Recent Advances in the Catalytic Conversion of Methane to Methanol: From the Challenges of Traditional Catalysts to the Use of Nanomaterials and Metal-Organic Frameworks
Abstract
:1. Introduction
2. Conversion of Methane to Methanol Routes
2.1. Direct and Indirect Routes
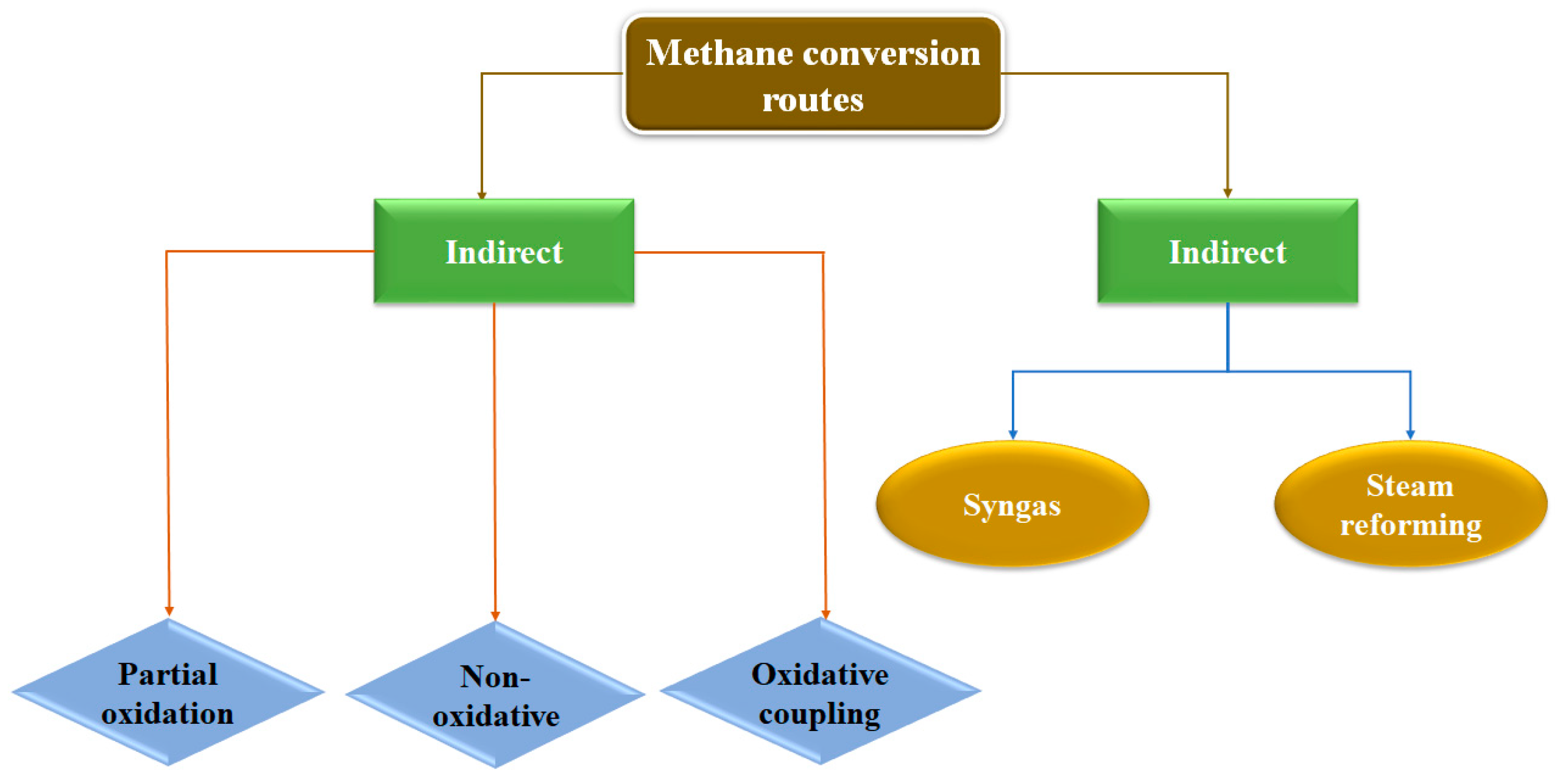
2.2. Challenging Parameters in Methane to Methanol Catalysis
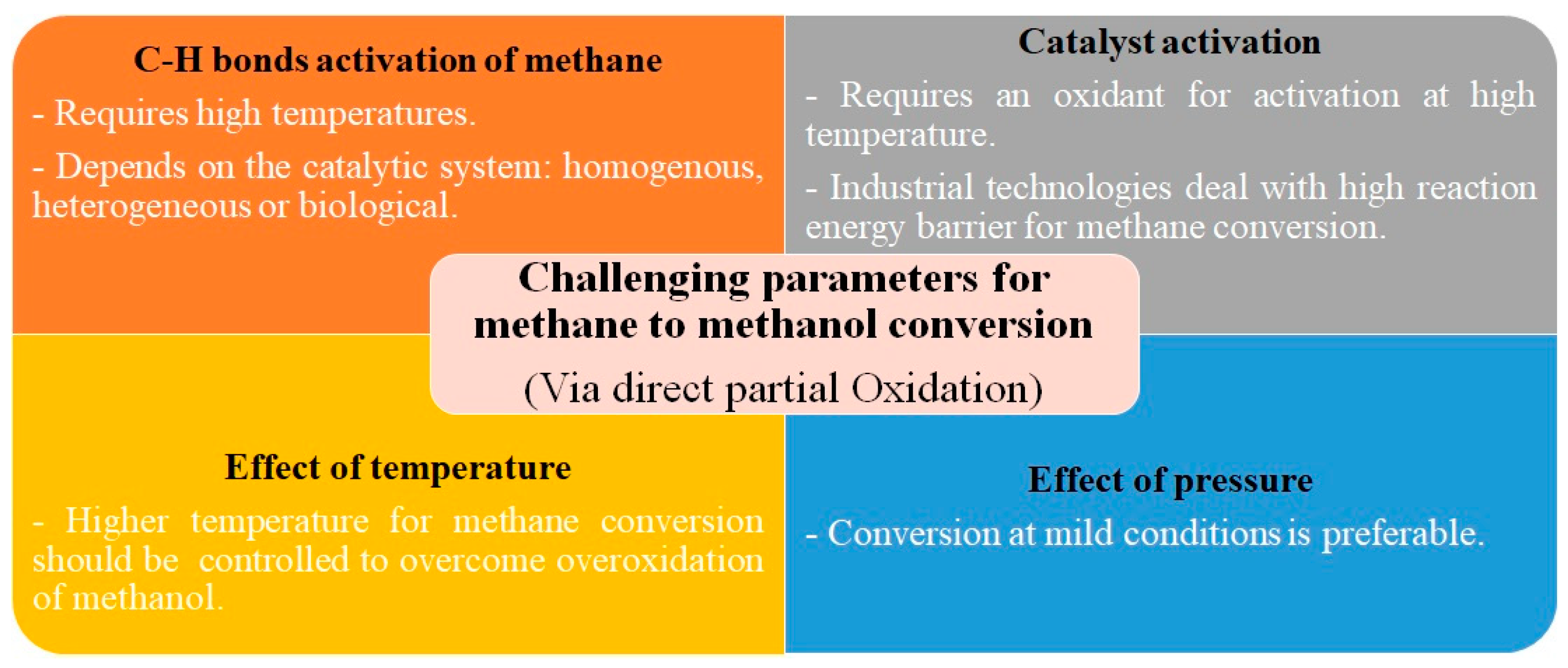
2.2.1. Activation of C-H Bonds and Its Connection to Selectivity
2.2.2. Activation of Catalyst
2.2.3. Temperature and Pressure
3. Traditional Catalysts
| Catalyst | Reaction Time (min) | Temp. (°C) | Pressure (bar) | Oxidant | Methanol Yield (µmol/gcat) | Selectivity (%) | Side Products | Refs. |
|---|---|---|---|---|---|---|---|---|
| ZSM-5 | 60 | 600-700 | 0.01 | O2 | - | 10 | CH2O CO2 O2 | [38] |
| FeHZSM-5 | 2.5 s (Contact time) | 630 | atmosphere | O2 | - | 16.51 | CO2 HCHO | [39] |
| FeNaZSM-5 | 0.5 s (Contact time) | 390 | atmosphere | O2 | - | 74.37 | CO2 HCHO | [39] |
| FeZSM-5 | 8–165 | 160 | 0.1 | N2O | 160 34 | 76 95 | C2H5OH C2H4O | [40] |
| Fe-ZSM-5 (84) | 30 | 50 | 30.5 | H2O2 | 74.4 | 10 | HCOOH CH3OOH | [44] |
| ZSM-5 (86) | 30 | 50 | 30.5 | H2O2 | 5.55 | 72 | HCOOH CH3OOH | [44] |
| Fe-silicalite-1 (86) | 30 | 50 | 30.5 | H2O2 | 65.18 | 19 | HCOOH CH3OOH | [44] |
| Fe-Cu-ZSM-5 (30) | Steady state = 60 min | 50 | 20 | H2O2 | 81 (µmol gcat−1 h−1) | 92.2 | CO2 | [42] |
| Cu-SSZ-13 | 60 | 200 | 0.3 | N2O | 13.1 | 24 | CO2 HCHO | [60] |
| Cu-MOR | 30 | 200 | 36 | O2 | 56 | 100 | - | [54] |
| Cu-MOR | 30 | 200 | 7 | H2O | 0.204 mol/molCu | 97 | H2O H2 | [25] |
| Cu-ZSM-5-Cl | 30 | 50 | 30 | H2O2 H2O | 5866 | 79.93 | CH3OOH HOCH2OOH | [61] |
| Cu-ZSM-5-N | 30 | 50 | 30 | H2O2 H2O | 3216 | 73.31 | CH3OOH HOCH2OOH | [61] |
| Cu-ZSM-5-Ac | 30 | 50 | 30 | H2O2 H2O | 2851 | 74.78 | CH3OOH HOCH2OOH | [61] |
| Cu-Fe(2/0.1)/ZSM-5 | 30 | 50 | 30 | H2O2 | 431 mol/molFe | 80 | HOCH2OOH CH3OOH CO2 | [48] |
4. Nanoparticles-Based Novel Catalysts

4.1. Nanomaterials Used with Zeolite
4.2. Graphene-Based Catalysts
4.3. Nanomaterials Used with MOFs
4.3.1. General Characteristics
4.3.2. Potentials and Limitations
4.4. Other Nanocatalysts
| Catalyst | Reaction Time (min) | Temp. (°C) | Pressure (bar) | Oxidant | Methanol Yield (µmol/gcat) | Methanol Selectivity (%) | Side Products | Refs. |
|---|---|---|---|---|---|---|---|---|
| Rh-ZSM-5 | 60 | 150 | 30 | O2 | 1224 | 8.78 | CH3COOH HCOOH | [67] |
| 1%Pd/HZS-5 (30) | 30 | 50 | 30.5 | H2O2 | 51.1 | 33.6 | CH3OOH HCOOH CO2 | [69] |
| Au/H-MOR | 60 | 150 | 30 | O2 | 1300 | 75 | CH3OOH HCOOH CO2 | [70] |
| MIL-53 (Fe, Al) | 60 | ≤60 | 30.5 | H2O2 | - | - | CH3OOH CH2O2 CO2 | [120] |
| CuxOy@UiO-bpy | 180 | 200 | 1 | O2 | 24 | 88.1 | C2H5OH | [121] |
| Uio-67-Pt-Z | 120 | 60 | 50 | H2O2 | - | 12.4 | C2H5OH CH3COOH | [122] |
| MOF derived IrO2/CuO | 180 | 150 | 3 | H2O | 872 | 95 | C2H5OH CH3COOH | [123] |
| AuPd@ZIF-8 | 30 | 90 | 15 | H2O2/O2 | 10.85 | 21.9 | CH3OOH HCOOH | [124] |
| Au@ZIF-8 | 30 | 90 | 15 | H2O2/O2 | 0.7 | - | CH3OOH HCOOH | [124] |
| Pd@ZIF-8 | 30 | 90 | 15 | H2O2/O2 | 1.2 | - | CH3OOH HCOOH | [124] |
| MOF-808-His-Cu | 60 | 150 | - | N2O | 31.7 | 100 | - | [9] |
| MOF-808-Iza-Cu | 60 | 150 | - | N2O | 61.8 | 100 | - | [9] |
| MOF-808-Bzz-Cu | 60 | 150 | - | N2O | 71.8 | 100 | - | [9] |
| CU-NU-1000 | 30-180 | 150-200 | 1-40 | O2 | 1.5–15.81 | 70–90 | C2H5OH CO2 | [125] |
| CU-NU-1000 | 180 | 200 | 1 | O2 | 17.7 | ≤46 | C2H5OH CO2 | [126] |
| MIL-100(Fe) | 120 | 200 | 0.015 | N2O | 0.2 | ≥98 | CO2 | [127] |
| Fe-ZSM-5@ZIF-8 | 300 | 150 | 1 | - | 0.12 | - | - | [128] |
| Pd/Pt core-shell | 30 | 50 | 30 | H2O2 | 83 mmol gcat−1 h−1 | 92.4 | CH3OOH HCOOH HOCH2OOH | [140] |
| Rh/TiO2 | 60 | 150 | 31 | H2O2 | - | 92 | [141] |
5. Stability and Reusability of Catalysts
6. Reactors Used for Methane to Methanol Catalysis
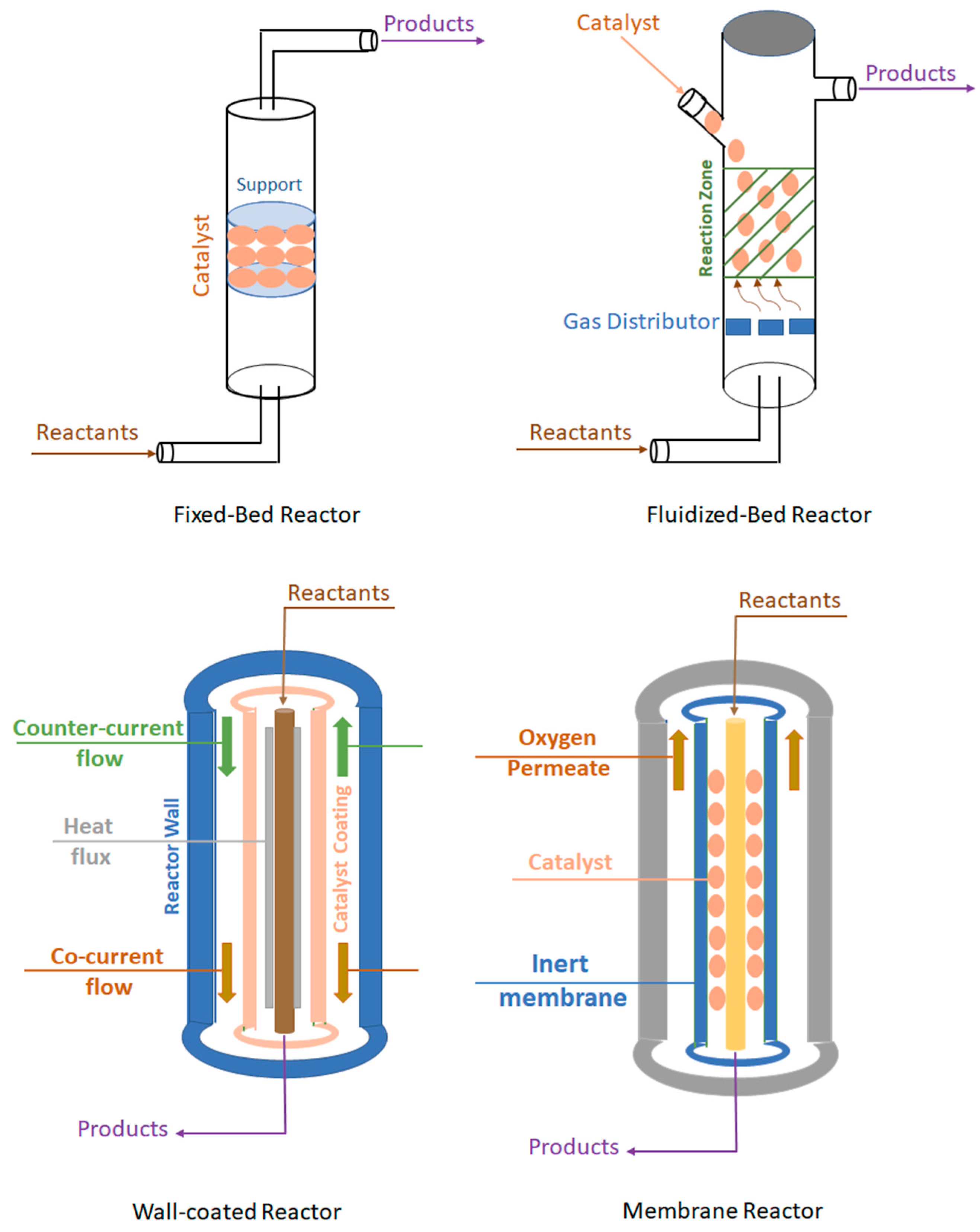
6.1. Fixed-Bed Reactor
6.2. Fluidized-Bed Reactor
6.3. Wall-Coated Reactors
6.3.1. Tubular Reactor Type
6.3.2. Monolithic Reactor Type
6.3.3. Plate-Type Reactor Type
6.3.4. Microchannel Plate Type Reactor
6.4. Membrane Reactor
7. Summaries and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Karl, T.R.; Trenberth, K.E. Modern Global Climate Change. Science 2003, 302, 1719–1723. [Google Scholar] [CrossRef] [PubMed]
- Vali, S.A.; Markeb, A.A.; Moral-Vico, J.; Font, X.; Sánchez, A. A Novel Cu-Based Catalyst Supported in Chitosan Nanoparticles for the Hydrogenation of Carbon Dioxide to Methanol: From the Optimization of the Catalyst Performance to the Reaction Mechanism. Catal. Commun. 2023, 182, 106747. [Google Scholar] [CrossRef]
