Abstract
Perovskite quantum dots (PQDs) have shown great promise in optoelectronic device applications. Typically, a traditional hot-injection method with heating and high vacuum pressure is used to synthesize these colloidal nanoparticles. In this article, we report a low-cost synthetic method for FAPbI3 PQDs in air at atmospheric pressure with the assistance of ZnI2. Compared with the FAPbI3 PQDs synthesized under vacuum/N2 condition, the air-synthesized Zn:FAPbI3 PQDs exhibit the same crystalline structure with a similar preferential crystallographic orientation but demonstrate higher colloidal stability and higher production yield. Furthermore, we examine the influence of ZnI2 during the synthesis process on morphologies and optoelectronic properties. The results show that the mean size of the obtained FAPbI3 PQDs is decreased by increasing the amount of added ZnI2. More importantly, introducing an optimal amount of ZnI2 into the Pb source precursor enables increasing the carrier lifetime of FAPbI3 PQDs, showing the potential beneficial effect on device performance.
1. Introduction
Organic–inorganic hybrid perovskite with a general formula of ABX3 has attracted much attention over the past decade, in which A is a monovalent cation (such as methylammonium (MA+), formamidinium (FA+), or cesium (Cs+)), B is an inorganic metal cation (usually lead (Pb2+) or tin (Sn2+)), and X is a halide anion (chlorine (Cl−), bromine (Br−), or iodine (I−)) [1]. Due to its superior semiconducting characteristics, such as high carrier mobility, long carrier diffusion length, and high defect tolerance, metal halide perovskite has shown great potential as the active material in optoelectronic applications [2]. Among them, formamidinium lead triiodide (FAPbI3) has emerged as one of the most promising materials for highly efficient and stable perovskite solar cells, having achieved the record power conversion efficiency (PCE) of perovskite solar cells exceeding 25% [3,4,5,6,7,8,9,10]. Beyond photovoltaics, FAPbI3 has also shown a large potential in lighting and displays, owing to the high brightness and good color purity [11]. More recently, the quantum dot (QD) form of FAPbI3 has emerged as a promising material for next optoelectronic devices [12,13,14]. It additionally possesses many unique features from quantum dots such as wide tunable absorption range, multiple exciton generation, and high compatibility with a large-scale fabrication process [15].
Colloidal FAPbI3 perovskite quantum dots (PQDs) are generally synthesized by a room-temperature ligand-assisted reprecipitation process (LARP) or a hot-injection method [16,17]. In LARP, since the surface capping ligands are commonly hydrophobic, polar solvents such as DMF and DMSO must be used to flocculate the PQDs, which are subsequently isolated via centrifugation. However, the high toxicity of such polar coordination solvents makes LARP inapplicable in industrial applications, according to ecological requirements. Furthermore, the synthesized PQDs are vulnerable to polar solvent that cannot be used especially in photovoltaic devices. Therefore, the hot-injection method, without using polar solvent, has so far been considered to be the only synthetic route for PQD-based photovoltaics, also in terms of production yield and surface ligand manipulation particularly with short-chain ligands. During the hot-injection synthetic route, a vacuum degassing process (usually <20 Pa) is first applied to the FA-oleate solution and the Pb-source precursor for removing moisture and volatile contaminants, which is typically unavoidable [17,18]. Then, the degassed FA-oleate solution is rapidly injected into the hot Pb-source precursor with a nonpolar solvent under inert atmosphere. Lastly, the mixture is cooled in an ice bath [17,19]. As can be seen above, the degassing process and maintaining the inert atmosphere are not industrially feasible due to their high costs and large time consumption. Thus, seeking a facile route for low-cost, large-scale, and controlled synthesis at atmospheric pressure in air is essential when exploring FAPbI3 PQD-related practical applications.
Introducing active ligands or additives during synthesis has been proven to be effective for attaining PQDs with reduced defect density and improved structural stability. Recently, Liu et al. used trioctylphosphine as a ligand to achieve a stable and highly reactive PbI2 precursor, and further obtained CsPbI3 PQDs with a superior photoluminescence quantum yield of up to 100% [20]. Moreover, doping with additives is a generally efficient approach that not only enhances the charge transport properties but also controls the size and uniformity. In particular, creating an iodine-rich chemical environment during the PQD nucleation/growth process is favorable for minimizing the defect density. In addition, the existence of metal halides in the Pb-precursor could avoid the requirement of excess PbI2 for achieving high-quality PQDs [21]. Herein, we develop an easy synthetic method for colloidal FAPbI3 PQDs under atmospheric pressure in air with the assistance of ZnI2 additives. The air-synthesized Zn:FAPbI3 PQDs exhibit high structural and colloidal stability. By increasing the amount of ZnI2 additives, the crystal size of the FAPbI3 PQDs is decreased, while no significant changes in the crystal shape and size dispersion are observed. Surprisingly, with the addition of an optimal amount of ZnI2, ~30 mol%, the yielded FAPbI3 PQDs possess a longer carrier lifetime than that obtained by the vacuum/N2-assisted method. This synthetic method described here provides an efficient, simple, and robust approach to the low-cost production of high-performance FAPbI3 PQDs.
2. Materials and Methods
Oleylamine (OAm; technical grade, 70%) and oleic acid (OA; technical grade, 90%) were purchased from Sigma-Aldrich, Co., St. Louis, MO, USA. 1-octadecene (1-ODE; technical grade, 90%) was bought from Acros Organics, Doral, NW, USA. Hexane (GC, ≥96%) and formamidinium acetate (FA-acetate, 99%) were purchased from Tokyo chemical industry, Fukaya, Japan. PbI2 (99.9%) and ZnI2 (99.99%) were purchased from Advanced Election Technology Co., Ltd., Yingkou, Liaoning, China and Aladdin Biochemical Technology Co., Ltd., Shanghai, China, respectively. Methyl acetate (MeOAc, Super Dry, 99%) was purchased from J&K Scientific., Beijing, China. All of them were used directly without further purification.
