Enhanced Pitting Corrosion Resistance of Nanostructured AISI 304 Stainless Steel via Pipe Inner Surface Grinding Treatment
Abstract
1. Introduction
2. Materials and Methods
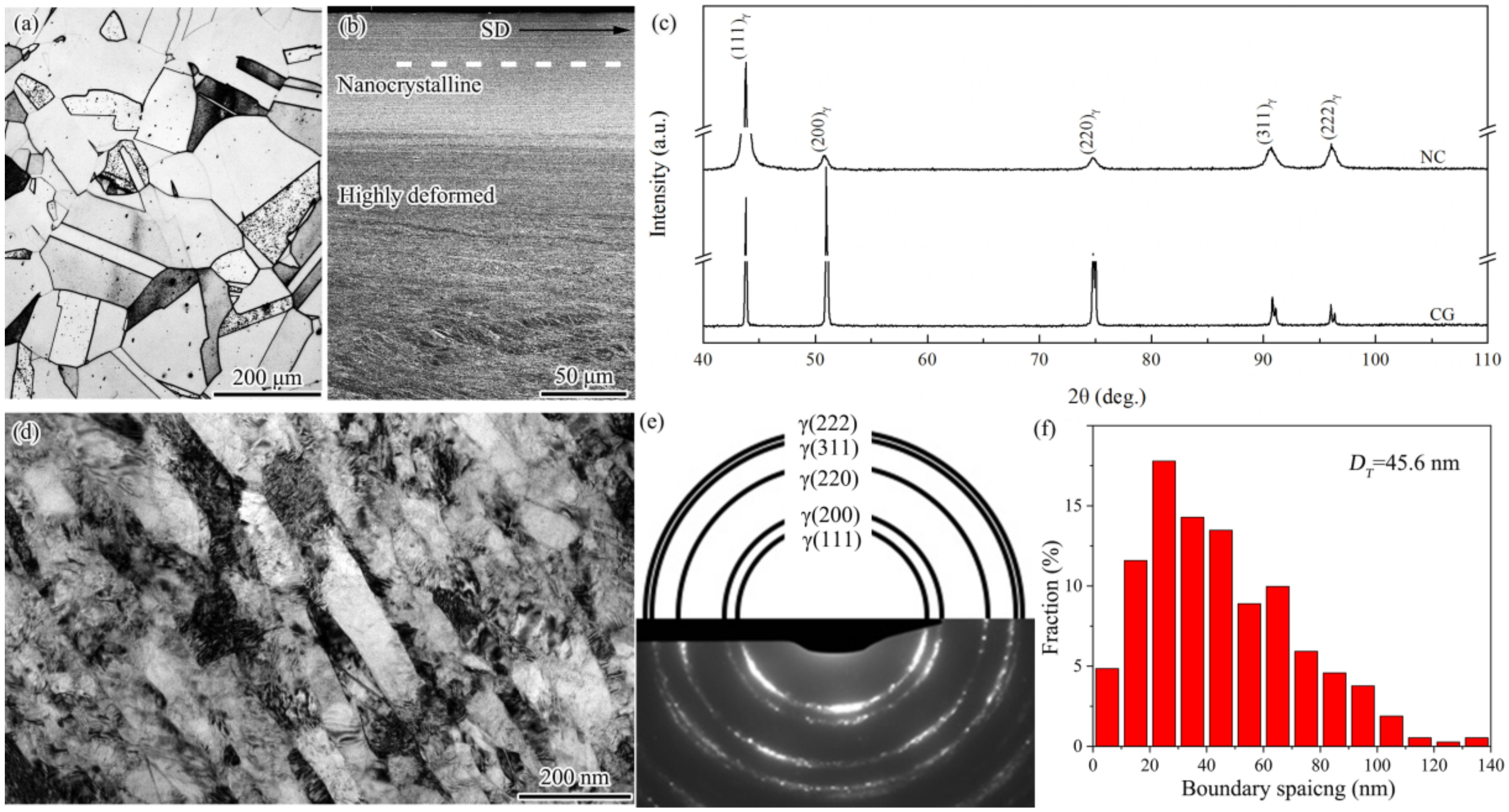
3. Results
4. Discussion
5. Conclusions
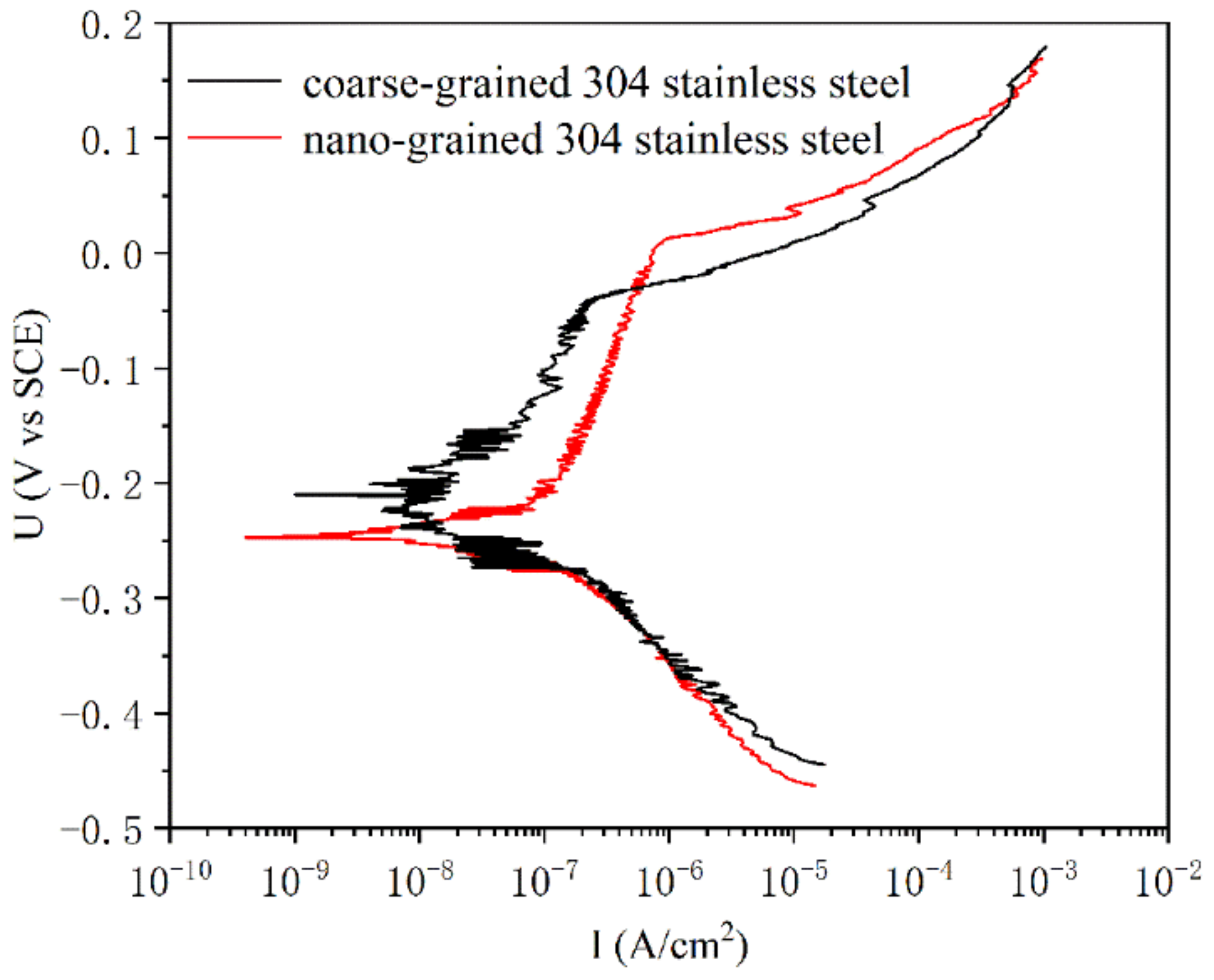
- Both nanostructured and coarse-grained austenite exhibit passivation behavior. However, the nanograined sample has a higher pitting potential (nanograined ~22 mV and coarse-grained ~−45 mV, respectively) and a wider passive interval (nanograined from ~−201 to 22 mV and coarse-grained from ~−141 to −45 mV, respectively) than the coarse-grained samples, indicating that the nanostructured sample has higher resistance to pitting corrosion.
