Abstract
Nanoparticles and nanotechnology developments continue to advance the livelihood of humankind. However, health challenges due to microorganisms and cancerous cells continue to threaten many people’s lives globally. Therefore, new technological interventions are of great importance. The phytochemicals present in medicinal plants are suggested as biocompatible, cost-effective, and regenerative sources that can be utilized for the green synthesis of nanoparticles. Different plant extracts with various phytochemical constituents can form nanoparticles with specific shapes, sizes, and optical properties. This review focuses on advances in green nanotechnology and provides details on reliable synthetic routes toward medically and biocompatible relevant metallic nanoparticles. We cover a wide range of applications that use phytonanoparticles with an in-depth look at what makes these materials interesting. The study also provides details of the literature on the interventions made in phytonanotechnology for the production of plant-mediated synthesis and capped metallic nanoparticles and their applications in various industries. It was observed that a variety of plants have been well studied, and detailed findings have been reported; however, the study of Phyllanthus is still in its early stages, and more needs to be uncovered.
1. Introduction
Nanotechnology is an emerging multidisciplinary research field described as the engineering, science, and technology of designing, fabricating, characterizing, and applying systems, structures, and devices at the nanoscale, typically in the range of 1 to 100 nm [1,2]. In the last decade, nanotechnology has made significant progress and shown great potential for application in the fields of physics, medicine, agriculture, biology, chemistry, and electronics [3,4,5]. Moreover, the integration of biotechnology and nanotechnology provides an environmentally friendly and green technology for the production, characterization, and application of nanomaterials [2,6]. Nanomaterials, especially metallic nanoparticles, have attracted great research interest due to their fascinating and unique optical, mechanical, and chemical properties related to their large surface-to-volume ratios [7,8].
Nanoparticles can be formulated by various methods, including physicochemical methods [9]. However, the disadvantages of physicochemical methods are mainly the handling of toxic chemicals, the need for high temperatures and pressures, and the generation of harmful toxic waste products [10,11]. Due to the aforementioned drawbacks, there is a need for less harmful, alternative methods for nanoparticle production. Consequently, there is an increasing demand for nontoxic, environmentally friendly, and cost-effective methods to produce metallic nanoparticles (MNPs) for their potential pharmaceutical and biomedical applications [12,13]. Figure 1 illustrates the different synthesis methods for the production of nanoparticles.
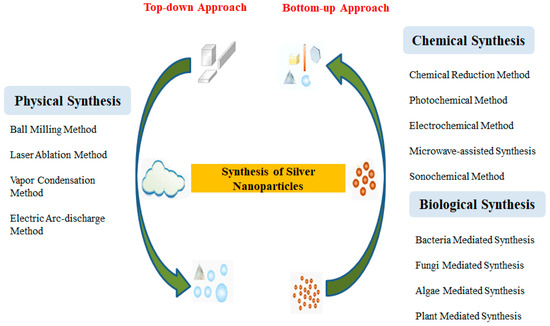
Figure 1.
Silver nanoparticle synthesis: a bottom-up and top-down approach, i.e., physical synthesis method, chemical synthesis method, and biological synthesis method, separately. The bottom-up approach refers to the growth of complex clusters and the synthesis of nanoparticles from molecular components. In contrast, the top-down approach refers to the formation of metal nanoparticles from bulk materials (adapted from [14]).
Green chemistry and green nanotechnology (also termed green synthesis modalities) utilize environmentally benign solvents and nontoxic precursor materials for the synthesis of nanomaterials. These processes are aimed at the elimination and/or reduction of toxic byproducts in reaction media that could harm our environment [14,15,16,17]. The remarkable advantage of green nanotechnology is the utilization of plant-derived phytochemical constituents, which serve as stabilizing, capping, and reducing agents for the transformation of metal ions to their metallic nanoparticle form [18].
The emergence of green synthesis of MNPs is a pioneering achievement in the field of nanobiotechnology. Therefore, the use of biological and natural resources such as plants [19,20], microorganisms [21,22,23,24,25], and algae [26,27] for the synthesis of MNPs has immense potential. Plant extracts have the added advantage of requiring less time to reduce metal ions [28]. This is because phytonanofabrication does not require the establishment of cell cultures and does not require long incubation times or high temperatures [29]. The rapid reduction of metal ions is due to the ability of plant constituents (functional groups) to donate electrons to metal ion complexes [30,31]. The synthesis of nanoparticles using green chemistry is the main reason that natural plant extracts are being considered for nanoparticle synthesis. The main advantage of green chemistry is that it allows the selection of environmentally friendly reducing agents, nontoxic materials for stabilization, and solvents [21,32]. A variety of compounds, such as amines, amides, alkaloids, flavonoids, phenols, terpenoids, proteins, and pigments, are present in plant extracts [23]. The aforementioned phytochemical constituents help in the stabilization and reduction of metal ions during the green synthesis of nanoparticles [29]. Therefore, this review aims to highlight the use of plant extracts from the genus Phyllanthus in the green synthesis of metallic nanoparticles and their potential applications in pharmaceutical and biomedical research.
2. Rationale
The utilization of green synthesis methods for the synthesis of metal nanoparticles (MNPs) has gained significant attention in recent years due to their eco-friendliness and sustainable nature [33]. Plant extracts, particularly those derived from the Phyllanthus genus, have shown great potential for the synthesis of MNPs due to their rich phytochemical composition. This rationale aims to explore the reasons behind utilizing Phyllanthus genus plant extracts for the synthesis of MNPs and their biomedical applications. The Phyllanthus genus comprises a wide variety of plant species that are abundantly available in various regions globally [34]. These plants are commonly found in tropical and subtropical zones, and their accessibility makes them an attractive choice for NPs synthesis [34]. The relative ease of acquiring Phyllanthus genus plant extracts ensures a consistent and reliable source for NP synthesis.
Phyllanthus genus plants are known to possess a diverse array of secondary metabolites, including polyphenols, flavonoids, alkaloids, terpenoids, and organic acids. These bioactive compounds have strong reducing, capping, and stabilizing potential, making them effective agents for the synthesis of MNPs. These phytochemicals exhibit strong reducing properties that facilitate the reduction of metal ions into NPs [34]. Additionally, the functional groups present in the extracts act as capping agents, imparting stability to the resulting NPs and preventing agglomeration. The synergistic effect of various phytochemicals present in Phyllanthus genus plant extracts enhances the efficiency and control over MNP synthesis. Different phytochemical constituents may contribute to distinct aspects of NP formation such as size, shape, and stability. The combined action of these compounds ensures the production of highly stable MNPs and improved performance in various applications. In contrast, the selection of Phyllanthus genus plant extracts as green agents for MNPs synthesis is well justified. Their abundance, phytochemical composition, reduction and stabilization mechanisms, synergistic effects, environmental benefits, biocompatibility, and cost-effectiveness render Phyllanthus genus plants highly suitable for the synthesis of MNPs. Exploring the vast potential of Phyllanthus genus plant extracts in nanotechnology applications contributes to sustainable and eco-friendly advancements in this field. This review highlights the benefits of Phyllanthus genus plant extracts in nanotechnology and their biodiverse applications in biomedical applications with a focus on antimicrobial and anticancer applications.
3. Review Methods
This review incorporates entries from the scientific literature. Data for this review were collected from databases such as Google Scholar, Springer, Elsevier, PubMed, ResearchGate, MDPI, and Hindawi from 2012 to 2021 for retrieving information on pertinent keywords like green synthesis, green nanotechnology, phytonanoparticles, genus Phyllanthus, and phytonanotechnology. Publications and scientific articles were selected from reputable journals and sorted out to extract scholarly information on the green synthesis of metallic nanoparticles from the genus Phyllanthus. Moreover, this review consists of the first research report on the use of the Phyllanthae genus for the synthesis of various plant-based metallic nanoparticles and their applications in a wide spectrum of industries.
4. Common Techniques and Characterization of NPs for Surface Chemistry Data Collection
In general, nanoparticles are characterized by their size, surface area, shape, and dispersity [29,35]. These properties are critical for many applications of nanoparticles. The most common techniques for characterizing nanoparticles are ultraviolet-visible spectroscopy (UV-vis), scanning electron microscopy (SEM), transmission electron microscopy (TEM), dynamic light scattering (DLS), Fourier transform infrared spectroscopy (FTIR), energy dispersive spectroscopy (EDS), and powder X-ray diffraction spectroscopy (XRD) [35,36,37].
UV-visible spectroscopy is the most commonly used technique [38]. Various metal nanoparticles in the size range of 2–100 nm are generally characterized in the wavelength range of 300–800 nm [39]. Spectrophotometric absorption bands in the wavelength ranges of 400–450 nm [40] and 500–550 nm [40] are assigned to silver and gold nanoparticles, respectively. Electron microscopy is another commonly used characterization technique [41]. Transmission electron microscopy and scanning electron microscopy are used for morphological characterization at nanometer and micrometer scales [42], but TEM has a 1000 times higher resolution than SEM [43]. Dynamic light scattering is used to characterize the size distribution and surface charge of particles suspended in a liquid solution [44].
FTIR spectroscopy is used to characterize the surface chemistry of the particles [39], detecting organic functional groups such as hydroxyl, carbonyl, etc., bound to the surface of the nanoparticles and chemical residues. The elemental composition of the metal nanoparticles is usually determined using energy-dispersive spectroscopy [45]. Similarly, phase identification of the crystal structure of the particles is performed using XRD [46]. X-rays penetrate the nanoparticles, and the resulting diffraction patterns are compared with reference standards to obtain the structural information of the nanoparticle [47].
Different scientific tools can be utilized to characterize the surface chemistry, morphology, and shape of the synthesized nanoparticles. As mentioned earlier, UV-visible spectroscopy can confirm the bioreduction of nanoparticles through the acquisition of surface plasmon resonance peaks. UV-visible spectroscopy can also provide crucial information about the width, shape, band, size, and possible aggregation state of nanoparticles [48]. Techniques such as AFM, SEM, TEM, and STM analysis can be utilized to study the NPs’ topology, morphology, size, surface roughness, shape, and texture. XRD is useful for the study of the crystal structure of the synthesized NPs [49,50]. Another useful technique is the DLS, which can be used to determine the size and aggregation nature of NPs. The functional groups and biomolecules of the stabilizing and reducing agents present on the NP’s surface can be identified with FTIR spectroscopy [51], respectively. Additionally, the purity and elemental composition of NPs can be studied with EDAX analysis [52]. Various other techniques can be used to verify the desired NPs; however, the listed tools are the basic ones and can offer useful information about the achieved synthetic nanomaterials.
5. Factors That Influence the Synthesis of Nanoparticles from Plants
There are various factors influencing the synthesis of nanoparticles from plant extracts. For example, the plant extract concentration needs to be optimized [53]. A correct quantity of plant extract enhances the size and shape of nanoparticles and increases their production [54]. Another critical factor is the reaction temperature, which directly influences the size and shape of nanoparticles. In addition, the temperature of the reaction directly affects the reaction rate, which affects nanoparticle characteristics. Consequently, one can customize the desired properties, including size, shape, growth, and particle distribution, by simply altering the temperature of the reaction [54,55,56].