X-ray diffraction (XRD) patterns were obtained with Rigaku Ultima IV (Tokyo, Japan, Cu Kα radiation, λ = 1.5418 Å) at room temperature in the air. For XRD measurements, the FAPbI3 PQD solution was concentrated by flowing nitrogen and dropped on the sample holder. Transmission electron microscope (TEM) was recorded with JEM-2800 (JEOL, Japan). UV-visible absorption spectra (UV-Vis) were measured with a Cary 100 spectrometer (Varian, Palo Alto, CA, USA) in absorbance mode. Steady-state photoluminescence (PL) emissions and time-resolved photoluminescence (TRPL) were carried out by Edinburgh FS5 (Edinburgh Instruments Ltd., Livingston, UK) with a monochromatized Xe lamp as the source excitation and the QD samples were excited at 475 nm.
3. Results and Discussion
FAPbI3 PQDs are commonly synthesized via a hot-injection method, as illustrated in Figure 1a. In detail, the FA-oleate precursor is prepared by mixing 0.521 g FAAc (5 mmol) with 10 mL OA and then the mixture is degassed under vacuum at 70 °C for 30 min. The temperature is subsequently increased to 110 °C and held to obtain a clear solution. Then, the FA-oleate precursor is maintained in nitrogen at 90 °C. Meanwhile, a mixture of 0.344 g PbI2 (0.75 mmol) and 20 mL 1-ODE is degassed under vacuum at 120 °C for 30 min in a three-necked round-bottom flask. Then, 4 mL OA and 2 mL OAm are heated to 120 °C for 20 min and added into the mixture of PbI2 and ODE. After PbI2 is fully dissolved, the temperature of the mixture is reduced to 80 °C under N2. For air-synthesized PQDs, the FA-oleate precursor and the Pb source are prepared by directly increasing the temperature to 80 °C in ambient air. Lastly, a 5 mL FA-oleate precursor solution is swiftly injected into the PbI2 mixture at 80 °C. After around 15 s, the reaction is quenched and cooled to room temperature using an ice water bath. For the purification of as-synthesized FAPbI3 PQDs, 9 mL MeOAc is added to the reaction mixture, which is subsequently centrifuged at 8000 rpm for 30 min. The precipitate is dispersed in 9 mL hexane, re-precipitated with 10 mL MeOAc, and centrifuged at 8000 rpm for 10 min. The final precipitate is dispersed in 2 mL octane and stored at room temperature.
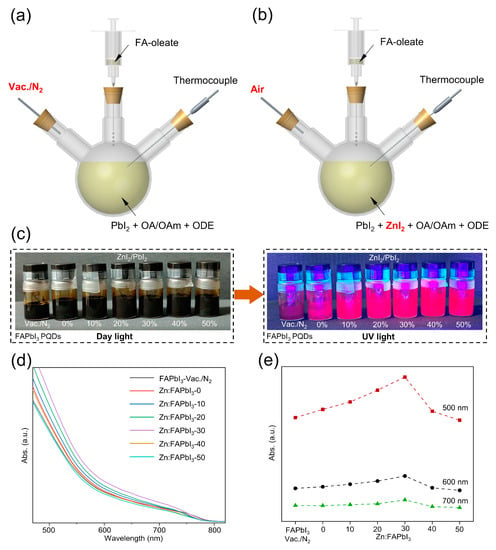
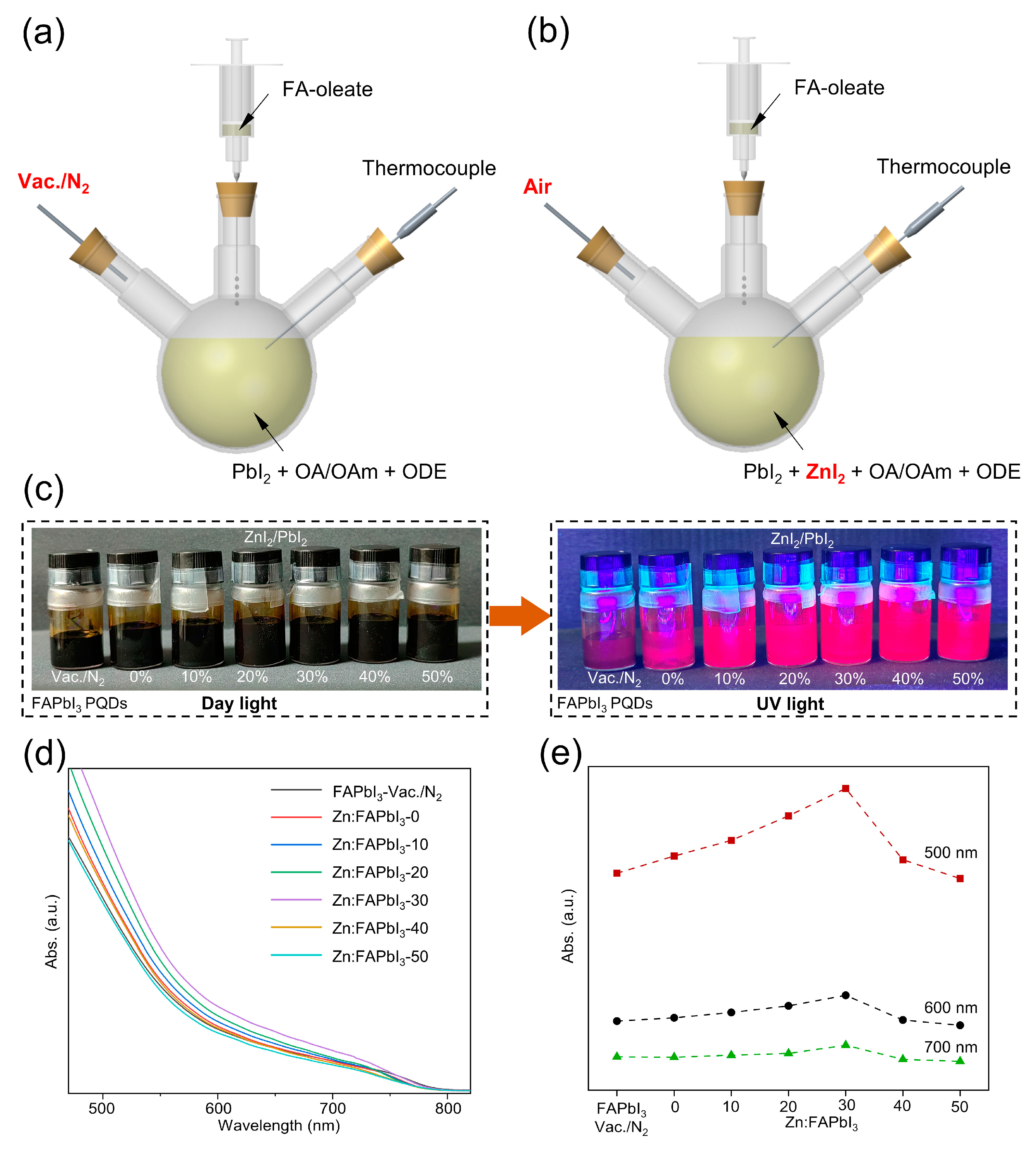
Figure 1.
