Solvent-Free Synthesis of Multifunctional Block Copolymer and Formation of DNA and Drug Nanocarriers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
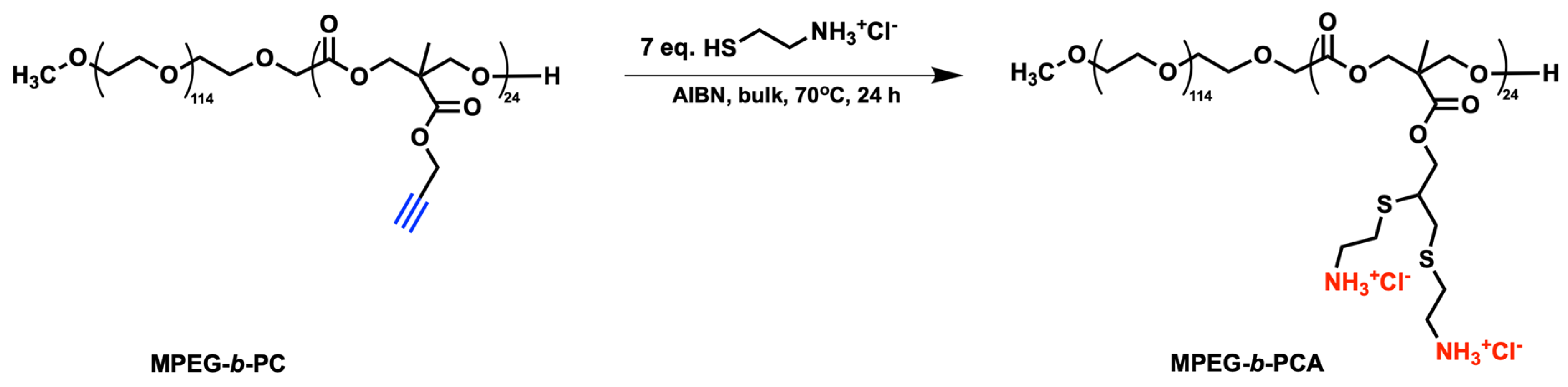
2.2. Synthesis of Double Hydrophilic Multi-Amine-Functional Block Copolymer MPEG-b-PCA
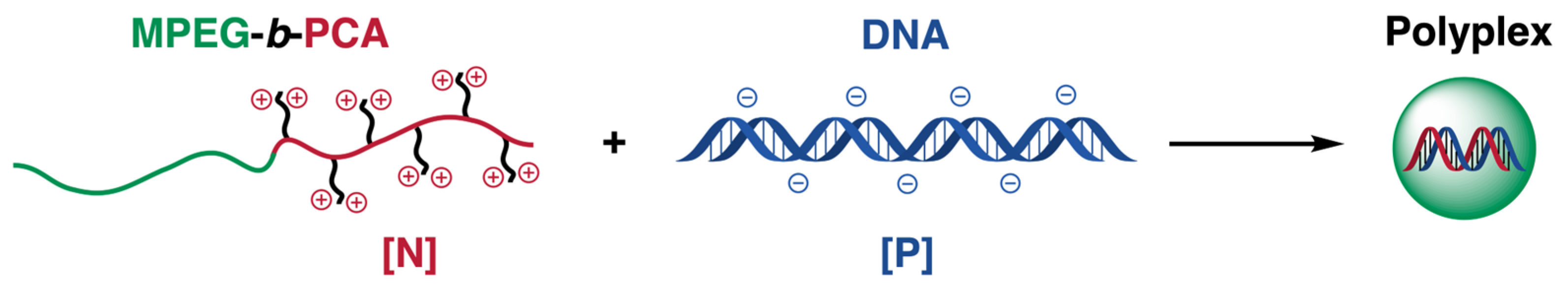
2.3. Polyplexes Formation
2.4. Ethidium Bromide (EtBr) Displacement Assay
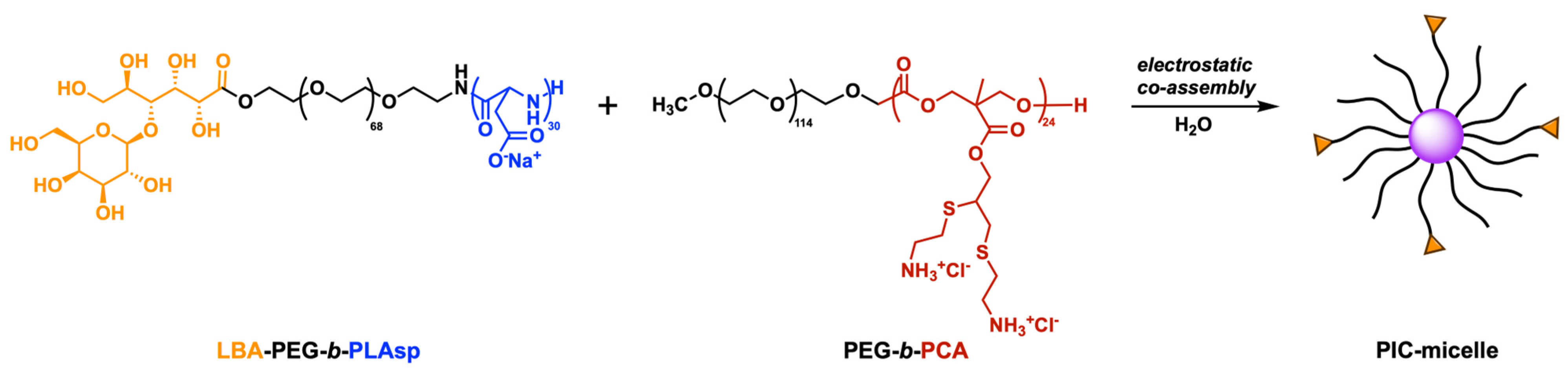
2.5. Formation of Polyion Complex (PIC) Micelles
2.6. Critical Micelle Concentration (CMC) Determination
2.7. Curcumin Loading Evaluation and Drug Release Studies
2.8. MTT Test
2.9. Characterization Methods
3. Results and Discussion
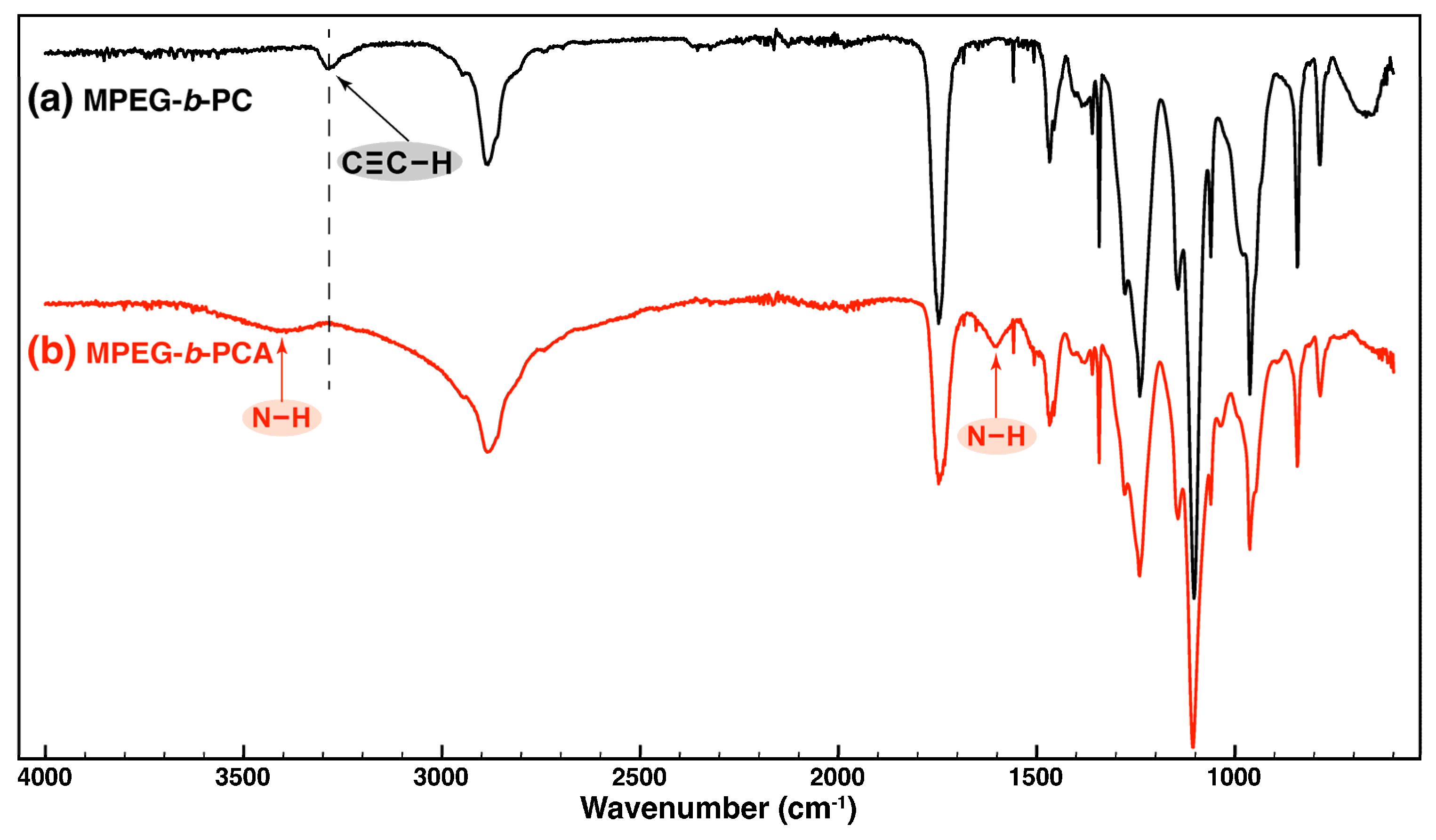
3.1. Solvent-Free Synthesis of Multi-Amino-Functional Diblock Copolymer MPEG-b-PCA
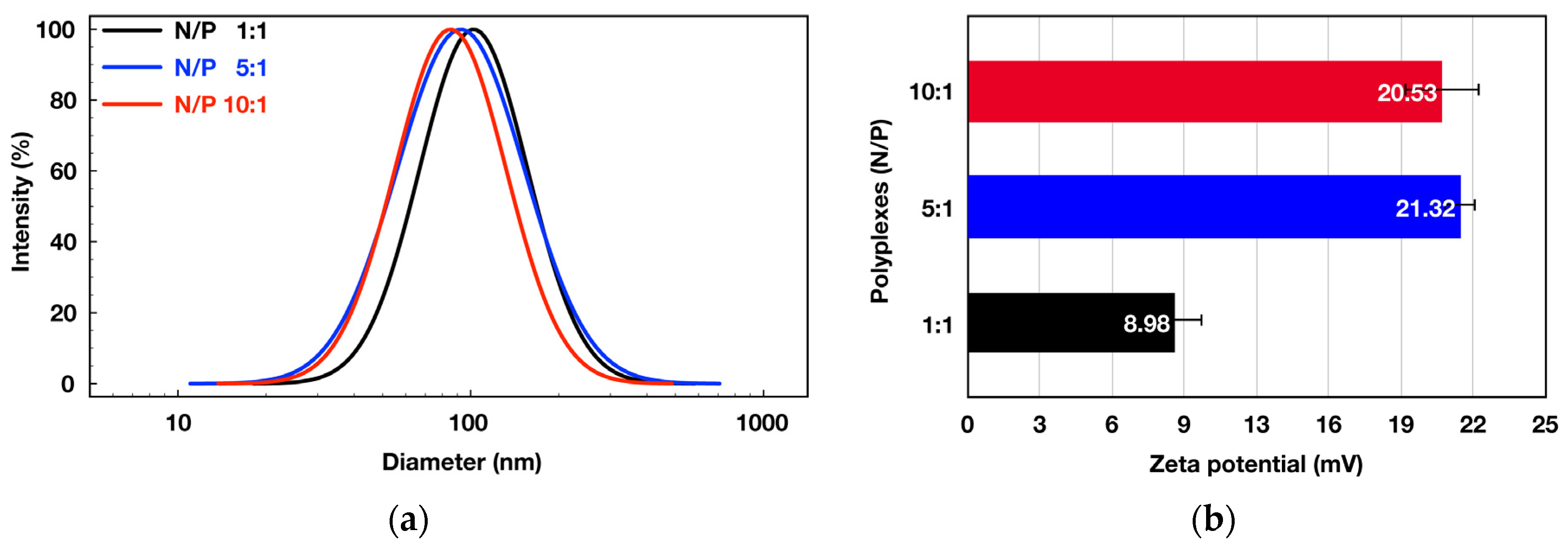
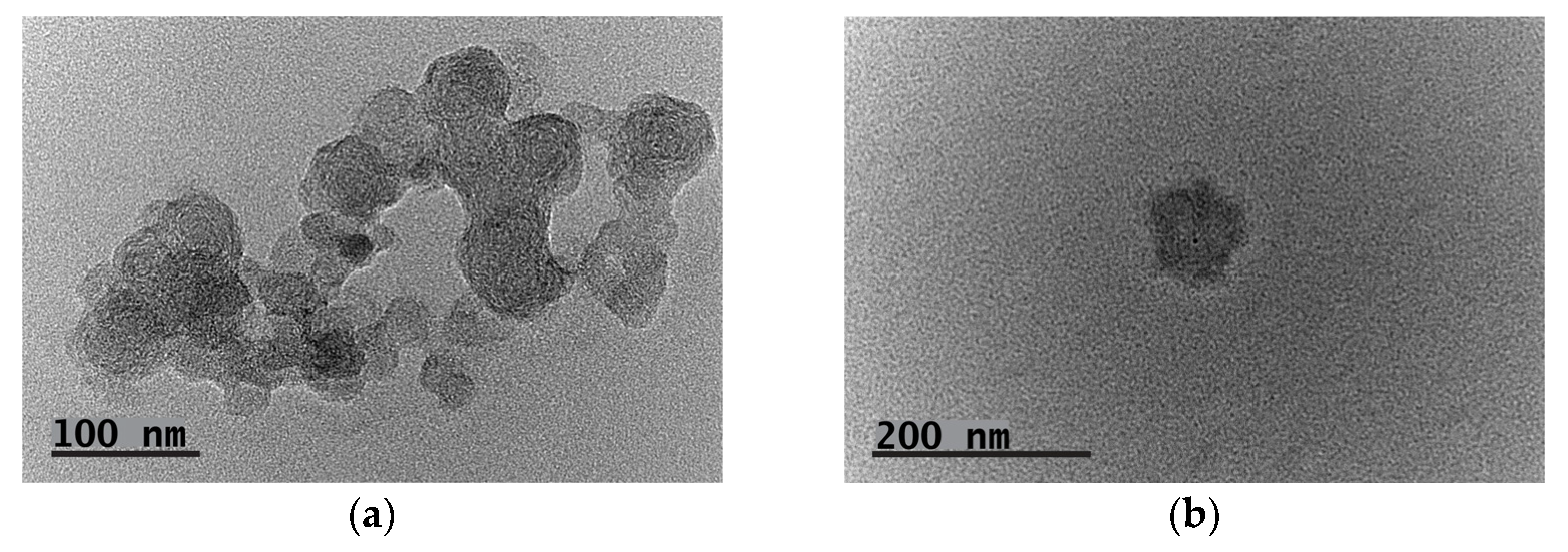
3.2. Formation and Evaluation of Polyplexes between the Double Hydrophilic Block Copolymer MPEG-b-PCA and DNA
3.3. Polyion Complex (PIC) Micelles Formation between the MPEG-b-PCA and LBA-PEG-b-PLAsp Functional Synthetic Block Copolymers Bearing Polycationic and Polyanionic Segments
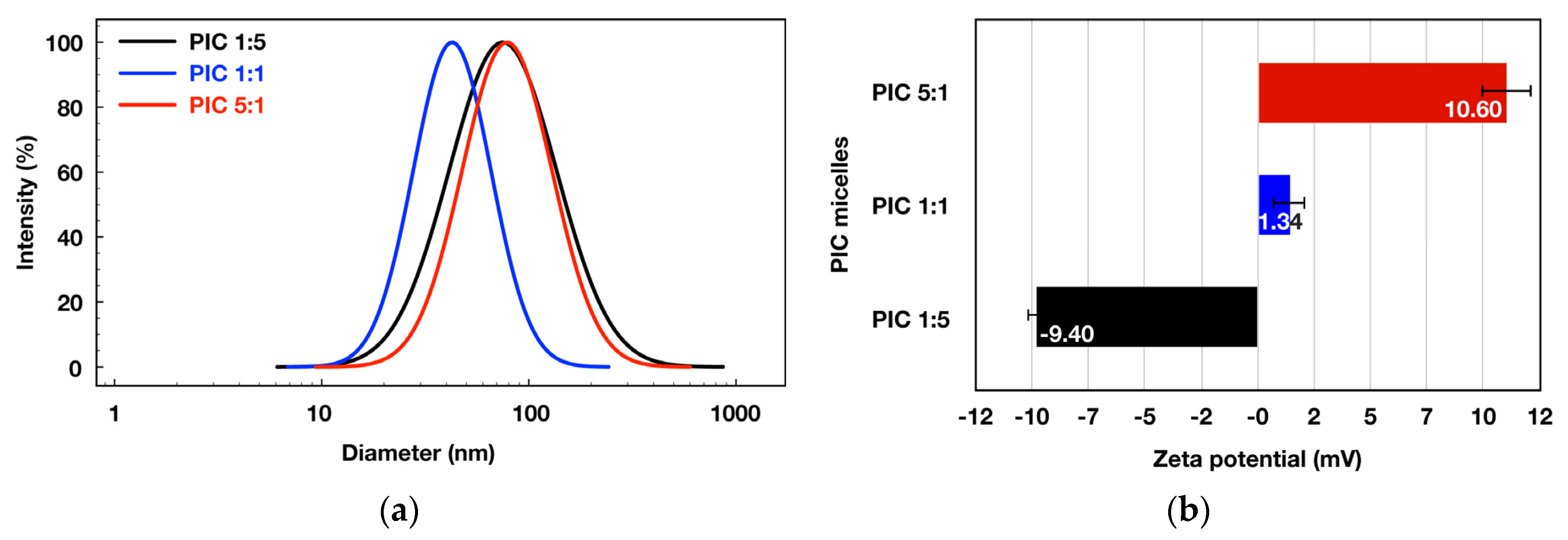
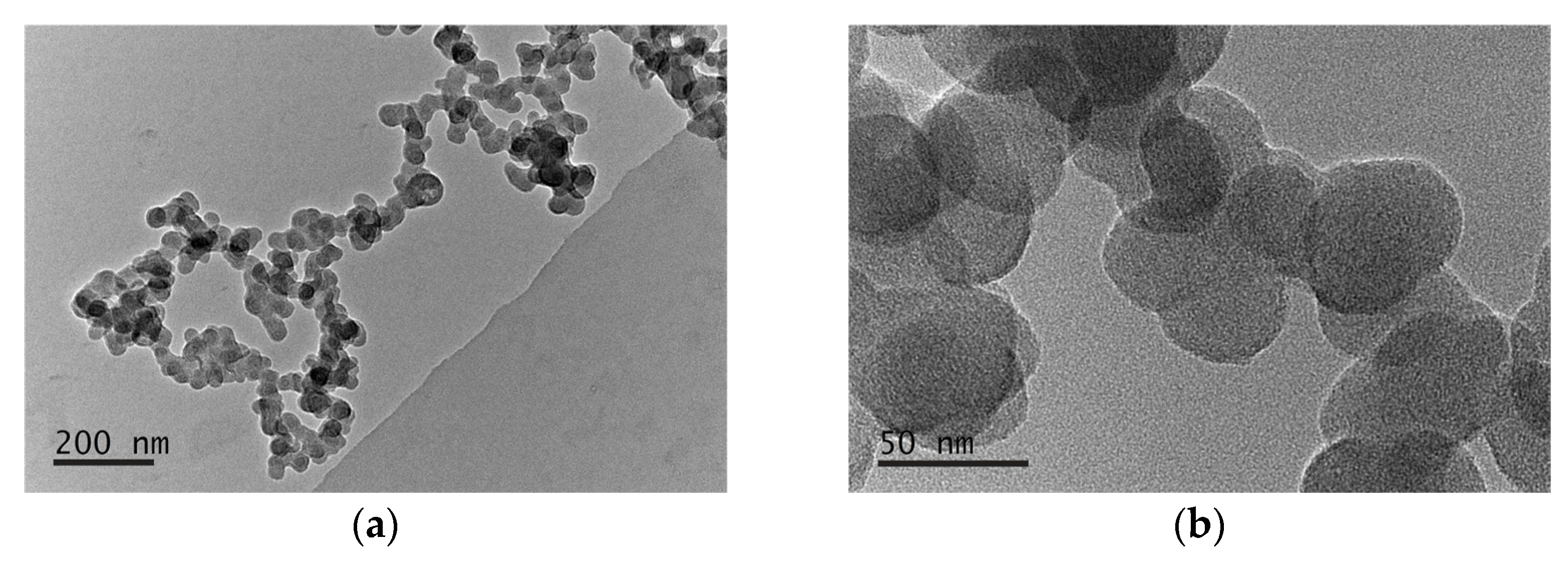
3.3.1. Physico-Chemical Characterization
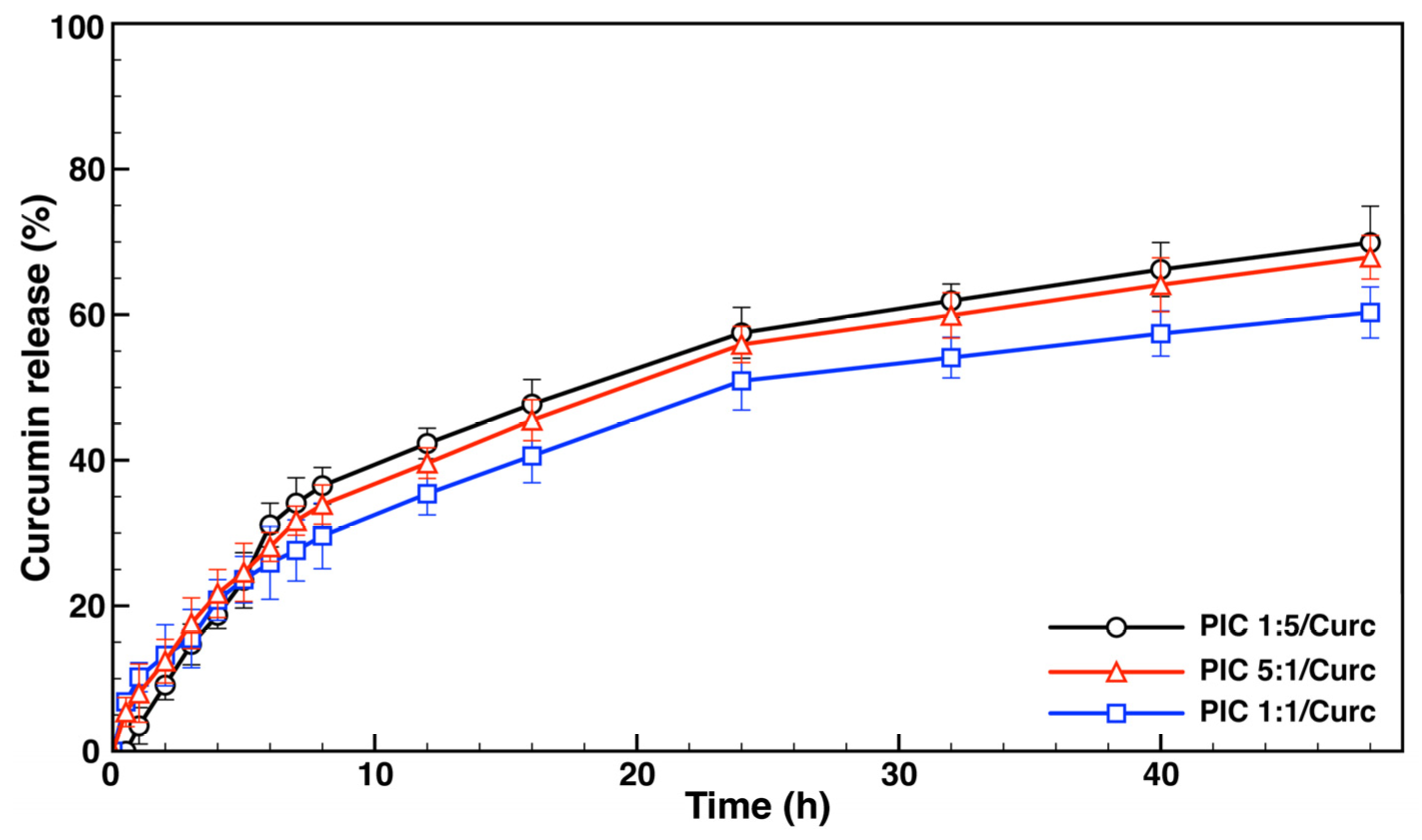
3.3.2. Curcumin Loading Evaluation and In Vitro Drug Release Studies
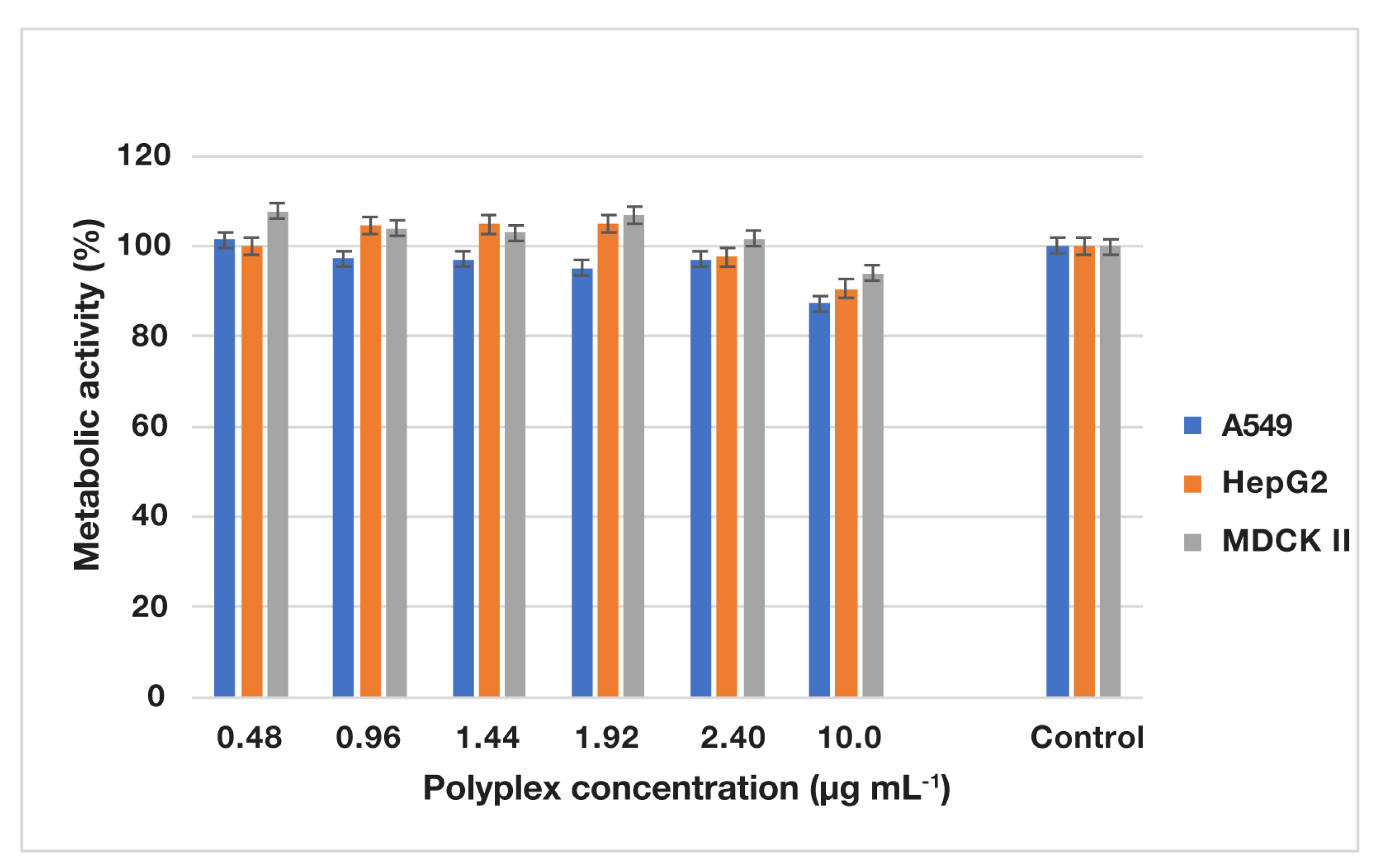
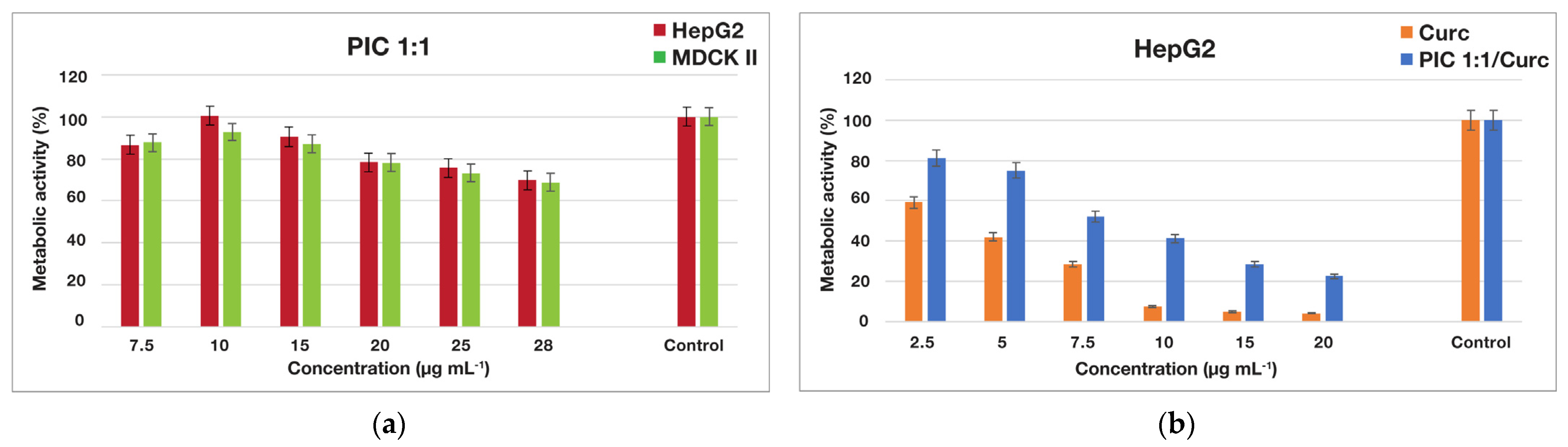