In addition, the pH of the solution influences the synthesis rate, size, and shape of NPs [57,58]. The nucleation centers increase with the increase in pH, transforming the metal ions into their solid metallic state. The solution’s pH enhances the reaction rate by affecting the activity of the functional groups present in the plant extract. Singh and coworkers (2010) [59] postulated that at lower pH, gold (Au) tends to aggregate to form bigger-sized NPs; however, more carbonyl and hydroxyl groups are available for Au binding at higher pH. Furthermore, in the synthesis of silver NPs (Ag NPs) from silver nitrate, with glucose used as a reducing agent, sodium hydroxide as an accelerator, and starch as a stabilizer, different surface plasmon resonance (SPR) was observed at different pH values [59].
Another crucial factor is the reaction time. In a study by Dwivedi and Gopal (2010) [60], they reported an increase in the sharpness of UV-vis absorption bands with an increase in contact time. They observed the formation of nanoparticles within 15 min of the reaction, and the synthesis rate increased to 2 h, respectively [60]. Furthermore, Dubey and coworkers (2010) [58] observed that the formation of Ag and AuNPs started within 10 min of the reaction time. They further observed that an increase in contact time was responsible for sharpening the absorption peaks for both Ag and AuNPs [58].
6. The Role of Plants in the Synthesis of Nanoparticles
Metallic nanoparticles have a wide range of commercial applications, which are constantly expanding. Many academics now embrace the use of biological synthesis since it is secure, economical, and ecologically beneficial [61]. Plants have been extensively researched in recent years as sources for the production of metallic nanoparticles from their inorganic metal ions [54]. The phytochemicals found in plants are also essential for the reduction of metal ions. Reduced maintenance and waste disposal costs, less toxic waste production, positive impacts on treatments and the action of the extracts as both reducing and stabilizing agents are benefits of using plant extracts in the synthesis of metal NPs [62].
Plant extract is combined with a metal precursor solution under various reaction conditions to create nanoparticles [63]. The rate of nanoparticle formation, as well as the yield and stability of the nanoparticles, are administered while taking into account the reaction parameters governing the conditions of the plant extract, such as (i) the metal salt concentration, (ii) the phytochemical concentration, (iii) the solution pH and temperature, and (iv) the type of phytochemical [64]. Also, compared to bacteria and fungi, which require longer incubation times, the phytochemicals found in plant extract have a higher tendency to reduce metal ions in a shorter amount of time [65]. As a result, plant extracts are thought to be great candidates for the creation of metal and metal oxide nanoparticles [66]. Plants have various concentrations of phytochemicals, which is another crucial factor in the creation of nanoparticles [67,68]. Sugars, carboxylic acids, flavones, terpenoids, amides, ketones, and aldehydes are the primary phytochemicals found in plants that are involved in the bioreduction of nanoparticles [67].
The numerous plant metabolites stated above are crucial to the bioreduction of metal ions into nanoparticles, as was hinted at above. The primary substances capable of reducing metal ions are shown in Figure 2 below.
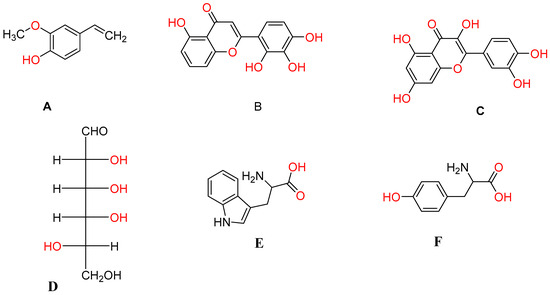
Figure 2.
Types of plant metabolites involved in the synthesis of metal nanoparticles: (A): terpenoids (eugenol); (B,C): flavonoids (luteolin, quercetin); (D): a hexose with the open-chain form; (E,F): amino acids (tryptophan (E) and tyrosine (F)) (adapted from [67]).
Alkaloids are basic nitrogenous compounds with pharmacological and definite physiological activity. Alkaloids are among the diverse phytochemicals of the Phyllanthus genus; at least 12 Phyllanthus species were found to contain about 22 different alkaloids of the securinene/norsecurinine type. These have a unique tricyclic skeleton with an α,β-unsaturated-γ-lactose ring. Securinine is arguably the first alkaloid found in many Phyllanthus species. Moreover, phyllanthine was the most common alkaloid and was isolated from three different phyllanthus species [62].
Terpenoids are a diverse class of organic polymers synthesized in plants formed from isoprene units and they display strong antioxidant activity. An initial study by Shankar et al. (2003) revealed that terpenoids play a pivotal role in transforming silver ions into nanoparticles in reactions using extracts from geranium leaves [62]. Eugenol, the main terpenoid, was found to play the principal role in the bioreduction of AgNO3 and HAuCl4 to yield nanoparticles [69]. According to Singh and coworkers (2010), the FTIR spectroscopic data suggested that dissociation of the eugenol proton (-OH) group results in the formation of a resonance structure capable of further oxidation. This process is accompanied by the active reduction of metal ions, followed by nanoparticle formation [59].
Flavonoids are polyphenolic compounds that comprise several classes such as flavanones, anthocyanins, flavonols, flavones, isoflavonoids, and chalcones, which actively reduce chelated metal ions into nanoparticles [70]. Various functional groups on flavonoids are capable of transforming metal ions into nanoparticles. The study by Ahmad and coworkers (2010) [69] postulated that the tautomeric transformations of flavonoids from the enol-form to the keto-form may release a reactive hydrogen atom capable of reducing metal ions to nanoparticles. Moreover, the conversion of ketones to carboxylic acids through the internal mechanism in flavonoids is likely involved in Au3+ ion reduction. Interestingly, some flavonoids can chelate metal ions with their π-electrons or carbonyl groups, respectively [69]. Scheme 1 shows the mechanism of the photochemical reduction of Ag ions to form nanoparticles, and Scheme 2 represents metal nanoparticle formation with plant extracts as reducing and stabilizing agents.
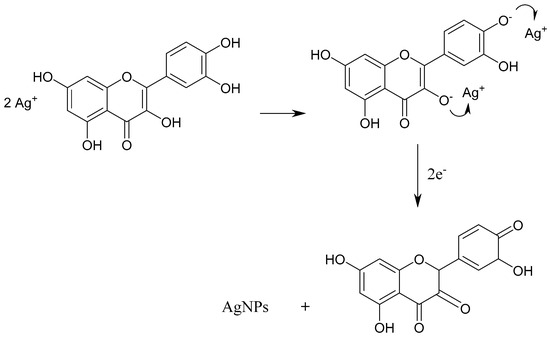
Scheme 1.
Reduction mechanisms of Ag ions to AgNPs by quercetin molecule (adapted from [71]).
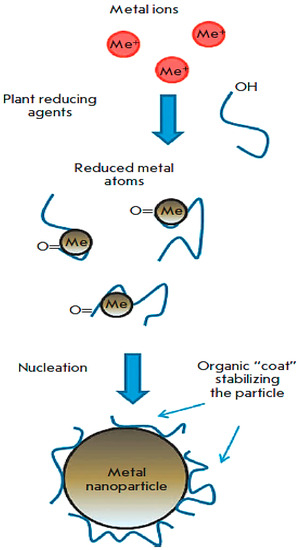
Scheme 2.
A representation of the plant-mediated synthesis of metal nanoparticles (adapted from [67]).
Scheme 2 shows metal ions binding to the stabilizing agents and reducing plant metabolites, and the ions are reduced to metal atoms. The resulting complexes interact with similar complexes, forming a small metal nanoparticle. This is followed by the growth and coalescence of separate small nanoparticles into larger ones that occur through the coarsening process. This process continues until the particles assume a stable shape and size for nanoparticles. Below is the scheme of nanoparticle formation with plant extract [72].
6.1. Green Synthesis Methods of MNPs Using Phyllanthus Plant Extracts
This section discusses the detailed synthesis of MNPs using the Phyllanthus plant extracts. Moreover, it also highlights detailed methods for obtaining reducing plant extract materials used in the synthesis from different Phyllanthus plants. As an illustration, Figure 3 demonstrates the synthesis route for MNPs from various plant extract. As observed from Figure 3, the plant materials (leaves in this case) are first chopped into smaller pieces and transferred into an extraction container. Then the plants in a solvent are heated at 60 °C for 1 h; the resulting solution is then filtered to collect the plant extracts. After filtration, the extracts are then used to synthesize Ag nanoparticles by adding silver nitrate into the plant extracts to form AgNPs. Figure 3 below illustrates the synthesis route for MNPs formation using plant extracts.
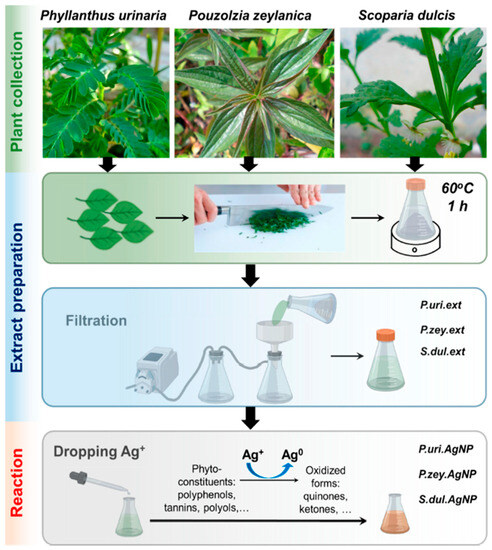
Figure 3.
Synthesis of metal nanoparticles using plant extracts (adapted from [73]).
6.1.1. Synthesis of AgNPs Using P. emblica Fruit Extracts by [74]
The study by Musam and co-workers (2019) reported the synthesis of AgNPs using P. emblica fruit extracts [74]. The fresh fruit extracts of P. emblica plants were prepared by following a procedure reported by [75]. Briefly, the fresh fruits were cleaned with sterilized double-deionized water and then chopped into small pieces, after which the seeds were removed. The sliced fruits were then finely macerated using a blender through sterile ddH2O to obtain 10% (w/v) fruit broth. The resulting extracts were then passed through a muslin cloth and then filtered using Whatman No. 1 filter paper and kept at 4 °C for later use. Furthermore, the NPs were synthesized as follows: In a 100 mL aqueous solution of AgNO3 (1 mM), various concentrations of aqueous fruit extracts (2.5, 5, 10, and 15 mL) were added and heated at 65 °C for 20 min and then kept at room temperature under dark conditions. The resulting NPs were then confirmed via color change and UV-Vis measurements.
For example, Figure 4 below demonstrates the synthesis of AgNPs from various fruit extracts. The NPs are further confirmed with various scientific techniques. As illustrated below, visual analysis was carried out using UV-vis to confirm surface plasmon of the NPs, FTIR to study the surface chemistry, FESEM to study the NP topology, TEM to confirm the NP size, Zeta potential to study the surface charge of the NPs, XRD to study the crystallinity and diffraction patterns of the NPs, AFM to study the surface roughness and topology, and lastly, EDX to confirm the elemental composition of the NPs. This is to demonstrate that after the synthesis of MNPs, different scientific techniques were employed to acquire more information about the synthesized MNPs. As a result, it can be confirmed from the acquired data in comparison to the scientific databases that the NPs formed are the desired materials under investigation. Figure 4 below is the illustration of the synthesis of MNPs and their characterization.
