Industrial Waste-Derived Carbon Materials as Advanced Electrodes for Supercapacitors
Abstract
:1. Introduction
2. Experimental
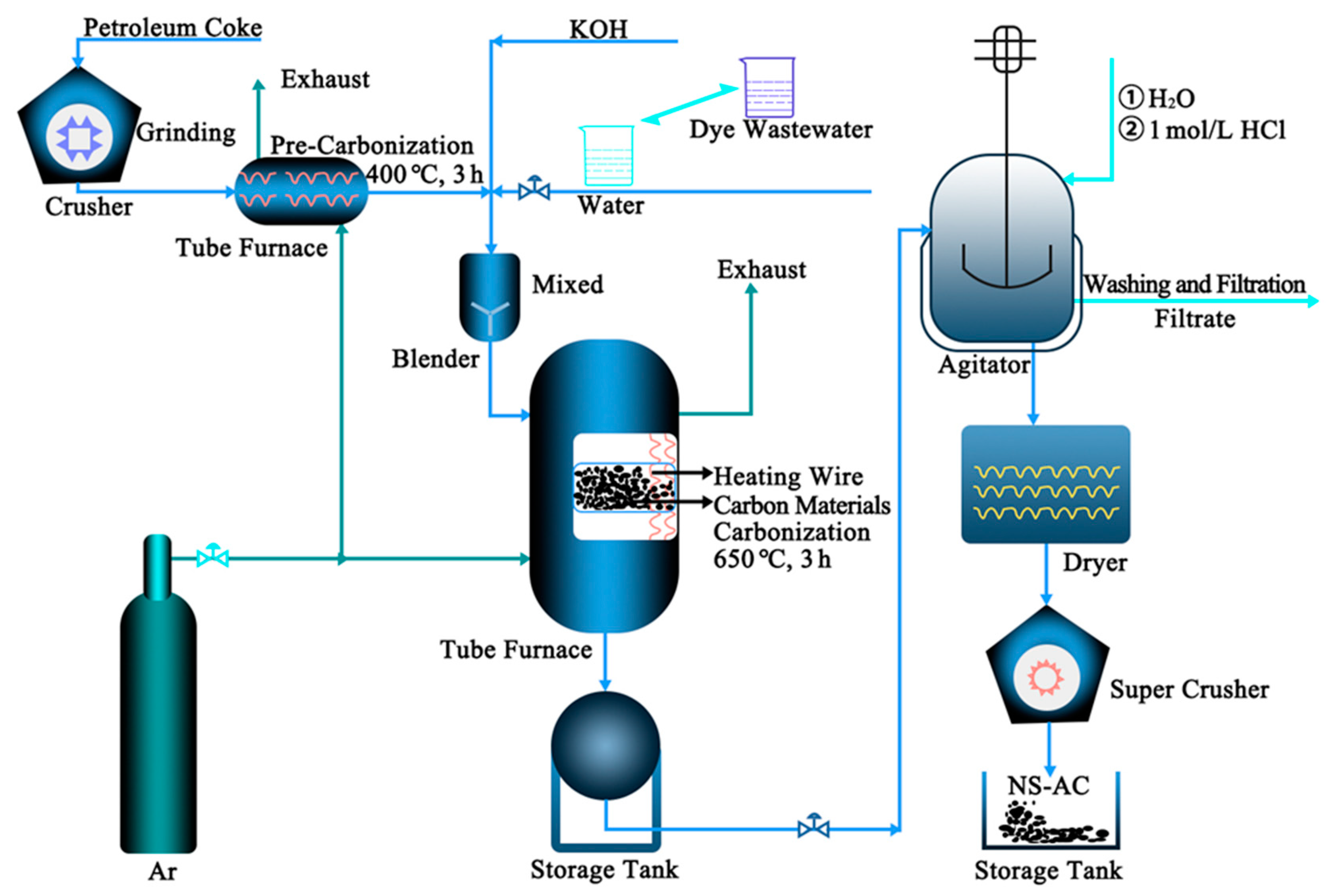
2.1. Preparation of Samples
2.2. Characterization
2.3. Electrochemical Test
2.3.1. Three-Electrode System
2.3.2. Coin Cell Supercapacitor
2.3.3. Soft-Packaged Supercapacitor
2.3.4. Electrochemical Measurements
3. Results and Discussion
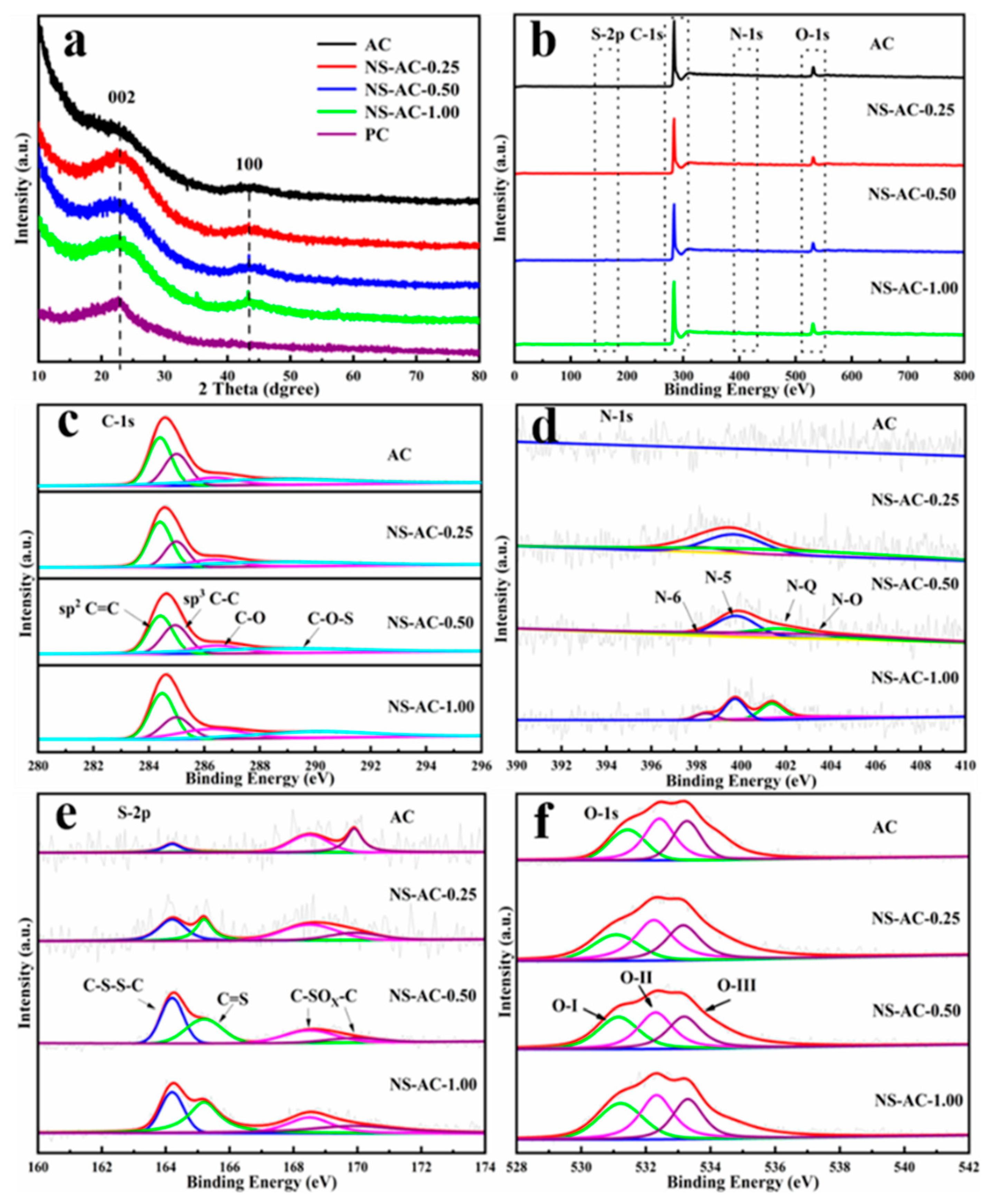
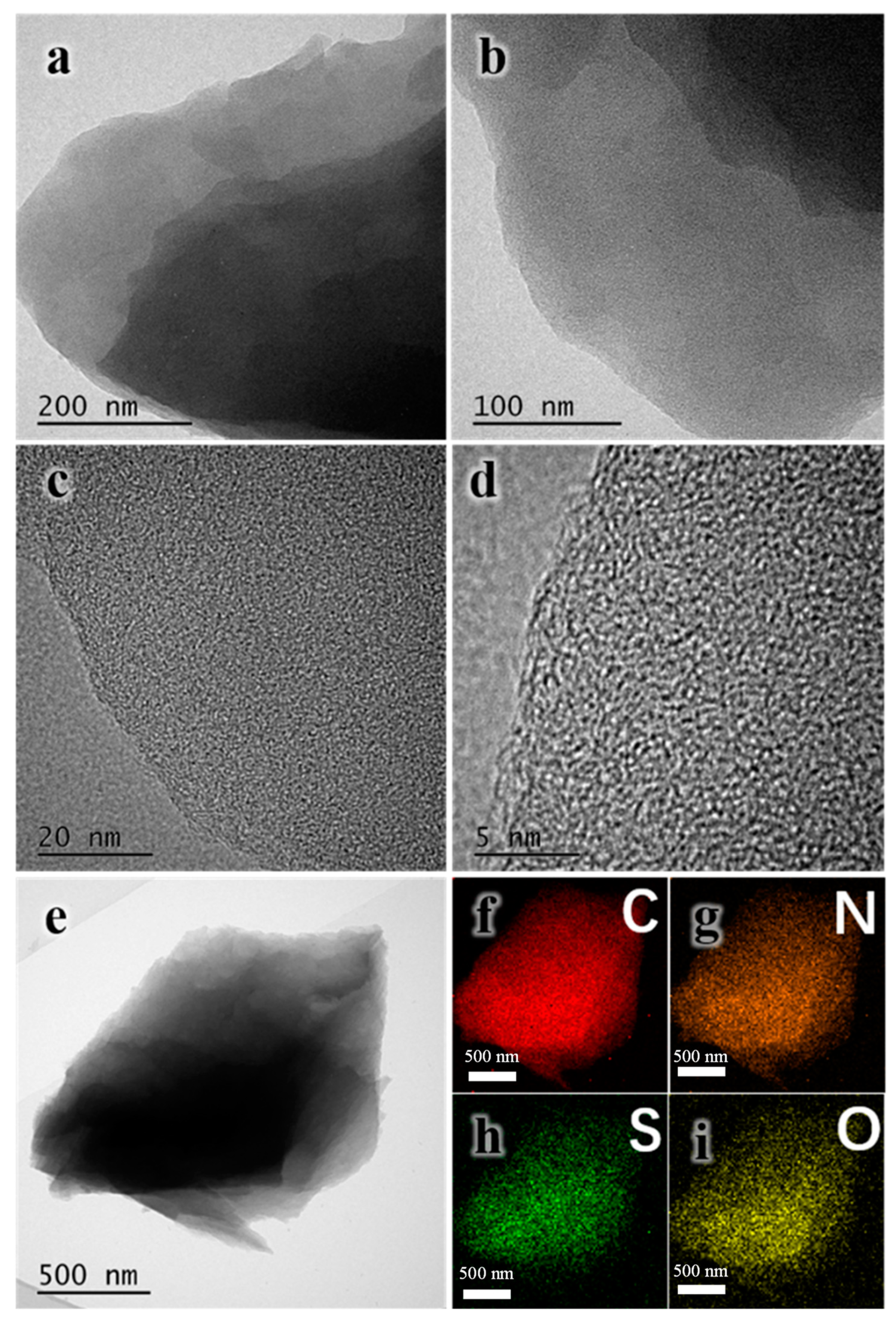
3.1. Structure and Morphology of the ACs
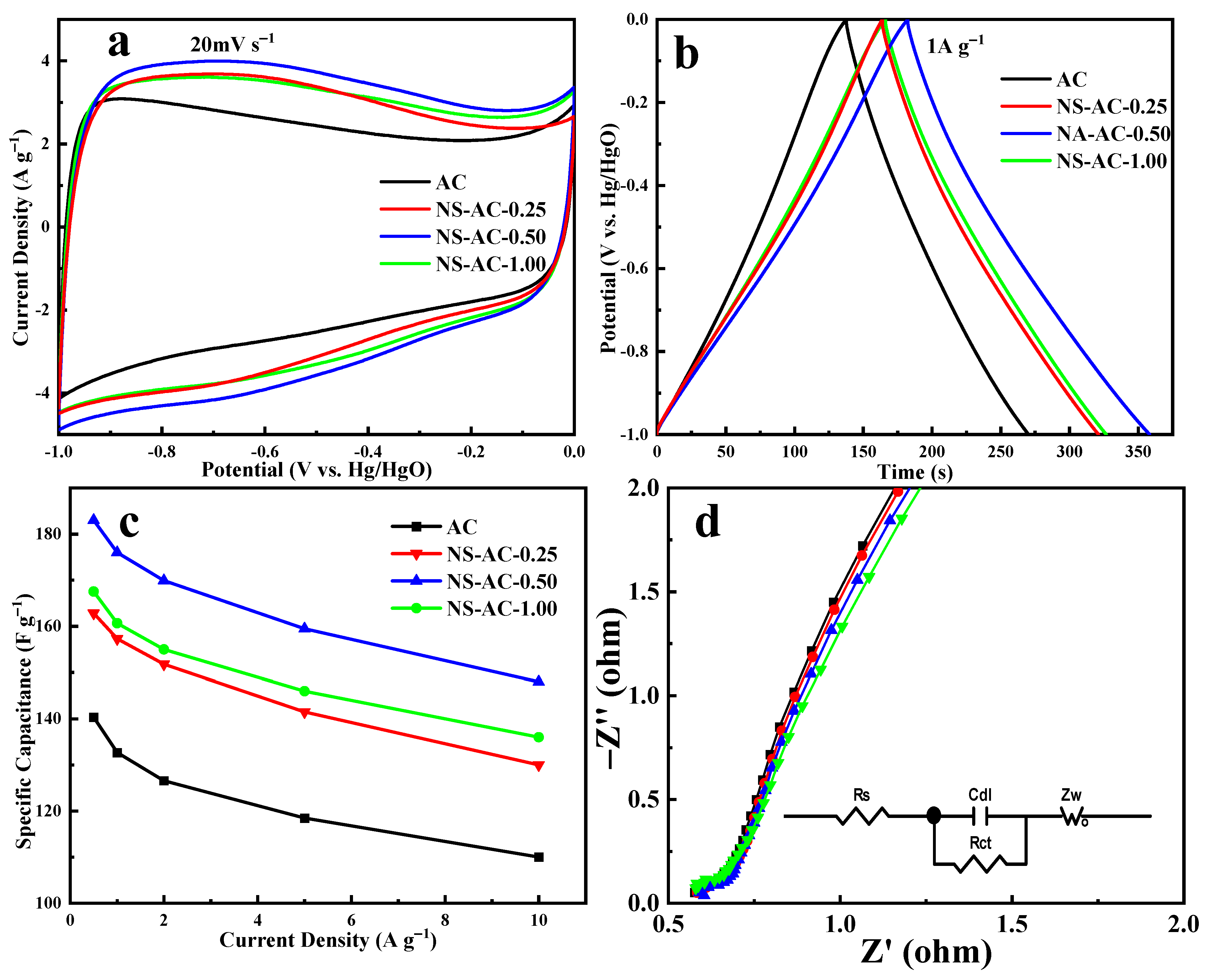
3.2. Electrochemical Performances
3.3. Structure and Morphology of the Scaled Sample NS-AC-0.5-S20
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon nanotube- and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Pothanamkandathil, V.; Fortunato, J.; Gorski, C.A. Electrochemical Desalination Using Intercalating Electrode Materials: A Comparison of Energy Demands. Environ. Sci. Technol. 2020, 54, 3653–3662. [Google Scholar] [CrossRef]
- Liu, C.-F.; Liu, Y.-C.; Yi, T.-Y.; Hu, C.-C. Carbon materials for high-voltage supercapacitors. Carbon 2019, 145, 529–548. [Google Scholar] [CrossRef]
- Kshetri, T.; Tran, D.T.; Nguyen, D.C.; Kim, N.H.; Lau, K.T.; Lee, J.H. Ternary graphene-carbon nanofibers-carbon nanotubes structure for hybrid supercapacitor. Chem. Eng. J. 2020, 380, 11. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhang, Y.Q.; Zhang, L.; Xie, F.X.; Vasileff, A.; Qiao, S.Z. Graphitic Carbon Nitride (g-C3N4)-Derived N-Rich Graphene with Tuneable Interlayer Distance as a High-Rate Anode for Sodium-Ion Batteries. Adv. Mater. 2019, 31, 10. [Google Scholar] [CrossRef]
- Kong, H.B.; Wu, Y.S.; Hong, W.Z.; Yan, C.S.; Zhao, Y.Y.; Chen, G. Structure-designed synthesis of Cu-doped Co3O4@N-doped carbon with interior void space for optimizing alkali-ion storage. Energy Storage Mater. 2020, 24, 610–617. