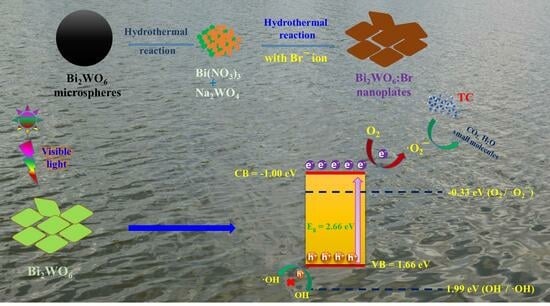
Bromine Ion-Intercalated Layered Bi2WO6 as an Efficient Catalyst for Advanced Oxidation Processes in Tetracycline Pollutant Degradation Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
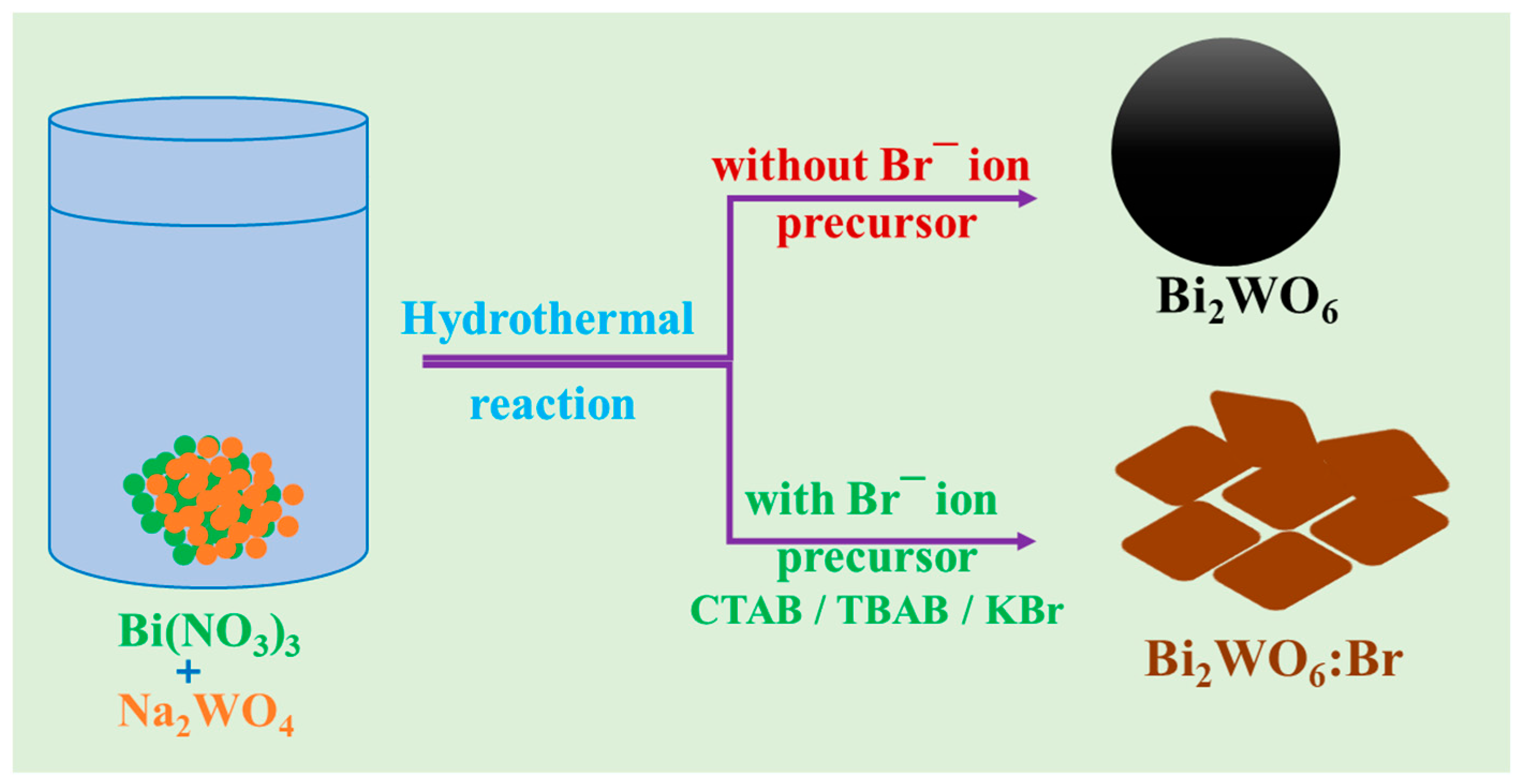
2.2. Synthesis of Various Bi2WO6 Nanostructures (BW NSs)
2.3. Physical Characterization Techniques
2.4. Photocatalytic Activity Experiments
3. Results and Discussion
3.1. Synthesis and Morphological Studies
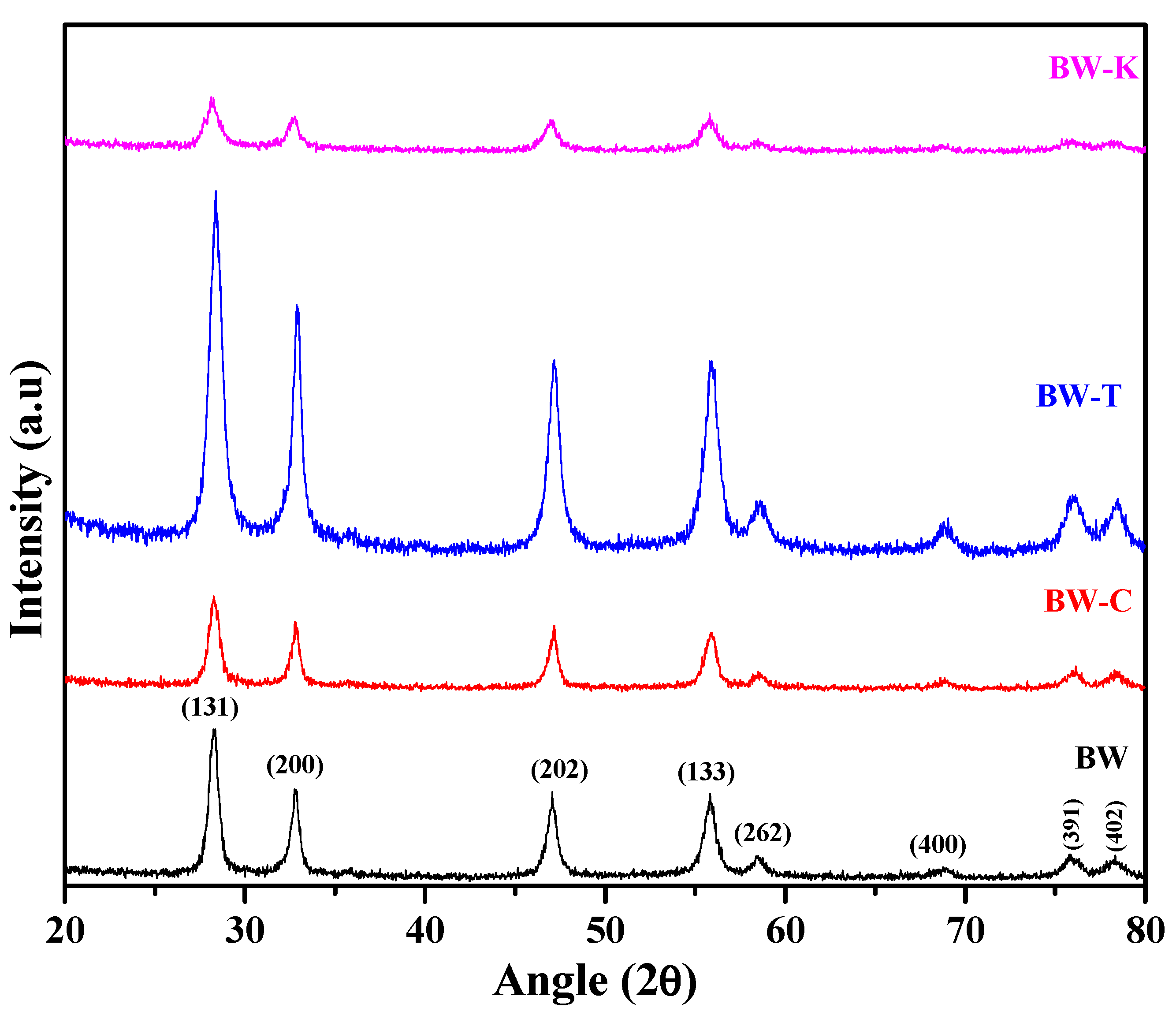
3.2. Structural Characterization
3.3. Optical Properties and Band Structure Studies
3.4. Photocatalytic Degradation of Tetracycline Pollutant Using BW NSs
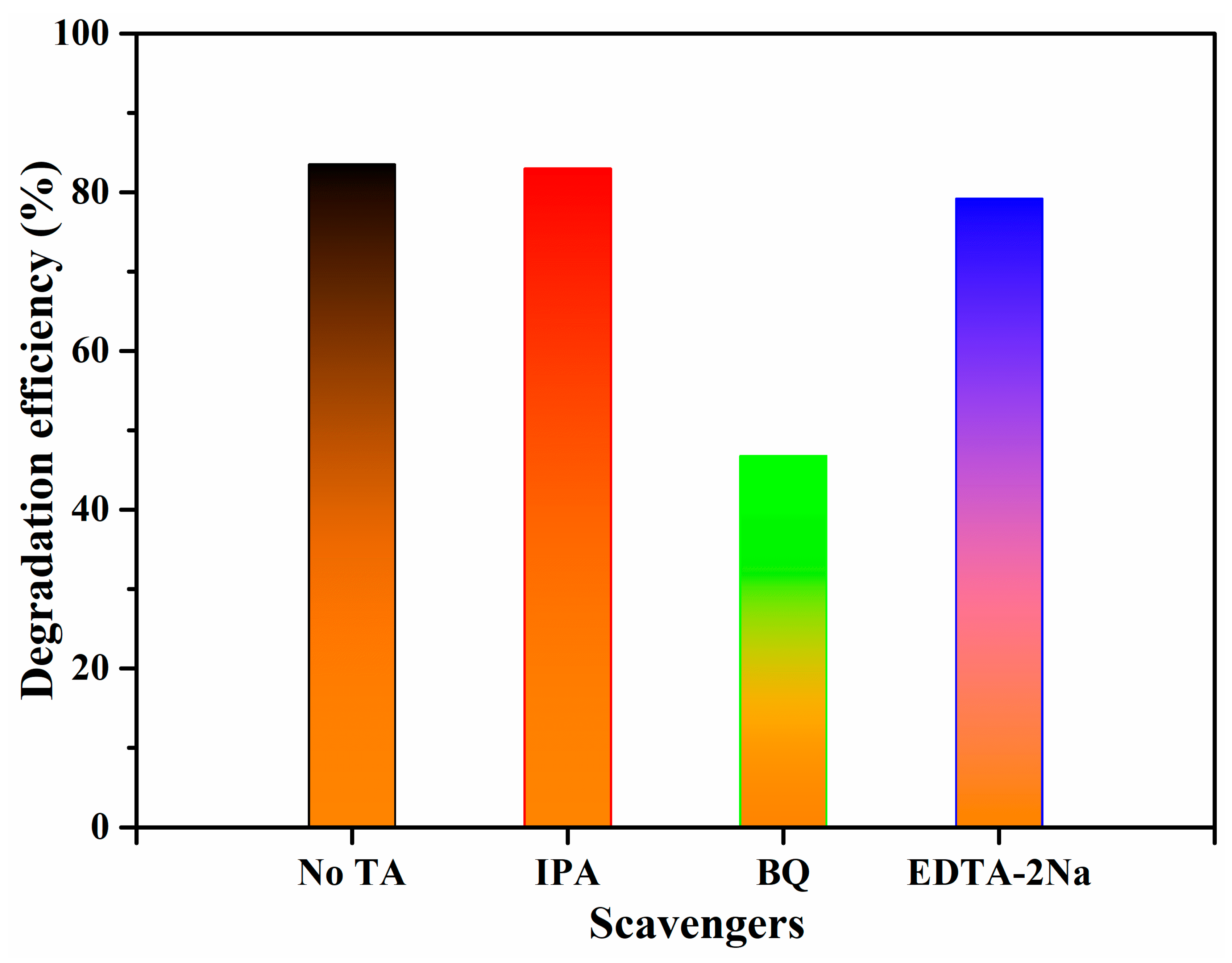
3.5. Photocatalytic Tetracycline Degradation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ren, H.T.; Qi, F.; Labidi, A.K.; Zhao, J.J.; Wang, H.; Xin, Y.; Luo, J.M.; Wang, C.Y. Chemically bonded carbon quantum dots/Bi2WO6 S-scheme heterojunction for boosted photocatalytic antibiotic degradation: Interfacial engineering and mechanism insight. App. Catal. B 2023, 330, 122587. [Google Scholar] [CrossRef]