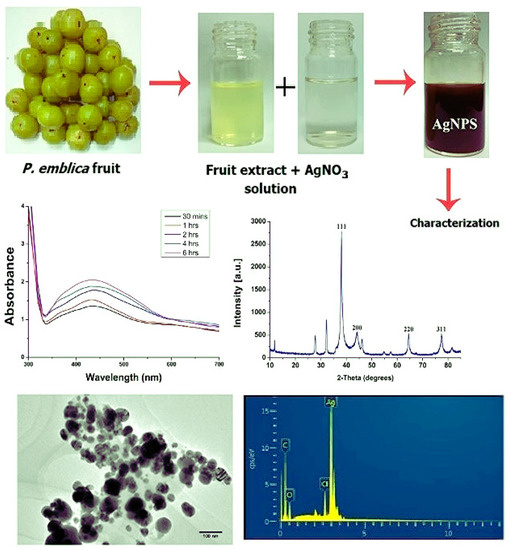
Figure 4.
The synthesis and characterization of MNPs using various aqueous fruit extracts were edited for this manuscript (adapted from [74]).
6.1.2. Synthesis of AgNPs Using P. emblica Methanol Fruit Extracts by [76]
Another study by Dhar and co-workers (2021) [76] reported the synthesis of AgNPs using P. emblica fruit extracts. The fruit extracts were prepared as follows: Fresh fruits of 80 g were cleaned carefully with DIH2O sliced into small pieces (3–5 mm in size) and dried at a specified temperature. Then, 20 g of chopped and dried fruit was placed into a 100 mL aqueous solution of 70% (v/v) methanol. The solution was then boiled at 60 °C while stirring for 30 min. The resulting solution was cooled at room temperature and filtered twice through Whatman filter paper (Qualitative, Φ18.5 cm) and stored at 4 °C for later use. NP synthesis was carried out as follows: In a reaction vial, 20 mL of fruit extracts of P. emblica were mixed with 180 mL of 1 mM solution of AgNO3. The mixture was stirred continuously at 65 °C for 1 h, after which a color change from light green to light brown was observed. The solution was then kept under incubation for 24 h and the color changed from light brown to dark brown, indicating the formation of AgNPs. The resulting NPs were confirmed with UV-Vis.
6.1.3. Synthesis of FeNPs Using P. niruri Plant Leaf Extracts by [72]
A study conducted by Kumar and Prem (2018) [72] reported the synthesis of FeNPs using the leaf extracts of P. niruni. The leaf extracts were prepared by cleaning under running water followed by DIH2O. About 25 g of leaves were boiled in 100 mL of DIH2O for 2 h. The extracts were then filtered through a Whatman No. 1 filter paper and cooled at room temperature. The resulting extract was stored for later use. Before the synthesis, a mixture of ammonium iron(III) sulfate decahydrate 9.6516 g was dissolved in 100 mL of DIH2O and 3.9213 g of ammonium iron(II) sulfate hexahydrate in 100 mL separately. Then, 5 mL of both solutions were mixed to have iron-salt mixture ratios of (1:2; 1:3; 1:5; and 2:3). The synthesis of FeNPs was performed by adding leaf extracts (1.2 mL) to 10 mL of iron salt mixtures, and the reactions were stirred for 30 min at 30 °C, after which the reddish-yellow solution turned to black-grey. The resulting FeNPs were separated with a magnet and confirmed with UV-Vis.
7. Nanoparticles Stability Test
The optimum in vivo and long-term stability of NPs is imperative for the use of NPs in biological applications. An in vitro stability study is performed on metal NPs by mixing the nanoparticles with biological media such as human serum albumin, cysteine, bovine serum albumin, and histidine in various pH solution ranges to mimic in vivo conditions [77]. The surface plasmon resonance peaks from NPs were monitored to see the stability of NPs mixed with various solutions. UV-visible was used to monitor the absorption spectra of the NPs to determine the stability of the NPs. In other studies by Kattumuri and co-workers (2007) [75], the introduction of an electrolyte method was used to study the stability of AuNPs produced using gum Arabic. Moreover, zeta-potentiometry was also used to provide the stability information of AuNPs because AuNPs with low surface charge tend to agglomerate, and over time the NPs gained a stable state.
8. Phytochemical Constituents with Metal Ion Reduction Capacity
Plant extracts, which are also known as secondary metabolites, contain a variety of phytochemicals that help to reduce and stabilize metal ions in nanoparticles (NPs), as was briefly mentioned in the section above [76,78]. Enzymes, polysaccharides, organic acids, proteins, amino acids, vitamins, etc., are only a few examples of bioactive phytochemicals. The role of a few secondary metabolites that are common in plants as reducing agents for metal ions is addressed below.
8.1. Sugars
It has been proven that plant sugar extracts can cause metal nanoparticles to become stable. The capping abilities of sugar extracts were recently shown by Fillipo and co-workers (2010) [78]; Pattnaik and co-workers (2023) [79] depending on the presence of non-soluble carbohydrates like starch. In their 2011 study, Shervani and Yamamoto [80] used the stabilizing and structure-directing agents’ soluble starch (polysaccharide) and β-α-glucose (monosaccharide) in the synthesis of spherical NPs and nanowires of gold and silver. The sugar concentration prevented the formation of NP agglomerates, which led to the discovery that the produced NPs were relatively stable. The C-6 position of the sugar was discovered to have oxidized to a carboxylic acid in contact with auric acid, producing AuNPs with glucoside substituents at the anomeric carbon center [79]. By anomerizing the released aldehyde and then oxidizing it, auric acid was reduced. Similar findings were reported by [67], who found that phenyl β-α-glucoside provided the maximum yield of mono-disperse cylindrical AuNPs in his investigation using 13C nuclear magnetic resonance (NMR) measurements.
8.2. Alkaloids
The indolizidine alkaloids, ergoline alkaloids, benzenoids, and phenolic chemicals identified in the extracts of I. pes-caprae roots demonstrated the stability and reduction capability of Ag ions to AgNPs in a study conducted by Subha and coworkers (2015) [81]. The team reported that the FTIR analysis of this extract in combination with silver salt revealed peaks at 1660, 1043, and 635 cm−1 that were attributed to the protein amide’s C=O stretching vibrations, cyclic alcohols’ C-OH stretch vibrations, and aromatic C-H vibrations that were attributed to the presence of free quinones derived from polyphenolic compounds in the extract [82]. The group called attention to the fact that only a small number of academic works discuss how alkaloids contribute to the conversion of metal ions into nanoparticles. More investigations are needed in this area in the future. Even though the scope of this research does not include noticeable reports on the synthesis of metal nanoparticles using alkaloids, other plant extracts have demonstrated the potential of alkaloids in the synthesis of MNPs. A study by Almadiy and Nenaah (2018) [83] has demonstrated the synthesis of AgNPs using potato steroidal alkaloids and their activity against phytopathogenic fungi. This goes on to illustrate the need for investigations of alkaloids of the genus Phyllanthus for the synthesis of MNPs. Furthermore, Almadiy and co-workers (2018) [83] also demonstrated the synthesis of AgNPs using harmala alkaloids. They also investigated NPs for their insecticidal activity against khapra beetles. Again, this indicates that alkaloids are capable of reducing metal ions to their NP counterparts.
8.3. Flavonoids
Many families of polyphenolic chemicals known as flavonoids avidly chelate and decrease metal ions to their NP form [72]. Due to their availability of important functional groups like carbonyl and hydroxyl moieties, metal ions can be reduced [84]. Consequently, it was demonstrated that a Rumex dentatus aqueous water extract with a high phenolic and flavonoid content allowed for the simple reduction of Ag+ ions to Ag0 [71]. According to a study by [85], the release of reactive hydrogen atoms from flavonoids during their transformation into keto-enol tautomers allows metallic ions to be reduced to their nanoparticle form. For instance, it was claimed that flavonoids and rosmarinic acid from Ocimum basilicum extract generate the keto-enol mechanism, which is essential for the development of NPs and is seen in the picture below [85]. Furthermore, the internal conversion of ketones to carboxylic acids in flavonoids is probably the cause of the reduction of Au3+ metal ions. Contrarily, flavonoids readily bind to the surfaces of developing NPs because they chelate metal ions such as Cu2+, Zn2+, Fe2+, Fe3+, Pb2+, Cr3+, and Co2+. This means that they have an impact on early nucleation, limit aggregation, and facilitate the bioreduction of metal ions [86]. As seen in Scheme 3 below, the hydrogen reacts with the Ag+ ion resulting in the keto-enol tautomerism mechanism of reaction for the reduction of Ag+ to Ag0 form. This mechanism has been shown to be the mode of AgNPs formation with phytochemicals as reducing and stabilizing agents.
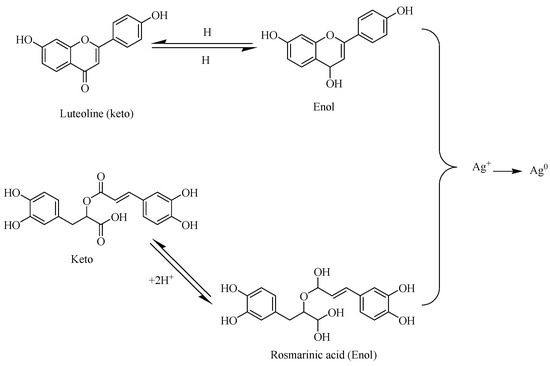
Scheme 3.
Keto-enol tautomerism and its effects on the synthesis of AgNPs (adapted from [84]).
8.4. Terpenoids
In a study by [87], they discovered that terpenoids are frequently linked to the formation of metallic NPs based on FTIR results. According to a study by Shankar and colleagues (2003) [62], the presence of terpenoids in leaves was responsible for the creation of AuNPs when geranium leaves and chloroaurate ions reacted. Also, the group came to a similar conclusion: the high content of eugenol in Cinnamomum zeylanisum (cinnamon) extracts was substantially responsible for the bioreduction of HAuCl4 and AgNO3 by the extracts. According to earlier research, deprotonation of the hydroxyl group in eugenol results in an anion that is further oxidized by metal ions, reducing the ions to metallic NPs [88]. Also, it was shown that the steroidal saponin diosgenin served as a capping and reducing agent during the creation of AgNPs; Scheme 4 below elaborates on the suggested reduction mechanism [78] where diosgenin red reacts with Ag+ ions to form the resulting AgNPs.
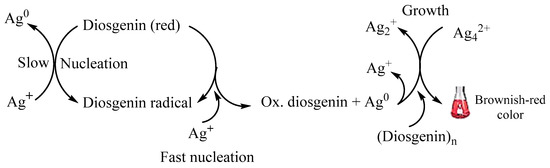
Scheme 4.
Proposed mechanism for AgNP formation by reduction with steroidal saponin diosgenin (adapted from [86]).
9. The Genus Phyllanthus and Its Phytochemical Constituents
The Phyllanthaceae, a large family of flowering plants with around 1301 species, includes the genus Phyllanthus. It is extensively dispersed in the tropical and subtropical regions of Africa, America, Asia, and Australia [89,90]. The most significant historically employed species of the Phyllanthaceae family for the treatment of various human maladies are the Phyllanthus Cicca and P. Kirganelia [36]. Phyllanthus plants contain a variety of phytochemical components that are significant in pharmacology. Among these phytochemicals are terpenoids, alkaloids, and polyphenolic substances such as phenolic acids, flavonoids, coumarins, lignins, stilbenes, and anthocyanins, among others [91].
Husnunnisa and colleagues (2022) [92] tabulated the phytochemical components of the Phyllanthus plant family, their pharmaceutical applications, and the biological activities of several Phyllanthus species [93]. The pharmacological and biological activity of chemical compounds isolated from diverse Phyllanthus plant species against common diseases were listed in a review study by Calixto and colleagues (1998) [93]. These chemical characteristics make the Phyllanthus genus a fascinating group of plants that should be researched further for possible medication development and future biological applications [91].
