An Evaluation of Experimental Calcium Ion-Leachable Nanocomposite Glass Ionomer Cements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Clay Modification via Ion Exchange Reaction
2.3. Preparation of Cement Pastes
2.4. Measurements
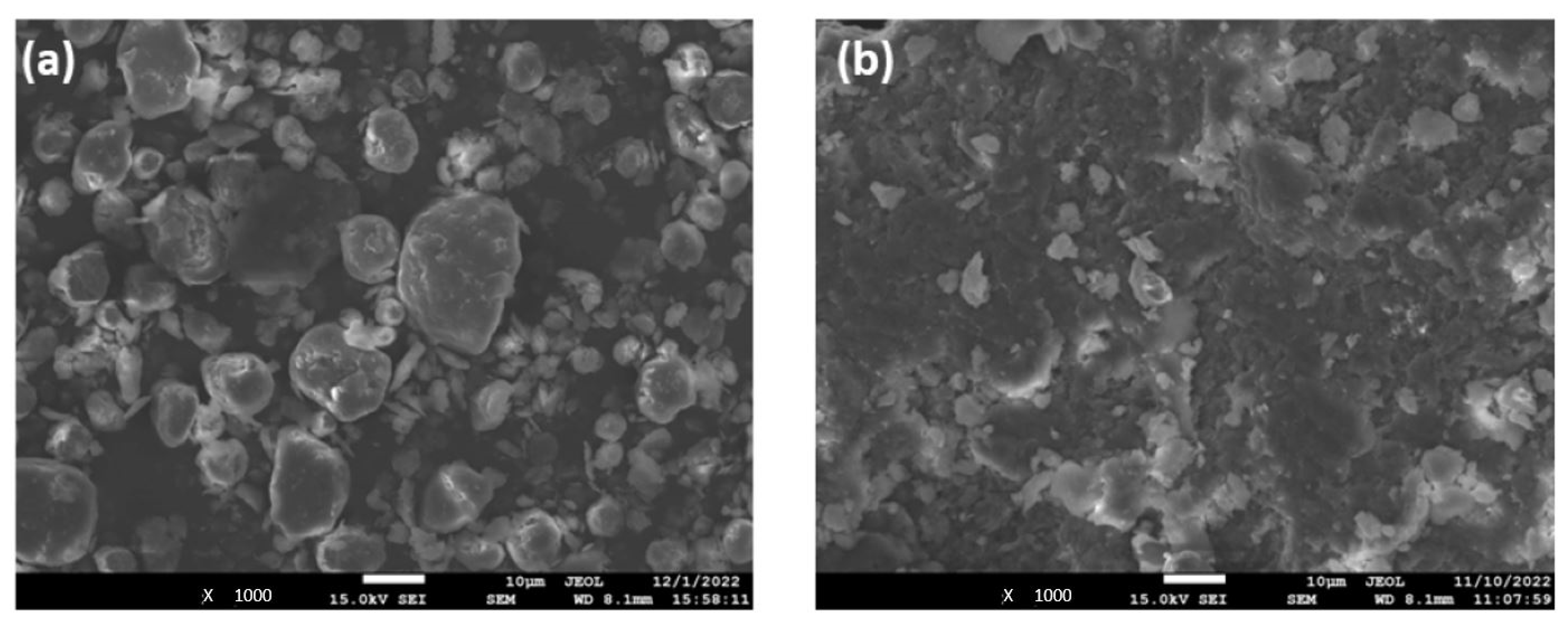
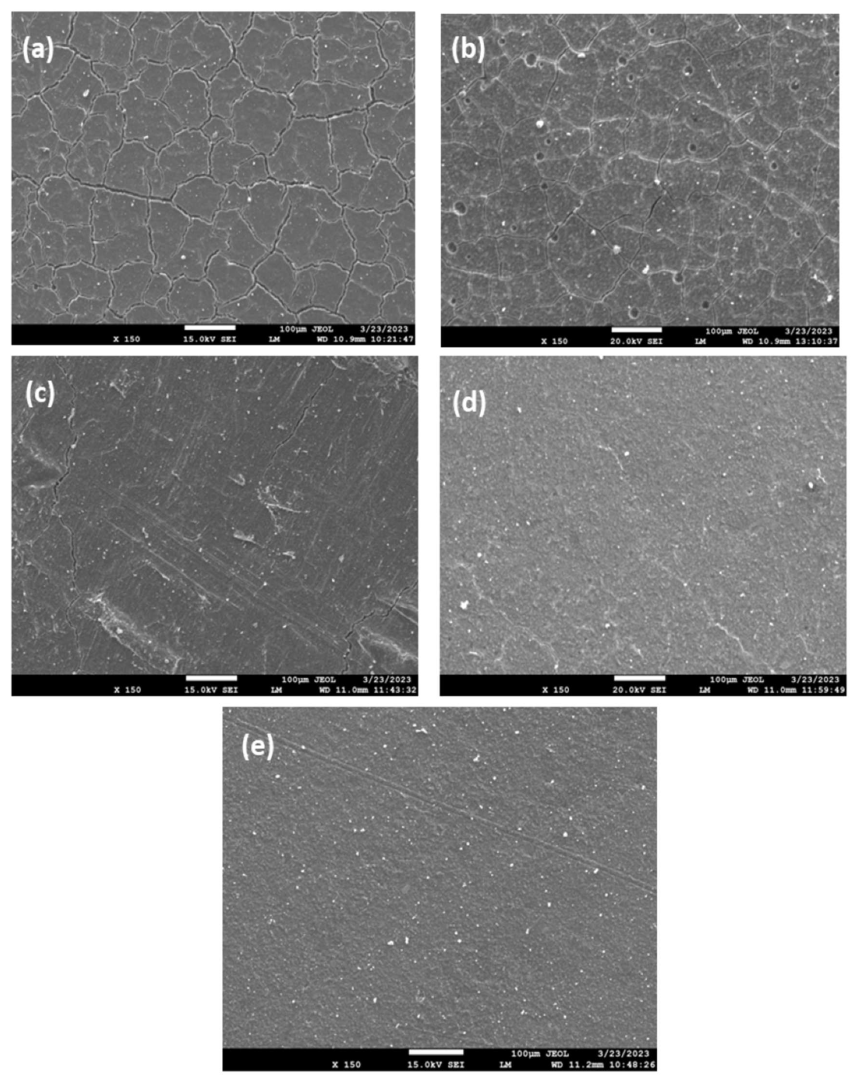
2.4.1. Characterization of GIC Nanocomposites Morphology
2.4.2. Assessment of the GIC Nanocomposites’ Setting Reaction Progress
2.4.3. Compression Tests
2.4.4. Determination of Calcium Ion Release by Means of Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
2.5. Statistical Analysis
3. Results and Discussion
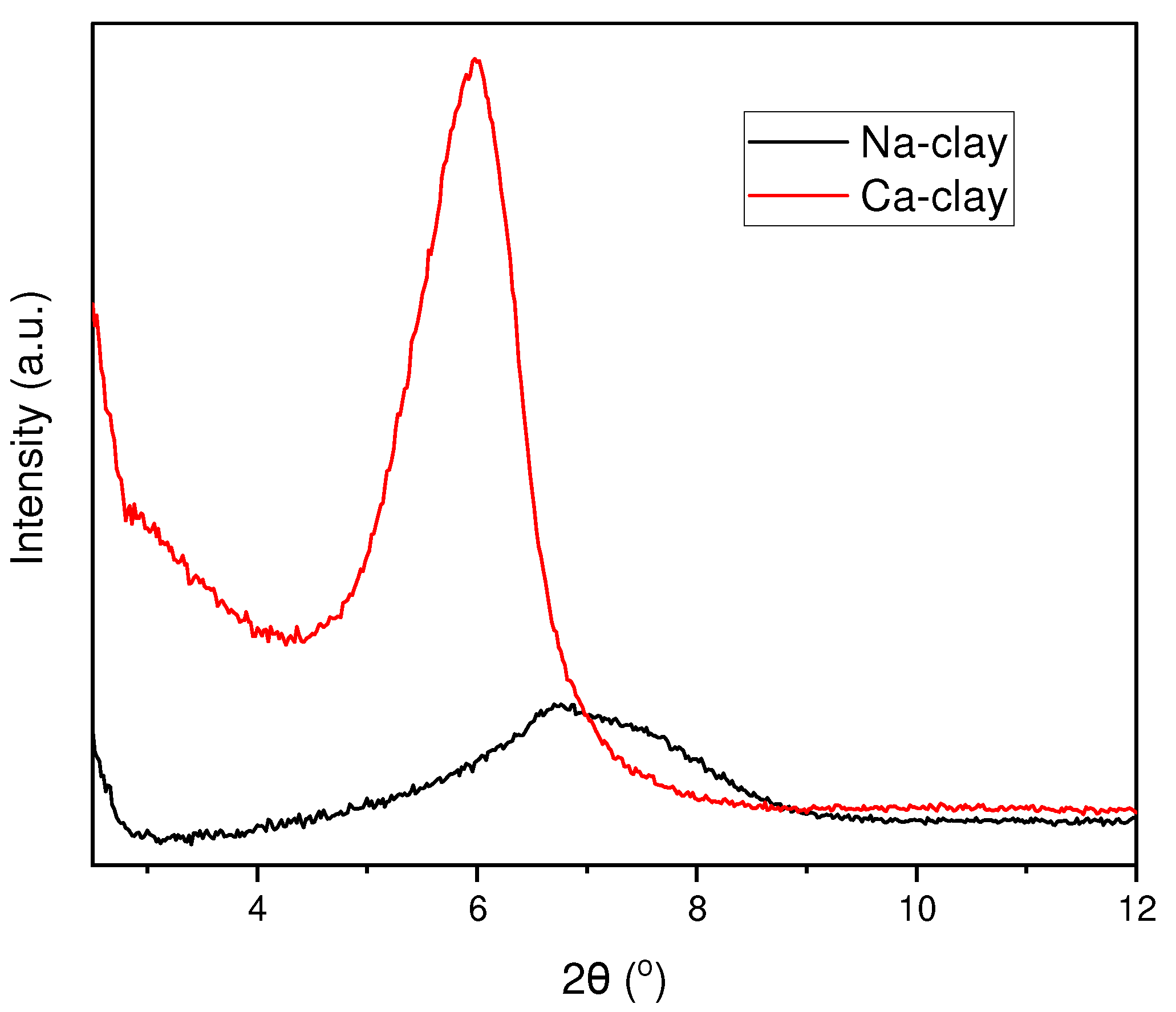
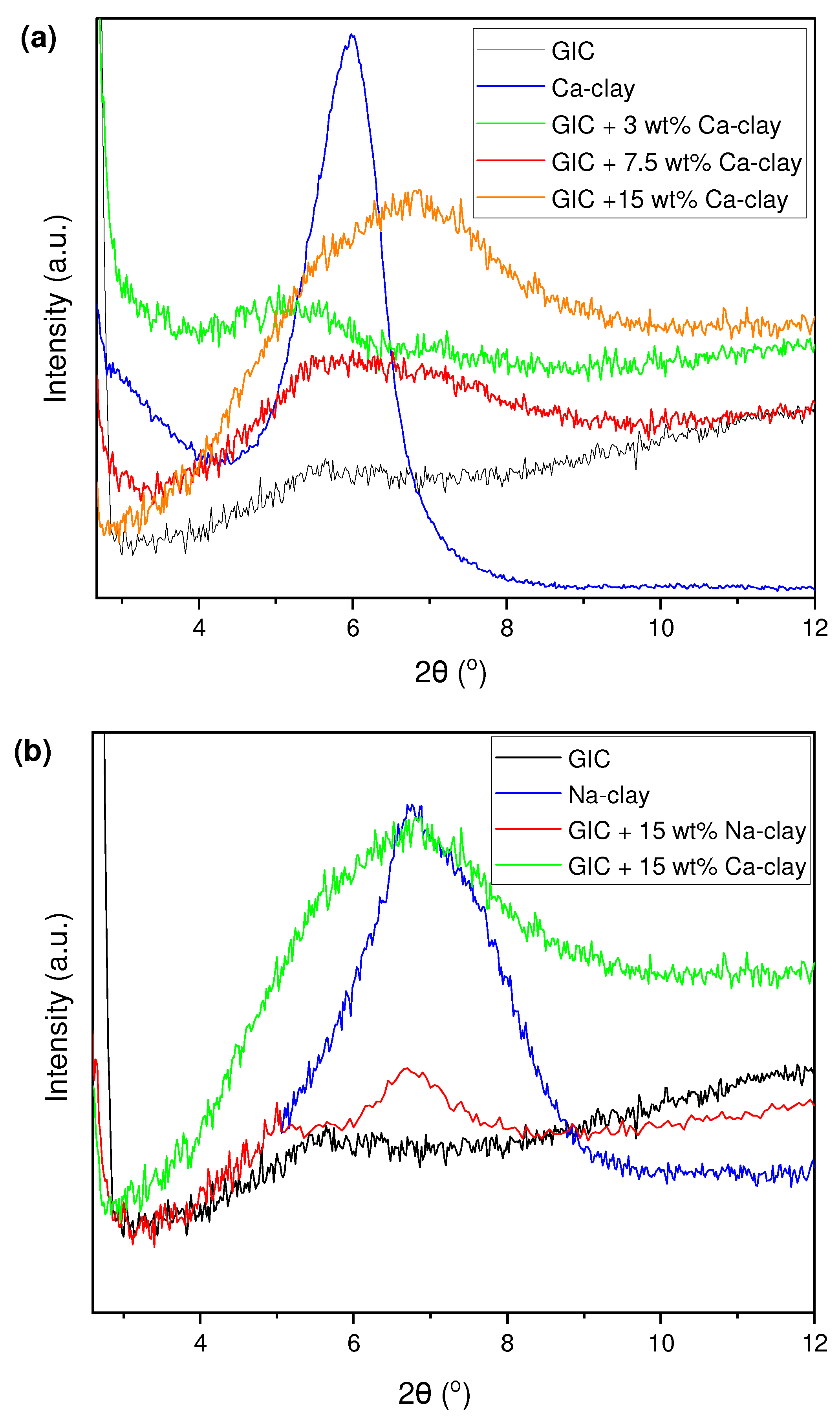
3.1. Verification of Ion Exchange Reaction in Clay Nanoparticles
3.2. Structural Characterization of GIC Nanocomposites
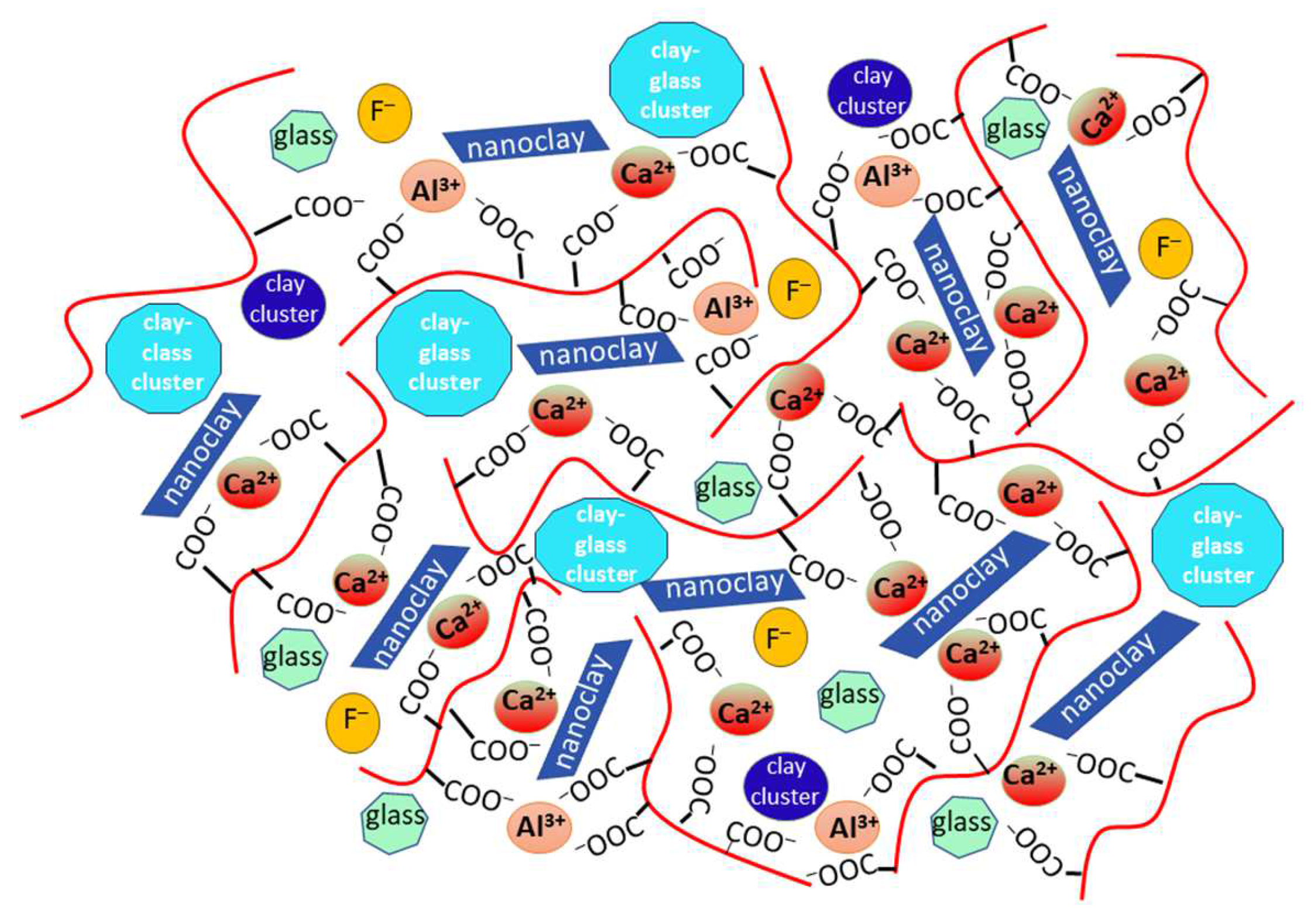
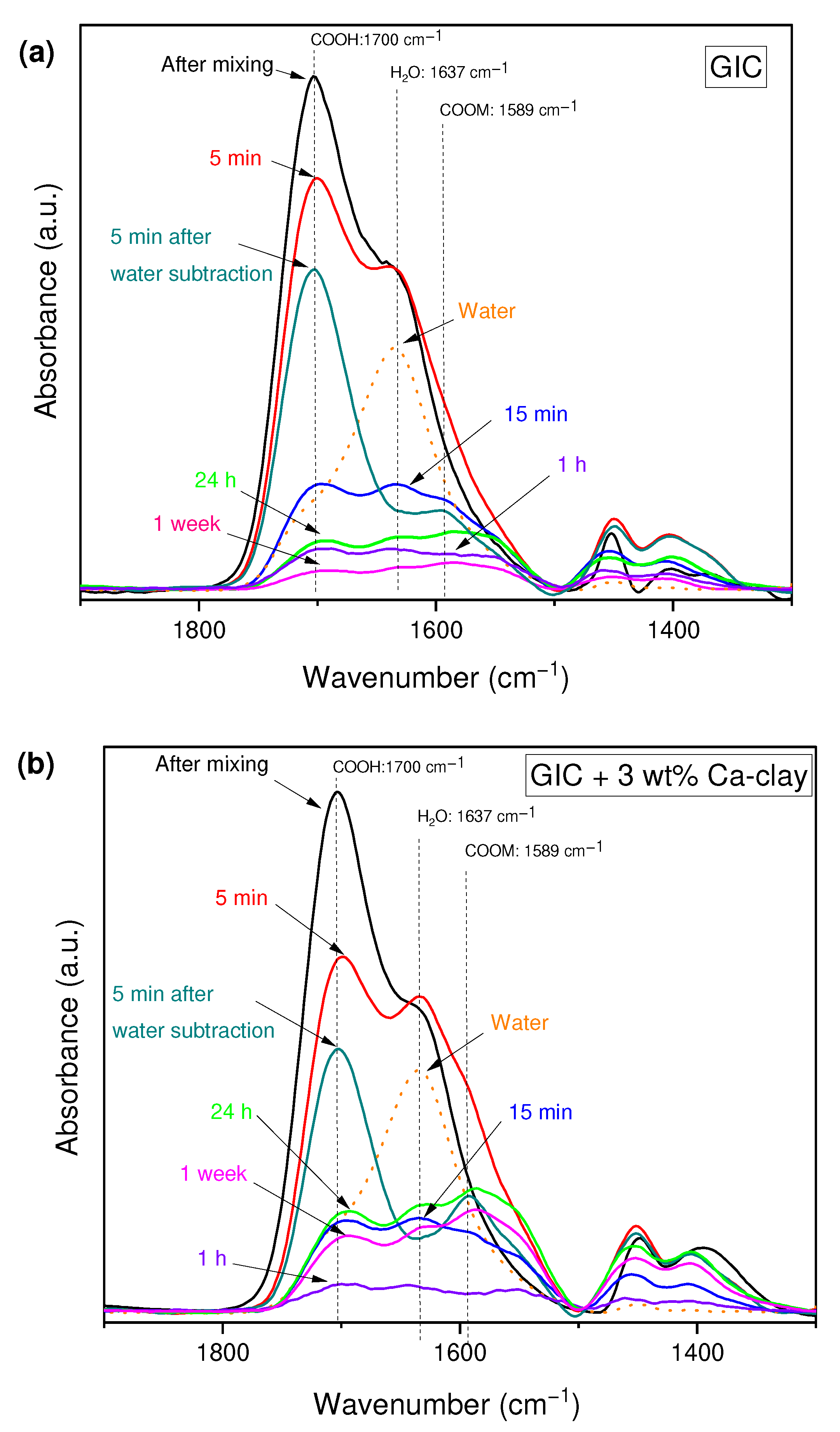
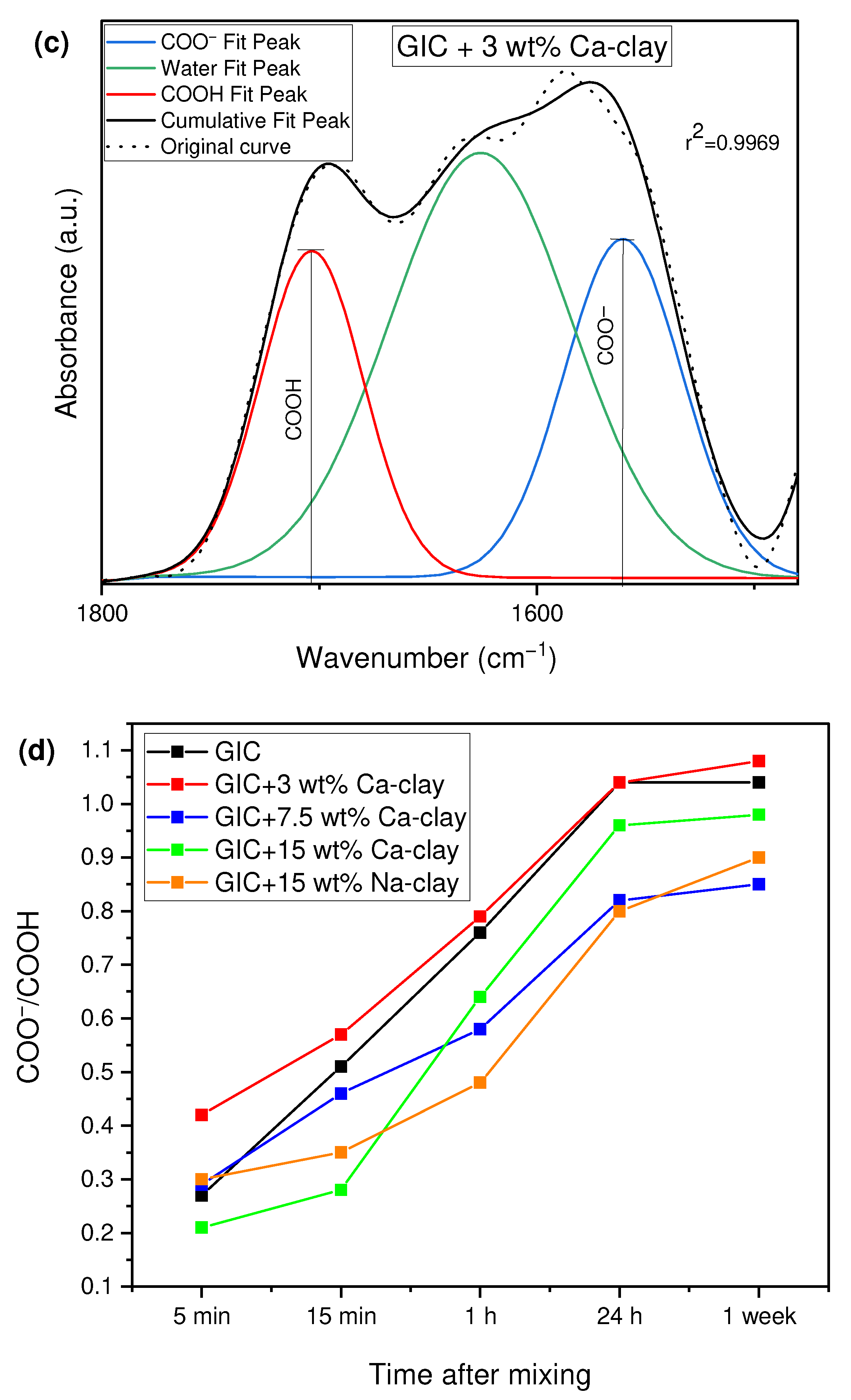
3.3. Evaluation of the Acid–Base Reaction
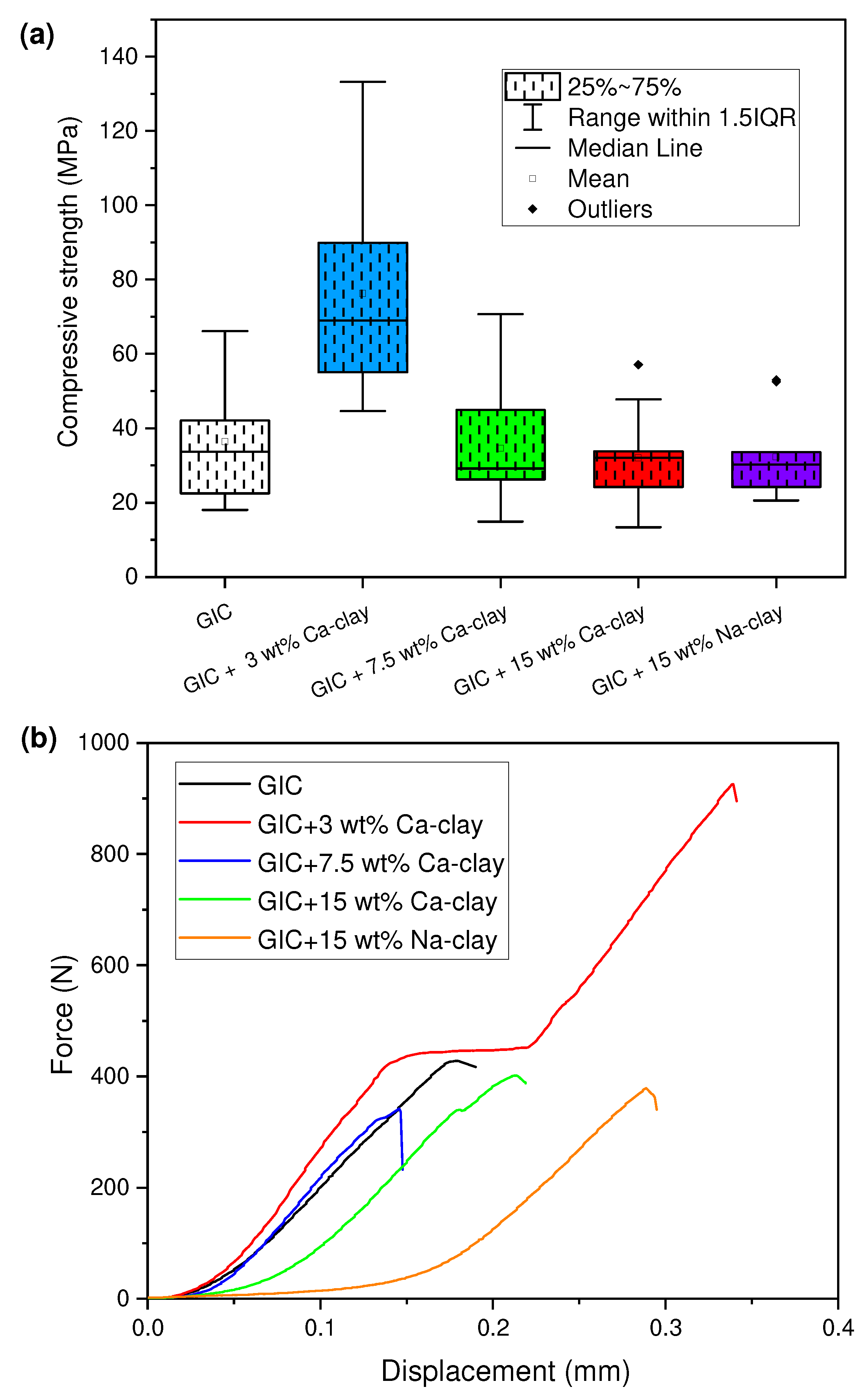
3.4. Evaluation of Mechanical Performance
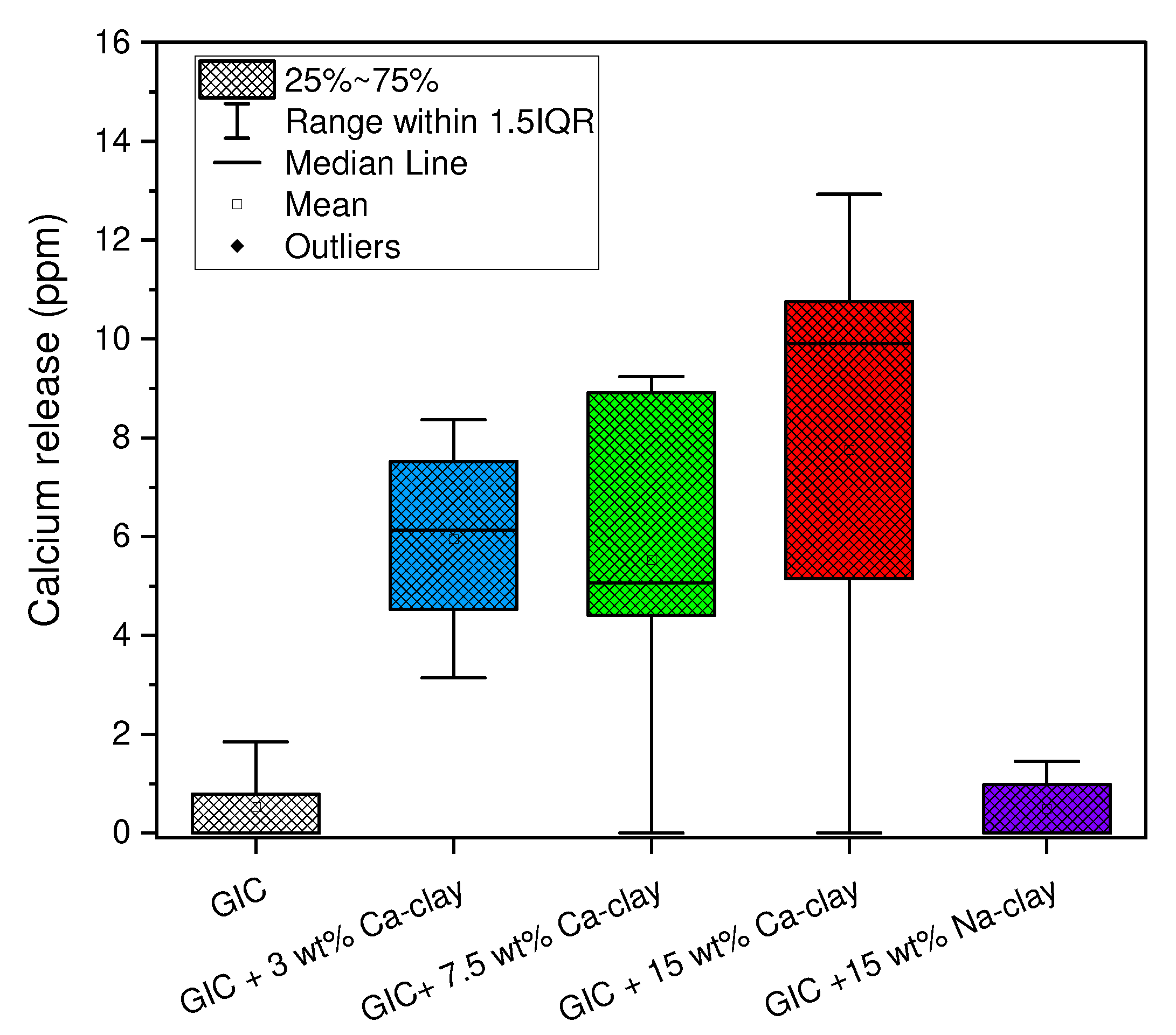
3.5. Assessment of Calcium Ion Release
3.6. Limitations of the Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, A.D.; Kent, B.E. The Glass-Ionomer Cement, a New Translucent Dental Filling Material. J. Appl. Chem. Biotechnol. 1971, 21, 313. [Google Scholar] [CrossRef]
- Almuhaiza, M. Glass-Ionomer Cements in Restorative Dentistry: A Critical Appraisal. J. Contemp. Dent. Pract. 2016, 17, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja Upadhya, P.; Kishore, G. Glass Ionomer Cement—The Different Generations. Trends Biomater. Artif. Organs 2005, 18, 158–165. [Google Scholar]