3.3.3. In Vitro Metabolic Activity Assessment of Various Cells Treated with Empty and Drug-Loaded PIC Micelles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Russell, T. Electroactive ionenes: Efficient interlayer materials in organic photovoltaics. Acc. Chem. Res. 2022, 55, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Ameduri, B. Copolymers of vinylidene fluoride with functional comonomers and applications therefrom: Recent developments, challenges and future trends. Prog. Polym. Sci. 2022, 133, 101591. [Google Scholar] [CrossRef]
- Kavand, A.; Anton, N.; Vandamme, T.; Serra, C.; Chan-Seng, D. Synthesis and functionalization of hyperbranched polymers for targeted drug delivery. J. Control. Release 2020, 321, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Finn, M.; Sharpless, K. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Keckeis, P.; Zeller, E.; Jung, C.; Besirske, P.; Kirner, F.; Ruiz-Agudo, C.; Schlaad, H.; Cölfen, H. Modular toolkit of multifunctional block copoly(2-oxazoline)s for the synthesis of nanoparticles. Chem. Eur. J. 2021, 27, 8283–8287. [Google Scholar] [CrossRef] [PubMed]
- García-Gallego, S.; Stenström, P.; Mesa-Antunez, P.; Zhang, Y.; Malkoch, M. Synthesis of heterofunctional polyester dendrimers with internal and external functionalities as versatile multipurpose platforms. Biomacromolecules 2020, 21, 4273–4279. [Google Scholar] [CrossRef]
- Rostovtsev, V.; Green, L.; Fokin, V.; Sharpless, K. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Escorihuela, J.; Marcelis, A.; Zuilhof, H. Metal-free click chemistry reactions on surfaces. Adv. Mater. Interfaces 2015, 2, 1500135. [Google Scholar] [CrossRef]