Scheme of the hot injection of FAPbI3 PQDs under N2 (a) and in air (b). Photographs of FAPbI3 PQDs solutions with same concentrations but different synthesis conditions under daylight and UV light (c). The UV-Vis spectra (d) and the absorbance at 500 nm, 600 nm, and 700 nm (e) for FAPbI3-vaccum/N2, Zn:FAPbI3-0, Zn:FAPbI3-10, Zn:FAPbI3-20, Zn:FAPbI3-30, Zn:FAPbI3-40, and Zn:FAPbI3-50 PQDs.
The FAPbI3 PQDs synthesized under vacuum and N2 are denoted by FAPbI3-vaccum/N2. The vacuum-free air-synthesized FAPbI3 PQDs obtained without the addition of ZnI2 are denoted by Zn:FAPbI3-0. When different amounts of ZnI2 are added into the PbI2 precursor with the ZnI2/PbI2 molar percentage of 10% (0.075 mmol ZnI2), 20% (0.15 mmol ZnI2), 30% (0.225 mmol ZnI2), 40% (0.3 mmol ZnI2), and 50% (0.375 mmol ZnI2), the air-synthesized FAPbI3 PQDs prepared by the same synthetic method (Figure 1b) are denoted by Zn:FAPbI3-10, Zn:FAPbI3-20, Zn:FAPbI3-30, Zn:FAPbI3-40, and Zn:FAPbI3-50, respectively. After 20 days’ storage, the Zn:FAPbI3 PQDs show high colloidal stability, as demonstrated in Figure 1c. Furthermore, compared with the FAPbI3-vaccum/N2 PQDs, the Zn:FAPbI3 PQDs demonstrate brighter emission under UV illumination (395 nm), as observed in Figure 1c, which suggests that the introduction of ZnI2 may lead to reduced non-radiative recombination. The higher colloidal stability of the air-synthesized FAPbI3 PQDs can be attributed to the iodine-rich negatively charged surfaces and the decreased crystal size induced by the addition of ZnI2, as discussed below. To compare the production yield of PQDs, we add 5 μL of PQD solutions under different synthetic conditions into 3 mL hexane and measure their UV-Vis absorbance, as presented in Figure 1d,e. The absorbance of PQDs is increased by increasing the molar percentage of ZnI2/PbI2 from 0% to 30%. When the molar percentage is further increased to 40% and 50%, a decrease in absorbance can be observed. To clearly demonstrate this trend, we plot the absorbance at 500 nm, 600 nm, and 700 nm for different molar ratios of ZnI2/PbI2 in Figure 1e. It can be found that the Zn:FAPbI3-30 PQDs exhibit a higher production yield than other conditions, which results from their higher colloidal stability. However, the excessive addition of ZnI2 might suppress the formation of [PbI6]4− octahedra by reducing the relative concentration of Pb2+ ions during QD nucleation and growth. The improved production yield and stability are helpful in reducing the cost of PQDs and the susceptibility to ambient environment which can be seen as the main obstacles for the further industrialization of PQDs, respectively.
