robert, T.; Ma, Y.; Shan, H.; Gu, X.; Shang, W.; Tao, P.; Song, C. Neighboring Pt atom sites in an ultrathin FePt nanosheet for the efficient and highly CO-tolerant oxygen reduction reaction. Nano Lett. 2018, 18, 5905–5912. [Google Scholar] [CrossRef]
- Zhang, K.; He, Y.; Guo, R.; Wang, W.; Zhan, Q.; Li, R.; He, T.; Wu, C.; Jin, M. Interstitial carbon-doped PdMo bimetallene for high-performance oxygen reduction reaction. ACS Energy Lett. 2022, 7, 3329–3336. [Google Scholar] [CrossRef]
- Ong, B.; Kamarudin, S.; Basri, S. Direct liquid fuel cells: A review. Int. J. Hydrog. Energy 2017, 42, 10142–10157. [Google Scholar] [CrossRef]
- Zhu, E.; Yan, X.; Wang, S.; Xu, M.; Wang, C.; Liu, H.; Huang, J.; Xue, W.; Cai, J.; Heinz, H. Peptide-assisted 2-D assembly toward free-floating ultrathin platinum nanoplates as effective electrocatalysts. Nano Lett. 2019, 19, 3730–3736. [Google Scholar] [CrossRef]
- Luo, X.; Liu, C.; Wang, X.; Shao, Q.; Pi, Y.; Zhu, T.; Li, Y.; Huang, X. Spin regulation on 2D Pd–Fe–Pt nanomeshes promotes fuel electrooxidations. Nano Lett. 2020, 20, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, L.; Noor, T.; Iqbal, N. A comprehensive and critical review of the recent progress in electrocatalysts for the ethanol oxidation reaction. Rsc Adv. 2021, 11, 16768–16804. [Google Scholar] [CrossRef] [PubMed]
- Yun, Q.; Lu, Q.; Li, C.; Chen, B.; Zhang, Q.; He, Q.; Hu, Z.; Zhang, Z.; Ge, Y.; Yang, N. Synthesis of PdM (M = Zn, Cd, ZnCd) nanosheets with an unconventional face-centered tetragonal phase as highly efficient electrocatalysts for ethanol oxidation. ACS Nano 2019, 13, 14329–14336. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, W.; Wang, F.; Li, J.; Ye, J.; Sheng, X.; Li, C.; Liang, X.; Liu, P.; Wang, X. Extraordinary p–d Hybridization Interaction in Heterostructural Pd-PdSe Nanosheets Boosts C−C Bond Cleavage of Ethylene Glycol Electrooxidation. Angew. Chem. Int. Ed. 2022, 61, e202200899. [Google Scholar] [CrossRef] [PubMed]
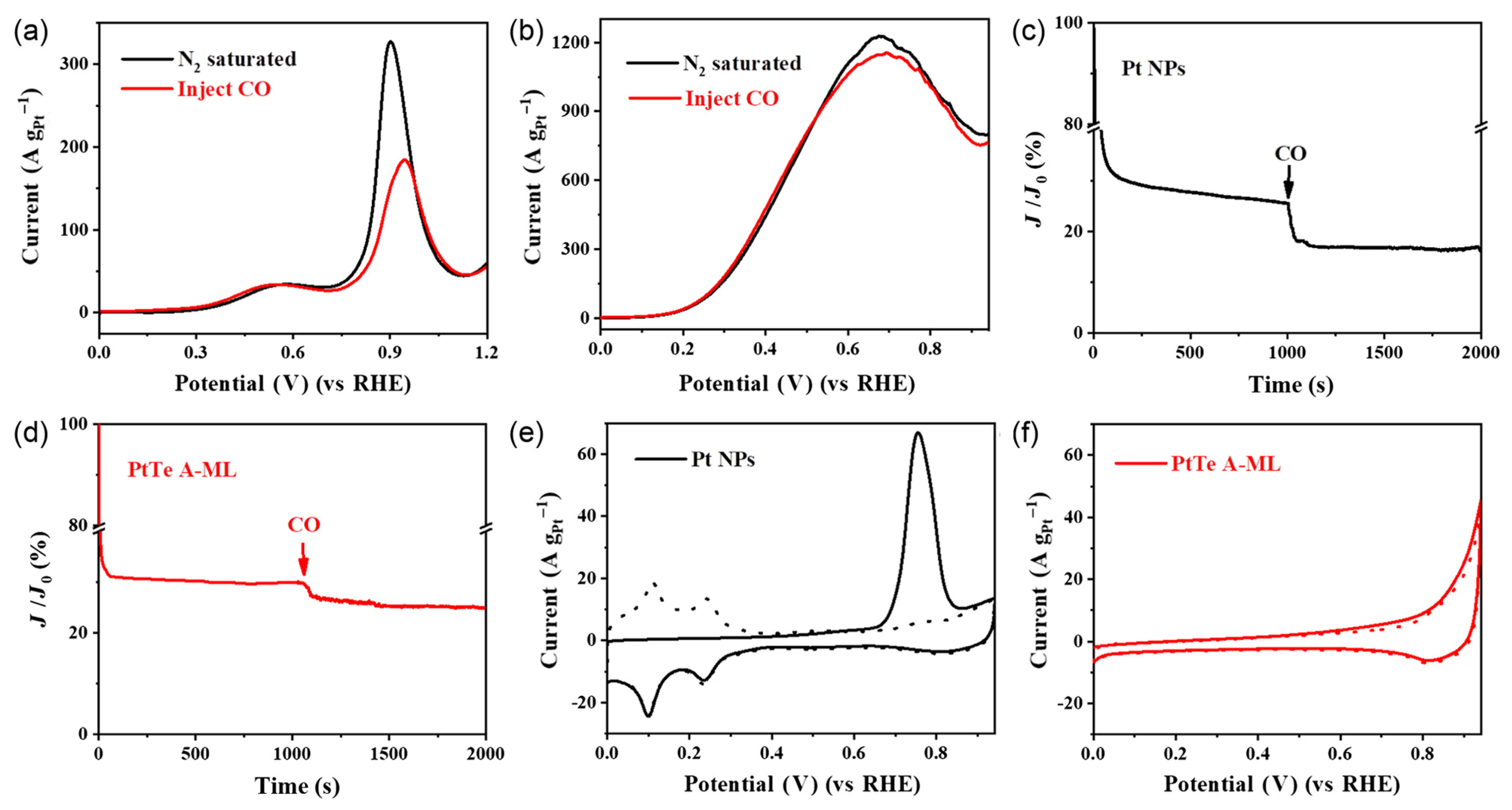
- Li, Y.-N.; Hong, Q.-L.; Miao, B.-Q.; Wang, T.-J.; Ding, Y.; Chen, Y. Platinum-tellurium alloy metallene toward formic acid oxidation reaction. Renewables 2023, 1, 90–99. [Google Scholar] [CrossRef]
- Solomon, S.; Plattner, G.-K.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef]
- Zheng, T.; Jiang, K.; Ta, N.; Hu, Y.; Zeng, J.; Liu, J.; Wang, H. Large-scale and highly selective CO2 electrocatalytic reduction on nickel single-atom catalyst. Joule 2019, 3, 265–278. [Google Scholar] [CrossRef]