- Pickens, C.; Johnson, S.; Pressnall, M.; Leon, M.; Berkland, C. Practical considerations, challenges, and limitations of bioconjugation via azide-alkyne cycloaddition. Bioconjug. Chem. 2018, 29, 686–701. [Google Scholar] [CrossRef]
- Tasdelen, M. Diels–Alder “click” reactions: Recent applications in polymer and material science. Polym. Chem. 2011, 2, 2133–2145. [Google Scholar] [CrossRef]
- Lowe, A. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis: A first update. Polym. Chem. 2014, 5, 4820–4870. [Google Scholar] [CrossRef]
- Lowe, A. Thiol-yne ‘click’/coupling chemistry and recent applications in polymer and materials synthesis and modification. Polymer 2014, 55, 5517–5549. [Google Scholar] [CrossRef]
- Massi, A.; Nanni, D. Thiol-yne coupling: Revisiting old concepts as a breakthrough for up-to-date applications. Org. Biomol. Chem. 2012, 10, 3791–3807. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboom, R. Thiol-yne chemistry: A powerful tool for creating highly functional materials. Angew. Chem. Int. Ed. 2010, 49, 3415–3417. [Google Scholar] [CrossRef]
- Sprafke, J.; Spruell, J.; Mattson, K.; Montarnal, D.; McGrath, A.; Pötzsch, R.; Miyajima, D.; Hu, J.; Latimer, A.; Voit, B.; et al. Revisiting thiol-yne chemistry: Selective and efficient monoaddition for block and graft copolymer formation. J. Polym. Sci. Pol. Chem. 2015, 53, 319–326. [Google Scholar] [CrossRef]
- Hensarling, R.; Doughty, V.; Chan, J.; Patton, D. “Clicking” polymer brushes with thiol-yne chemistry: Indoors and out. J. Am. Chem. Soc. 2009, 131, 14673–14675. [Google Scholar] [CrossRef]