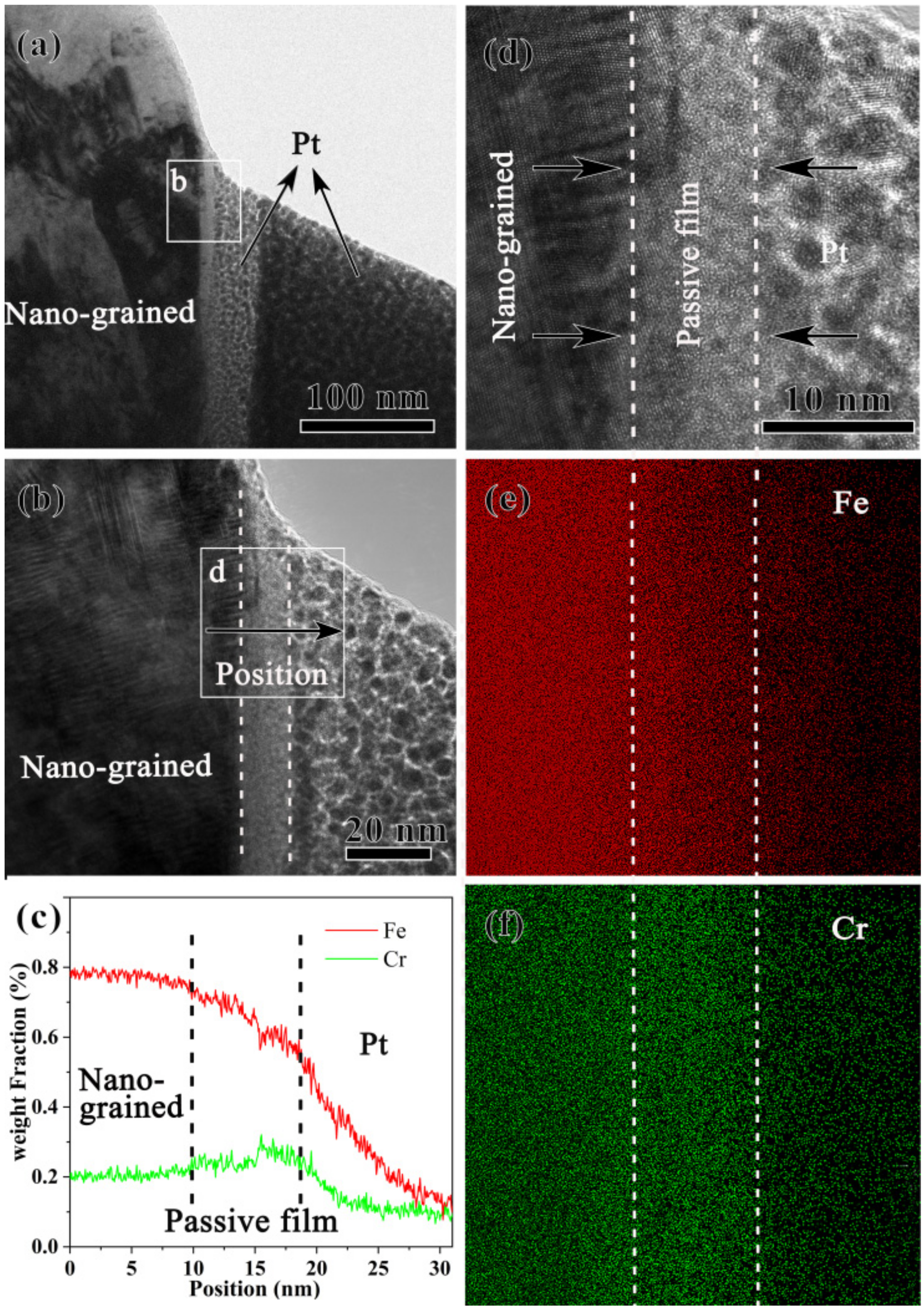
- The nanostructured sample forms a passive film of 8 nm enriched with Cr but depleted of Fe, which is thicker than the passive film of 4 nm of coarse-grained samples. The nanostructured sample forms a thicker passive film since Cr diffusion was enhanced. The effective diffusion coefficient (Deff) of Cr in the surface nanograined sample is estimated to be ~2.9 × 103 times higher than that in the coarse-grained sample.
- Here, the positive effect of grain refinement was highlighted, and the side effects on corrosion resistance from surface roughness and the second phase should be deliberately considered.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.Y.; Lu, L.; Li, J.G.; Zhang, X.; Gao, H.J. Mechanical properties and deformation mechanisms of gradient nanostructured metals and alloys. Nat. Rev. Mater. 2020, 57, 706–723. [Google Scholar] [CrossRef]
- Han, X.L.; Li, C.J.; Chen, C.H.; Zhang, X.D.; Zhang, H.W. Fabrication of Low Roughness Gradient Nanostructured Inner Surface on an AISI 304 Stainless Steel Pipe via Ultra-Sonic Rolling Treatment (USRT). Nanomaterials 2021, 11, 1769. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Birbilis, N. The influence of nanocrystalline structure and processing route on corrosion of stainless steel: A review. Corros. Sci. 2014, 92, 1–15. [Google Scholar] [CrossRef]
- Lu, A.Q.; Zhang, Y.; Li, Y.; Liu, G.; Zang, Q.H.; Liu, C.M. Effect of Nanocrystalline and Twin Boundaries on Corrosion Behavior of 316L Stainless Steel Using SMAT. Acta Metall. Sin.-Engl. Lett. 2006, 19, 183–189. [Google Scholar] [CrossRef]
- Raja, K.S.; Namjoshi, S.A.; Misra, M. Improved corrosion resistance of Ni-22Cr-13Mo-4W Alloy by surface nanocrystallization. Mater. Lett. 2005, 59, 570–574. [Google Scholar] [CrossRef]
- Schino, A.D.; Kenny, J.M. Effects of the grain size on the corrosion behavior of refined AISI 304 austenitic stainless steels. J. Mater. Sci. Lett. 2002, 21, 1631–1634. [Google Scholar] [CrossRef]