- Kumar, B.; Atla, V.; Brian, J.P.; Kumari, S.; Nguyen, T.Q.; Sunkara, M.; Spurgeon, J.M. Reduced SnO2 porous nanowires with a high density of grain boundaries as catalysts for efficient electrochemical CO2-into-HCOOH conversion. Angew. Chem. Int. Ed. 2017, 56, 3645–3649. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Lee, J. Formic acid from carbon dioxide on nanolayered electrocatalyst. Electrocatalysis 2010, 1, 108–115. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, X.; Yang, W.; Jia, C.; Chen, X.; Ren, W.; Smith, S.C.; Zhao, C. Surface reconstruction of ultrathin palladium nanosheets during electrocatalytic CO2 reduction. Angew. Chem. Int. Ed. 2020, 59, 21493–21498. [Google Scholar] [CrossRef]
- Liu, S.; Tao, H.; Zeng, L.; Liu, Q.; Xu, Z.; Liu, Q.; Luo, J.-L. Shape-dependent electrocatalytic reduction of CO2 to CO on triangular silver nanoplates. J. Am. Chem. Soc. 2017, 139, 2160–2163. [Google Scholar] [CrossRef]
- DiMeglio, J.L.; Rosenthal, J. Selective conversion of CO2 to CO with high efficiency using an inexpensive bismuth-based electrocatalyst. J. Am. Chem. Soc. 2013, 135, 8798–8801. [Google Scholar] [CrossRef]
- Medina-Ramos, J.; DiMeglio, J.L.; Rosenthal, J. Efficient reduction of CO2 to CO with high current density using in situ or ex situ prepared Bi-based materials. J. Am. Chem. Soc. 2014, 136, 8361–8367. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, D.; Simbeck, D.; Karp, A.; Dickenson, R. Syngas production for gas-to-liquids applications: Technologies, issues and outlook. Fuel Process. Technol. 2001, 71, 139–148. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.; Fu, X.; Zhu, S.; Min, Y.; Xu, Q.; Li, Q. Curved porous PdCu metallene as a high-efficiency bifunctional electrocatalyst for oxygen reduction and formic acid oxidation. ACS Appl. Mater. Interfaces 2023, 15, 5198–5208. [Google Scholar] [CrossRef]
- Waldvogel, S.R.; Janza, B. Renaissance of electrosynthetic methods for the construction of complex molecules. Angew. Chem. Int. Ed. 2014, 53, 7122–7123. [Google Scholar] [CrossRef]
- McClymont, K.S.; Wang, F.-Y.; Minakar, A.; Baran, P.S. Total synthesis of (−)-maximiscin. J. Am. Chem. Soc. 2020, 142, 8608–8613. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.N.; Surendranath, Y. Molecular control of heterogeneous electrocatalysis through graphite conjugation. Acc. Chem. Res. 2019, 52, 3432–3441. [Google Scholar] [CrossRef]
- Lee, K.J.; Elgrishi, N.; Kandemir, B.; Dempsey, J.L. Electrochemical and spectroscopic methods for evaluating molecular electrocatalysts. Nat. Rev. Chem. 2017, 1, 0039. [Google Scholar] [CrossRef]
- Savéant, J.-M. Molecular catalysis of electrochemical reactions. Mechanistic aspects. Chem. Rev. 2008, 108, 2348–2378. [Google Scholar] [CrossRef] [PubMed]




| Method | Advantages | Disadvantages |
|---|---|---|