- Kumar, J.; Bousquet, A.; Stenzel, M. Thiol-alkyne chemistry for the preparation of micelles with glycopolymer corona: Dendritic surfaces versus linear glycopolymer in their ability to bind to lectins. Macromol. Rapid Commun. 2011, 32, 1620–1626. [Google Scholar] [CrossRef]
- El Jundi, A.; Buwalda, S.; Bakkour, Y.; Garric, X.; Nottelet, B. Double hydrophilic block copolymers self-assemblies in biomedical applications. Adv. Colloid Interface Sci. 2020, 283, 102213. [Google Scholar] [CrossRef]
- Abolmaali, S.; Tamaddon, A.; Salmanpour, M.; Mohammadi, S.; Dinarvand, R. Block ionomer micellar nanoparticles from double hydrophilic copolymers, classifications and promises for delivery of cancer chemotherapeutics. Eur. J. Pharm. Sci. 2017, 104, 393–405. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Formation of polyion complex micelles in an aqueous milieu from a pair of oppositely-charged block copolymers with poly(ethylene glycol) segments. Macromolecules 1995, 28, 5294–5299. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Polyion complex micelle formation from double-hydrophilic block copolymers composed of charged and non-charged segments in aqueous media. Polym. J. 2018, 50, 95–100. [Google Scholar] [CrossRef]
- Ita, K. Polyplexes for gene and nucleic acid delivery: Progress and bottlenecks. Eur. J. Pharm. Sci. 2020, 150, 105358. [Google Scholar] [CrossRef] [PubMed]
- Vetter, V.; Wagner, E. Targeting nucleic acid-based therapeutics to tumors: Challenges and strategies for polyplexes. J. Control. Release 2022, 346, 110–135. [Google Scholar] [CrossRef]