- Bradforf, M.C.J.; Vannice, M.A. CO2 Reforming of CH4. Catal. Rev. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Bitter, J.H.; Seshan, K.; Lercher, J.A. Mono and Bifunctional Pathways of CO2/CH4 Reforming over Pt and Rh Based Catalysts. J. Catal. 1998, 176, 93–101. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Arutyunov, V. Low-scale direct methane to methanol—Modern status and future prospects. Catal. Today 2013, 215, 243–250. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y. Nanostructured Perovskite Oxides as Promising Substitutes of Noble Metals Catalysts for Catalytic Combustion of Methane. Chin. Chem. Lett. 2018, 29, 252–260. [Google Scholar] [CrossRef]
- Cihlar, J.; Vrba, R.; Castkova, K.; Cihlar, J. Effect of Transition Metal on Stability and Activity of La-Ca-M-(Al)-O (M = Co, Cr, Fe and Mn) Perovskite Oxides during Partial Oxidation of Methane. Int. J. Hydrogen Energy 2017, 42, 19920–19934. [Google Scholar] [CrossRef]
- Beckner, M.; Dailly, A. A Pilot Study of Activated Carbon and Metal-Organic Frameworks for Methane Storage. Appl. Energy 2016, 162, 506–514. [Google Scholar] [CrossRef]
- Baek, J.; Rungtaweevoranit, B.; Pei, X.; Park, M.; Fakra, S.C.; Liu, Y.S.; Matheu, R.; Alshmimri, S.A.; Alshehri, S.; Trickett, C.A.; et al. Bioinspired Metal-Organic Framework Catalysts for Selective Methane Oxidation to Methanol. J. Am. Chem. Soc. 2018, 140, 18208–18216. [Google Scholar] [CrossRef]
- Aseem, A.; Jeba, G.G.; Conato, M.T.; Rimer, J.D.; Harold, M.P. Oxidative Coupling of Methane over Mixed Metal Oxide Catalysts: Steady State Multiplicity and Catalyst Durability. Chem. Eng. J. 2018, 331, 132–143. [Google Scholar] [CrossRef]
- Alizadeh, R.; Jamshidi, E.; Zhang, G. Transformation of Methane to Synthesis Gas over Metal Oxides without Using Catalyst. J. Nat. Gas. Chem. 2009, 18, 124–130. [Google Scholar] [CrossRef]
- Hu, Y.; Higashimoto, S.; Takahashi, S.; Nagai, Y.; Anpo, M. Selective Photooxidation of Methane into Methanol by Nitric Oxide over V-MCM-41 Mesoporous Molecular Sieves. Catal. Lett. 2005, 100, 35–37. [Google Scholar] [CrossRef]
- Kaliaguine, S.L.; Shelimov, B.N.; Kazansky, V.B. Reactions of Methane and Ethane with Hole Centers O−. J. Catal. 1978, 55, 384–393. [Google Scholar] [CrossRef]
- Ward, M.D.; Brazdil, J.F.; Mehandru, S.P.; Anderson, A.B. Methane Photoactivation on Copper Molybdate: An Experimental and Theoretical Study. J. Phys. Chem. 1987, 91, 6515–6521. [Google Scholar] [CrossRef]
- Xie, J.; Jin, R.; Li, A.; Bi, Y.; Ruan, Q.; Deng, Y.; Zhang, Y.; Yao, S.; Sankar, G.; Ma, D.; et al. Highly Selective Oxidation of Methane to Methanol at Ambient Conditions by Titanium Dioxide-Supported Iron Species. Nat. Catal. 2018, 1, 889–896. [Google Scholar] [CrossRef]
- Ahlquist, M.; Nielsen, R.J.; Periana, R.A.; Goddard, W.A. Product Protection, the Key to Developing High Performance Methane Selective Oxidation Catalysts. J. Am. Chem. Soc. 2009, 131, 17110–17115. [Google Scholar] [CrossRef]
- Otsuka, K.; Wang, Y. Direct conversion of methane into oxygenates. Appl. Catal. A-Gen. 2001, 222, 145–161. [Google Scholar] [CrossRef]
- Sirajuddin, S.; Rosenzweig, A.C. Enzymatic Oxidation of Methane. Biochemistry 2015, 54, 2283–2294. [Google Scholar] [CrossRef]
- White, R.J.; Luque, R.; Budarin, V.L.; Clark, J.H.; Macquarrie, D.J. Supported Metal Nanoparticles on Porous Materials. Methods and Applications. Chem. Soc. Rev. 2009, 38, 481–494. [Google Scholar] [CrossRef]
- Goel, S.; Wu, Z.; Zones, S.I.; Iglesia, E. Synthesis and Catalytic Properties of Metal Clusters Encapsulated within Small-Pore (SOD, GIS, ANA) Zeolites. J. Am. Chem. Soc. 2012, 134, 17688–17695. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.L.; Xu, Q. Immobilization of Ultrafine Metal Nanoparticles to High-Surface-Area Materials and Their Catalytic Applications. Chem 2016, 1, 220–245. [Google Scholar] [CrossRef]
- Tomkins, P.; Ranocchiari, M.; van Bokhoven, J.A. Direct Conversion of Methane to Methanol under Mild Conditions over Cu-Zeolites and Beyond. Acc. Chem. Res. 2017, 50, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.; Forde, M.M.; Ab Rahim, M.H.; Thetford, A.; He, Q.; Jenkins, R.L.; Dimitratos, N.; Lopez-Sanchez, J.A.; Dummer, N.F.; Murphy, D.M.; et al. Direct Catalytic Conversion of Methane to Methanol in an Aqueous Medium by Using Copper-Promoted Fe-ZSM-5. Angew. Chem.-Int. Ed. 2012, 51, 5129–5133. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Palagin, D.; Ranocchiari, M.; van Bokhoven, J.A. Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 2017, 356, 523–527. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, L.; Zuidema, E.; Mondal, K.; Zhang, M.; Zhang, J.; Wang, C.; Meng, X.; Yang, H.; Mesters, C.; et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science 2020, 367, 193–197. [Google Scholar] [CrossRef]
- Chawdhury, P.; Bhargavi, K.V.S.S.; Subrahmanyam, C. A Single-Stage Partial Oxidation of Methane to Methanol: A Step Forward in the Synthesis of Oxygenates. Sustain. Energy Fuels 2021, 5, 3351–3362. [Google Scholar] [CrossRef]
- Sogukkanli, S.; Moteki, T.; Ogura, M. Selective Methanol FormationviaCO-Assisted Direct Partial Oxidation of Methane over Copper-Containing CHA-Type Zeolites Prepared by One-Pot Synthesis. Green. Chem. 2021, 23, 2148–2154. [Google Scholar] [CrossRef]