| Top-Down Synthesis | Mechanical cleavage: basic and straightforward technique. | Mechanical cleavage: Challenges in controlling factors like morphology and thickness. Not suitable for large-scale production without further exploration. |
| Ultrasonic exfoliation: produces highly pure metallenes with stable morphologies for layered metals. | Ultrasonic exfoliation: struggles to achieve similar effects with non-layered metals. | |
| Electrochemical exfoliation: suitable for layered metals, providing quick cycles, high yields, and efficiency. | Electrochemical exfoliation: requires further investigation for application to non-layered materials. | |
| Plasma-assisted processes: controllable morphology and purity. | Plasma-assisted processes: room for improvement in addressing tough reaction conditions and low yields. | |
| Bottom-Up Synthesis | MBE and CVD: high purity, controlled morphology, and good yields. | MBE and CVD: demand harsh reaction conditions, including high temperatures and pressures, contributing to elevated production costs. |
| Wet chemical method: uniform, controllable material morphology, high yield, flexibility in reaction conditions and time. | Wet chemical method: Might not guarantee complete purity, leaving behind reaction intermediates. A common challenge in this method. | |
| Summary of Methods | Exfoliation methods: Simplicity, scalability, and relatively low cost. Struggles with precise control over nanosheet properties and yielding high-quality monolayers. | Surfactant-directed synthesis: Tailored structures and improved uniformity through surfactant-guided self-assembly. Challenges with surfactant removal and scalability. |
| Vapor deposition techniques: Controlled growth and high-purity products. Specialized equipment, demanding conditions, and elevated costs pose challenges. | ||
| Considerations for Choice | Practical applications: appropriate method selection based on the intended purpose and unique attributes of each technique. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basyooni-M. Kabatas, M.A. A Comprehensive Review on Electrocatalytic Applications of 2D Metallenes. Nanomaterials 2023, 13, 2966. https://doi.org/10.3390/nano13222966
Basyooni-M. Kabatas MA. A Comprehensive Review on Electrocatalytic Applications of 2D Metallenes. Nanomaterials. 2023; 13(22):2966. https://doi.org/10.3390/nano13222966
Chicago/Turabian StyleBasyooni-M. Kabatas, Mohamed A. 2023. "A Comprehensive Review on Electrocatalytic Applications of 2D Metallenes" Nanomaterials 13, no. 22: 2966. https://doi.org/10.3390/nano13222966
APA StyleBasyooni-M. Kabatas, M. A. (2023). A Comprehensive Review on Electrocatalytic Applications of 2D Metallenes. Nanomaterials, 13(22), 2966. https://doi.org/10.3390/nano13222966