Indeed, many plants of Phyllanthus species have long been used as traditional medicines in the treatment of various diseases, mainly kidney, urinary bladder disturbances, intestinal infections, diabetes, and hepatitis B. The medicinal value of these plants, however, is influenced by some chemical substances that produce definite physiological actions in the human body. Given the healing properties of species in the genus, much interest has been focused on the chemical components of these plants. The preclinical and clinical studies carried out with the extracts and purified compounds from these species support most of their reported uses in folk medicine for the treatment of a wide variety of pathological conditions. The species in this genus have been reported to contain terpenes, alkaloids, lignans, flavonoids, and tannins with various kinds of bioactivities. The phytochemical constituents of the Phyllathus genus are summarized in Figure 5 and Figure 6.
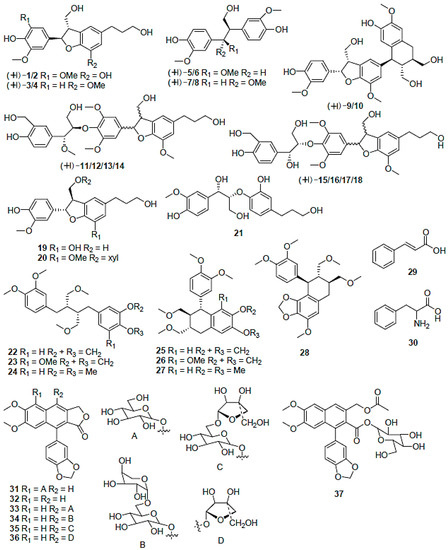
Figure 5.
Phenylpropanoids from various Phyllanthus species (adapted from [89]).
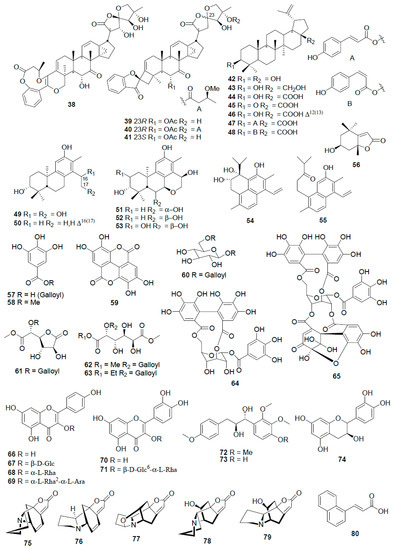
Figure 6.
Flavonoids, alkaloids, and other compounds from various Phyllanthus species (adapted from [89]).
In addition, phytochemical compounds play a vital role in MNP synthesis as reducing agents and stabilizers. Polyphenol compounds, proteins, and reducing sugars are the main phytochemical compounds that are responsible for the reduction, stability, and synthesis of MNPs [90]. Moreover, green synthesis is a method of synthesizing nanoparticles using natural materials that are environmentally friendly and safe. The use of green chemistry methods to synthesize NPs has attracted wide research interest due to its potential advantages [94], such as environmental friendliness, low energy consumption, cost-effectiveness, non-toxic, lack of pollution, and greater sustainability [94,95,96]. Lastly, NPs produced using green chemistry methods are relatively more stable and safer than those produced using traditional physicochemical methods [82,83].
10. Plant-Mediated Synthesis of Nanoparticles Using the Genus Phyllanthus
Nanoparticles in nature can be fabricated using two distinct approaches: the bottom-up method or the top-down approach (Figure 1) to obtain nanostructures with desired functionalities, shapes, and sizes [97]. The top-down approach requires diverse synthesis approaches like lithographic techniques, ball milling, sputtering, and etching to generate nanomaterials [98]. However, these techniques result in undesirable disadvantages like the production of hazardous byproducts, excessive energy use, chemicals harmful to the environment, etc. Furthermore, the bottom-up approach is the most widely used and efficient method for synthesizing nanoparticles, which involves aggressive reducing agents, volatile solvents, and capping agents. These two methods effectively produce pure and well-defined metallic nanoparticles, but their high production costs hinder their use [99].
The development of nanoparticles from plants has drawn tremendous attention and gained much importance in recent years owing to its eco-friendliness and simplicity [95]. In a review article by Gour and Jain (2019) [95], they elaborated on the synthesis of various metallic and oxide nanoparticles using different plants. Moreover, their study demonstrated various green synthetic methods of nanoparticle synthesis from enzymes, vitamins, microwave-assisted synthesis, bacteria and actinomycetes, yeast and fungi, algae, and plants, and their characterization and applications in pharmaceuticals [99]. Elsewhere, Yadi and coworkers (2018) [99] demonstrated the green synthesis of various nanoparticles, including Pd NPs, ZnO NPs, CuO NPs, CeO2 NPs, Ag NPs, and Au NPs (particle size not highlighted) from different plants [100]. Many studies have reported that plant extract-mediated metal NPs not only are biocompatible and non-toxic with normal human cells but also afford targeted drug delivery due to the localization of NPs in particular areas; they also exhibit anticancer, antimicrobial, and antiviral properties [101,102].
Table 1 below summarizes the green synthesis of Ag NPs, Au NPs, Cu NPs, Fe2O3 NPs, MgO, and Pt NPs from Phyllanthus plant extracts and their potential biological application against microorganisms. From the studies tabled below, it can be shown that phytonanoparticles hold great potential to serve as future bactericidal and fungicidal agents. However, most research by various groups did not carry out the minimum inhibitory concentration studies, which would eventually quantify their findings. Minimum inhibitory concentration studies are of paramount importance when studying drugs and/or materials to monitor their activity and also confirm microorganism resistance toward the studied materials. Therefore, the limitation of most of the researched work is that the groups performed screening (disc diffusion) studies only to report their work, which in our view does not give evidence of their activity.

Table 1.
Biosynthesis of NPs using Phyllanthus plant extracts and their antibacterial applications.
However, the reported results indicate that even at lower concentrations (50 mg/mL to 200 mg/mL), the tested phytonanoparticles could enhance microbial cell death with a zone of inhibition of more than 12 mm. These findings could have propelled the research works to determine the MIC for the studied NPs. Furthermore, it can be observed that AgNPs dominate the most in terms of activity toward antimicrobial activity, with a MIC value of 10 µg/mL against most known pathogenic microorganisms. It is also observed from the research findings that the studied NPs are effective in reducing cell mortality even at very low concentrations (between 20 to 200 mg/mL), which is an ideal consideration of interest for their biological applications in the future over their synthetic counterparts. The reason for these great findings is that NPs can penetrate microbial cells and release reactive oxygen species (ROS), which promote apoptotic cell death.
Similar observations can be made in Table 2. This is because a similar mechanism of activity takes place where NPs promote cell penetration and the release of ROS and cell damage. Additionally, AgNPs also dominate cell mortality. The NPs demonstrated strong activity against HeLa cell lines, with IC50 values of 15–50 µg/mL. Another interesting observation of these studies is that most of the reports showed that the NPs were not toxic toward normal cell lines, which is one of the attributes desired for a prominent drug and/or material. As a consequence, further studies in the field of phytonanotechnology need to be investigated to overcome the challenges reported about synthetic drugs’ counterparts. This suggests that there is great potential for natural product discovery with major efficacy on cancer cells while sparing normal cells. Therefore, more research emphases should be interrogated and significant findings will be made shortly that will improve the quality of life for many people globally. Phytonanotechnology holds all the desired solutions to human being’s known challenges today.

Table 2.
Biosynthesis of NPs using Phyllanthus plant extracts and their anticancer and antifungal applications.
11. Nanoparticle Uptake and Interaction with Cell Mechanisms
A NP’s mode of cell penetration can be through ion exchange and cellular pores in the cell membrane and is dependent on the NP’s size. The uptake of NPs by cells is not solely dependent on membrane cell receptors but can also be through Van der Waals forces, electrostatic interaction, or steric interactions [122]. The size of the NP triggers different cellular effects based on the localization of the NPs in the cell. Some small metallic NPs at high concentrations are readily endocytosed by cellular vesicles. Furthermore, macropinocytosis and phagocytosis are carried out by neutrophils and macrophages [123]. Moreover, when protein-coated NPs interact with neutrophils and macrophages at the site of inflammation, the protein corona on the NP’s surface comes into contact with the cell surface receptors first [124]. The protein corona has serum proteins that act as ligands for receptors on the M2 macrophages, which then activate the anti-inflammatory M2 macrophages. These M2 macrophages play a pivotal role in NP uptake by cells [122].
In a study conducted by Binnemars-Postma and co-workers (2016), they found that in the presence of a serum protein, M2 macrophages exhibit rapid and high NP uptake compared to M1 macrophages. A phagocytosis gene array study on M1 and M2 cells exhibited an increase in the expression levels of receptors for complement factors (FCGR2B and CD36 receptors) and immunoglobulins in M2 macrophages when compared to M1. These observations led to the conclusion that M2-induced receptors bind to the protein corona [125]. This concludes that the adsorption of serum proteins (complement factors and immunoglobulin) enhances the uptake of NPs by M2 macrophages. Furthermore, neutrophils from extracellular traps (NETs) around M2 macrophages respond to endogenous stimuli like cholesterol or uric acid and exogenous stimuli like foreign particles or pathogenic microbes. The NET formation depends on receptor-interacting protein kinase-3 (RIPK-3) enzymes as well as reactive oxygen species (ROS) radicals [125]. ROS are highly reactive and unstable as they contain unpaired electrons in their outermost shells. They are formed by lipid peroxide formation, which causes cell membrane damage [126].
12. Application of Plant-Mediated Synthesized Nanoparticles
Phytonanoparticles for Antimicrobial Activity
The green synthesis of nanoparticles using phytochemicals as mentioned before offers a wide range of advantages for their application in nanomedicine. A study conducted by Balachandar and coworkers (2019) [102] successfully demonstrated the synthesis of silver nanoparticles using stem extracts of Phyllanthus pinnatus. This group revealed that phytochemicals such as alkaloids, alcohols, saponins, terpenes, phenols, and proteins are responsible for the photosynthesis of AgNPs. The nanoparticles’ surface plasmon resonance (SPR) λmax absorption peak was observed at 490 nm. The nanoparticles’ shape and surface morphology demonstrated that the nanoparticles were cuboidal and, to a lesser extent, spherical and triangular.
Furthermore, Balachanda and coworkers tested the synthesized nanoparticles for their antimicrobial efficacy against Vibrio cholera, Shigella flexneri, Pseudomonas aeruginosa, Mycobacterium smegmatis, Proteus vulgaris, and Bacillus subtilis. They found that all bacterial pathogens exhibited dose-dependent inhibition. The highest measured zone of inhibition was at 1.8 mm at a concentration of 40 µL. The study was somewhat inconclusive in that the group did not perform further studies to measure the minimum inhibitory concentration, giving concrete evidence of their antibacterial activity. However, it was highlighted that these studies would be carried out later [104].