- Sidhu, S.K.; Nicholson, J.W. A Review of Glass-Ionomer Cements for Clinical Dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef]
- Nomoto, R.; McCabe, J.F. Effect of Mixing Methods on the Compressive Strength of Glass Ionomer Cements. J. Dent. 2001, 29, 205–210. [Google Scholar] [CrossRef]
- Croll, T.P.; Bar-Zion, Y.; Segura, A.; Donly, K.J. Clinical Performance of Resin-Modified Glass Ionomer Cement Restorations in Primary Teeth: A Retrospective Evaluation. J. Am. Dent. Assoc. 2001, 132, 1110–1116. [Google Scholar] [CrossRef]
- Xie, D.; Brantley, W.A.; Culbertson, B.M.; Wang, G. Mechanical Properties and Microstructures of Glass-Ionomer Cements. Dent. Mater. 2000, 16, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Brzović Rajić, V.; Ivanišević Malčić, A.; Bilge Kütük, Z.; Gurgan, S.; Jukić Krmek, S.; Miletic, I. Compressive Strength of New Glass Ionomer Cement Technology Based Restorative Materials after Thermocycling and Cyclic Loading. Acta Stomatol. Croat. 2019, 53, 318–325. [Google Scholar] [CrossRef]
- Fareed, M.A.; Stamboulis, A. Effect of Nanoclay Dispersion on the Properties of a Commercial Glass Ionomer Cement. Int. J. Biomater. 2014, 2014, 685389. [Google Scholar] [CrossRef]
- Howard, L.; Weng, Y.; Xie, D. Preparation and Evaluation of a Novel Star-Shaped Polyacid-Constructed Dental Glass-Ionomer System. Dent. Mater. 2014, 30, 644–653. [Google Scholar] [CrossRef]