- Hu, J.D.; Chen, D.Y.; Mo, Z.; Li, N.J.; Xu, Q.F.; Li, H.; He, J.H.; Xu, H.; Lu, J.M. Z-Scheme 2D/2D Heterojunction of Black Phosphorus/Monolayer Bi2WO6 Nanosheets with Enhanced Photocatalytic Activities. Angew. Chem. Int. Ed. 2019, 58, 2073–2077. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, J.; Li, Y.; Huang, L.; Zhang, H.; Chen, L. A novel Pd decorated polydopamine-SiO2/PVA electrospun nanofiber membrane for highly efficient degradation of organic dyes and removal of organic chemicals and oils. J. Cleaner Prod. 2020, 275, 122937. [Google Scholar] [CrossRef]
- Bian, L.; Shen, C.; Song, C.; Zhang, S.; Cui, Z.; Yan, F.; He, B.; Li, J. Compactness tailored hollow fiber loose nanofiltration separation layers based on “chemical crosslinking and metal ion coordination” for selective dye separation. J. Membr. Sci. 2021, 620, 118948. [Google Scholar] [CrossRef]
- Hashem, A.H.; Saied, E.; Hasanin, M.S. Green and ecofriendly bio-removal of methylene blue dye from aqueous solution using biologically activated banana peel waste. Sustain. Chem. Pharm. 2020, 18, 100333. [Google Scholar] [CrossRef]
- Mojtabavi, S.; Khoshayand, M.R.; Fazeli, M.R.; Samadi, N.; Faramarzi, M.A. Combination of thermal and biological treatments for bio-removal and detoxification of some recalcitrant synthetic dyes by betaine-induced thermostabilized laccase. Environ. Technol. Innov. 2020, 20, 101046. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, C.; Chen, W.; Xie, Q.; Guo, Q.; Huang, B. Electrochemical-driven nanoparticulate catalysis for highly efficient dechlorination of chlorinated environmental pollutant. J. Catal. 2021, 395, 362–374. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Mohammadkhani, R.; Ahmadipouya, S.; Shokrgozar, A.; Rezakazemi, M.; Molavi, H.; Aminabhavi, T.M.; Arjmand, M. Superior chemical stability of UiO-66 metal-organic frameworks (MOFs) for selective dye adsorption. Chem. Eng. J. 2020, 399, 125346. [Google Scholar] [CrossRef]
- Siakavelas, G.I.; Charisiou, N.D.; Alkhoori, A.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Yentekakis, I.V.; Polychronopoulou, K.; Goula, M.A. Cerium oxide catalysts for oxidative coupling of methane reaction: Effect of lithium, samarium and lanthanum dopants. J. Env. Chem. Eng. 2022, 10, 107259. [Google Scholar] [CrossRef]
- Siakavelas, G.I.; Charisiou, N.D.; Alkhoori, A.; Gaber, S.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Yentekakis, I.V.; Polychronopoulou, K.; Goula, M.A. Oxidative coupling of methane on Li/CeO2 based catalysts: Investigation of the effect of Mg- and La-doping of the CeO2 support. Mol. Catal. 2022, 520, 112157. [Google Scholar] [CrossRef]