A study conducted by Sivakumar and coworkers (2017) [127] also demonstrated the phytosynthesis of AgNPs from a leaf extract of Phyllanthus urinaria L. They speculated that polyols such as (flavones and catechins) might be involved in the reduction of silver ions to form AgNPs. In addition to that, the group also observed the disappearance of the C-O band at 1226 cm−1 in the IR spectra ascribed to the polyols. This corroborated the findings of other research groups [62,74,76,78]. Furthermore, the synthesized nanoparticles were cuboidal and orthorhombic in shape, ranging from 15 nm to 80 nm, respectively. The antibacterial activity was tested against five bacterial pathogens, i.e., Escherichia coli, Salmonella typhi, Vibrio cholera, Pseudomonas aeruginosa, and Proteus mirabilis. The phytosynthesized AgNPs showed good bactericidal activity at all concentrations (100 to 400 µg) against all five tested microorganisms. The nanoparticles exhibited a maximum zone of inhibition of 18 mm at 400 µL concentration against V. cholera and a minimum level of bacterial activity of 8 mm at 100 µg against P. mirabilis. Further studies need to be performed to examine the efficacy of the nanoparticles by determining the MIC values to quantify the results obtained by the group [127].
Another study conducted by Acharyulu and coworkers (2014) [107] demonstrated the synthesis of CuONPs using Phyllanthus amarus leaf extract. The CuONPs showed a SPR band at 285 nm, which is ascribed to Cu NPs crystalline structures according to the (JCPDS 45-0397) database. Moreover, the NP size was calculated to be between 22 nm and 50 nm using the Sherrer formula and SEM images, respectively. The group postulated that the bactericidal properties of CuONPs are characterized by the size, stability, and concentration of the NPs added to the inoculum solution. The antibacterial properties of the NPs showed a significant inhibition effect towards both the Gram-negative (P. aeruginosa and E. coli) and Gram-positive (S. aureus and B. subtilis) bacterial strains investigated in the study. In comparing the zone of inhibition study against the positive control (the antibiotic Rifampicin), the group observed that the CuONPs demonstrated an efficacy of about 55% more than the positive control used in this study. Moreover, the MIC showed that the NP’s efficacy was 22% higher than that of Rifampicin against Gram-negative bacteria and 32% more effective against the antibiotic under study. These findings emphasize the significance of phytonanotechnology’s effectiveness against pathogenic microorganisms, which needs more exploration [107].
Recently, Dharshini and coworkers (2021) [128] demonstrated the biosynthesis of FeNPs using Phyllanthus reticulatus leaf extract. The phytosynthesized FeNPs showed SPR bands at 229 nm, ascribed to FeNPs. Moreover, the synthesized NPs showed some functional groups assigned to phytochemical constituents responsible for reducing Fe ions to FeNPs. These findings are in agreement with other results mentioned above [67,77,129,130]. The size of the FeNPs was found to be in the range of 65 to 230 nm with an irregular spherical shape. Dharshini’s group studied the antimicrobial activity of the FeNPs against pathogenic bacteria (Gram-negative: Proteus vulgaris, Vibrio chlorae, Shigellaflexneri, Salmonella typhi, Klebsiella pneumonia, and Pseudomonas aeruginosa; Gram-positive: Staphylococcus aureus, Streptococcus epidermis). The activity of the NPs was measured using the zone of inhibition for different microorganisms. The NPs exhibited a maximum of 32 mm and a minimum of 25 mm of zone inhibition against Gram-negative bacteria.
In comparison, a maximum of 17 mm and a minimum of 15 mm for Gram-positive bacteria were observed. The group further investigated the antifungal properties of the FeNPs against (Trichoderma viridae, Aspergillus niger, Aspergillus fumigatus, and Aspergillus flavus). The NPs demonstrated a maximum of 30 mm and a minimum of 18 mm zone of inhibition and about a 55% improvement compared to the crude extract alone. This significant improvement suggests that NPs enhance the antimicrobial properties of the extracts [128].
14. Other Innovative Applications of Phytonanotechnology
In recent years, nano-based products have seen a wide growth in industrial applications in our day-to-day lives. To date, there are various commercial eco-friendly nanoproducts with high efficiency on the market [131]. For example, silica NPs, silver NPs, and platinum NPs have found use in various cosmetics and personal care products. They are used as active ingredients in products such as toothpaste, mouthwash, sunscreens, hair care products, perfumes, and anti-aging products. Moreover, silica and modified silica NPs have been used as excellent pesticide controls in the agricultural industry [132].
Metallic silver is a high heat-conducting material; due to this property, nano-silver is used in diverse mechanical devices. It is mainly used in instruments such as PCR lids and UV-visible spectrophotometers. Some parts of these instruments are made from nano-silver coating material, which is highly stable at high temperatures and does not interfere with the samples [133]. In the food industry, food products are prone to contamination with microorganisms during the processing, manufacturing, and shipping of raw materials. Therefore, metallic NPs have been used in biosensors to detect pathogens and monitor different stages of contaminants at low cost [133].
In the past few decades, high growth in antimicrobial resistance to antibiotics has been witnessed, and it has resulted in high mortality rates in humans. The re-emergence of pathogenic antibiotic-resistant Gram-negative and Gram-positive bacteria became a major public health concern globally [127,134,135]. Therefore, green NPs have found great use as inhibitory activity enhancers and as antimicrobial efficient agents [133].
Furthermore, in the agriculture sector, the current major drawback of livestock stock additives and nutrient supplements is to increase the production of livestock while maintaining product quality, protecting the environment from hazardous substances, and providing food security [111]. Nanotechnology can deliver novel vehicles for nutrient supplementation while improving the functionality of feed molecules, respectively. Additionally, minerals in the form of NPs bypass the intestinal wall and cells more easily due to their size, thus enhancing their bioavailability [102]. For example, the addition of ZnNPs to livestock feed has been found to improve immunity, increase growth, and increase milk production and reproduction in cows [112]. A study by Yang and Sun [136] reported a significant drop in diarrhea incidences by supplying a graded dose of ZnONPs in the feed diet. Moreover, Fondevilain (2010) [137] reported that the addition of AgNPs in animal feed enhanced antimicrobial efficacy and selectively combated potential pathogens. Therefore, NPs are a future nutrient tool in animal feed to improve production performance, enhance bioavailability, and increase the immunity of livestock [138]. These are a few attributes that brought about NPs’ influence in various sectors to improve our livelihood.
15. Future Innovative Prospects of Phytonanotechnology
Recently, a wide range of limitations in conventional therapies, such as toxicity and high cost, have made it mandatory to develop and design novel drugs. Accordingly, the opportunity to apply various eco-friendly nanosized NPs in a greener way has opened doors for new frontiers in phytonanotechnology [120,122]. Over the past decades, advancements in nanotechnology have promoted industrial and biomedical applications of phytonanoparticles in industries including bioimaging, drug delivery and detection, etc. [121]. From various relevant studies, it is evident that bio-derived nanomaterials have been investigated in vitro, while there are limited data on their in vivo applications. Therefore, there is a great need for in vivo trial studies to be investigated to understand the in vivo toxicity mechanisms of phytonanoparticles through tests to generate data depicting nanomaterial behavior before clinical research [139,140]. Shortly, the application of plant-derived nanomaterials will see significant growth, which is expected to shed light on green nanotechnology’s long-term effects on animals, plants, humans, and the environment [141]. Some of the laboratory experiments by various research groups have shown beneficial effects of green nanomaterial applications in various sectors. However, there are limited data related to the use of the Phyllanthus genus as a capping and reducing agent for the biofabrication of phytonanoparticles. There is a lack of data in the literature regarding the use of the genus Phyllanthae as a potential plant material for the synthesis of cost-effective, nontoxic, bioavailable, and safe nanomaterials for various applications. Therefore, this review intends to shed light, bring a broad perspective, and highlight the need for further investigations in the future for the synthesis and production of various nanomaterials using the Phyllanthus genus.
16. Conclusions
This review presents information about the eco-friendly biosynthesis of metallic nanoparticles with particular stress on the understanding of underlying mechanisms for biosynthesis on NPs. It particularly emphasizes the biomolecules of plant extracts from the Phyllanthus genus that are involved in the reduction of metal ions to their NP forms and their consequent applications in various industrial sectors. Moreover, this review also highlights the significance of phytonanotechnology as a supplementary method for developing nanoparticles with bioactive properties over traditional methods. Moreover, we show the significance of the green synthesis approach as a feasible alternative method for phytonanoparticle production with enhanced activity against microorganisms and cancers alike. In addition, this review also demonstrates the potential of the genus Phyllanthus in reducing metal ions to their nanoparticle forms. The Phyllanthus family is known for its population of medicinal plants, and there are also significant findings by various groups that nanoparticles reduced with Phyllanthus lead to more great medicinal applications. Moreover, the synthesized NPs reported in this review demonstrated strong cell interaction with phytonanoparticles, leading to apoptotic cell death. However, more work needs to be performed based on phytonanotechnology using members of the Phyllanthus genus to unpack their potency as bactericidal, fungicidal, and anticancer agents.
Author Contributions
Investigation; resources, methodology, data curation, and writing-original draft preparation, M.T.; Conceptualization, methodology, funding and supervision, A.-L.E.M., N.S.M.-F. and J.V.T.; Conceptualization, reviewing and editing, project administration, and visualization, N.P.D. and D.K. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the National Research Foundation of South Africa (Grant No. 129468), the Tshwane University of Technology Research and Innovation (Grant No. 117898TTK), and the University of Pretoria for institutional and financial support (Grant No. 95674).
Data Availability Statement
Data is openly available and no new data were created for this study.
Acknowledgments
This study was supported by the Department of Chemistry, Tshwane University of Technology, and the Postdoctoral Research Fellowship program. The authors thank the University of Pretoria for the financial support for this study and give extended thanks and gratitude to NRF South Africa for funding this study.
Conflicts of Interest
Authors declare no conflict of interest.