- Luo, L.; Luo, J.; Li, H.; Ren, F.; Zhang, Y.; Liu, A.; Li, W.X.; Zeng, J. Water Enables Mild Oxidation of Methane to Methanol on Gold Single-Atom Catalysts. Nat. Commun. 2021, 12, 1218. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, D.; Bao, X. Catalysis for Selected C1 Chemistry. Chem 2020, 6, 2497–2514. [Google Scholar] [CrossRef]
- Ikbal, S.A.; Colomban, C.; Zhang, D.; Delecluse, M.; Brotin, T.; Dufaud, V.; Dutasta, J.-P.; Sorokin, A.B.; Martinez, A. Bioinspired Oxidation of Methane in the Confined Spaces of Molecular Cages. Inorg. Chem. 2019, 58, 7220–7228. [Google Scholar] [CrossRef] [PubMed]
- Dinh, K.T.; Sullivan, M.M.; Serna, P.; Meyer, R.J.; Dincǎ, M.; Román-Leshkov, Y. Viewpoint on the Partial Oxidation of Methane to Methanol Using Cu- and Fe-Exchanged Zeolites. ACS Catal. 2018, 8, 8306–8313. [Google Scholar] [CrossRef]
- Gunsalus, N.J.; Koppaka, A.; Park, S.H.; Bischof, S.M.; Hashiguchi, B.G.; Periana, R.A. Homogeneous Functionalization of Methane. Chem. Rev. 2017, 117, 8521–8573. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.v.; Jerome, S.v.; Cramer, C.J.; Truhlar, D.G. Charge Model 5: An Extension of Hirshfeld Population Analysis for the Accurate Description of Molecular Interactions in Gaseous and Condensed Phases. J. Chem. Theory Comput. 2012, 8, 527–541. [Google Scholar] [CrossRef]
- Latimer, A.A.; Kakekhani, A.; Kulkarni, A.R.; Nørskov, J.K. Direct Methane to Methanol: The Selectivity-Conversion Limit and Design Strategies. ACS Catal. 2018, 8, 6894–6907. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, T.Y.; Kwon, G.; Yi, J.; Lee, H. Selective Activation of Methane on Single-Atom Catalyst of Rhodium Dispersed on Zirconia for Direct Conversion. J. Am. Chem. Soc. 2017, 139, 17694–17699. [Google Scholar] [CrossRef]
- Sastre, F.; Fornés, V.; Corma, A.; García, H. Selective, Room-Temperature Transformation of Methane to C1 Oxygenates by Deep UV Photolysis over Zeolites. J. Am. Chem. Soc. 2011, 133, 17257–17261. [Google Scholar] [CrossRef]
- Kudo, H.; Ono, T. Partial oxidation of CH4 over ZSM-5 catalysts. Appl. Surf. Sci. 1997, 121, 413–416. [Google Scholar] [CrossRef]
- Michalkiewicz, B. Partial Oxidation of Methane to Formaldehyde and Methanol Using Molecular Oxygen over Fe-ZSM-5. Appl. Catal. A Gen. 2004, 277, 147–153. [Google Scholar] [CrossRef]
- Starokon, E.v.; Parfenov, M.v.; Arzumanov, S.S.; Pirutko, L.v.; Stepanov, A.G.; Panov, G.I. Oxidation of Methane to Methanol on the Surface of FeZSM-5 Zeolite. J. Catal. 2013, 300, 47–54. [Google Scholar] [CrossRef]
- Parfenov, M.v.; Starokon, E.v.; Pirutko, L.v.; Panov, G.I. Quasicatalytic and Catalytic Oxidation of Methane to Methanol by Nitrous Oxide over FeZSM-5 Zeolite. J. Catal. 2014, 318, 14–21. [Google Scholar] [CrossRef]
- Xu, J.; Armstrong, R.D.; Shaw, G.; Dummer, N.F.; Freakley, S.J.; Taylor, S.H.; Hutchings, G.J. Continuous Selective Oxidation of Methane to Methanol over Cu- and Fe-Modified ZSM-5 Catalysts in a Flow Reactor. Catal. Today 2016, 270, 93–100. [Google Scholar] [CrossRef]
- Starokon, E.v.; Parfenov, M.v.; Pirutko, L.v.; Abornev, S.I.; Panov, G.I. Room-Temperature Oxidation of Methane by α-Oxygen and Extraction of Products from the FeZSM-5 Surface. J. Phys. Chem. C 2011, 115, 2155–2161. [Google Scholar] [CrossRef]
- Hammond, C.; Dimitratos, N.; Lopez-Sanchez, J.A.; Jenkins, R.L.; Whiting, G.; Kondrat, S.A.; Ab Rahim, M.H.; Forde, M.M.; Thetford, A.; Hagen, H.; et al. Aqueous-Phase Methane Oxidation over Fe-MFI Zeolites; Promotion through Isomorphous Framework Substitution. ACS Catal. 2013, 3, 1835–1844. [Google Scholar] [CrossRef]
- Xiao, P.; Wang, Y.; Nishitoba, T.; Kondo, J.N.; Yokoi, T. Selective Oxidation of Methane to Methanol with H2O2 over an Fe-MFI Zeolite Catalyst Using Sulfolane Solvent. Chem. Commun. 2019, 55, 2896–2899. [Google Scholar] [CrossRef]
- Kang, J.; Park, E.D. Selective oxidation of methane over Fe-Zeolites by In situ generated H2O2. Catal 2020, 10, 299. [Google Scholar] [CrossRef]
- Fang, Z.; Murayama, H.; Zhao, Q.; Liu, B.; Jiang, F.; Xu, Y.; Tokunaga, M.; Liu, X. Selective Mild Oxidation of Methane to Methanol or Formic Acid on Fe-MOR Catalysts. Catal. Sci. Technol. 2019, 9, 6946–6956. [Google Scholar] [CrossRef]
- Yu, T.; Li, Z.; Lin, L.; Chu, S.; Su, Y.; Song, W.; Wang, A.; Weckhuysen, B.M.; Luo, W. Highly Selective Oxidation of Methane into Methanol over Cu-Promoted Monomeric Fe/ZSM-5. ACS Catal. 2021, 11, 6684–6691. [Google Scholar] [CrossRef]
- Tao, L.; Lee, I.; Khare, R.; Jentys, A.; Fulton, J.L.; Sanchez-Sanchez, M.; Lercher, J.A. Speciation of Cu-Oxo Clusters in Ferrierite for Selective Oxidation of Methane to Methanol. Chem. Mater. 2021, 34, 4355–4363. [Google Scholar] [CrossRef]
- Koishybay, A.; Shantz, D.F. Water Is the Oxygen Source for Methanol Produced in Partial Oxidation of Methane in a Flow Reactor over Cu-SSZ-13. J. Am. Chem. Soc. 2020, 142, 11962–11966. [Google Scholar] [CrossRef]