- Mallmann, A.; Oliveira Ataíde, J.C.; Amoeda, R.; Rocha, P.V.; Jacques, L.B. Compressive Strength of Glass Ionomer Cements Using Different Specimen Dimensions. Braz. Oral Res. 2007, 21, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, F.M.; Pascotto, R.C.; Malacarne, L.C.; Bento, A.C.; Medina Neto, A.; de Castro-Hoshino, L.V.; Souza, M.; Nicholson, J.W.; Baesso, M.L. Studies of the Early Stages of the Dynamic Setting Process of Chemically Activated Restorative Glass-Ionomer Cements. Biomater. Investig. Dent. 2021, 8, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.K.; Watson, T.F. Resin-Modified Glass Ionomer Materials. A Status Report for the American Journal of Dentistry. Am. J. Dent. 1995, 8, 59–67. [Google Scholar] [PubMed]
- Ching, H.S.; Luddin, N.; Kannan, T.P.; Ab Rahman, I.; Abdul Ghani, N.R.N. Modification of Glass Ionomer Cements on Their Physical-Mechanical and Antimicrobial Properties. J. Esthet. Restor. Dent. 2018, 30, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, R.C. Incorporation of Nanomaterials in Glass Ionomer Cements-Recent Developments and Future Perspectives: A Narrative Review. Nanomaterials 2022, 12, 3827. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.; Garcia, L.d.F.R.; Volpato, C.A.M. Bioactive Materials for Direct and Indirect Restorations: Concepts and Applications. Front. Dent. Med. 2021, 2, 647267. [Google Scholar] [CrossRef]
- PAS (Publicly Available Specification) 132. Terminology for the Bio-Nano Interface; BSI (British Standards Institution): London, UK, 2007. [Google Scholar]