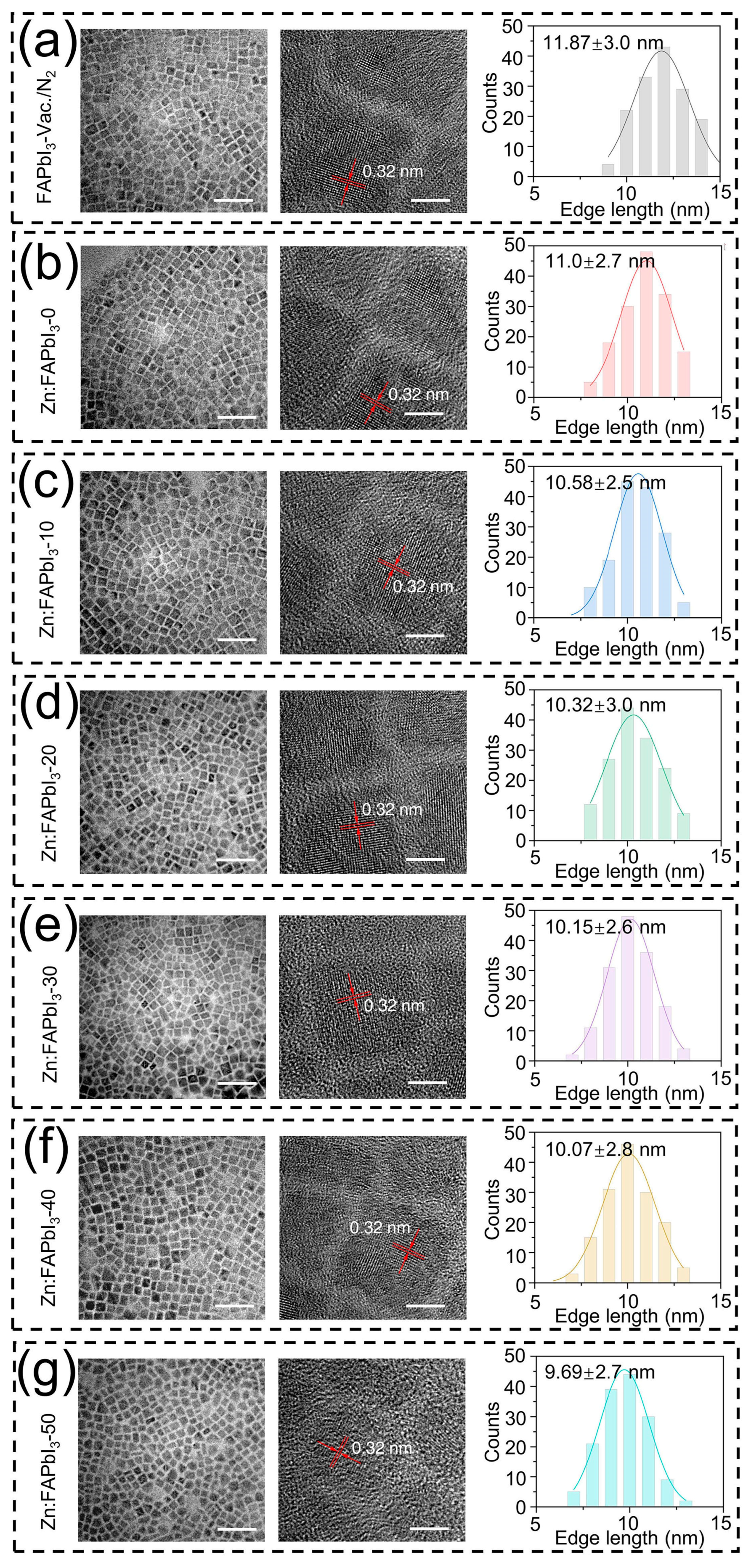
First, we investigate the effect of ZnI2 additives on the morphologies of the FAPbI3 PQDs. The TEM measurements are performed and used to characterize the mean size and size distribution of the PQDs. As presented in Figure 2, the mean size is decreased by increasing the amount of added ZnI2, while there are no observable changes in the crystal shape and size dispersion of the FAPbI3 PQDs. Similarly, it has been reported for the CsPbX3 QDs that the particle size is decreased with increasing the X-to-Pb ratio in the reaction mixture, where the excess halide ions, X−, are able to suppress the further growth of QDs [21,22]. Therefore, the introduced ZnI2 can be seen as an excess source of I−, leading to the reduced PQD size. Nevertheless, it should be noted that when the added Zn salts have different halide ions than the PQDs, the over-all size changes may occur in an opposite trend. For example, during the synthesis of the CsPbI3 PQDs, the increase of the ZnCl2 additive amount results in a noticeable increase in crystal size, exhibiting a different influence of ZnI2 additives on the QD nucleation/growth mechanism [23]. Interestingly, we also observed that the ZnI2-free air-synthesized FAPbI3 PQDs (Zn:FAPbI3-0) demonstrate a smaller crystal size than the vacuum/N2-synthesized FAPbI3 PQDs. This reduction of QD size might be attributed to the existence of the water in the reaction solution as an antisolvent that is not removed in the air-synthesis process, thus facilitating the supersaturation of the PQDs.
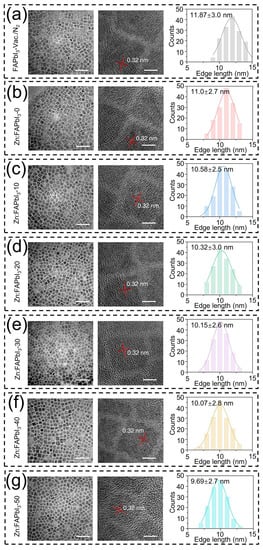
Figure 2.
TEM images and size distributions with Gauss fitting of FAPbI3-vaccum/N2 (a), Zn:FAPbI3-0 (b), Zn:FAPbI3-10 (c), Zn:FAPbI3-20 (d), Zn:FAPbI3-30 (e), Zn:FAPbI3-40 (f), and Zn:FAPbI3-50 (g) PQDs. The scale bars in the left and right TEM images are 50 nm and 5 nm, respectively. The size distributions are obtained by counting 150 particles.
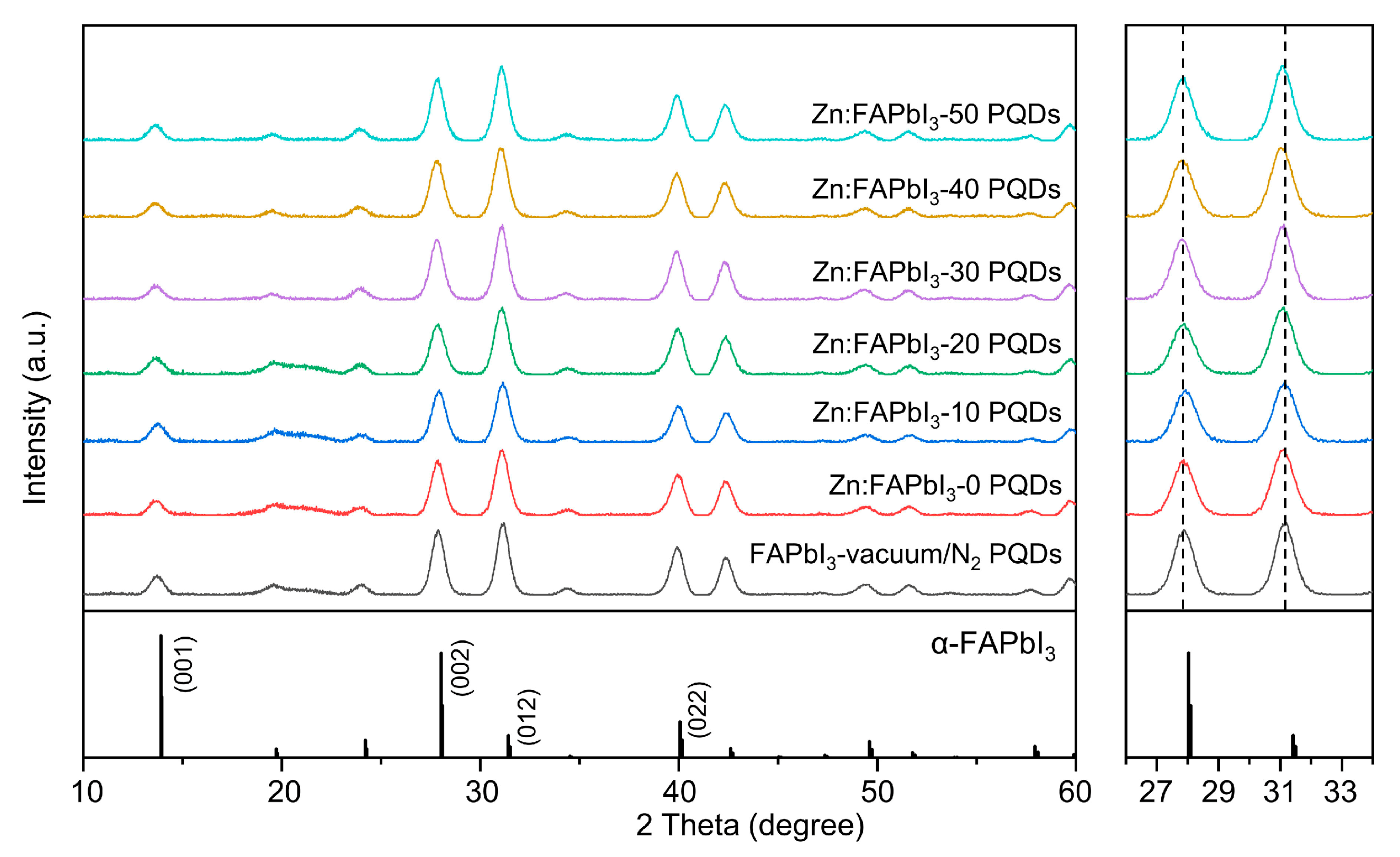
The influence of the ZnI2 additive on the crystal structure of the FAPbI3 PQD is identified by the XRD measurements. In Figure 3, the vacuum/N2-synthesized FAPbI3 PQDs show the diffraction peaks at 13.7°, 27.9°, 31.1°, 39.9°, and 42.4° that can be assigned to (001), (002), (012), (022), and (003) plane of cubic α-phase perovskite, respectively [17]. The air-synthesized Zn:FAPbI3 PQDs do not show any new diffraction peaks (the left panel in Figure 3), while no significant peak shift is observed when the ZnI2 additive is