- Jeong, Y.R.; Jung, H.; Kang, J.; Han, J.W.; Park, E.D. Continuous Synthesis of Methanol from Methane and Steam over Copper-Mordenite. ACS Catal. 2021, 11, 1065–1070. [Google Scholar] [CrossRef]
- Le, H.v.; Parishan, S.; Sagaltchik, A.; Ahi, H.; Trunschke, A.; Schomäcker, R.; Thomas, A. Stepwise Methane-to-Methanol Conversion on CuO/SBA-15. Chem.-A Eur. J. 2018, 24, 12592–12599. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, J.; Hirayama, A.; Tsuchimura, Y.; Kondou, N.; Yoshida, H.; Machida, M.; Nishimura, S.; Kato, K.; Miyazato, I.; Takahashi, K. Catalytic Direct Oxidation of Methane to Methanol by Redox of Copper Mordenite. Catal. Sci. Technol. 2021, 11, 3437–3446. [Google Scholar] [CrossRef]
- Tomkins, P.; Mansouri, A.; Bozbag, S.E.; Krumeich, F.; Park, M.B.; Alayon, E.M.C.; Ranocchiari, M.; Vanbokhoven, J.A. Isothermal Cyclic Conversion of Methane into Methanol over Copper-Exchanged Zeolite at Low Temperature. Angew. Chem.-Int. Ed. 2016, 55, 5467–5471. [Google Scholar] [CrossRef]
- Álvarez, M.; Marín, P.; Ordóñez, S. Harnessing of Diluted Methane Emissions by Direct Partial Oxidation of Methane to Methanol over Cu/Mordenite. Ind. Eng. Chem. Res. 2021, 60, 9409–9417. [Google Scholar] [CrossRef]
- Knorpp, A.J.; Pinar, A.B.; Newton, M.A.; Sushkevich, V.L.; van Bokhoven, J.A. Copper-Exchanged Omega (MAZ) Zeolite: Copper-Concentration Dependent Active Sites and Its Unprecedented Methane to Methanol Conversion. ChemCatChem 2018, 10, 5593–5596. [Google Scholar] [CrossRef]
- Knorpp, A.J.; Newton, M.A.; Pinar, A.B.; van Bokhoven, J.A. Conversion of Methane to Methanol on Copper Mordenite: Redox Mechanism of Isothermal and High-Temperature-Activation Procedures. Ind. Eng. Chem. Res. 2018, 57, 12036–12039. [Google Scholar] [CrossRef]
- Dinh, K.T.; Sullivan, M.M.; Narsimhan, K.; Serna, P.; Meyer, R.J.; Dincǎ, M.; Román-Leshkov, Y. Continuous Partial Oxidation of Methane to Methanol Catalyzed by Diffusion-Paired Copper Dimers in Copper-Exchanged Zeolites. J. Am. Chem. Soc. 2019, 141, 11641–11650. [Google Scholar] [CrossRef]
- Zhu, J.; Sushkevich, V.L.; Knorpp, A.J.; Newton, M.A.; Mizuno, S.C.M.; Wakihara, T.; Okubo, T.; Liu, Z.; van Bokhoven, J.A. Cu-Erionite Zeolite Achieves High Yield in Direct Oxidation of Methane to Methanol by Isothermal Chemical Looping. Chem. Mater. 2020, 32, 1448–1453. [Google Scholar] [CrossRef]
- Ipek, B.; Lobo, R.F. Catalytic Conversion of Methane to Methanol on Cu-SSZ-13 Using N2O as Oxidant. Chem. Commun. 2016, 52, 13401–13404. [Google Scholar] [CrossRef]
- Fang, Z.; Huang, M.; Liu, B.; Jiang, F.; Xu, Y.; Liu, X. Identifying the Crucial Role of Water and Chloride for Efficient Mild Oxidation of Methane to Methanol over a [Cu2(μ-O)]2+-ZSM-5 Catalyst. J. Catal. 2022, 405, 1–14. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous Single-Atom Catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Cuenya, B.R. Synthesis and Catalytic Properties of Metal Nanoparticles: Size, Shape, Support, Composition, and Oxidation State Effects. Thin Solid. Film. 2010, 518, 3127–3150. [Google Scholar] [CrossRef]
- Li, Z.; Ji, S.; Liu, Y.; Cao, X.; Tian, S.; Chen, Y.; Niu, Z.; Li, Y. Well-Defined Materials for Heterogeneous Catalysis: From Nanoparticles to Isolated Single-Atom Sites. Chem. Rev. 2020, 120, 623–682. [Google Scholar] [CrossRef]
- Weisz, P.B. Molecular Diffusion in Microporous Materials, Formalisms and Mechanisms. Ind. Eng. Chem. Res. 1995, 34, 2692–2699. [Google Scholar] [CrossRef]
- Cui, T.; Ke, W.; Zhang, W.; Wang, H.; Li, X.; Chen, J. Encapsulating Palladium Nanoparticles Inside Mesoporous MFI Zeolite Nanocrystals for Shape-Selective Catalysis. Angew. Chem. 2016, 128, 9324–9328. [Google Scholar] [CrossRef]
- Shan, J.; Li, M.; Allard, L.F.; Lee, S.; Flytzani-Stephanopoulos, M. Mild Oxidation of Methane to Methanol or Acetic Acid on Supported Isolated Rhodium Catalysts. Nature 2017, 551, 605–608. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.; Fung, V.; Jiang, D.E.; Huang, W.; Zhang, S.; Iwasawa, Y.; Sakata, T.; Nguyen, L.; Zhang, X.; et al. Single Rhodium Atoms Anchored in Micropores for Efficient Transformation of Methane under Mild Conditions. Nat. Commun. 2018, 9, 1231. [Google Scholar] [CrossRef]
- Lewis, R.J.; Bara-Estaun, A.; Agarwal, N.; Freakley, S.J.; Morgan, D.J.; Hutchings, G.J. The Direct Synthesis of H2O2 and Selective Oxidation of Methane to Methanol Using HZSM-5 Supported AuPd Catalysts. Catal. Lett. 2019, 149, 3066–3075. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, W.; Tang, Y.; Cao, W.; Docherty, S.R.; Wu, F.; Cheng, K.; Zhang, Q.; Copéret, C.; Wang, Y. Selective Oxidation of Methane to Methanol over Au/H-MOR. J. Am. Chem. Soc. 2023, 145, 12928–12934. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Xiao, F.S. Metal@zeolite Hybrid Materials for Catalysis. ACS Cent. Sci. 2020, 6, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Wehling, T.O.; Novoselov, K.S.; Morozov, S.v.; Vdovin, E.E.; Katsnelson, M.I.; Geim, A.K.; Lichtenstein, A.I. Molecular Doping of Graphene. Nano Lett. 2008, 8, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Blake, P.; Hill, E.W.; Castro Neto, A.H.; Novoselov, K.S.; Jiang, D.; Yang, R.; Booth, T.J.; Geim, A.K. Making graphene visible. Appl. Phys. Lett. 2007, 91, 063124. [Google Scholar] [CrossRef]