References
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Fakruddin, M.; Hossain, Z.; Afroz, H. Prospects and applications of nanobiotechnology: A medical perspective. J. Nanobiotechnol. 2012, 10, 31. [Google Scholar] [CrossRef]
- Chahardoli, A.; Karimi, N.; Fattahi, A. Biosynthesis, Characterization, Antimicrobial and Cytotoxic Effects of Silver Nanoparticles Using Nigella arvensis Seed Extract. Iran. J. Pharm. Res. 2017, 16, 1167–1175. [Google Scholar] [PubMed]
- Barabadi, H.; Tajani, B.; Moradi, M.; Damavandi Kamali, K.; Meena, R.; Honary, S.; Mahjoub, M.A.; Saravanan, M. Penicillium Family as Emerging Nanofactory for Biosynthesis of Green Nanomaterials: A Journey into the World of Microorganisms. J. Clust. Sci. 2019, 30, 843–856. [Google Scholar] [CrossRef]
- Boomi, D.P.; Poorani, G.; Subramanian, P.; Selvam, D.S.; Ramanathan, G.; Sundaram, R.; Barabadi, H.; Halliah, G.P.; Jeyaraman, J.; Muthupandian, S. Evaluation of Antibacterial and Anticancer Potential of Polyaniline-Bimetal Nanocomposites Synthesized from Chemical Reduction Method. J. Clust. Sci. 2019, 30, 715–726. [Google Scholar] [CrossRef]
- Barabadi, H. Nanobiotechnology: A promising scope of gold biotechnology. Cell. Mol. Biol. 2017, 63, 3–4. [Google Scholar] [CrossRef]
- Salari, S.; Esmaeilzadeh Bahabadi, S.; Samzadeh-Kermani, A.; Yosefzaei, F. In-vitro Evaluation of Antioxidant and Antibacterial Potential of GreenSynthesized Silver Nanoparticles Using Prosopis farcta Fruit Extract. Iran. J. Pharm. Res. 2019, 18, 430–455. [Google Scholar]
- Sarebanhassanabadi, M. Designing a Hydrogen Peroxide Biosensor Using Catalase and Modified Electrode with Magnesium Oxide Nanoparticles. Int. J. Electrochem. Sci. 2014, 9, 257–271. [Google Scholar]
- Ramezani, T.; Nabiuni, M.; Baharara, J.; Parivar, K.; Namvar, F. Sensitization of Resistance Ovarian Cancer Cells to Cisplatin by Biogenic Synthesized Silver Nanoparticles through p53 Activation. Iran. J. Pharm. Res. 2019, 18, 222–231. [Google Scholar]
- Rajan, P.I.; Vijaya, J.J.; Jesudoss, S.K.; Kaviyarasu, K.; Kennedy, L.J.; Jothiramalingam, R.; Al-Lohedan, H.A.; Vaali-Mohammed, M.-A. Green-fuel-mediated synthesis of self-assembled NiO nano-sticks for dual applications—Photocatalytic activity on Rose Bengal dye and antimicrobial action on bacterial strains. Mater. Res. Express 2017, 4, 085030. [Google Scholar] [CrossRef]
- Arasu, M.V.; Arokiyaraj, S.; Viayaraghavan, P.; Kumar, T.S.J.; Duraipandiyan, V.; Al-Dhabi, N.A.; Kaviyarasu, K. One step green synthesis of larvicidal, and azo dye degrading antibacterial nanoparticles by response surface methodology. J. Photochem. Photobiol. B 2019, 190, 154–162. [Google Scholar] [CrossRef]
- Barabadi, H.; Ovais, M.; Shinwari, Z.K.; Saravanan, M. Anti-cancer green bionanomaterials: Present status and future prospects. Green Chem. Lett. Rev. 2017, 10, 285–314. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Raza, A.; Khan, M.A.; Ahmad, I.; Islam, N.U.; Saravanan, M.; Ubaid, M.F.; Ali, M.; Shinwari, Z.K. Green synthesis of silver nanoparticles via plant extracts: Beginning a new era in cancer theranostics. Nanomedicine 2016, 11, 3157–3177. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Seema, K. Living Nano-factories: An Eco-friendly Approach Towards Medicine and Environment. In Bio-Manufactured Nanomaterials: Perspectives and Promotion; Springer International Publishing: Chem, Switzerland, 2021; pp. 95–124. [Google Scholar]
- Tangthong, T.; Piroonpan, T.; Thipe, V.C.; Khoobchandani, M.; Katti, K.; Katti, K.V.; Pasanphan, W. Water-Soluble Chitosan Conjugated DOTA-Bombesin Peptide Capped Gold Nanoparticles as a Targeted Therapeutic Agent for Prostate Cancer. Nanotechnol. Sci. Appl. 2021, 14, 69–89. [Google Scholar] [CrossRef]
- Khoobchandani, M.; Khan, A.; Katti, K.K.; Thipe, V.C.; Al-Yasiri, A.Y.; MohanDoss, D.K.D.; Nicholl, M.B.; Lugão, A.B.; Hans, C.P.; Katti, K.V. Green nanotechnology of MGF-AuNPs for immunomodulatory intervention in prostate cancer therapy. Sci. Rep. 2021, 11, 16797. [Google Scholar] [CrossRef] [PubMed]
- Sibuyi, N.R.S.; Thipe, V.C.; Panjtan-Amiri, K.; Meyer, M.; Katti, K.V. Green synthesis of gold nanoparticles using Acai berry and Elderberry extracts and investigation of their effect on prostate and pancreatic cancer cells. Nanobiomedicine 2021, 8, 1849543521995310. [Google Scholar] [CrossRef] [PubMed]
- Eckelman, M.J.; Zimmerman, J.B.; Anastas, P.T. Toward Green Nano. J. Ind. Ecol. 2008, 12, 316–328. [Google Scholar] [CrossRef]
- Maham, M.; Karami-Osboo, R. Extraction of Sulfathiazole from Urine Samples Using Biosynthesized Magnetic Nanoparticles. Iran. J. Pharm. Res. 2017, 16, 462–470. [Google Scholar]
- Kasithevar, M.; Muthupandian, S.; Periakaruppan, P.; Kumar, H.; Ovais, M.; Barabadi, H.; Shinwari, Z. Green synthesis of silver nanoparticles using Alysicarpus monilifer leaf extract and its antibacterial activity against MRSA and CoNS isolates in HIV patients. J. Interdiscip. Nanomed. 2017, 2, 131–141. [Google Scholar] [CrossRef]
- Rezvani Amin, Z.; Khashyarmanesh, Z.; Fazly Bazzaz, B.S.; Sabeti Noghabi, Z. Does Biosynthetic Silver Nanoparticles Are More Stable with Lower Toxicity than Their Synthetic Counterparts? Iran. J. Pharm. Res. 2019, 18, 210–221. [Google Scholar] [PubMed]
- Saravanan, M.; Barik, S.K.; MubarakAli, D.; Prakash, P.; Pugazhendhi, A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb. Pathog. 2018, 116, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, M.; Kannabiran, K. Actinomycetes-mediated biogenic synthesis of metal and metal oxide nanoparticles: Progress and challenges. Lett. Appl. Microbiol. 2017, 64, 401–408. [Google Scholar] [CrossRef]
- Niknejad, F.; Nabili, M.; Daie Ghazvini, R.; Moazeni, M. Green synthesis of silver nanoparticles: Advantages of the yeast Saccharomyces cerevisiae model. Curr. Med. Mycol. 2015, 1, 17–24. [Google Scholar] [CrossRef]
- Barabadi, H.; Honary, S. Biofabrication of gold and silver nanoparticles for pharmaceutical applications. Pharm. Biomed. Res. 2016, 2, 1. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Mechouet, M.; Wijesekera, K.; Filipe, C.D.M.; Sicard, C.; Bazylinski, D.A.; Jeffryes, C. Algae-mediated biosynthesis of inorganic nanomaterials as a promising route in nanobiotechnology—A review. Green Chem. 2017, 19, 552–587. [Google Scholar] [CrossRef]
- Arya, A.; Gupta, K.; Chundawat, T.S.; Vaya, D. Biogenic Synthesis of Copper and Silver Nanoparticles Using Green Alga Botryococcus braunii and Its Antimicrobial Activity. Bioinorg. Chem. Appl. 2018, 2018, 7879403. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Kuča, K.; Kalia, A.; Nepovimova, E.; Verma, R.; Kumar, D. Flower-Based Green Synthesis of Metallic Nanoparticles: Applications beyond Fragrance. Nanomaterials 2020, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Soni, V.; Raizada, P.; Singh, P.; Cuong, H.N.; S, R.; Saini, A.; Saini, R.V.; Le, Q.V.; Nadda, A.K.; Le, T.T.; et al. Sustainable and green trends in using plant extracts for the synthesis of biogenic metal nanoparticles toward environmental and pharmaceutical advances: A review. Environ. Res. 2021, 202, 111622. [Google Scholar] [CrossRef] [PubMed]
- El-Kassas, H.Y.; Ghobrial, M.G. Biosynthesis of metal nanoparticles using three marine plant species: Anti-algal efficiencies against “Oscillatoria simplicissima”. Environ. Sci. Pollut. Res. Int. 2017, 24, 7837–7849. [Google Scholar] [CrossRef]
- Palomo, J.M.; Filice, M. Biosynthesis of Metal Nanoparticles: Novel Efficient Heterogeneous Nanocatalysts. Nanomaterials 2016, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Haque, T.; Muhsin, M.; Akhter, T.; Haq, M.; Begum, R.; Chowdhury, S. Antimicrobial and analgesic activity of leaf extracts of Phyllanthus reticulatus Poir. (Family-Euphorbiaceae). Jahangirnagar Univ. J. Biol. Sci. 2016, 5, 81. [Google Scholar] [CrossRef]
- Mao, X.; Wu, L.-F.; Guo, H.-L.; Chen, W.-J.; Cui, Y.-P.; Qi, Q.; Li, S.; Liang, W.-Y.; Yang, G.-H.; Shao, Y.-Y.; et al. The Genus Phyllanthus: An Ethnopharmacological, Phytochemical, and Pharmacological Review. Evid.-Based Complement. Altern. Med. 2016, 2016, 7584952. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Oberdörster, G.; Biswas, P.J. Characterization of Size, Surface Charge, and Agglomeration State of Nanoparticle Dispersions for Toxicological Studies. J. Nanoparticle Res. 2008, 11, 77–89. [Google Scholar] [CrossRef]
- Sepeur, S. Nanotechnology: Technical Basics and Applications; Vincentz Network GmbH & Co KG: Hannover Deutschland, Germany, 2008. [Google Scholar]
- Fedlheim, D.L.; Foss, C.A. Metal Nanoparticles: Synthesis, Characterization, and Applications; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Shahverdi, A.R.; Shakibaie, M.; Nazari, P. Basic and Practical Procedures for Microbial Synthesis of Nanoparticles. In Metal Nanoparticles in Microbiology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 177–195. [Google Scholar]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yang, X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: A green method. Carbohydr. Res. 2004, 339, 2627–2631. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef]
- Cao, G. Nanostructures & Nanomaterials: Synthesis, Properties & Applications; Imperial College Press: London, UK, 2004. [Google Scholar]
- Schaffer, B.; Hohenester, U.; Trügler, A.; Hofer, F. High-resolution surface plasmon imaging of gold nanoparticles by energy-filtered transmission electron microscopy. Phys. Rev. B 2009, 79, 041401. [Google Scholar] [CrossRef]