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.G.; Wang, Y.G.; Zhang, D.Y.; Lu, Y.; Luo, R.J.; Wang, Y.B.; Liu, X.M.; Kim, J.K.; Luo, Y.S. Vertically aligned ultrathin MoS2 nanosheets grown on graphene-wrapped hollow carbon microtubes derived from loofah sponge as advanced anodes for highly reversible lithium storage. Electrochim. Acta 2019, 296, 989–998. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y.J.; Chen, H.B.; Yang, M.; Li, H.M. MnO2 Nanostructures Deposited on Graphene-Like Porous Carbon Nanosheets for High-Rate Performance and High-Energy Density Asymmetric Supercapacitors. Acs Sustain. Chem. Eng. 2019, 7, 3101–3110. [Google Scholar] [CrossRef]
- Kamath, D.; Arsenault, R.; Kim, H.C.; Anctil, A. Economic and Environmental Feasibility of Second-Life Lithium-Ion Batteries as Fast-Charging Energy Storage. Environ. Sci. Technol. 2020, 54, 6878–6887. [Google Scholar] [CrossRef]
- Lin, Y.; Zhao, H.X.; Yu, F.; Yang, J.F. Design of an Extended Experiment with Electrical Double Layer Capacitors: Electrochemical Energy Storage Devices in Green Chemistry. Sustainability 2018, 10, 3630. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, J.; Fan, Z.J. Carbon materials for high volumetric performance supercapacitors: Design, progress, challenges and opportunities. Energy Environ. Sci. 2016, 9, 729–762. [Google Scholar] [CrossRef]
- Mo, R.J.; Zhao, Y.; Zhao, M.M.; Wu, M.; Wang, C.; Li, J.P.; Kuga, S.; Huang, Y. Graphene-like porous carbon from sheet cellulose as electrodes for supercapacitors. Chem. Eng. J. 2018, 346, 104–112. [Google Scholar] [CrossRef]
- Lin, Y.N.; Chen, H.; Shi, Y.L.; Wang, G.; Chen, L.; Wang, F.; Li, S.Q.; Yu, F.; Zhang, L.L. Nitrogen and Sulfur Co-Doped Graphene-Like Carbon from Industrial Dye Wastewater for Use as a High-Performance Supercapacitor Electrode. Glob. Chall. 2019, 3, 9. [Google Scholar] [CrossRef]
- Tang, Z.J.; Pei, Z.X.; Wang, Z.F.; Li, H.F.; Zeng, J.; Ruan, Z.H.; Huang, Y.; Zhu, M.S.; Xue, Q.; Yu, J.; et al. Highly anisotropic, multichannel wood carbon with optimized heteroatom doping for supercapacitor and oxygen reduction reaction. Carbon 2018, 130, 532–543. [Google Scholar] [CrossRef]