- Gandolfi, M.G.; Taddei, P.; Siboni, F.; Modena, E.; Ciapetti, G.; Prati, C. Development of the Foremost Light-Curable Calcium-Silicate MTA Cement as Root-End in Oral Surgery. Chemical-Physical Properties, Bioactivity and Biological Behavior. Dent. Mater. 2011, 27, e134–e157. [Google Scholar] [CrossRef]
- Hamdy, T.M. Bioactivity: A New Buzz in Dental Materials. EC Dent. Sci. 2018, 17, 115592001. [Google Scholar]
- Ladino, L.G.; Bernal, A.; Calderón, D.; Cortés, D. Bioactive Materials in Restorative Dentistry: A Literature Review. SVOA Dent. 2021, 2, 74–81. [Google Scholar]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive Glass Applications in Dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef]
- Spagnuolo, G. Bioactive Dental Materials: The Current Status. Materials 2022, 15, 2016. [Google Scholar] [CrossRef]
- Fernando, D.; Attik, N.; Pradelle-Plasse, N.; Jackson, P.; Grosgogeat, B.; Colon, P. Bioactive Glass for Dentin Remineralization: A Systematic Review. Mater. Sci. Eng. C 2017, 76, 1369–1377. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Siboni, F.; Botero, T.; Bossù, M.; Riccitiello, F.; Prati, C. Calcium Silicate and Calcium Hydroxide Materials for Pulp Capping: Biointeractivity, Porosity, Solubility and Bioactivity of Current Formulations. J. Appl. Biomater. Funct. Mater. 2015, 13, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Barot, T.; Rawtani, D.; Kulkarni, P. Nanotechnology-Based Materials as Emerging Trends for Dental Applications. Rev. Adv. Mater. Sci. 2021, 60, 173–189. [Google Scholar] [CrossRef]
- Sasalawad, S.S.; Naik, S.N.; Shashibhushan, K.K.; Poornima, P. Nanodentistry: The next Big Thing Is Small. Int. J. Contemp. Dent. Med. Rev. 2014, 2014, 101114. [Google Scholar]