- Eppler, A.; Rupprechter, G.; Anderson, E.; Somorjai, G. Thermal and Chemical Stability and Adhesion Strength of Pt Nanoparticle Arrays Supported on Silica Studied by Transmission Electron Microscopy and Atomic Force Microscopy. J. Phys. Chem. B 2000, 104, 7286–7292. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef]
- Sun, S.; Murray, C.B.; Weller, D.; Folks, L.; Moser, A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 2000, 287, 1989–1992. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Santhoshkumar, J.; Rajeshkumar, S.; Venkat Kumar, S. Phyto-assisted synthesis, characterization and applications of gold nanoparticles—A review. Biochem. Biophys. Rep. 2017, 11, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Jain, P.; Chandrashekhar Hariharapura, R.; Narayanan, K.; Bhat, K.U.; Udupa, N.; Rao, J.V. Biosynthesis of copper nanoparticles using copper-resistant Bacillus cereus, a soil isolate. Process Biochem. 2016, 51, 1348–1356. [Google Scholar] [CrossRef]
- Agarwal, H.; Kumar, V.; Shanmugam, R. Antidiabetic effect of silver nanoparticles synthesized using lemongrass (Cymbopogon citratus) through conventional heating and microwave irradiation approach. J. Microbiol. Biotechnol. Food Sci. 2018, 7, 371–376. [Google Scholar] [CrossRef]
- Lv, Q.; Zhang, B.; Xing, X.; Zhao, Y.; Cai, R.; Wang, W.; Gu, Q. Biosynthesis of copper nanoparticles using Shewanella loihica PV-4 with antibacterial activity: Novel approach and mechanisms investigation. J. Hazard. Mater. 2018, 347, 141–149. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Folorunso, A.S.; Folorunso, F.A.; Oyebamiji, A.K. Green synthesis of copper oxide nanoparticles for biomedical application and environmental remediation. Heliyon 2020, 6, e04508. [Google Scholar] [CrossRef]
- Maniam, G.P.; Govindan, N.; Rahim, M.H.; Yusoff, M. Plant extracts: Nanoparticle sources. In Phytonanotechnology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 41–49. [Google Scholar]
- Jiang, X.C.; Chen, W.M.; Chen, C.Y.; Xiong, S.X.; Yu, A.B. Role of Temperature in the Growth of Silver Nanoparticles Through a Synergetic Reduction Approach. Nanoscale Res. Lett. 2011, 6, 32. [Google Scholar] [CrossRef]
- Gericke, M.; Pinches, A. Biological synthesis of metal nanoparticles. Hydrometallurgy 2006, 83, 132–140. [Google Scholar] [CrossRef]
- Gamez, G.; Gardea-Torresdey, J.; Tiemann, K.J.; Parsons, J.; Dokken, K.; Yacaman, M. Recovery of gold(III) from multi-elemental solutions by alfafa biomass. Adv. Environ. Res. 2003, 7, 563–571. [Google Scholar] [CrossRef]
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010, 45, 1065–1071. [Google Scholar] [CrossRef]
- Singh, A.; Talat, M.; Singh, D.; Srivastava, O.N. Biosynthesis of gold and silver nanoparticles by natural precursor clove and their functionalization with amine group. J. Nanoparticle Res. 2010, 12, 1667–1675. [Google Scholar] [CrossRef]
- Dwivedi, A.D.; Gopal, K. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf. A Physicochem. Eng. Asp. 2010, 369, 27–33. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K.; Kumar, V.; Prasad, K. Biosynthesis of silver nanoparticles using Eclipta leaf. Biotechnol. Prog. 2009, 25, 1476–1479. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Pasricha, R.; Sastry, M. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J. Mater. Chem. 2003, 13, 1822–1826. [Google Scholar] [CrossRef]
- Malik, P.; Shankar, R.; Malik, V.; Sharma, N.; Mukherjee, T.K. Green Chemistry Based Benign Routes for Nanoparticle Synthesis. J. Nanoparticles 2014, 2014, 302429. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Chen, Z.-S.; Chen, G. Biosynthesis of Nanoparticles by Microorganisms and Their Applications. J. Nanomater. 2011, 2011, 270974. [Google Scholar] [CrossRef]
- S, M.; Seetharaman, B. Cashew Apple Juice (Anacardium occidentale L.) Speeds Up the Synthesis of Silver Nanoparticles. Int. J. Green Nanotechnol. 2012, 4, 71–79. [Google Scholar] [CrossRef]
- Tc, P.; Mathew, L.; Chandrasekaran, N.; Raichur, A.; Mukherjee, A. Biomimetic synthesis of nanoparticles: Science, technology & applicability. In Biomimetics Learning from Nature; Intechopen: London, UK, 2010; pp. 1–20. [Google Scholar]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Naturae 2014, 6, 35–44. [Google Scholar] [CrossRef]
- Nahar, D.L.; Sarker, S.; Delazar, A.; Nahar, L.; Sarker, S.D.; Delazar, A. Phytochemistry of the genus Phyllanthus. In Phyllanthus Species: Scientific Evaluation and Medicinal Applications; Harikumar, K.B., Kuttan, R., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 72–91. [Google Scholar]
- Ahmad, N.; Sharma, S.; Alam, M.K.; Singh, V.N.; Shamsi, S.F.; Mehta, B.R.; Fatma, A. Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloids Surf. B Biointerfaces 2010, 81, 81–86. [Google Scholar] [CrossRef]
- Ramesh, P.S.; Kokila, T.; Geetha, D. Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Emblica officinalis fruit extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 142, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Mehata, M.S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and their Enhanced Antibacterial Property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef] [PubMed]
- V G, V.; Prem, A. Green Synthesis and Characterization of Iron Oxide Nanoparticles Using Phyllanthus Niruri Extract. Orient. J. Chem. 2018, 34, 2583–2589. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Lee, J.S.; Park, K.D.; Ching, Y.C.; Nguyen, X.T.; Phan, V.H.G.; Hoang Thi, T.T. Green Silver Nanoparticles Formed by Phyllanthus urinaria, Pouzolzia zeylanica, and Scoparia dulcis Leaf Extracts and the Antifungal Activity. Nanomaterials 2020, 10, 542. [Google Scholar] [CrossRef] [PubMed]
- Masum, M.M.I.; Siddiqa, M.M.; Ali, K.A.; Zhang, Y.; Abdallah, Y.; Ibrahim, E.; Qiu, W.; Yan, C.; Li, B. Biogenic Synthesis of Silver Nanoparticles Using Phyllanthus emblica Fruit Extract and Its Inhibitory Action Against the Pathogen Acidovorax oryzae Strain RS-2 of Rice Bacterial Brown Stripe. Front. Microbiol. 2019, 10, 820. [Google Scholar] [CrossRef]
- Kattumuri, V.; Katti, K.; Bhaskaran, S.; Boote, E.J.; Casteel, S.W.; Fent, G.M.; Robertson, D.J.; Chandrasekhar, M.; Kannan, R.; Katti, K.V. Gum arabic as a phytochemical construct for the stabilization of gold nanoparticles: In vivo pharmacokinetics and X-ray-contrast-imaging studies. Small 2007, 3, 333–341. [Google Scholar] [CrossRef]
- Dhar, S.; Chowdhury, R.; Das, S.; Nahian, M.K.; Islam, D.; Gafur, M. Plant-mediated green synthesis and characterization of silver nanoparticles using Phyllanthus emblica fruit extract. Mater. Today Proc. 2021, 42, 1867–1871. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Muthukumaran, U.; Hoti, S.L.; Khater, H.F.; Benelli, G. Single-step biosynthesis and characterization of silver nanoparticles using Zornia diphylla leaves: A potent eco-friendly tool against malaria and arbovirus vectors. J. Photochem. Photobiol. B 2016, 161, 482–489. [Google Scholar] [CrossRef]
- Filippo, E.; Serra, A.; Buccolieri, A.; Manno, D. Green synthesis of silver nanoparticles with sucrose and maltose: Morphological and structural characterization. J. Non-Cryst. Solids 2010, 356, 344–350. [Google Scholar] [CrossRef]
- Pattnaik, C.; Mishra, R.; Sahu, A.K.; Sahoo, L.N.; Sahoo, N.K.; Tripathy, S.K.; Sahoo, S. Green synthesis of glucose-capped stable silver nanoparticles: A cost-effective sensor for the selective detection of Hg2+ ions in aqueous solutions. Sens. Diagn. 2023, 2, 647–656. [Google Scholar] [CrossRef]
- Shervani, Z.; Yamamoto, Y. Carbohydrate-directed synthesis of silver and gold nanoparticles: Effect of the structure of carbohydrates and reducing agents on the size and morphology of the composites. Carbohydr. Res. 2011, 346, 651–658. [Google Scholar] [CrossRef]
- Subha, V.; Ramaswami Sachidanandan, E.R.; Sruthi, P. An eco-friendly approach for synthesis of silver nanoparticles using Ipomoea pes-caprae root extract and their antimicrobial properties. Asian J. Pharm. Clin. Res. 2015, 8, 104–107. [Google Scholar]
- Almadiy, A.; Nenaah, G.E. Ecofriendly Synthesis of Silver Nanoparticles Using Potato Steroidal Alkaloids and Their Activity Against Phytopathogenic Fungi. Braz. Arch. Biol. Technol. 2018, 61, e18180013. [Google Scholar] [CrossRef]
- Almadiy, A.; Nenaah, G.E.; Shawer, D. Facile synthesis of silver nanoparticles using harmala alkaloids and their insecticidal and growth inhibitory activities against the khapra beetle. J. Pest Sci. 2018, 91, 727–737. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.M.; Saeed, A.; Shah, A.; Shah, R.; Iftikhar, F.J.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H.; et al. Metal nanoparticles fabricated by green chemistry using natural extracts: Biosynthesis, mechanisms, and applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef] [PubMed]
- El Shahaby, O. Evaluation of Antimicrobial Activity of Water Infusion Plant-Mediated Silver Nanoparticles. J. Nanomed. Nanotechnol. 2013, 4, 2. [Google Scholar] [CrossRef]
- Hussain, S.; Bashir, O.; Khan, Z.; Al-thabaiti, S. Steroidal saponin based extracellular biosynthesis of AgNPs. J. Mol. Liq. 2014, 199, 489–494. [Google Scholar] [CrossRef]
- Annamalai, J.; Nallamuthu, T. Green synthesis of silver nanoparticles: Characterization and determination of antibacterial potency. Appl. Nanosci. 2016, 6, 259–265. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnology 2018, 16, 84. [Google Scholar] [CrossRef]
- Nisar, M.F.; He, J.; Ahmed, A.; Yang, Y.; Li, M.; Wan, C. Chemical Components and Biological Activities of the Genus Phyllanthus: A Review of the Recent Literature. Molecules 2018, 23, 2567. [Google Scholar] [CrossRef]
- GBIF Backbone Taxonomy; Phyllanthus L. in GBIF Secretariat. Checklist Dataset; GBIF Secretariat: Copenhagen, Denmark, 2021.