- Lu, H.; Kim, K.; Kwon, Y.; Sun, X.M.; Ryoo, R.; Zhao, X.S. Zeolite-templated nanoporous carbon for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 10388–10394. [Google Scholar] [CrossRef]
- Miao, L.; Zhu, D.Z.; Liu, M.X.; Duan, H.; Wang, Z.W.; Lv, Y.K.; Xiong, W.; Zhu, Q.J.; Li, L.C.; Chai, X.L.; et al. Cooking carbon with protic salt: Nitrogen and sulfur self-doped porous carbon nanosheets for supercapacitors. Chem. Eng. J. 2018, 347, 233–242. [Google Scholar] [CrossRef]
- Zheng, X.J.; Wu, J.; Cao, X.C.; Abbott, J.; Jin, C.; Wang, H.B.; Strasser, P.; Yang, R.Z.; Chen, X.; Wu, G. N-, P-, and S-doped graphene-like carbon catalysts derived from onium salts with enhanced oxygen chemisorption for Zn-air battery cathodes. Appl. Catal. B-Environ. 2019, 241, 442–451. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Luo, J.D.; Qi, X.T.; Yu, J.; Cai, J.X.; Wei, J.C.; Yang, Z.Y. A Chemical Blowing Strategy to Fabricate Biomass-Derived Carbon-Aerogels with Graphene-Like Nanosheet Structures for High-Performance Supercapacitors. Chemsuschem 2019, 12, 2462–2470. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Liu, H.Z.; Sun, H.H.; Hua, W.; Wang, H.W.; Liu, X.R.; Wei, B.Q. One-pot synthesis of nitrogen-doped ordered mesoporous carbon spheres for high-rate and long-cycle life supercapacitors. Carbon 2018, 127, 85–92. [Google Scholar] [CrossRef]
- Evans, A.E.V.; Mateo-Sagasta, J.; Qadir, M.; Boelee, E.; Ippolito, A. Agricultural water pollution: Key knowledge gaps and research needs. Curr. Opin. Environ. Sustain. 2019, 36, 20–27. [Google Scholar] [CrossRef]
- Li, W.; Mu, B.N.; Yang, Y.Q. Feasibility of industrial-scale treatment of dye wastewater via bio-adsorption technology. Bioresour. Technol. 2019, 277, 157–170. [Google Scholar] [CrossRef]
- Hao, X.D.; Li, J.; van Loosdrecht, M.C.M.; Jiang, H.; Liu, R.B. Energy recovery from wastewater: Heat over organics. Water Res. 2019, 161, 74–77. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhu, M.Y.; Li, Y.C.; Zhang, M.J.; Xue, X.Y.; Shi, Y.L.; Dai, B.; Guo, X.H.; Yu, F. Heteroatom-doped porous carbon from methyl orange dye wastewater for oxygen reduction. Green Energy Environ. 2018, 3, 172–178. [Google Scholar] [CrossRef]