- Jarak, I.; Pereira-Silva, M.; Santos, A.; Veiga, F.; Cabral, H.; Figueiras, A. Multifunctional polymeric micelle-based nucleic acid delivery: Current advances and future perspectives. Appl. Mater. Today 2021, 25, 101217. [Google Scholar] [CrossRef]
- van den Berg, A.; Yun, C.-O.; Schiffelers, R.; Hennink, W. Polymeric delivery systems for nucleic acid therapeutics: Approaching the clinic. J. Control. Release 2021, 331, 121–141. [Google Scholar] [CrossRef]
- Pippa, N.; Kalinova, R.; Dimitrov, I.; Pispas, S.; Demetzos, C. Insulin/poly(ethylene glycol)-block-poly(L-Lysine) complexes: Physicochemical properties and protein encapsulation. J. Phys. Chem. B. 2015, 119, 6813–6819. [Google Scholar] [CrossRef]
- Chen, F.; Stenzel, M. Polyion complex micelles for protein delivery. Aust. J. Chem. 2018, 71, 768–780. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, A.; Yuan, J.; Gao, Q. Preparation, characterization and drug release behavior of polyion complex micelles. Int. J. Pharm. 2009, 374, 139–144. [Google Scholar] [CrossRef]
- Luo, Y.-L.; Yuan, J.-F.; Shi, J.-H.; Gao, Q.-Y. Synthesis and characterization of polyion complex micelles and their controlled release of folic acid. J. Colloid Interface Sci. 2010, 350, 140–147. [Google Scholar] [CrossRef]