- Sokołowski, K.; Szynkowska, M.I.; Pawlaczyk, A.; Łukomska-Szymańska, M.; Sokołowski, J. The Impact of Nanosilver Addition on Element Ions Release Form Light-Cured Dental Composite and Compomer into 0.9% NaCl. Acta Biochim. Pol. 2014, 61, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Mantri, S.S.; Mantri, S.P. The Nano Era in Dentistry. J. Nat. Sci. Biol. Med. 2013, 4, 39–44. [Google Scholar] [CrossRef]
- Katti, K.S.; Jasuja, H.; Jaswandkar, S.V.; Mohanty, S.; Katti, D.R. Nanoclays in Medicine: A New Frontier of an Ancient Medical Practice. Mater. Adv. 2022, 3, 7484–7500. [Google Scholar] [CrossRef]
- Sandomierski, M.; Zielińska, M.; Adamska, K.; Patalas, A.; Voelkel, A. Calcium Montmorillonite as a Potential Carrier in the Release of Bisphosphonates. New J. Chem. 2022, 46, 3401–3408. [Google Scholar] [CrossRef]
- Ritto, F.P.; da Silva, E.M.; Borges, A.L.S.; Borges, M.A.P.; Sampaio-Filho, H.R. Fabrication and Characterization of Low-Shrinkage Dental Composites Containing Montmorillonite Nanoclay. Odontology 2022, 110, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Sandomierski, M.; Buchwald, Z.; Voelkel, A. Calcium Montmorillonite and Montmorillonite with Hydroxyapatite Layer as Fillers in Dental Composites with Remineralizing Potential. Appl. Clay Sci. 2020, 198, 105822. [Google Scholar] [CrossRef]
- Wang, R.; Li, H.; Guangxu, G.; Dai, N.; Jinsong, R.; Ran, H.; Zhang, Y. Montmorillonite-Based Two-Dimensional Nanocomposites: Preparation and Applications. Molecules 2021, 26, 2521. [Google Scholar] [CrossRef]