introduced (the right panel in Figure 3). Since the ionic radius of Zn2+ (74 pm) is smaller than that of Pb2+ (119 pm), the replacement of Pb2+ by Zn2+ will bring a reduced lattice constant and thus higher-angle diffraction peaks [22,24,25]. However, if the added Zn2+ resides in an interstitial lattice position, this will lead to a lattice expansion, demonstrating a lower-angle diffraction peaks [26]. Both circumstances have been reported and the differences are probably attributed to the introduced ions or the types of A-site cations. It will definitely be interesting to identify the dynamic and thermodynamic processes during the Zn salt-involved PQD synthesis. However, based on the XRD results here, we state that the introduced ZnI2 would not induce the significant lattice distortion in the PQDs, rather only reside at the surface.
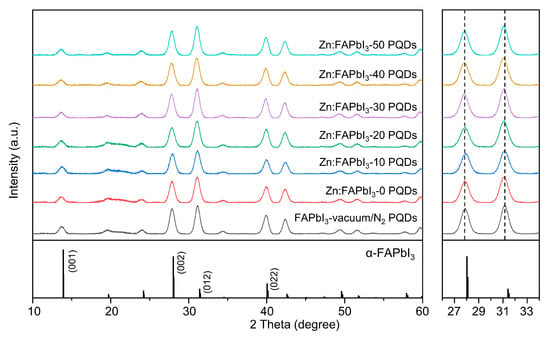
Figure 3.
XRD patterns of the FAPbI3-vaccum/N2, Zn:FAPbI3-0, Zn:FAPbI3-10, Zn:FAPbI3-20, Zn:FAPbI3-30, Zn:FAPbI3-40, and Zn:FAPbI3-50 PQDs. The right panel presents the enlarged XRD patterns in the range of 26 to 34 degrees.
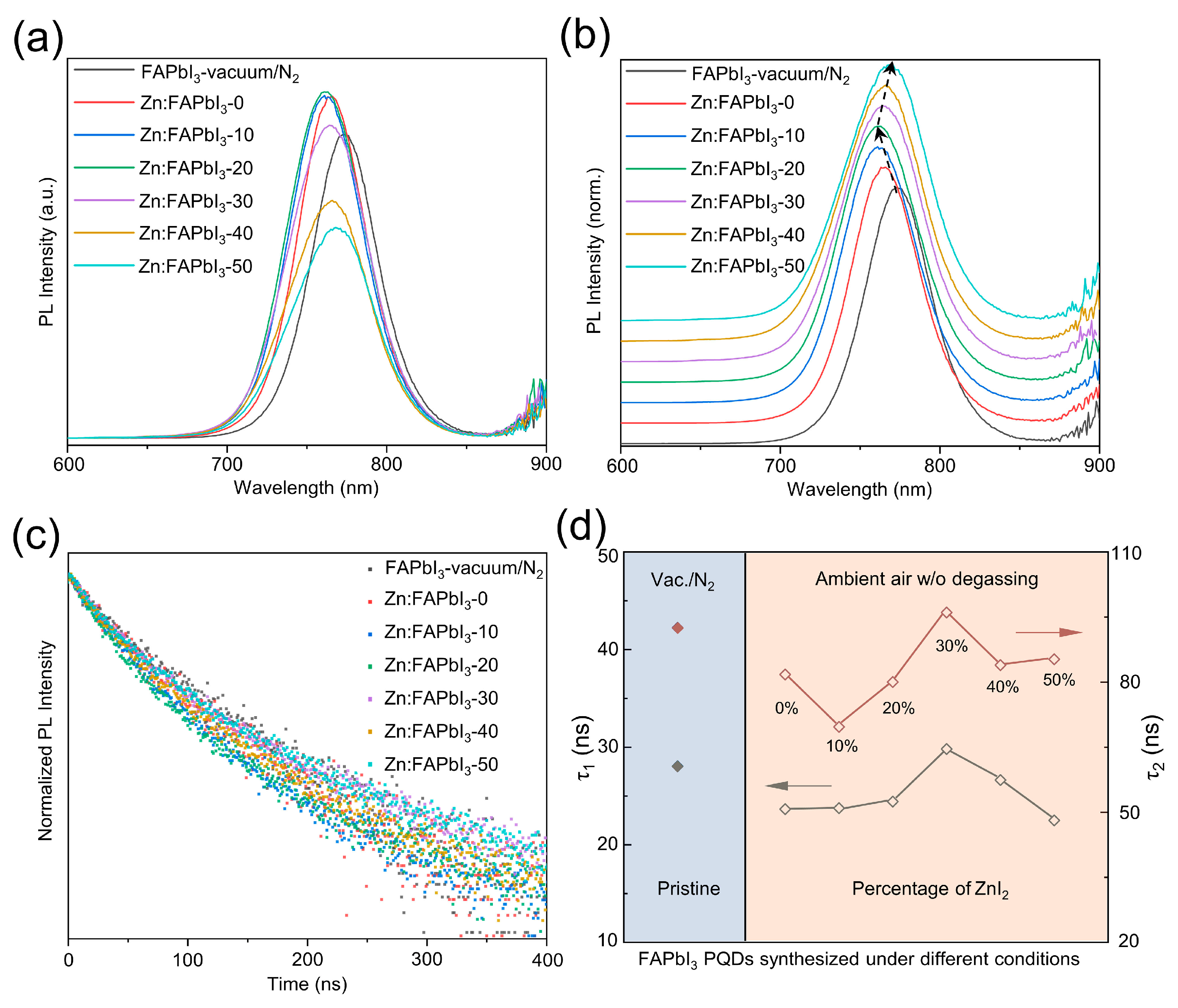
Lastly, we compare the optical and optoelectronic properties of these synthesized PQDs using different methods. The variations of PL emission intensity are presented in Figure 4a: Zn:FAPbI3-20 > Zn:FAPbI3-10 ≈ Zn:FAPbI3-0 > Zn:FAPbI3-30 > FAPbI3-vaccum/N2 > Zn:FAPbI3-40 > Zn:FAPbI3-50 PQDs. The PL emission intensity of the colloidal QDs is influenced by many factors: solution concentration, particle size, particle dispersity, ligand binding, and defect density. As observed in Figure 4a, the variation in PL intensity for different FAPbI3 PQDs is complex and is a result of competitions among these factors. Thus, we are only able to presume that the better colloidal stability and decreased iodine-vacancy states are helpful in enhancing the PL intensity, while the weakened ligand binding and formed impurities induced by excessive addition of ZnI2 result in a decrease in PL intensity. Therefore, the higher intensity of Zn:FAPbI3-0, Zn:FAPbI3-10, Zn:FAPbI3-20, and Zn:FAPbI3-30 than the pristine FAPbI3-vaccum/N2 PQDs may be due to suppressed non-radiative recombination and improved particle dispersity. However, when excessive ZnI2 is used, the Zn ions start to negatively impact the ligand binding with OA and OAm, inducing the defects on the PQD surface, further resulting in a decrease in PL intensity. In addition to intensity variation, the peak position shifts are also observed in Figure 4b. The blue shift can be attributed