- Kiran, K.R.; Swathy, P.S.; Paul, B.; Shama Prasada, K.; Radhakrishna Rao, M.; Joshi, M.B.; Rai, P.S.; Satyamoorthy, K.; Muthusamy, A. Untargeted metabolomics and DNA barcoding for discrimination of Phyllanthus species. J. Ethnopharmacol. 2021, 273, 113928. [Google Scholar] [CrossRef] [PubMed]
- Husnunnisa, H.; Hartati, R.; Mauludin, R.; Insanu, M. A review of the Phyllanthus genus plants: Their phytochemistry, traditional uses, and potential inhibition of xanthine oxidase. Pharmacia 2022, 69, 681–687. [Google Scholar] [CrossRef]
- Calixto, J.B.; Santos, A.R.; Cechinel Filho, V.; Yunes, R.A. A review of the plants of the genus Phyllanthus: Their chemistry, pharmacology, and therapeutic potential. Med. Res. Re.v 1998, 18, 225–258. [Google Scholar] [CrossRef]
- Bhardwaj, B.; Singh, P.; Kumar, A.; Kumar, S.; Budhwar, V. Eco-Friendly Greener Synthesis of Nanoparticles. Adv. Pharm. Bull. 2020, 10, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Qi, L. Recent progress in plant-gold nanoparticles fabrication methods and bio-applications. Talanta 2021, 223, 121396. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, R.; Beulaja, M.; Thiagarajan, R.; Palanisamy, S.; Goutham, G.; Koodalingam, A.; Prabhu, N.M.; Kannapiran, E.; Basu, M.J.; Arulvasu, C.; et al. Biosynthesis of silver nanoparticles using aqueous extract of Phyllanthus acidus L. fruits and characterization of its anti-inflammatory effect against H2O2 exposed rat peritoneal macrophages. Process Biochem. 2017, 55, 172–181. [Google Scholar] [CrossRef]
- Rahimzadeh, C.Y.; Barzinjy, A.A.; Mohammed, A.S.; Hamad, S.M. Green synthesis of SiO2 nanoparticles from Rhus coriaria L. extract: Comparison with chemically synthesized SiO2 nanoparticles. PLoS ONE 2022, 17, e0268184. [Google Scholar] [CrossRef]
- Yadi, M.; Mostafavi, E.; Saleh, B.; Davaran, S.; Aliyeva, I.; Khalilov, R.; Nikzamir, M.; Nikzamir, N.; Akbarzadeh, A.; Panahi, Y.; et al. Current developments in green synthesis of metallic nanoparticles using plant extracts: A review. Artif. Cells Nanomed. Biotechnol. 2018, 46, S336–S343. [Google Scholar] [CrossRef]
- Muñoz, L.E.; Bilyy, R.; Biermann, M.H.C.; Kienhöfer, D.; Maueröder, C.; Hahn, J.; Brauner, J.M.; Weidner, D.; Chen, J.; Scharin-Mehlmann, M.; et al. Nanoparticles size-dependently initiate self-limiting NETosis-driven inflammation. Proc. Natl. Acad. Sci. USA 2016, 113, E5856–E5865. [Google Scholar] [CrossRef]
- Arunachalam, A.; Christinaa, V.L.P.; Christinaa, V.; Ptv, L. Green synthesis and characterisation of Ag NPs using aqueous extract of Phyllanthus maderaspatensis L. J. Exp. Nanosci. 2014, 9, 113–119. [Google Scholar] [CrossRef]
- Balachandar, R.; Gurumoorthy, P.; Karmegam, N.; Barabadi, H.; Subbaiya, R.; Anand, K.; Boomi, P.; Saravanan, M. Plant-Mediated Synthesis, Characterization and Bactericidal Potential of Emerging Silver Nanoparticles Using Stem Extract of Phyllanthus pinnatus: A Recent Advance in Phytonanotechnology. J. Clust. Sci. 2019, 30, 1481–1488. [Google Scholar] [CrossRef]
- Sundarrajan, M.; Jeelani, A.; Santhanam, V.; Durgadevi, S.; Abirami, S. Effect of Concentration, pH and Time on the Morphology of Silver Nanoparticles Synthesized by Green Method using Phyllanthus Niruri and Solanum Nigrum Leaf Extracts. Int. J. Curr. Res. Rev. 2018, 10, 25–29. [Google Scholar] [CrossRef]
- Chellappa, J.; Badhusha, S. Green synthesis of ZnO nanoparticles using Phyllanthus embilica stem extract and their antibacterial activity. Der Pharm. Lett. 2016, 8, 218–223. [Google Scholar]
- Singh, K.; Panghal, M.; Kadyan, S.; Chaudhary, U.; Yadav, J.P. Green silver nanoparticles of Phyllanthus amarus: As an antibacterial agent against multi drug resistant clinical isolates of Pseudomonas aeruginosa. J. Nanobiotechnology 2014, 12, 40. [Google Scholar] [CrossRef]
- Kollur, S.P.; Patra, A. Green synthesis of MnO2 nanorods using Phyllanthus amarus plant extract and their fluorescence studies. Green Process. Synth. 2017, 6, 549–554. [Google Scholar] [CrossRef]
- Acharyulu, N.; Dubey, D.R.S.; Kollu, P.; Swaminadham, V.; Kalyani, R.; Pammi, S.V.N. Green Synthesis of CuO Nanoparticles using Phyllanthus Amarus Leaf Extract and their Antibacterial Activity Against Multidrug Resistance Bacteria Green synthesis of CuO nanoparticles. Int. J. Eng. Res. Technol. 2014, 3, 639. [Google Scholar]
- Ramasamy, S.; Lavanya, R.; Selvapriya, K.; Selvam, M. Green Synthesis of Silver Nanoparticles from Phyllanthus amarus and their Antibacterial and Antioxidant Properties. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 600–606. [Google Scholar]
- Sowmya, C.; Lavakumar, V.; Venkateshan, N.; Ravichandiran, V.; Saigopal, D.V.R. Exploration of Phyllanthus acidus mediated silver nanoparticles and its activity against infectious bacterial pathogen. Chem. Cent. J. 2018, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Renuka, R.; Devi, K.; Sivakami, M.; Thilagavathi, T.; Uthrakumar, R. Biosynthesis of silver nanoparticles using phyllanthus emblica fruit extract for antimicrobial application. Biocatal. Agric. Biotechnol. 2020, 24, 101567. [Google Scholar] [CrossRef]
- Kaur, T.; Putatunda, C.; Vyas, A.; Kumar, G. Zinc oxide nanoparticles inhibit bacterial biofilm formation via altering cell membrane permeability. Prep. Biochem. Biotechnol. 2021, 51, 309–319. [Google Scholar] [CrossRef]
- Ramanujam, K.; Sundrarajan, M. Antibacterial effects of biosynthesized MgO nanoparticles using ethanolic fruit extract of Emblica officinalis. J. Photochem. Photobiol. B 2014, 141, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Vasantharaj, S.; Sripriya, N.; Shanmugavel, M.; Manikandan, E.; Gnanamani, A.; Senthilkumar, P. Surface active gold nanoparticles biosynthesis by new approach for bionanocatalytic activity. J. Photochem. Photobiol. B Biol. 2018, 179, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, M.; Roopan, S.M.; Selvaraj, C.I.; Arunachalam, P. Phyllanthus emblica seed extract mediated synthesis of PdNPs against antibacterial, heamolytic and cytotoxic studies. J. Photochem. Photobiol. B 2017, 167, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Ananda, A.; Ramakrishnappa, T.; Archana, S.; Reddy Yadav, L.S.; Shilpa, B.M.; Nagaraju, G.; Jayanna, B.K. Green synthesis of MgO nanoparticles using Phyllanthus emblica for Evans blue degradation and antibacterial activity. Mater. Today: Proc. 2022, 49, 801–810. [Google Scholar] [CrossRef]
- Pathak, M.C. Cytotoxic action of silver nanoparticles synthesized from Phyllanthus fraternus on hepatic and breast cancer cell lines: A green approach. Int. J. Green Pharm. 2019, 13, 229–235. [Google Scholar]
- Thoidingjam, S.; Tiku, A.B. Therapeutic efficacy of Phyllanthus emblica-coated iron oxide nanoparticles in A549 lung cancer cell line. Nanomedicine 2019, 14, 2355–2371. [Google Scholar] [CrossRef]
- Karuppannan, K.; Nagaraj, E.; Sujatha, V. Green Synthesis and Biological Applications of Silver Nanoparticles Using Phyllanthus maderaspatensis L. Root Extract. Smart Sci. 2016, 4, 180–189. [Google Scholar] [CrossRef]
- Wang, R.; Xu, X.; Puja, A.M.; Perumalsamy, H.; Balusamy, S.R.; Kim, H.; Kim, Y.J. Gold Nanoparticles Prepared with Phyllanthus emblica Fruit Extract and Bifidobacterium animalis subsp. lactis Can Induce Apoptosis via Mitochondrial Impairment with Inhibition of Autophagy in the Human Gastric Carcinoma Cell Line AGS. Nanomater. 2021, 11, 1260. [Google Scholar] [CrossRef]
- Thulasidevi, R.; Shah, G.; Snima, K.S.; Kamath, C.R.; Nair, S.; Lakshmanan, V.-K. Enhanced Delivery of Phyllanthus Niruri Nanoparticles for Prostate Cancer Therapy. J. Bionanosciences 2014, 8, 101–107. [Google Scholar] [CrossRef]
- Maheswari, P.; Harish, S.; Ponnusamy, S.; Muthamizhchelvan, C. A novel strategy of nanosized herbal Plectranthus amboinicus, Phyllanthus niruri and Euphorbia hirta treated TiO(2) nanoparticles for antibacterial and anticancer activities. Bioprocess Biosyst. Eng. 2021, 44, 1593–1616. [Google Scholar] [CrossRef] [PubMed]
- Simko, M.; Fiedeler, U.; Gazsó, A.; Nentwich, M. The impact of nanoparticles on cellular functions. Nanotrust Doss. 2011, 7, 1–4. [Google Scholar]
- Kuhn, D.A.; Vanhecke, D.; Michen, B.; Blank, F.; Gehr, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J. Nanotechnol. 2014, 5, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Binnemars-Postma, K.A.; Ten Hoopen, H.W.; Storm, G.; Prakash, J. Differential uptake of nanoparticles by human M1 and M2 polarized macrophages: Protein corona as a critical determinant. Nanomedicine 2016, 11, 2889–2902. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 2009, 84, 151–157. [Google Scholar] [CrossRef]
- Sivakumar, S.; Tamizhazhagan, A.; Abdhul, K. Synthesis, Characterization and Anti-Bacterial Activity of Silver Nanoparticles from Leaf Extract of Phyllanthus urinaria L. Eur. J. Biomed. Pharm. Sci. 2017, 4, 544–553. [Google Scholar]
- Dharshini, R.S.; Poonkothai, M.; Srinivasan, P.; Mythili, R.; Syed, A.; Elgorban, A.M.; Selvankumar, T.; Kim, W. Nano-decolorization of methylene blue by Phyllanthus reticulatus iron nanoparticles: An eco-friendly synthesis and its antimicrobial, phytotoxicity study. Appl. Nanosci. 2023, 13, 2527–2537. [Google Scholar] [CrossRef]
- Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary Metabolites in the Green Synthesis of Metallic Nanoparticles. Materials 2018, 11, 940. [Google Scholar] [CrossRef]
- Kavitha, S.; Dhamodaran, M.; Prasad, R.; Ganesan, M. Synthesis and characterisation of zinc oxide nanoparticles using terpenoid fractions of Andrographis paniculata leaves. Int. Nano Lett. 2017, 7, 141–147. [Google Scholar] [CrossRef]
- Schröfel, A.; Kratošová, G.; Šafařík, I.; Šafaříková, M.; Raška, I.; Shor, L.M. Applications of biosynthesized metallic nanoparticles—A review. Acta Biomater. 2014, 10, 4023–4042. [Google Scholar] [CrossRef]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, T.M.; Al-Rajhi, A.M.H.; Al Abboud, M.A.; Alawlaqi, M.M.; Ganash Magdah, A.; Helmy, E.A.M.; Mabrouk, A.S. Recent Advances in Green Synthesis of Silver Nanoparticles and Their Applications: About Future Directions. A Review. BioNanoScience 2018, 8, 5–16. [Google Scholar] [CrossRef]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Singh, K. Evaluation of Antimicrobial Activity of Synthesized Silver Nanoparticles using Phyllanthus amarus and Tinospora cordifolia Medicinal Plants. J. Nanomed. Nanotechnol. 2014, 5, 1–5. [Google Scholar] [CrossRef]
- Yang, Z.P.; Sun, L.P. Effects of nanometer ZnO on growth performance of early weaned piglets. J. Shanxi Agric. Sci. 2006, 3, 24. [Google Scholar]
- Fondevilain, M. Silver Nanoparticles. Peres, D.P., Ed.; InTech: Rijeka, Croatia, 2010; pp. 325–334. [Google Scholar]
- Kamalzadeh, A.; Rajabbaigy, M.; Kiasat, A. Livestock production systems and trends in livestock Industry in Iran. J. Agric. Soc. Sci. 2008, 4, 1813–223504. [Google Scholar]
- Maru, G. Isolation and analyses of polymeric polyphenol fractions from Black tea. Food Chem. 2006, 94, 331–340. [Google Scholar] [CrossRef]
- Zuhrotun, A.; Oktaviani, D.J.; Hasanah, A.N. Biosynthesis of Gold and Silver Nanoparticles Using Phytochemical Compounds. Molecules 2023, 28, 3240. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Rafa, N.; Chowdhury, A.T.; Chowdhury, S.; Nahrin, M.; Islam, A.; Ong, H.C. Green approaches in synthesising nanomaterials for environmental nanobioremediation: Technological advancements, applications, benefits and challenges. Environ. Res. 2022, 204, 111967. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).