- De Menezes, L.R.; Da Silva, E.O. The Use of Montmorillonite Clays as Reinforcing Fillers for Dental Adhesives. Mater. Res. 2016, 19, 236–242. [Google Scholar] [CrossRef]
- Dowling, A.H.; Stamboulis, A.; Fleming, G.J.P. The Influence of Montmorillonite Clay Reinforcement on the Performance of a Glass Ionomer Restorative. J. Dent. 2006, 34, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Giannakas, A.; Tsagkalias, I.; Achilias, D.S.; Ladavos, A. A Novel Method for the Preparation of Inorganic and Organo-Modified Montmorillonite Essential Oil Hybrids. Appl. Clay Sci. 2017, 146, 362–370. [Google Scholar] [CrossRef]
- Xue, Z.; Ma, J.; Hao, W.; Bai, X.; Kang, Y.; Liu, J.; Li, R. Synthesis and Characterization of Ordered Mesoporous Zeolite LTA with High Ion Exchange Ability. J. Mater. Chem. 2012, 22, 2532–2538. [Google Scholar] [CrossRef]
- Eliades, G.; Palaghias, G. In Vitro Characterization of Visible Light-Cured Glass Ionomer Liners. Dent. Mater. 1993, 9, 198–203. [Google Scholar] [CrossRef]
- Kakaboura, A.; Eliades, G.; Palaghias, G. An FTIR Study on the Setting Mechanism of Resin-Modified Glass Ionomer Restoratives. Dent. Mater. 1996, 12, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.W.; Brookman, P.J.; Lacy, O.M.; Wilson, A.D. Fourier Transform Infrared Spectroscopic Study of the Role of Tartaric Acid in Glass-Ionomer Dental Cements. J. Dent. Res. 1988, 67, 1451–1454. [Google Scholar] [CrossRef]
- ISO 9917-1:2007; Dentistry—Water-Based Cements—Part 1: Powder/Liquid Acid-Base Cements. ISO: Geneva, Switzerland, 2017.