- Zeiger, W.; Scharnweber, M.; Worth, H. Passivity and pitting corrosion of a nanocrystalline FeAl8-alloy. Mater. Sci. Forum 1998, 269, 833–836. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, Q.; Zheng, Z.; Gao, Y. The negative effect of high-intensity shot-peening on the intergranular corrosion behavior of the Super304H austenitic stainless steel. Corr. Sci. 2018, 143, 390–402. [Google Scholar] [CrossRef]
- Maruda, R.W.; Krolczyk, G.M.; Wojciechowski, S.; Powalka, B.; Klos, S.; Szczotkarz, N.; Matuszak, M.; Khanna, N. Evaluation of turning with different cooling-lubricating techniques in terms of surface integrity and tribologic properties. Tribol. Int. 2020, 148, 1–14. [Google Scholar] [CrossRef]
- Zhang, H.W.; Zhao, Y.M.; Wang, Y.H.; Yu, H.X.; Zhang, C.L. Fabrication of nanostructure in inner-surface of AISI 304 stainless steel pipe with surface plastic deformation. J. Mater. Sci. Technol. 2018, 34, 2125–2130. [Google Scholar] [CrossRef]
- Zhang, H.W.; Zhao, Y.M.; Wang, Y.H.; Zhang, C.L.; Peng, Y. On the microstructural evolution pattern toward nano-scale of an AISI 304 stainless steel during high strain rate surface deformation. J. Mater. Sci. Technol. 2020, 44, 148–159. [Google Scholar] [CrossRef]
- Guo, P.F.; Lin, X.; Liu, J.R.; Xu, J.J.; Li, J.Q.; Zhang, Y.F.; Lu, X.F.; Qu, N.S.; Lan, H.B.; Huang, W.D. Passive behavior of nickel-based superalloys prepared by high-deposition-rate laser solid forming additive manufacturing. Corr. Sci. 2020, 177, 109036. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Wu, B.; Guo, X.W.; Wang, Y.J.; Chen, D.; Zhang, Y.C.; Du, K.; Oguzie, E.E.; Ma, X.L. Unmasking chloride attack on the passive film of metals. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Li, W.; Li, D.Y. Influence of surface morphology on corrosion and electronic behavior. Acta Mater. 2006, 54, 445–452. [Google Scholar] [CrossRef]
- Sasakik, K.; Burstein, G.T. The generation of surface roughness during slurry erosion-corrosion and its effect. Corr. Sci. 1996, 38, 2111–2120. [Google Scholar] [CrossRef]
- Nagumo, M. Effect of surface roughness on early stages of pitting corrosion of type 301 stainless Steel. Corr. Sci. 1997, 39, 1665–1672. [Google Scholar]
- Hao, Y.W.; Deng, B.; Chong, C.; Jiang, Y.M.; Li, J. Effect of Surface Mechanical Attrition Treatment on Corrosion Behavior of 316 Stainless Steel. J. Iron Steel Res. Int. 2009, 16, 68–72. [Google Scholar] [CrossRef]