to the strong quantum confinement that is demonstrated by the FAPbI3 PQDs with a smaller crystal size (as illustrated in Figure 2). The smaller size of PQDs typically results in stronger quantum confinement effects, showing a higher bandgap (a higher-energy PL emission) [27]. Zn:FAPbI3-30, Zn:FAPbI3-40, and Zn:FAPbI3-50 PQDs exhibit red shifts in a peak position (a lower-energy PL emission), which is likely caused by the weakened electronic coupling between QDs and the formation of impurities of related Zn salts on PQD surfaces owing to the excessive Zn2+ [28]. Furthermore, TRPL measurements are carried out to examine the carrier lifetime, as demonstrated in Figure 4c. Two TRPL lifetimes, τ1 and τ2, are plotted with different synthesis conditions of FAPbI3 PQDs in Figure 4d, which are drawn from the fitting results using a two-exponential decay function. The fast decay component τ1 and the slow decay component τ2 are ascribed to a trap-assisted recombination at the QD surfaces and an intrinsic radiative recombination inside the QDs, respectively [4,29]. It should be noted that the average lifetime is not presented here, because the average lifetime basically has no real physical meaning. Both τ1 and τ2 for the air-synthesized FAPbI3 PQDs are decreased, compared with the PQDs obtained using degassing (vacuum) and N2 protection, which can be ascribed to the moisture and volatile contaminants in the reaction mixture inducing large amounts of defects in the PQDs. However, the lifetime of the photogenerated charge carrier, both τ1 and τ2, can be prolonged by introducing the ZnI2 additive, as illustrated in Figure 4d. The Zn:FAPbI3-30 PQDs exhibit 29.81 ns for τ1 and 96.04 ns for τ2, comparable with, or slightly higher compared with, the pristine FAPbI3-vaccum/N2 PQDs (28.05 ns for τ1 and 92.54 ns for τ2). The iodine-rich environment provided by the addition of ZnI2 inhibits the formation of iodine-vacancy trap states in the FAPbI3 PQDs, which is also beneficial to the structural stability [4]. Nevertheless, when the percentage of ZnI2 is increased to 40% and 50%, the increased surface-to-volume ratio (due to the decrease in size) and the abundant Zn ions that can be seen as new contaminants, lead to a new defect state formation inside and on the PQD surfaces, which is responsible for the decreased TRPL lifetimes shown in Figure 4d. For practical device applications, the enhanced carrier lifetime of PQDs may lead to increased current density in solar cells, increased external quantum efficiency in LEDs, and improved responsivity in photodetectors, etc.