- Chen, J.; Ding, J.; Zhang, Y.; Xiao, C.; Zhuang, X.; Chen, X. Polyion complex micelles with gradient pH-sensitivity for adjustable intracellular drug delivery. Polym. Chem. 2015, 6, 397–405. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, X.; Yu, J.; Zeng, J.; Chang, P.; Xu, H.; Huang, J. Fabrication and reduction-sensitive behavior of polyion complex nano-micelles based on PEG-conjugated polymer containing disulfide bonds as a potential carrier of anti-tumor paclitaxel. Colloids Surf. B 2013, 110, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, L.; Shen, Y.; Tang, L.; Sun, R.; Shi, D.; Webster, T.; Tu, J.; Sun, C. Electrostatic interactions between polyglutamic acid and polylysine yields stable polyion complex micelles for deoxypodophyllotoxin delivery. Int. J. Nanomed. 2017, 12, 7963–7977. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiao, Y.; Ge, Y.; Liu, G.; Xu, W.; Li, L. The study of tacrolimus-loaded polyion complex micelles for oral delivery. J. Biomed. Nanotechnol. 2017, 13, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Kalinova, R.; Dimitrov, I. Functional Polyion complex micelles for potential targeted hydrophobic drug delivery. Molecules 2022, 27, 2178. [Google Scholar] [CrossRef] [PubMed]
- Kalinova, R.; Grancharov, G.; Doumanov, J.; Mladenova, K.; Petrova, S.; Dimitrov, I. Green synthesis and the evaluation of a functional amphiphilic block copolymer as a micellar curcumin delivery system. Int. J. Mol. Sci. 2023, 24, 10588. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarth, J.; Hatton, T. Micellization of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in aqueous solutions: Thermodynamics of copolymer association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Babikova, D.; Kalinova, R.; Zhelezova, I.; Momekova, D.; Konstantinov, S.; Momekov, G.; Dimitrov, I. Functional block copolymer nanocarriers for anticancer drug delivery. RSC Adv. 2016, 6, 84634–84644. [Google Scholar] [CrossRef]