- Kantovitz, K.R.; Carlos, N.R.; Silva, I.A.P.S.; Braido, C.; Costa, B.C.; Kitagawa, I.L.; Nociti, F.H., Jr.; Basting, R.T.; de Figueiredo, F.K.P.; Lisboa-Filho, P.N. TiO2 Nanotube-Based Nanotechnology Applied to High-Viscosity Conventional Glass-Ionomer Cement: Ultrastructural Analyses and Physicochemical Characterization. Odontology, 2023; ahead-of-print. [Google Scholar] [CrossRef]
- Gjorgievska, E.; Nicholson, J.W.; Gabrić, D.; Guclu, Z.A.; Miletić, I.; Coleman, N.J. Assessment of the Impact of the Addition of Nanoparticles on the Properties of Glass-Ionomer Cements. Materials 2020, 13, 276. [Google Scholar] [CrossRef]
- Duarte, A.C.A.; Pereira, R.D.F.C.; de Carvalho, S.M.; da Silva, A.G.; de Araújo, C.T.P.; Galo, R.; Dumont, V.C. Enhancing Glass Ionomer Cement Features by Using the Calcium Phosphate Nanocomposite. Braz. Dent. J. 2022, 33, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Ivanišević, A.; Rajić, V.B.; Pilipović, A.; Par, M.; Ivanković, H.; Baraba, A. Compressive Strength of Conventional Glass Ionomer Cement Modified with TiO2 Nano-Powder and Marine-Derived HAp Micro-Powder. Materials 2021, 14, 4964. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.L.; Carvalho, C.N.; Carvalho, E.M.; Bauer, J.; Leal, A.M.A. The Influence of Mixing Methods on the Compressive Strength and Fluoride Release of Conventional and Resin-Modified Glass Ionomer Cements. Int. J. Dent. 2019, 2019, 6834931. [Google Scholar] [CrossRef]
- Fareed, M.A.; Stamboulis, A. Nanoclay Addition to a Conventional Glass Ionomer Cements: Influence on Physical Properties. Eur. J. Dent. 2014, 8, 456–463. [Google Scholar] [CrossRef]
- Nicholson, J.W.; Coleman, N.J.; Sidhu, S.K. Kinetics of Ion Release from a Conventional Glass-Ionomer Cement. J. Mater. Sci. Mater. Med. 2021, 32, 30. [Google Scholar] [CrossRef]
- Awosanya, K.; Nicholson, J.W. A Study of Phosphate Ion Release from Glass-Ionomer Dental Cements. Ceram.-Silik. 2014, 58, 210–214. [Google Scholar]
- Sayyedan, F.S.; Fathi, M.; Edris, H.; Doostmohammadi, A.; Mortazavi, V.; Shirani, F. Fluoride Release and Bioactivity Evaluation of Glass Ionomer: Forsterite Nanocomposite. Dent. Res. J. 2013, 10, 452–459. [Google Scholar]
- Gandolfi, M.G.; Zamparini, F.; Degli Esposti, M.; Chiellini, F.; Fava, F.; Fabbri, P.; Taddei, P.; Prati, C. Highly Porous Polycaprolactone Scaffolds Doped with Calcium Silicate and Dicalcium Phosphate Dihydrate Designed for Bone Regeneration. Mater. Sci. Eng. C 2019, 102, 341–361. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Zamparini, F.; Valente, S.; Parchi, G.; Pasquinelli, G.; Taddei, P.; Prati, C. Green Hydrogels Composed of Sodium Mannuronate/Guluronate, Gelatin and Biointeractive Calcium Silicates/Dicalcium Phosphate Dihydrate Designed for Oral Bone Defects Regeneration. Nanomaterials 2021, 11, 3439. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Relationship between in Vitro Apatite-Forming Ability Measured Using Simulated Body Fluid and in Vivo Bioactivity of Biomaterials. Mater. Sci. Eng. C 2014, 35, 134–143. [Google Scholar] [CrossRef] [PubMed]
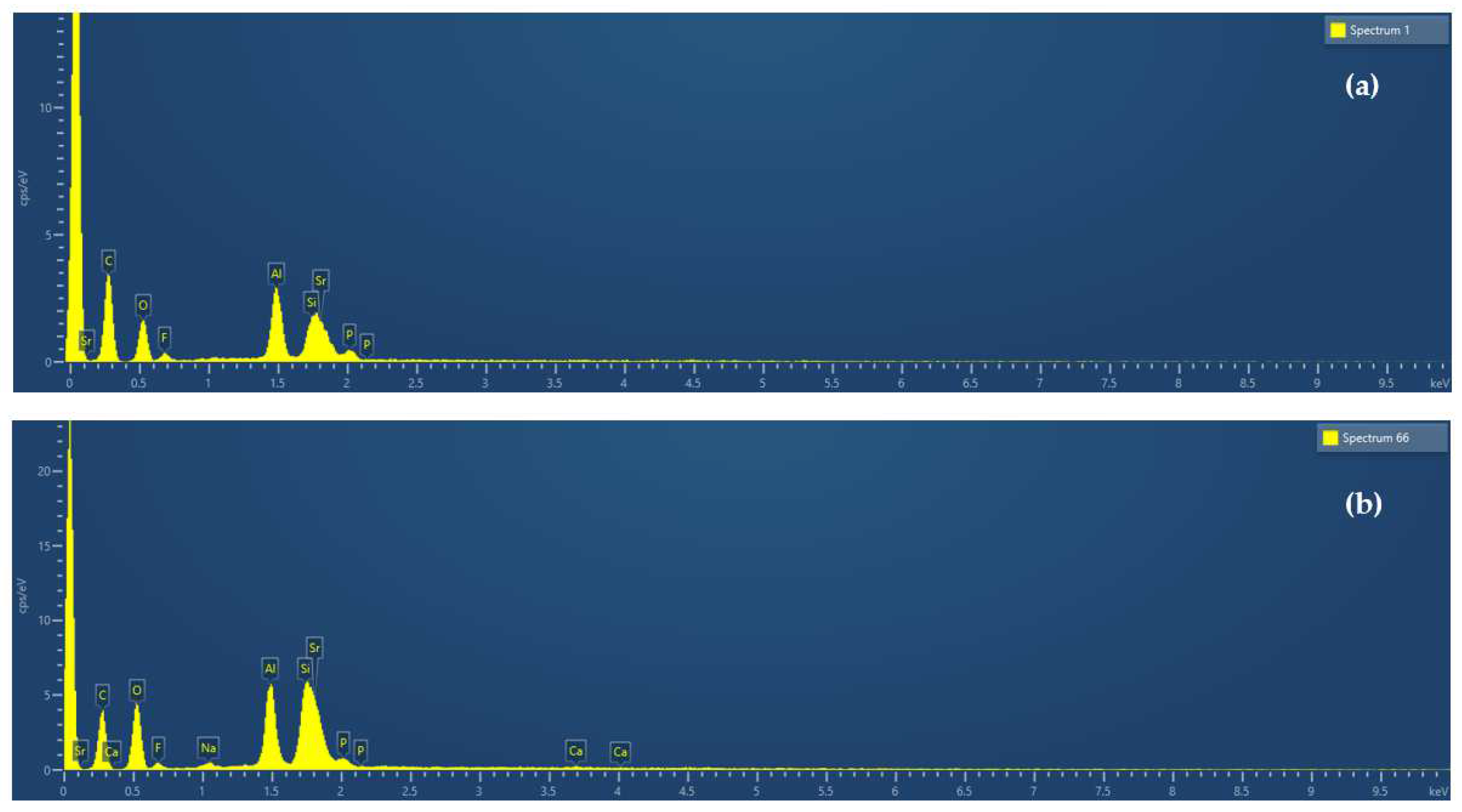
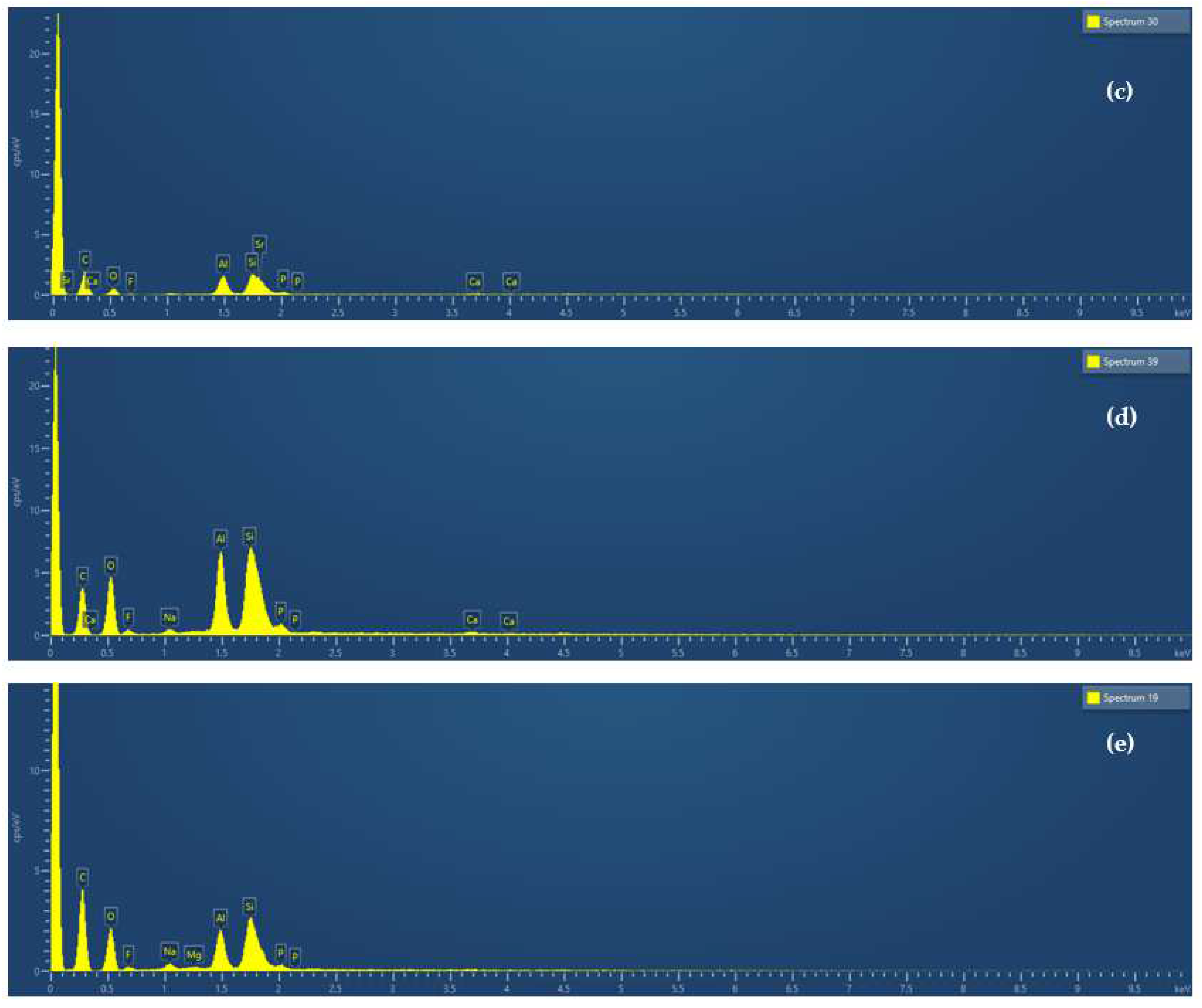
| Νο. | GIC Composition * | Abbreviated Name | ||
|---|---|---|---|---|