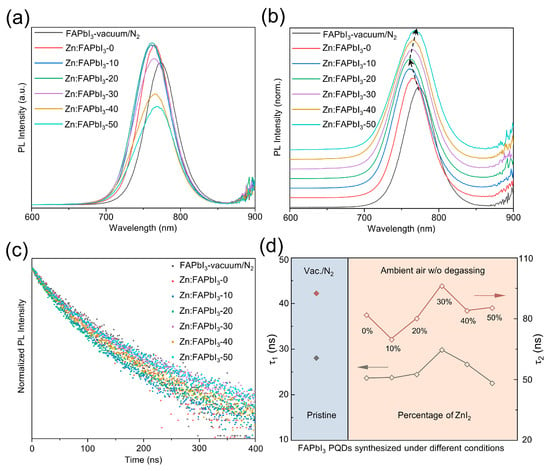
Figure 4.
PL (a), normalized PL (b) spectra, and TRPL data (c) for the FAPbI3-vaccum/N2, Zn:FAPbI3-0, Zn:FAPbI3-10, Zn:FAPbI3-20, Zn:FAPbI3-30, Zn:FAPbI3-40, and Zn:FAPbI3-50 PQDs. Summary of TRPL decay times (d) for FAPbI3 PQDs obtained under different synthetic conditions.
4. Conclusions
In summary, an easy and effective synthetic method by introducing ZnI2 additives is developed to achieve high-quality FAPbI3 PQDs without degassing and N2 protection. Compared with the pristine vacuum/N2-FAPbI3 PQDs, the air-synthesized Zn:FAPbI3 PQDs exhibit higher structural and colloidal stability, as well as higher production yield. As the amount of added ZnI2 in the reaction mixture is increased, the crystal size of the FAPbI3 PQDs is decreased, while no significant changes in the crystal structure, shape, and size dispersion are observed. The introduced ZnI2 would not induce the significant lattice distortion in the PQDs, but may only reside at the surfaces. Thus, the excessive addition of ZnI2 induces the formation of impurities of related Zn salts on PQD surfaces, negatively impacts the ligand binding with OA and OAm, and results in defects as well as weakened electronic coupling between QDs. However, importantly, with assistance of an optimal amount of ZnI2 additives, ~30 mol%, these air-synthesized FAPbI3 PQDs demonstrate slightly longer carrier lifetimes than vacuum/N2-assisted FAPbI3 PQDs. This vacuum-free air-synthesis route offers an industrial-friendly method for producing low-cost colloidal FAPbI3 PQDs for high-performance PV applications.
Author Contributions
Conceptualization, S.W. and Q.Z.; methodology, S.W. and S.L.; validation, S.W. and Q.Z.; formal analysis, S.W. and Q.Z.; investigation, S.W. and Q.Z.; data curation, S.W. and S.L.; writing—original draft preparation, S.W. and Q.Z.; writing—review and editing, S.W., S.L. and Q.Z.; supervision, Q.Z.; project administration, Q.Z.; funding acquisition, Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
this research was funded by the National Natural Science Foundation of China (52102266) and the China Postdoctoral Science Foundation (2020M680861).
Data Availability Statement
Data that support the plots within this work are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, S.; Yousefi Amin, A.A.; Wu, L.; Cao, M.; Zhang, Q.; Ameri, T. Perovskite Nanocrystals: Synthesis, Stability, and Optoelectronic Applications. Small Struct. 2021, 2, 2000124. [Google Scholar] [CrossRef]
- Li, N.; Niu, X.; Chen, Q.; Zhou, H. Towards Commercialization: The Operational Stability of Perovskite Solar Cells. Chem. Soc. Rev. 2020, 49, 8235–8286. [Google Scholar] [CrossRef] [PubMed]
- Milić, J.V.; Zakeeruddin, S.M.; Grätzel, M. Layered Hybrid Formamidinium Lead Iodide Perovskites: Challenges and Opportunities. Acc. Chem. Res. 2021, 54, 2729–2740. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, Y.; Wang, H.; Zhan, Q.; Liu, Q.; Xia, Z. Postsynthetic Surface Trap Removal of CsPbX3 (X = Cl, Br, or I) Quantum Dots via a ZnX2/Hexane Solution toward an Enhanced Luminescence Quantum Yield. Chem. Mater. 2018, 30, 8546–8554. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-halide Anion Engineering for α-FAPbI3 Perovskite Solar Cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef]
- Huang, Y.; Lei, X.; He, T.; Jiang, Y.; Yuan, M. Recent Progress on Formamidinium-Dominated Perovskite Photovoltaics. Adv. Energy Mater. 2022, 12, 2100690. [Google Scholar] [CrossRef]
- Hanul, M.; Do, Y.-L.; Junu, K.; Gwisu, K.; Kyoung, S.-L.; Jongbeom, K.; Min, J.-P.; Young, K.-K.; Kwang, S.-K.; Min, G.-K.; et al. Perovskite Solar Cells with Atomically Coherent Interlayers on SnO2 Electrodes. Nature 2021, 598, 444–450. [Google Scholar]
- Chen, Z.; Zhang, H.; Yao, F.; Tao, C.; Fang, G.; Li, G. Room Temperature Formation of Semiconductor Grade α-FAPbI3 Films for Efficient Perovskite Solar Cells. Cell Rep. Phys. Sci. 2020, 1, 100205. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Zhang, T.; Liu, X.; Wang, X.; Zhao, Y. Advances to High-Performance Black-Phase FAPbI3 Perovskite for Efficient and Stable Photovoltaics. Small Struct. 2021, 2, 2000130. [Google Scholar] [CrossRef]
- Tang, Y.; Gu, Z.-K.; Fu, C.-P.; Xiao, Q.; Zhang, S.-S.; Zhang, Y.-Q.; Song, Y.-L. FAPbI3 Perovskite Solar Cells: From Film Morphology Regulation to Device Optimization. Sol. RRL 2022, 6, 2200120. [Google Scholar] [CrossRef]