- Lei, Y.B.; Wang, Z.B.; Zhang, B.; Lou, Z.P.; Lu, J.; Lu, K. Enhanced mechanical properties and corrosion resistance of 316L stainless steel by pre-forming a gradient nanostructured surface layer and annealing. Acta Mater. 2020, 208, 1–18. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, D.Y. Mechanical, electrochemical and tribological properties of nano-crystalline surface of 304 stainless steel. Wear 2003, 255, 836–845. [Google Scholar] [CrossRef]
- Xie, S.L.; Wang, Z.B.; Lu, K. Diffusion behavior of Cr in gradient nanolaminated surface layer on an interstitial-free steel. J. Mater. Sci. Technol. 2019, 35, 460–464. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.B.; Lu, K. Chromizing behaviors of a low carbon steel processed by means of surface mechanical attrition treatment. Acta Mater. 2005, 53, 2081–2089. [Google Scholar]
- Bowen, A.W.; Leak, G.M. Solute Diffusion in Alpha- and Gamma-Iron. Metall. Trans. 1970, 1, 1695–1700. [Google Scholar] [CrossRef]
- Wang, Z.B.; Tao, N.R.; Tong, W.P.; Lu, J.; Lu, K. Diffusion of chromium in nanocrystalline iron produced by means of surface mechanical attrition treatment. Acta Mater. 2003, 51, 4319–4329. [Google Scholar] [CrossRef]
- Chen, X.D.; Li, Y.S.; Zhu, Y.T.; Yang, B. Enhanced irradiation and corrosion resistance of 316LN stainless steel with high densities of dislocations and twins. J. Nucl. Mater. 2019, 517, 234–240. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Wang, S.G.; Wang, F.H. Electrochemical corrosion behavior of nanocrystallized bulk 304 stainless steel. Electrochim. Acta 2006, 52, 760–765. [Google Scholar] [CrossRef]
- Zou, J.Y.; Wang, Z.W.; Ma, Y.L.; Yan, Y.; Qiao, L.J. Role of gradient nano-structured surface in collapsed pitting corrosion on AISI 316L stainless steel during tribocorrosion. Corr. Sci. 2022, 197, 110043. [Google Scholar] [CrossRef]
- Tiamiyu, A.A.; Eduok, U.; Odeshi, A.G.; Szpunar, J.A. Effect of prior plastic deformation and deformation rate on the corrosion resistance of AISI 321 austenitic stainless steel. Mater. Sci. Eng. A 2019, 745, 1–9. [Google Scholar] [CrossRef]