- Cui, M.H.; Cui, D.; Gao, L.; Cheng, H.Y.; Wang, A.J. Analysis of electrode microbial communities in an up-flow bioelectrochemical system treating azo dye wastewater. Electrochim. Acta 2016, 220, 252–257. [Google Scholar] [CrossRef]
- Bommier, C.; Surta, T.W.; Dolgos, M.; Ji, X.L. New Mechanistic Insights on Na-Ion Storage in Nongraphitizable Carbon. Nano Lett. 2015, 15, 5888–5892. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Li, Z.S.; Lin, L.; Wang, X.Y.; Wang, Y.F.; Zhang, C.H.; Wang, H.Q. Facile synthesis of activated carbon/carbon nanotubes compound for supercapacitor application. Chem. Eng. J. 2010, 156, 500–504. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Kong, L.N.; Song, A.L.; Qin, X.J.; Shao, G.J. Phosphorus-doped 3D hierarchical porous carbon for high-performance supercapacitors: A balanced strategy for pore structure and chemical composition. Carbon 2018, 127, 557–567. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.L. Simultaneous Electrochemical Dual-Electrode Exfoliation of Graphite toward Scalable Production of High-Quality Graphene. Adv. Funct. Mater. 2019, 29, 14. [Google Scholar] [CrossRef]
- Yu, M.L.; Wang, Z.Y.; Wang, Y.W.; Dong, Y.F.; Qiu, J.S. Freestanding Flexible Li2S Paper Electrode with High Mass and Capacity Loading for High-Energy Li–S Batteries. Adv. Energy Mater. 2017, 7, 10. [Google Scholar] [CrossRef]
- Liu, W.; Niu, H.; Yang, J.; Cheng, K.; Ye, K.; Zhu, K.; Wang, G.L.; Cao, D.X.; Yan, J. Ternary Transition Metal Sulfides Embedded in Graphene Nanosheets as Both the Anode and Cathode for High-Performance Asymmetric Supercapacitors. Chem. Mater. 2018, 30, 1055–1068. [Google Scholar] [CrossRef]