- Fairbanks, B.; Scott, T.; Kloxin, C.; Anseth, K.; Bowman, C. Thiol−Yne Photopolymerizations: Novel mechanism, kinetics, and step-growth formation of highly cross-linked networks. Macromolecules 2009, 42, 211–217. [Google Scholar] [CrossRef]
- Suk, J.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Hama, S.; Itakura, S.; Nakai, M.; Nakayama, K.; Morimoto, S.; Suzuki, S.; Kogure, K. Overcoming the polyethylene glycol dilemma via pathological environment-sensitive change of the surface property of nanoparticles for cellular entry. J. Control. Release 2015, 206, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kah, G.; Chandran, R.; Abrahamse, H. Curcumin a natural phenol and its therapeutic role in cancer and photodynamic therapy: A review. Pharmaceutics 2023, 15, 639. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, K.; Bhaskaran, M.; Selvam, C.; Thilagavathi, R. Nano formulation approaches for curcumin delivery—A review. J. Drug Deliv. Sci. Technol. 2023, 82, 104326. [Google Scholar] [CrossRef]
- Chang, R.; Chen, L.; Qamar, M.; Wen, Y.; Li, L.; Zhang, J.; Li, X.; Assadpour, E.; Esatbeyoglu, T.; Kharazmi, M.; et al. The bioavailability, metabolism and microbial modulation of curcumin-loaded nanodelivery systems. Adv. Colloid Interface Sci. 2023, 318, 102933. [Google Scholar] [CrossRef] [PubMed]