- Vollmer, A.; Feng, X.L.; Wang, X.; Zhi, L.J.; Müllen, K.; Koch, N.; Rabe, J.P. Electronic and Structural Properties of Graphene-Based Transparent and Conductive Thin Film Electrodes. Appl. Phys. A Mater. Sci. Process. 2009, 94, 1–4. [Google Scholar] [CrossRef]
- Murugan, A.V.; Muraliganth, T.; Manthiram, A. Rapid, Facile Microwave-Solvothermal Synthesis of Graphene Nanosheets and Their Polyaniline Nanocomposites for Energy Strorage. Chem. Mater. 2009, 21, 5004–5006. [Google Scholar] [CrossRef]
- Yoo, E.J.; Kim, J.; Hosono, E.; Zhou, H.S.; Kudo, T.; Honma, I. Large Reversible Li Storage of Graphene Nanosheet Families for Use in Rechargeable Lithium Ion Batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef]
- Wang, S.; Yu, D.; Dai, L.; Chang, D.W.; Baek, J.B. Polyelectrolyte-Functionalized Graphene as Metal-Free Electrocatalysts for Oxygen Reduction. ACS Nano 2011, 5, 6202–6209. [Google Scholar] [CrossRef]
- Guo, B.; Fang, L.; Zhang, B.; Gong, J.R. Graphene Doping: A Review. Insci. J. 2011, 1, 80–89. [Google Scholar] [CrossRef]
- Wang, D.W.; Su, D. Heterogeneous Nanocarbon Materials for Oxygen Reduction Reaction. Energy Env. Sci. 2014, 7, 576–591. [Google Scholar] [CrossRef]
- Kyriakou, G.; Boucher, M.B.; Jewell, A.D.; Lewis, E.A.; Lawton, T.J.; Baber, A.E.; Tierney, H.L.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Isolated Metal Atom Geometries as a Strategy for Selective Heterogeneous Hydrogenations. Science 2012, 335, 1209–1212. [Google Scholar] [CrossRef]
- Moses-Debusk, M.; Yoon, M.; Allard, L.F.; Mullins, D.R.; Wu, Z.; Yang, X.; Veith, G.; Stocks, G.M.; Narula, C.K. CO Oxidation on Supported Single Pt Atoms: Experimental and Ab Initio Density Functional Studies of CO Interaction with Pt Atom on θ-Al 2O3(010) Surface. J. Am. Chem. Soc. 2013, 135, 12634–12645. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.G.; Wen, M.; Wu, Q.S.; Fang, H. Ni/Graphene Nanostructure and Its Electron-Enhanced Catalytic Action for Hydrogenation Reaction of Nitrophenol. J. Phys. Chem. C 2014, 118, 6307–6313. [Google Scholar] [CrossRef]
- Santos, E.J.G.; Ayuela, A.; Fagan, S.B.; Mendes Filho, J.; Azevedo, D.L.; Souza Filho, A.G.; Sánchez-Portal, D. Switching on magnetism in Ni-doped graphene, Density functional calculations. Phys. Rev. B 2008, 78, 195420. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, J.M.; Xu, K.W.; Ji, V. A First-Principles Study on Gas Sensing Properties of Graphene and Pd-Doped Graphene. Appl. Surf. Sci. 2015, 343, 121–127. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, G.; Gauquelin, N.; Chen, N.; Zhou, J.; Yang, S.; Chen, W.; Meng, X.; Geng, D.; Banis, M.N.; et al. Single-atom catalysis using Pt/graphene achieved through atomic layer deposition. Sci. Rep. 2013, 3, 1775. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Yu, G.; Chen, W.; Chen, Z. CO Catalytic Oxidation on Iron-Embedded Graphene: Computational Quest for Low-Cost Nanocatalysts. J. Phys. Chem. C 2010, 114, 6250–6254. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Saeidi, N.; Nematollahi, P. Si-Doped Graphene: A Promising Metal-Free Catalyst for Oxidation of SO2. Chem. Phys. Lett. 2016, 649, 37–43. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.C.; Liu, Y.J.; Zhao, J.X.; Cai, Q.H.; Wang, X.Z. Can Si-Doped Graphene Activate or Dissociate O2 Molecule? J. Mol. Graph Model 2013, 39, 126–132. [Google Scholar] [CrossRef]
- Li, R.; Wei, Z.; Gou, X.; Xu, W. Phosphorus-Doped Graphene Nanosheets as Efficient Metal-Free Oxygen Reduction Electrocatalysts. RSC Adv. 2013, 3, 9978–9984. [Google Scholar] [CrossRef]
- He, Z.; He, K.; Robertson, A.W.; Kirkland, A.I.; Kim, D.; Ihm, J.; Yoon, E.; Lee, G.-D.; Warner, J.H. Atomic Structure and Dynamics of Metal Dopant Pairs in Graphene. Nano Lett. 2014, 14, 3766–3772. [Google Scholar] [CrossRef]
- Usachov, D.; Vilkov, O.; Grüneis, A.; Haberer, D.; Fedorov, A.; Adamchuk, V.K.; Preobrajenski, A.B.; Dudin, P.; Barinov, A.; Oehzelt, M.; et al. Nitrogen-Doped Graphene: Efficient Growth, Structure, and Electronic Properties. Nano Lett. 2011, 11, 5401–5407. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-Doped Graphene by Chemical Vapor Deposition and Its Electrical Properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Kattel, S.; Atanassov, P.; Kiefer, B. Stability, Electronic and Magnetic Properties of in-Plane Defects in Graphene: A First-Principles Study. J. Phys. Chem. C 2012, 116, 8161–8166. [Google Scholar] [CrossRef]
- Cho, Y.J.; Kim, H.S.; Baik, S.Y.; Myung, Y.; Jung, C.S.; Kim, C.H.; Park, J.; Kang, H.S. Selective Nitrogen-Doping Structure of Nanosize Graphitic Layers. J. Phys. Chem. C 2011, 115, 3737–3744. [Google Scholar] [CrossRef]