- Zhu, H.; Xiao, C.; Cheng, H.; Grote, F.; Zhang, X.D.; Yao, T.; Li, Z.; Wang, C.M.; Wei, S.Q.; Lei, Y.; et al. Magnetocaloric effects in a freestanding and flexible graphene-based superlattice synthesized with a spatially confined reaction. Nat. Commun. 2014, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dai, F.; Xiao, Q.F.; Yang, L.; Shen, J.M.; Zhang, C.M.; Cai, M. Nitrogen-doped activated carbon for a high energy hybrid supercapacitor. Energy Environ. Sci. 2016, 9, 102–106. [Google Scholar] [CrossRef]
- Liu, W.J.; Tian, K.; Ling, L.L.; Yu, H.Q.; Jiang, H. Use of Nutrient Rich Hydrophytes to Create N,P-Dually Doped Porous Carbon with Robust Energy Storage Performance. Environ. Sci. Technol. 2016, 50, 12421–12428. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.R.; Yao, B.; Wei, X.J.; Liu, T.Y.; Kou, T.Y.; Xiao, P.; Zhang, Y.H.; Li, Y. Pore and Heteroatom Engineered Carbon Foams for Supercapacitors. Adv. Energy Mater. 2019, 9, 9. [Google Scholar] [CrossRef]
- Wang, M.; Liu, H.; Zhai, D.D.; Chen, X.Y.; Zhang, Z.J. In-situ synthesis of highly nitrogen, sulfur co-doped carbon nanosheets from melamine-formaldehyde-thiourea resin with improved cycling stability and energy density for supercapacitors. J. Power Sources 2019, 416, 79–88. [Google Scholar] [CrossRef]
- Huang, G.G.; Wang, Y.; Zhang, T.Y.; Wu, X.X.; Cai, J.J. High-performance hierarchical N-doped porous carbons from hydrothermally carbonized bamboo shoot shells for symmetric supercapacitors. J. Taiwan Inst. Chem. E 2019, 96, 672–680. [Google Scholar] [CrossRef]
- Li, Z.S.; Zhang, L.; Chen, X.; Li, B.L.; Wang, H.Q.; Li, Q.Y. Three-dimensional graphene-like porous carbon nanosheets derived from molecular precursor for high-performance supercapacitor application. Electrochim. Acta 2019, 296, 8–17. [Google Scholar] [CrossRef]
- Liu, H.Y.; Wang, K.P.; Teng, H.S. A simplified preparation of mesoporous carbon and the examination of the carbon accessibility for electric double layer formation. Carbon 2005, 43, 559–566. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Z.Y.; Yu, C.Y.; Zhong, W.B. Heteroatom-Doped Sheet-Like and Hierarchical Porous Carbon Based on Natural Biomass Small Molecule Peach Gum for High-Performance Supercapacitors. Acs Sustain. Chem. Eng. 2019, 7, 3389–3403. [Google Scholar] [CrossRef]