- Miao, Y.; Ke, Y.; Wang, N.; Zou, W.; Xu, M.; Cao, Y.; Sun, Y.; Yang, R.; Wang, Y.; Tong, Y.; et al. Stable and Bright Formamidinium-based Perovskite Light-emitting Diodes with High Energy Conversion Efficiency. Nat. Commun. 2019, 10, 3624. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-C.; Chao, L.-W.; Xu, C.-Y.; Hsu, C.-H.; Lee, Y.-T.; Xu, Z.-M.; Lin, C.-C.; Tseng, Z.-L. Room-Temperature Synthesis of Air-Stable Near-Infrared Emission in FAPbI3 Nanoparticles Embedded in Silica. Biosensors 2021, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, H.; Jin, L.; Wen, C.; Zhao, Q.; Zhao, C.; Guo, J.; Cheng, C.; Wang, H.; Zhang, L.; et al. Ligand-Assisted Coupling Manipulation for Efficient and Stable FAPbI3 Colloidal Quantum Dot Solar Cells. Angew. Chem. Int. Ed. Engl. 2022, e202214241. [Google Scholar] [CrossRef]
- Ding, S.; Hao, M.; Fu, C.; Lin, T.; Baktash, A.; Chen, P.; He, D.; Zhang, C.; Chen, W.; Whittaker, A.K.; et al. In Situ Bonding Regulation of Surface Ligands for Efficient and Stable FAPbI3 Quantum Dot Solar Cells. Adv. Sci. 2022, 9, 2204476. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jia, D.; Johansson, E.M.J.; Hagfeldt, A.; Zhang, X. Emerging Perovskite Quantum Dot Solar Cells: Feasible Approaches to Boost Performance. Energy Environ. Sci. 2021, 14, 224–261. [Google Scholar] [CrossRef]
- Minh, D.N.; Kim, J.; Hyon, J.; Sim, J.H.; Sowlih, H.H.; Seo, C.; Nam, J.; Eom, S.; Suk, S.; Lee, S.; et al. Room-Temperature Synthesis of Widely Tunable Formamidinium Lead Halide Perovskite Nanocrystals. Chem. Mater. 2017, 29, 5713–5719. [Google Scholar] [CrossRef]
- Hazarika, A.; Zhao, Q.; Gaulding, E.A.; Christians, J.A.; Dou, B.; Marshall, A.R.; Moot, T.; Berry, J.J.; Johnson, J.C.; Luther, J.M. Perovskite Quantum Dot Photovoltaic Materials beyond the Reach of Thin Films: Full-Range Tuning of A-Site Cation Composition. ACS Nano 2018, 12, 10327–10337. [Google Scholar] [CrossRef]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum Dot–induced Phase Stabilization of α-CsPbI3 Perovskite for High-efficiency Photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef]
- Zhao, Q.; Hazarika, A.; Chen, X.; Harvey, S.P.; Larson, B.W.; Teeter, G.R.; Liu, J.; Song, T.; Xiao, C.; Shaw, L.; et al. High Efficiency Perovskite Quantum Dot Solar Cells with Charge Separating Heterostructure. Nat. Commun. 2019, 10, 2842. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Ding, C.; Kobayashi, S.; Izuishi, T.; Nakazawa, N.; Toyoda, T.; Ohta, T.; Hayase, S.; Minemoto, T.; et al. Highly Luminescent Phase-Stable CsPbI3 Perovskite Quantum Dots Achieving Near 100% Absolute Photoluminescence Quantum Yield. ACS Nano 2017, 11, 10373–10383. [Google Scholar] [CrossRef]
- Dong, Y.; Qiao, T.; Kim, D.; Parobek, D.; Rossi, D.; Son, D.H. Precise Control of Quantum Confinement in Cesium Lead Halide Perovskite Quantum Dots via Thermodynamic Equilibrium. Nano Lett. 2018, 18, 3716–3722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kang, C.; Zhang, G.; Pan, Z.; Huang, Z.; Xu, S.; Rao, H.; Liu, H.; Wu, S.; Wu, X.; et al. All-Inorganic CsPbI3 Quantum Dot Solar Cells with Efficiency over 16% by Defect Control. Adv. Funct. Mater. 2021, 31, 2005930. [Google Scholar] [CrossRef]
- Bi, C.; Sun, X.; Huang, X.; Wang, S.; Yuan, J.; Wang, J.X.; Pullerits, T.; Tian, J. Stable CsPb1–xZnxI3 Colloidal Quantum Dots with Ultralow Density of Trap States for High-Performance Solar Cells. Chem. Mater. 2020, 32, 6105–6113. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Kershaw, S.V.; Li, T.; Wang, C.; Zhang, X.; Wang, W.; Li, D.; Wang, Y.; Lu, M.; et al. Zn-Alloyed CsPbI3 Nanocrystals for Highly Efficient Perovskite Light-Emitting Devices. Nano Lett. 2019, 19, 1552–1559. [Google Scholar] [CrossRef]
- Thapa, S.; Adhikari, G.C.; Zhu, H.; Grigoriev, A.; Zhu, P. Zn-Alloyed All-Inorganic Halide Perovskite-Based White Light-Emitting Diodes with Superior Color Quality. Sci. Rep. 2019, 9, 18636. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, J.; Xu, L.; Liu, S.; Lan, S.; Li, X.; Song, J. A Zinc Non-halide Dopant Strategy enables Efficient Perovskite CsPbI3 Quantum Dot-based Light-emitting Diodes. Mater. Chem. Front. 2020, 4, 1444–1453. [Google Scholar] [CrossRef]
- Zhao, Q.; Hazarika, A.; Schelhas, L.T.; Liu, J.; Gaulding, E.A.; Li, G.; Zhang, M.; Toney, M.F.; Sercel, P.C.; Luther, J.M. Size-Dependent Lattice Structure and Confinement Properties in CsPbI3 Perovskite Nanocrystals: Negative Surface Energy for Stabilization. ACS Energy Lett. 2020, 5, 238–247. [Google Scholar] [CrossRef]
- Baranov, D.; Toso, S.; Imran, M.; Manna, L. Investigation into the Photoluminescence Red Shift in Cesium Lead Bromide Nanocrystal Superlattices. J. Phys. Chem. Lett. 2019, 10, 655–660. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Jiang, Q.; Wang, P.; Yin, Z.; Zhang, X.; Tan, H.; Yang, Y.M.; Wei, M.; Sutherland, B.R.; et al. Ultra-bright and highly efficient inorganic based perovskite light-emitting diodes. Nat. Commun. 2017, 8, 15640. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).