- Wang, S.; Xin, Y.; Zhang, W.; Wang, L. Conversion of Methane to Methanol on Cobalt-Embedded Graphene: A Theoretical Perspective. Catal. Lett. 2022, 152, 1331–1337. [Google Scholar] [CrossRef]
- Impeng, S.; Khongpracha, P.; Warakulwit, C.; Jansang, B.; Sirijaraensre, J.; Ehara, M.; Limtrakul, J. Direct Oxidation of Methane to Methanol on Fe-O Modified Graphene. RSC Adv. 2014, 4, 12572–12578. [Google Scholar] [CrossRef]
- Impeng, S.; Khongpracha, P.; Sirijaraensre, J.; Jansang, B.; Ehara, M.; Limtrakul, J. Methane Activation on Fe- and FeO-Embedded Graphene and Boron Nitride Sheet: Role of Atomic Defects in Catalytic Activities. RSC Adv. 2015, 5, 97918–97927. [Google Scholar] [CrossRef]
- Sahoo, S.; Suib, S.L.; Alpay, S.P. Graphene Supported Single Atom Transition Metal Catalysts for Methane Activation. ChemCatChem 2018, 10, 3229–3235. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, W.; Li, X.; Yang, J. A High Performance Catalyst for Methane Conversion to Methanol: Graphene Supported Single Atom Co. Chem. Commun. 2018, 54, 2284–2287. [Google Scholar] [CrossRef]
- Chang, C.C.; Liu, C.Y.; Sun, Y.C. Effective Methane Conversion to Methanol on Bi-Functional Graphene-Oxide-Supported Platinum Nanoclusters (Pt5)-a DFT Study. Phys. Chem. Chem. Phys. 2020, 22, 4967–4973. [Google Scholar] [CrossRef]
- Cui, X.; Li, H.; Wang, Y.; Hu, Y.; Hua, L.; Li, H.; Han, X.; Liu, Q.; Yang, F.; He, L.; et al. Room-Temperature Methane Conversion by Graphene-Confined Single Iron Atoms. Chem 2018, 4, 1902–1910. [Google Scholar] [CrossRef]
- He, Y.; Luan, C.; Fang, Y.; Feng, X.; Peng, X.; Yang, G.; Tsubaki, N. Low-Temperature Direct Conversion of Methane to Methanol over Carbon Materials Supported Pd-Au Nanoparticles. Catal. Today 2020, 339, 48–53. [Google Scholar] [CrossRef]
- Deria, P.; Mondloch, J.E.; Karagiaridi, O.; Bury, W.; Hupp, J.T.; Farha, O.K. Beyond Post-Synthesis Modification: Evolution of Metal-Organic Frameworks via Building Block Replacement. Chem. Soc. Rev. 2014, 43, 5896–5912. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Marshall, R.J.; Forgan, R.S. Postsynthetic Modification of Zirconium Metal-Organic Frameworks. Eur. J. Inorg. Chem. 2016, 2016, 4310–4331. [Google Scholar] [CrossRef]
- Rogge, S.M.J.; Bavykina, A.; Hajek, J.; Garcia, H.; Olivos-Suarez, A.I.; Sepúlveda-Escribano, A.; Vimont, A.; Clet, G.; Bazin, P.; Kapteijn, F.; et al. Metal-Organic and Covalent Organic Frameworks as Single-Site Catalysts. Chem. Soc. Rev. 2017, 46, 3134–3184. [Google Scholar] [CrossRef] [PubMed]
- Tranchemontagne, D.J.; Tranchemontagne, J.L.; O’keeffe, M.; Yaghi, O.M. Secondary Building Units, Nets and Bonding in the Chemistry of Metal–Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1257–1283. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.H.; Rutledge, W.; Li, J.R.; Zhou, H.C. Zr-Based Metal-Organic Frameworks: Design, Synthesis, Structure, and Applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- Lu, W.; Wei, Z.; Gu, Z.Y.; Liu, T.F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle, T.; et al. Tuning the Structure and Function of Metal-Organic Frameworks via Linker Design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef]
- Schoedel, A.; Li, M.; Li, D.; O’Keeffe, M.; Yaghi, O.M. Structures of Metal-Organic Frameworks with Rod Secondary Building Units. Chem. Rev. 2016, 116, 12466–12535. [Google Scholar] [CrossRef]
- Cheetham, A.K.; Bennett, T.D.; Coudert, F.X.; Goodwin, A.L. Defects and Disorder in Metal Organic Frameworks. Dalton Trans. 2016, 45, 4113–4126. [Google Scholar] [CrossRef] [PubMed]
- Sholl, D.S.; Lively, R.P. Defects in Metal-Organic Frameworks: Challenge or Opportunity? J. Phys. Chem. Lett. 2015, 6, 3437–3444. [Google Scholar] [CrossRef]
- Fang, Z.; Bueken, B.; de Vos, D.E.; Fischer, R.A. Defektmanipulierte Metall-Organische Gerüste. Angew. Chem. 2015, 127, 7340–7362. [Google Scholar] [CrossRef]
- Dissegna, S.; Epp, K.; Heinz, W.R.; Kieslich, G.; Fischer, R.A. Defective Metal-Organic Frameworks. Adv. Mater. 2018, 30, 1704501. [Google Scholar] [CrossRef] [PubMed]
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water Stability and Adsorption in Metal-Organic Frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Keser Demir, N.; Chen, J.P.; Li, K. Applications of Water Stable Metal-Organic Frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar] [CrossRef]
- Silva, P.; Vilela, S.M.F.; Tomé, J.P.C.; Almeida Paz, F.A. Multifunctional Metal-Organic Frameworks: From Academia to Industrial Applications. Chem. Soc. Rev. 2015, 44, 6774–6803. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New Synthetic Routes towards MOF Production at Scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef]
- Ren, J.; Dyosiba, X.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Liao, S. Review on the Current Practices and Efforts towards Pilot-Scale Production of Metal-Organic Frameworks (MOFs). Coord. Chem. Rev. 2017, 352, 187–219. [Google Scholar] [CrossRef]