| 1 | Fuji II GC | Liquid | Poly(acrylic acid) Polybasic carboxylic acid: 10–20% Water: 50% | GIC |
| Powder | Glass Oxide Poly(acrylic acid) | |||
| 2 | Fuji II GC + 3 wt% Ca-clay | GIC + 3 wt% Ca-clay | ||
| 3 | Fuji II GC + 7.5 wt% Ca-clay | GIC + 7.5 wt% Ca-clay | ||
| 4 | Fuji II GC + 15 wt% Ca-clay | GIC + 15 wt% Ca-clay | ||
| 5 | Fuji II GC + 15 wt% Na-clay | GIC + 15 wt% Na-clay | ||
| Nano-Powder | Median (IQR) (%) | 2θ (deg) | d001 (nm) |
|---|---|---|---|
| Na-clay | 0.73 (0.43) | 6.71 | 1.32 |
| Ca-clay | 2.05 (0.63) | 5.98 | 1.48 |
| GIC Nanocomposite | Ca (%) Median (IQR) | d001 (nm) |
|---|---|---|
| GIC | - | - |
| GIC + 3 wt% Ca-clay | 0.65 (1.09) | 1.75 |
| GIC + 7.5 wt% Ca-clay | 0.11 (0.76) | 1.61 |
| GIC + 15 wt% Ca-clay | 0.54 (0.87) | 1.30 |
| GIC + 15 wt% Na-clay | - | 1.32 |
| GIC Nanocomposite | Time after Powder-Liquid Mixing | ||||
|---|---|---|---|---|---|
| 5 min | 15 min | 1 h | 24 h | 1 week | |
| GIC | 0.27 (0.05) | 0.51 (0.10) | 0.76 (0.22) | 1.04 (0.03) | 1.04 (0.08) |
| GIC + 3 wt% Ca-clay | 0.42 (0.08) | 0.57 (0.11) | 0.79 (0.21) | 1.04 (0.10) | 1.08 (0.12) |
| GIC + 7.5 wt% Ca-clay | 0.29 (0.16) | 0.46 (0.23) | 0.58 (0.18) | 0.82 (0.04) | 0.85 (0.05) |
| GIC + 15 wt% Ca-clay | 0.21 (0.09) | 0.28 (0.15) | 0.64 (0.24) | 0.96 (0.14) | 0.98 (0.13) |
| GIC + 15 wt% Na-clay | 0.30 (0.10) | 0.35 (0.13) | 0.48 (0.15) | 0.80 (0.16) | 0.90 (0.18) |
| GIC Nanocomposite | Compressive Strength (MPa) Median (IQR) |
|---|---|
| GIC | 33.65 (22.48, 42.08) a |
| GIC + 3 wt% Ca-clay | 68.97 (55.08, 89.88) b |
| GIC + 7.5 wt% Ca-clay | 29.19 (26.25, 44.89) a |
| GIC + 15 wt% Ca-clay | 32.09 (24.25, 33.81) a |
| GIC + 15 wt% Na-clay | 30.26 (24.29, 33.62) a |
| GIC Nanocomposite | Calcium Release (ppm) Median (Range) |
|---|---|
| GIC | 0 (0, 1.85) a |
| GIC + 3 wt% Ca-clay | 6.13 (3.15, 8.37) a |
| GIC + 7.5 wt% Ca-clay | 5.06 (0, 9.24) a |
| GIC + 15 wt% Ca-clay | 9.91 (0, 12.93) a |
| GIC + 15 wt% Na-clay | 0 (0, 1.45) a |
| Glass Ionomer Cement Nanocomposite | Median (IQR) (%) |
|---|---|
| GIC | - |
| GIC + 3 wt% Ca-clay | 0.37 (0.18) |
| GIC + 7.5 wt% Ca-clay | 1.45 (0.7) |
| GIC + 15 wt% Ca-clay | 1.48 (0.52) |
| GIC + 15 wt% Na-clay | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsolianos, I.; Nikolaidis, A.K.; Koulaouzidou, E.A.; Achilias, D.S. An Evaluation of Experimental Calcium Ion-Leachable Nanocomposite Glass Ionomer Cements. Nanomaterials 2023, 13, 2690. https://doi.org/10.3390/nano13192690
Tsolianos I, Nikolaidis AK, Koulaouzidou EA, Achilias DS. An Evaluation of Experimental Calcium Ion-Leachable Nanocomposite Glass Ionomer Cements. Nanomaterials. 2023; 13(19):2690. https://doi.org/10.3390/nano13192690
Chicago/Turabian StyleTsolianos, Ioannis, Alexandros K. Nikolaidis, Elisabeth A. Koulaouzidou, and Dimitris S. Achilias. 2023. "An Evaluation of Experimental Calcium Ion-Leachable Nanocomposite Glass Ionomer Cements" Nanomaterials 13, no. 19: 2690. https://doi.org/10.3390/nano13192690
APA StyleTsolianos, I., Nikolaidis, A. K., Koulaouzidou, E. A., & Achilias, D. S. (2023). An Evaluation of Experimental Calcium Ion-Leachable Nanocomposite Glass Ionomer Cements. Nanomaterials, 13(19), 2690. https://doi.org/10.3390/nano13192690