| Non-Loaded PIC Micelles | Curcumin-Loaded PIC Micelles | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Code | CMC a (mg mL−1) | d b (nm) | PdI b | ζ b (mV) | d b (nm) | PdI b | ζ b (%) | DLE c (%) | DLC c (%) |
| PIC 1:5 | 0.041 | 74.21 ± 1.47 | 0.416 | −9.40 ± 0.36 | 79.43 ± 0.69 | 0.060 | −8.65 ± 0.56 | 66 | 7.3 |
| PIC 1:1 | 0.016 | 41.04 ± 1.53 | 0.223 | 1.34 ± 0.66 | 47.81 ± 1.20 | 0.088 | 1.96 ± 0.85 | 76 | 6.9 |
| PIC 5:1 | 0.034 | 78.52 ± 2.24 | 0.282 | 10.60 ± 1.09 | 86.62 ± 1.57 | 0.091 | 12.10 ± 1.29 | 56 | 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinova, R.; Mladenova, K.; Petrova, S.; Doumanov, J.; Dimitrov, I. Solvent-Free Synthesis of Multifunctional Block Copolymer and Formation of DNA and Drug Nanocarriers. Nanomaterials 2023, 13, 2936. https://doi.org/10.3390/nano13222936
Kalinova R, Mladenova K, Petrova S, Doumanov J, Dimitrov I. Solvent-Free Synthesis of Multifunctional Block Copolymer and Formation of DNA and Drug Nanocarriers. Nanomaterials. 2023; 13(22):2936. https://doi.org/10.3390/nano13222936
Chicago/Turabian StyleKalinova, Radostina, Kirilka Mladenova, Svetla Petrova, Jordan Doumanov, and Ivaylo Dimitrov. 2023. "Solvent-Free Synthesis of Multifunctional Block Copolymer and Formation of DNA and Drug Nanocarriers" Nanomaterials 13, no. 22: 2936. https://doi.org/10.3390/nano13222936
APA StyleKalinova, R., Mladenova, K., Petrova, S., Doumanov, J., & Dimitrov, I. (2023). Solvent-Free Synthesis of Multifunctional Block Copolymer and Formation of DNA and Drug Nanocarriers. Nanomaterials, 13(22), 2936. https://doi.org/10.3390/nano13222936