- Li, B.; Chrzanowski, M.; Zhang, Y.; Ma, S. Applications of Metal-Organic Frameworks Featuring Multi-Functional Sites. Coord. Chem. Rev. 2016, 307, 106–129. [Google Scholar] [CrossRef]
- Huang, Y.B.; Liang, J.; Wang, X.S.; Cao, R. Multifunctional Metal-Organic Framework Catalysts: Synergistic Catalysis and Tandem Reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef] [PubMed]
- Herbst, A.; Janiak, C. MOF Catalysts in Biomass Upgrading towards Value-Added Fine Chemicals. CrystEngComm 2017, 19, 4092–4117. [Google Scholar] [CrossRef]
- Trickett, C.A.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The chemistry of metal-organic frameworks for CO2 capture, regeneration and conversion. Nat. Rev. Mater. 2017, 2, 17045. [Google Scholar] [CrossRef]
- Maina, J.W.; Pozo-Gonzalo, C.; Kong, L.; Schütz, J.; Hill, M.; Dumée, L.F. Metal Organic Framework Based Catalysts for CO2 Conversion. Mater. Horiz. 2017, 4, 345–361. [Google Scholar] [CrossRef]
- Vali, S.A.; Moral-Vico, J.; Font, X.; Sánchez, A. Adsorptive Removal of Siloxanes from Biogas: Recent Advances in Catalyst Reusability and Water Content Effect. Biomass Convers. Biorefin. 2023, 1–15. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Corma, A.; García, H.; Llabrés I Xamena, F.X. Engineering Metal Organic Frameworks for Heterogeneous Catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.K.; Roberts, J.M.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal—Organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal-Organic Frameworks: Opportunities for Catalysis. Angew. Chem.-Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Jiang, H.L. Metal-Organic Frameworks Meet Metal Nanoparticles: Synergistic Effect for Enhanced Catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef]
- Osadchii, D.Y.; Olivos-Suarez, A.I.; Szécsényi, Á.; Li, G.; Nasalevich, M.A.; Dugulan, I.A.; Crespo, P.S.; Hensen, E.J.M.; Veber, S.L.; Fedin, M.v.; et al. Isolated Fe Sites in Metal Organic Frameworks Catalyze the Direct Conversion of Methane to Methanol. ACS Catal. 2018, 8, 5542–5548. [Google Scholar] [CrossRef]
- Ren, M.; Shi, Q.; Mi, L.; Liang, W.; Yuan, M.; Wang, L.; Gao, Z.; Huang, W.; Huang, J.; Zuo, Z. Isothermal Conversion of Methane to Methanol over CuxOy@UiO-Bpy. Mater. Today Sustain. 2021, 11–12, 100061. [Google Scholar] [CrossRef]
- Xia, M.; Qiu, L.; Li, Y.; Shen, T.; Sui, Z.; Feng, L.; Chen, Q. A Metal-Organic Frameworks Composite Catalyst Containing Platinum and Polyoxometalate for Direct Conversion of Methane. Mater. Lett. 2022, 307, 131078. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.; Ma, R.; You, R.; Zeng, H.; Rui, Z. Metal-Organic Framework-Derived IrO2/CuO Catalyst for Selective Oxidation of Methane to Methanol. ACS Energy Lett. 2019, 4, 2945–2951. [Google Scholar] [CrossRef]
- Xu, G.; Yu, A.; Xu, Y.; Sun, C. Selective oxidation of methane to methanol using AuPd@ZIF-8. Catal. Commun. 2021, 158, 106338. [Google Scholar] [CrossRef]
- Zheng, J.; Ye, J.; Ortuño, M.A.; Fulton, J.L.; Gutiérrez, O.Y.; Camaioni, D.M.; Motkuri, R.K.; Li, Z.; Webber, T.E.; Mehdi, B.L.; et al. Selective Methane Oxidation to Methanol on Cu-Oxo Dimers Stabilized by Zirconia Nodes of an NU-1000 Metal-Organic Framework. J. Am. Chem. Soc. 2019, 141, 9292–9304. [Google Scholar] [CrossRef]
- Ikuno, T.; Zheng, J.; Vjunov, A.; Sanchez-Sanchez, M.; Ortuño, M.A.; Pahls, D.R.; Fulton, J.L.; Camaioni, D.M.; Li, Z.; Ray, D.; et al. Methane Oxidation to Methanol Catalyzed by Cu-Oxo Clusters Stabilized in NU-1000 Metal-Organic Framework. J. Am. Chem. Soc. 2017, 139, 10294–10301. [Google Scholar] [CrossRef]
- Hall, J.N.; Bollini, P. Low-Temperature, Ambient Pressure Oxidation of Methane to Methanol Over Every Tri-Iron Node in a Metal–Organic Framework Material. Chem.-A Eur. J. 2020, 26, 16639–16643. [Google Scholar] [CrossRef]
- Imyen, T.; Znoutine, E.; Suttipat, D.; Iadrat, P.; Kidkhunthod, P.; Bureekaew, S.; Wattanakit, C. Methane Utilization to Methanol by a Hybrid Zeolite@Metal-Organic Framework. ACS Appl. Mater. Interfaces 2020, 12, 23812–23821. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.; Peres, L.; Collière, V.; Philippot, K.; Lecante, P.; Chen, Y.; Yan, N. Oxidation of Methane to Methanol over Pd@Pt Nanoparticles under Mild Conditions in Water. Catal. Sci. Technol. 2021, 11, 3493–3500. [Google Scholar] [CrossRef]
- Gu, F.; Qin, X.; Li, M.; Xu, Y.; Hong, S.; Ouyang, M.; Giannakakis, G.; Cao, S.; Peng, M.; Xie, J.; et al. Selective Catalytic Oxidation of Methane to Methanol in Aqueous Medium over Copper Cations Promoted by Atomically Dispersed Rhodium on TiO2. Angew. Chem. Int. Ed. 2022, 61, e202201540. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.; Kamarudin, S.K. Direct Conversion Technologies of Methane to Methanol: An Overview. Renew. Sustain. Energy Rev. 2016, 65, 250–261. [Google Scholar] [CrossRef]