- Lin, X.Q.; Yang, N.; Lu, Q.F.; Liu, R. Self-Nitrogen-Doped Porous Biocarbon from Watermelon Rind: A High-Performance Supercapacitor Electrode and Its Improved Electrochemical Performance Using Redox Additive Electrolyte. Energy Technol. 2019, 7, 12. [Google Scholar] [CrossRef]
- Ganfoud, N.; Sene, A.; Haefele, M.; Marin-Lafleche, A.; Daffos, B.; Taberna, P.L.; Salanne, M.; Simon, P.; Rotenberg, B. Effect of the carbon microporous structure on the capacitance of aqueous supercapacitors. Energy Storage Mater. 2019, 21, 190–195. [Google Scholar] [CrossRef]
- Xu, H.; Wu, C.K.; Wei, X.J.; Gao, S.Y. Hierarchically porous carbon materials with controllable proportion of micropore area by dual-activator synthesis for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 15340–15347. [Google Scholar] [CrossRef]
- Dong, S.; He, X.J.; Zhang, H.F.; Xie, X.Y.; Yu, M.X.; Yu, C.; Xiao, N.; Qiu, J.S. Surface modification of biomass-derived hard carbon by grafting porous carbon nanosheets for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 15954–15960. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Minakshi, M. Status on electrodeposited manganese dioxide and biowaste carbon for hybrid capacitors: The case of high-quality oxide composites, mechanisms, and prospects. J. Energy Storage 2022, 56, 106099. [Google Scholar] [CrossRef]
- Wu, Y.G.; Liu, Z.; Ran, F. New comprehensions on structure superiority of asymmetric carbon membrane and controlled construction of advanced hierarchical inner-structure for high performance supercapacitors. Micropor. Mesopor. Mater. 2019, 275, 14–25. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Z.Y.; Chang, J.L.; Liu, X.; Wu, D.P.; Xu, F.; Guo, Y.M.; Jiang, K. Nitrogen-Doped Porous Carbons As Electrode Materials for High-Performance Supercapacitor and Dye-Sensitized Solar Cell. Acs Appl. Mater. Inter. 2015, 7, 20234–20244. [Google Scholar] [CrossRef]