- Man, C.; Dong, C.; Kong, D.; Wang, L.; Li, X. Beneficial effect of reversed austenite on the intergranular corrosion resistance of martensitic stainless steel. Corr. Sci. 2019, 151, 108–121. [Google Scholar] [CrossRef]
- Jelliti, S.; Richard, C.; Retraint, D.; Roland, T.; Chemkhi, M.; Demangel, C. Effect of surface nanocrystallization on the corrosion behavior of Ti-6Al-4V titanium alloy. Surf. Coat. Technol. 2013, 224, 82–87. [Google Scholar] [CrossRef]
- Mathur, S.; Vyas, R.; Sachdev, K.; Sharma, S.K. XPS characterization of corrosion films formed on the crystalline, amorphous and nanocrystalline states of the alloy Ti60Ni40. J. Non-Cryst. Solids. 2011, 357, 1632–1635. [Google Scholar] [CrossRef]
- Maurice, V.; Yang, W.P.; Marcus, P. X-Ray Photoelectron Spectroscopy and Scanning Tunneling Microscopy Study of Passive Films Formed on (100) Fe-18Cr-13Ni Single-Crystal Surfaces. J. Electrochem. Soc. 1998, 145, 909–920. [Google Scholar] [CrossRef]
- Xu, L.J.; Wu, P.G.; Zhu, X.J.; Zhao, G.C.; Ren, X.L.; Wei, Q.F.; Xie, L.L. Structural characteristics and chloride intrusion mechanism of passive film. Corr. Sci. 2022, 207, 110563. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Wei, P.; Zhao, Y.; Wang, Z.; Li, C.; Wu, X.; Zhang, H. Enhanced Pitting Corrosion Resistance of Nanostructured AISI 304 Stainless Steel via Pipe Inner Surface Grinding Treatment. Nanomaterials 2023, 13, 318. https://doi.org/10.3390/nano13020318
Han X, Wei P, Zhao Y, Wang Z, Li C, Wu X, Zhang H. Enhanced Pitting Corrosion Resistance of Nanostructured AISI 304 Stainless Steel via Pipe Inner Surface Grinding Treatment. Nanomaterials. 2023; 13(2):318. https://doi.org/10.3390/nano13020318
Chicago/Turabian StyleHan, Xiaolei, Ping Wei, Yiming Zhao, Zuohua Wang, Changji Li, Xinqiang Wu, and Hongwang Zhang. 2023. "Enhanced Pitting Corrosion Resistance of Nanostructured AISI 304 Stainless Steel via Pipe Inner Surface Grinding Treatment" Nanomaterials 13, no. 2: 318. https://doi.org/10.3390/nano13020318
APA StyleHan, X., Wei, P., Zhao, Y., Wang, Z., Li, C., Wu, X., & Zhang, H. (2023). Enhanced Pitting Corrosion Resistance of Nanostructured AISI 304 Stainless Steel via Pipe Inner Surface Grinding Treatment. Nanomaterials, 13(2), 318. https://doi.org/10.3390/nano13020318