- De Smet, C.R.H.; De Croon, M.H.J.M.; Berger, R.J.; Marin, G.B.; Schouten, J.C. Design of adiabatic fixed-bed reactors for the partial oxidation of methane to synthesis gas. Application to production of methanol and hydrogen-for-fuel-cells. Chem. Eng. Sci. 2001, 56, 4849–4861. [Google Scholar] [CrossRef]
- He, L.; Fan, Y.; Bellettre, J.; Yue, J.; Luo, L. A Review on Catalytic Methane Combustion at Low Temperatures: Catalysts, Mechanisms, Reaction Conditions and Reactor Designs. Renew. Sustain. Energy Rev. 2020, 119, 109589. [Google Scholar] [CrossRef]
- Gosiewski, K.; Pawlaczyk, A.; Jaschik, M. Energy Recovery from Ventilation Air Methane via Reverse-Flow Reactors. Energy 2015, 92, 13–23. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, P.; Zhang, L.; Guo, M.; Yan, Y. Investigation of Low Concentration Methane Combustion in a Fluidized Bed with Pd/Al2O3 as Catalytic Particles. RSC Adv. 2014, 4, 59418–59426. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, P.; Zhang, L.; Guo, M.; Ran, J. Experiment and Modeling of Low-Concentration Methane Catalytic Combustion in a Fluidized Bed Reactor. Appl. Therm. Eng. 2016, 93, 660–667. [Google Scholar] [CrossRef]
- Seo, Y.S.; Yu, S.P.; Cho, S.J.; Song, K.S. The catalytic heat exchanger using catalytic fin tubes. Chem. Eng. Sci. 2003, 58, 43–53. [Google Scholar] [CrossRef]
- Ismagilov, Z.R.; Pushkarev, V.v.; Podyacheva, O.Y.; Koryabkina, N.A.; Veringa, H. A catalytic heat-exchanging tubular reactor for combining of high temperature exothermic and endothermic reactions. Chem. Eng. J. 2001, 82, 355–360. [Google Scholar] [CrossRef]
- Govender, S.; Friedrich, H.B. Monoliths, A review of the basics, preparation methods and their relevance to oxidation. Catalysts 2017, 7, 62. [Google Scholar] [CrossRef]
- Lyubovsky, M.; Karim, H.; Menacherry, P.; Boorse, S.; LaPierre, R.; Pfefferle, W.C.; Roychoudhury, S. Complete and partial catalytic oxidation of methane over substrates with enhanced transport properties. Catal. Today 2003, 83, 183–197. [Google Scholar] [CrossRef]
- Kolios, G.; Gritsch, A.; Morillo, A.; Tuttlies, U.; Bernnat, J.; Opferkuch, F.; Eigenberger, G. Heat-Integrated Reactor Concepts for Catalytic Reforming and Automotive Exhaust Purification. Appl. Catal. B 2007, 70, 16–30. [Google Scholar] [CrossRef]
- Guo, X.; Fan, Y.; Luo, L. Multi-Channel Heat Exchanger-Reactor Using Arborescent Distributors: A Characterization Study of Fluid Distribution, Heat Exchange Performance and Exothermic Reaction. Energy 2014, 69, 728–741. [Google Scholar] [CrossRef]
- O’Connell, M.; Kolb, G.; Zapf, R.; Men, Y.; Hessel, V. Bimetallic Catalysts for the Catalytic Combustion of Methane Using Microreactor Technology. Catal. Today 2009, 144, 306–311. [Google Scholar] [CrossRef]
- Rodrigues, J.M.; Ribeiro, M.F.; Fernandes, E.C. Catalytic Activity of Electrodeposited Cobalt Oxide Films for Methane Combustion in a Micro-Channel Reactor. Fuel 2018, 232, 51–59. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, S.; Ganguly, S.; Patwardhan, A.v. Steam Reforming of Methane and Methanol in Simulated Macro & Micro-Scale Membrane Reactors: Selective Separation of Hydrogen for Optimum Conversion. J. Nat. Gas. Sci. Eng. 2014, 18, 286–295. [Google Scholar]
- Hu, T.; Zhou, H.; Peng, H.; Jiang, H. Nitrogen production by efficiently removing oxygen from air using a perovskite hollow-fiber membrane with porous catalytic layer. Front. Chem. 2018, 6, 329. [Google Scholar] [CrossRef]
- Habib, M.A.; Nemitallah, M.A. Design of an Ion Transport Membrane Reactor for Application in Fire Tube Boilers. Energy 2015, 81, 787–801. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vali, S.A.; Markeb, A.A.; Moral-Vico, J.; Font, X.; Sánchez, A. Recent Advances in the Catalytic Conversion of Methane to Methanol: From the Challenges of Traditional Catalysts to the Use of Nanomaterials and Metal-Organic Frameworks. Nanomaterials 2023, 13, 2754. https://doi.org/10.3390/nano13202754
Vali SA, Markeb AA, Moral-Vico J, Font X, Sánchez A. Recent Advances in the Catalytic Conversion of Methane to Methanol: From the Challenges of Traditional Catalysts to the Use of Nanomaterials and Metal-Organic Frameworks. Nanomaterials. 2023; 13(20):2754. https://doi.org/10.3390/nano13202754
Chicago/Turabian StyleVali, Seyed Alireza, Ahmad Abo Markeb, Javier Moral-Vico, Xavier Font, and Antoni Sánchez. 2023. "Recent Advances in the Catalytic Conversion of Methane to Methanol: From the Challenges of Traditional Catalysts to the Use of Nanomaterials and Metal-Organic Frameworks" Nanomaterials 13, no. 20: 2754. https://doi.org/10.3390/nano13202754
APA StyleVali, S. A., Markeb, A. A., Moral-Vico, J., Font, X., & Sánchez, A. (2023). Recent Advances in the Catalytic Conversion of Methane to Methanol: From the Challenges of Traditional Catalysts to the Use of Nanomaterials and Metal-Organic Frameworks. Nanomaterials, 13(20), 2754. https://doi.org/10.3390/nano13202754