- Tan, M.H.; Zheng, J.T.; Li, P.; Noritatsu, T.; Wu, M.B. Preparation and modification of high performance porous carbons from petroleum coke for use as supercapacitor electrodes. New Carbon Mater. 2016, 31, 343–351. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Song, J.H.; Yang, W.; Wang, M.S.; Zhu, C.Z.; Zhao, W.G.; Zheng, J.M.; Lin, Y.H. Self-supporting activated carbon/carbon nanotube/reduced graphene oxide flexible electrode for high performance supercapacitor. Carbon 2018, 129, 236–244. [Google Scholar] [CrossRef]
- Yang, J.; Wu, H.L.; Zhu, M.; Ren, W.J.; Lin, Y.; Chen, H.B.; Pan, F. Optimized mesopores enabling enhanced rate performance in novel ultrahigh surface area meso-/microporous carbon for supercapacitors. Nano Energy 2017, 33, 453–461. [Google Scholar] [CrossRef]
- Samal, R.; Dash, B.; Sarangi, C.K.; Sanjay, K.; Subbaiah, T.; Senanayake, G.; Minakshi, M. Influence of Synthesis Temperature on the Growth and Surface Morphology of Co(3)O(4) Nanocubes for Supercapacitor Applications. Nanomaterials 2017, 7, 356. [Google Scholar] [CrossRef]
- Maqsood, M.F.; Latif, U.; Sheikh, Z.A.; Abubakr, M.; Rehman, S.; Khan, K.; Khan, M.A.; Kim, H.; Ouladsmane, M.; Rehman, M.A.; et al. A comprehensive study of Bi2Sr2Co2Oy misfit layered oxide as a supercapacitor electrode material. Inorg. Chem. Commun. 2023, 158, 111487. [Google Scholar] [CrossRef]
- Chodankar, N.R.; Patil, S.J.; Hwang, S.-K.; Shinde, P.A.; Karekar, S.V.; Raju, G.S.R.; Ranjith, K.S.; Olabi, A.G.; Dubal, D.P.; Huh, Y.S.; et al. Refurbished carbon materials from waste supercapacitors as industrial-grade electrodes: Empowering electronic waste. Energy Storage Mater. 2022, 49, 564–574. [Google Scholar] [CrossRef]
- Sheikh, Z.A.; Katkar, P.K.; Kim, H.; Rehman, S.; Khan, K.; Chavan, V.D.; Jose, R.; Khan, M.F.; Kim, D.-K. Transition metal chalcogenides, MXene, and their hybrids: An emerging electrochemical capacitor electrodes. J. Energy Storage 2023, 71, 107997. [Google Scholar] [CrossRef]



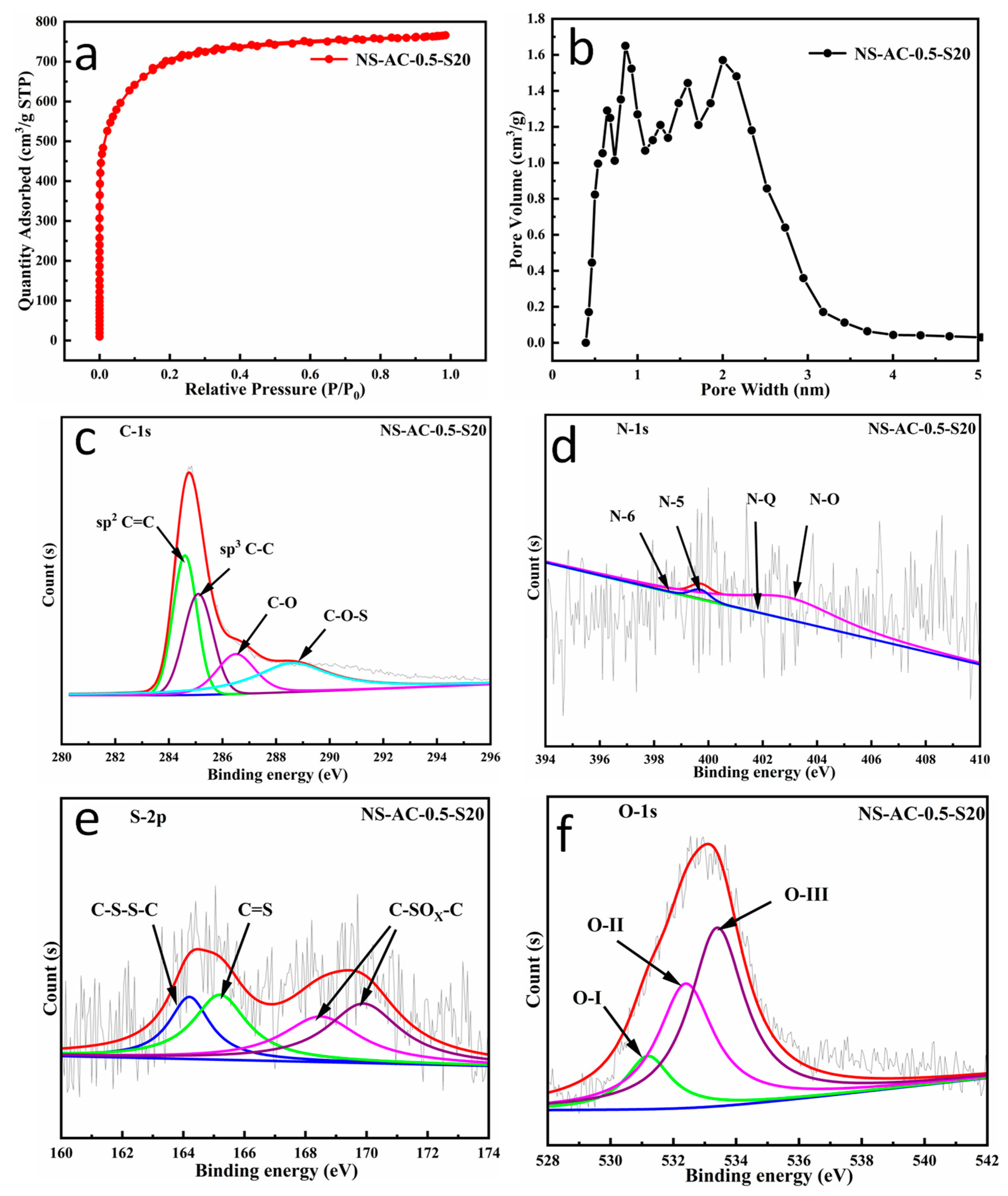
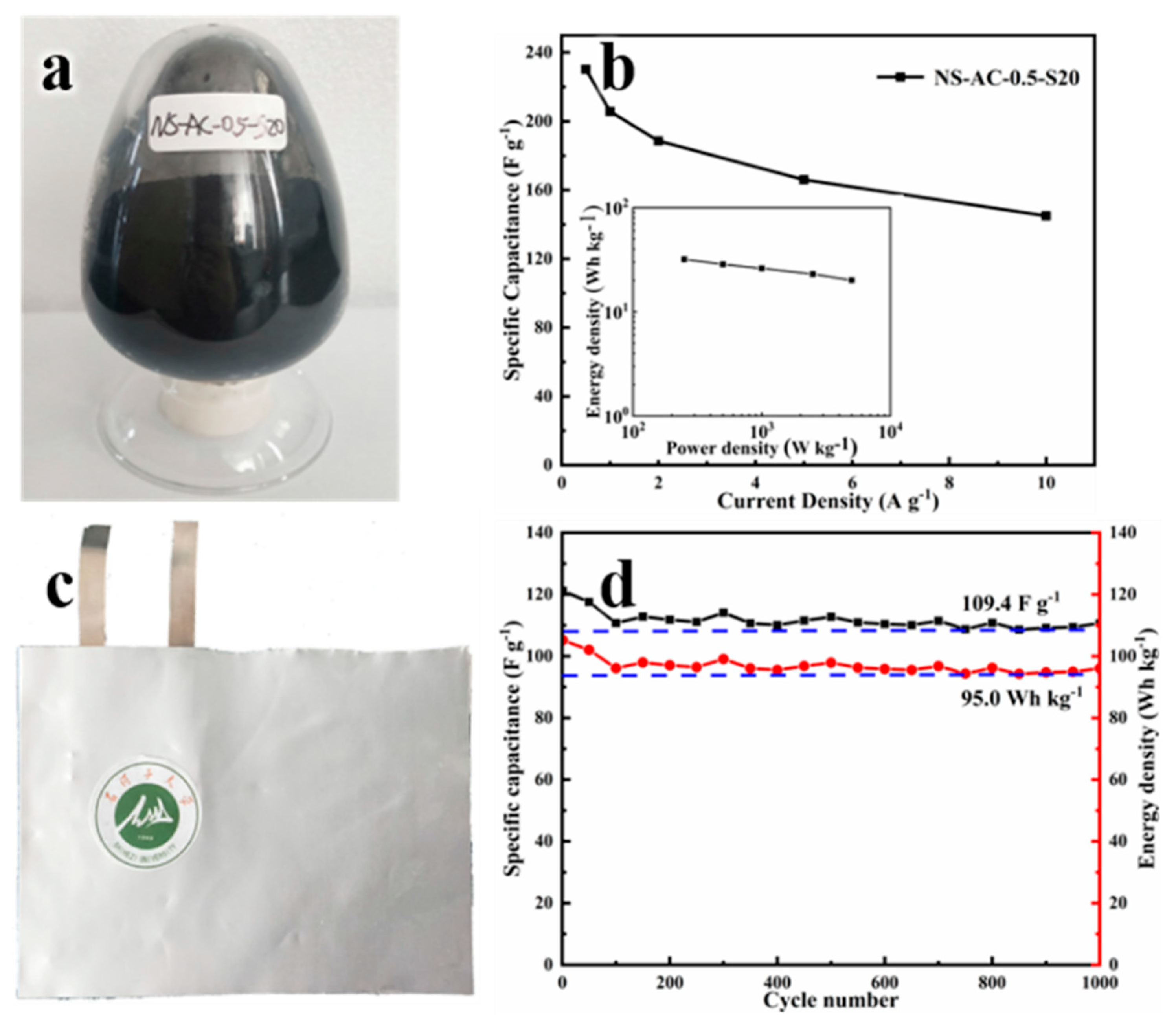
| ACs | XPS (at.%) | |||
|---|---|---|---|---|
| C | N | O | S | |
| AC | 92.97 | - | 6.86 | 0.17 |
| NS-AC-0.25 | 90.69 | 1.76 | 7.22 | 0.33 |
| NS-AC-0.50 | 89.95 | 2.18 | 6.96 | 0.91 |
| NS-AC-1.00 | 89.36 | 1.99 | 7.75 | 0.90 |
| NS-AC-0.5-S20 | 90.39 | 1.60 | 7.46 | 0.54 |
| ACs | SBET, (m2 g−1) | Pore Volume, (cm3 · g−1) | Vmic/Vt, (%) | Vmes/Vt, (%) | Dap, (nm) | ||
|---|---|---|---|---|---|---|---|
| Vt | Vmic | Vmes | |||||
| AC | 1903.0 | 0.89 | 0.81 | 0.08 | 91.0 | 9.00 | 1.87 |
| NS-AC-0.25 | 1488.9 | 0.64 | 0.58 | 0.06 | 90.6 | 9.40 | 1.72 |
| NS-AC-0.50 | 1445.3 | 0.63 | 0.57 | 0.06 | 90.5 | 9.50 | 1.72 |
| NS-AC-1.00 | 1070.3 | 0.46 | 0.42 | 0.04 | 91.3 | 8.70 | 1.71 |
| NS-AC-0.5-S20 | 2581.8 | 1.19 | 1.09 | 0.10 | 91.6 | 8.40 | 1.84 |
| Samples | Solution Resistance Rs (Ω) | Charge Transfer Resistance Rct (Ω) |
|---|---|---|
| AC | 9.56 | 0.26 |
| NS-AC-0.25 | 9.56 | 0.25 |
| NS-AC-0.50 | 9.56 | 0.23 |
| NS-AC-1.0 | 9.58 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, G.; Guo, W.; Wang, G.; Dai, B.; Liu, L.; Zhang, L.; Yu, F. Industrial Waste-Derived Carbon Materials as Advanced Electrodes for Supercapacitors. Nanomaterials 2023, 13, 2924. https://doi.org/10.3390/nano13222924
Bai G, Guo W, Wang G, Dai B, Liu L, Zhang L, Yu F. Industrial Waste-Derived Carbon Materials as Advanced Electrodes for Supercapacitors. Nanomaterials. 2023; 13(22):2924. https://doi.org/10.3390/nano13222924
Chicago/Turabian StyleBai, Ge, Wen Guo, Gang Wang, Bin Dai, Lu Liu, Lili Zhang, and Feng Yu. 2023. "Industrial Waste-Derived Carbon Materials as Advanced Electrodes for Supercapacitors" Nanomaterials 13, no. 22: 2924. https://doi.org/10.3390/nano13222924
APA StyleBai, G., Guo, W., Wang, G., Dai, B., Liu, L., Zhang, L., & Yu, F. (2023). Industrial Waste-Derived Carbon Materials as Advanced Electrodes for Supercapacitors. Nanomaterials, 13(22), 2924. https://doi.org/10.3390/nano13222924