- Sgroi, M.; Snyder, S.A.; Roccaro, P. Comparison of AOPs at pilot scale: Energy costs for micro-pollutants oxidation, disinfection by-products formation and pathogens inactivation. Chemosphere 2021, 273, 128527. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Long, M.; Yang, Y.; Chen, C.; Cai, W.; Zhou, B. A highly active bimetallic oxides catalyst supported on Al-containing MCM-41 for Fenton oxidation of phenol solution. Appl. Catal. B 2011, 110, 118–125. [Google Scholar] [CrossRef]
- Sinha, R.; Ghosal, P.S. A comprehensive appraisal on status and management of remediation of DBPs by TiO2 based-photocatalysts: Insights of technology, performance and energy efficiency. J. Environ. Manag. 2023, 328, 17011. [Google Scholar] [CrossRef]
- Hunag, T.T.; Li, Y.H.; Wu, X.F.; Lv, K.L.; Li, Q.; Li, M.; Du, D.Y.; Ye, H.P. In-situ transformation of Bi2WO6 to highly photoreactive Bi2WO6@Bi2S3 nanoplate via ion exchange. Chin. J. Catal. 2018, 39, 718–727. [Google Scholar] [CrossRef]
- Chava, R.K.; Son, N.G.; Kim, Y.S.; Kang, M.S. Controlled Growth and Bandstructure Properties of One Dimensional Cadmium Sulfide Nanorods for Visible Photocatalytic Hydrogen Evolution Reaction. Nanomaterials 2020, 10, 619. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, X.R.; Liu, C.X.; Qian, X.X.; Wen, Y.R.; Yang, Q.A.; Sun, T.; Chang, W.Y.; Liu, X.; Chen, Z. Facile Construction of All-Solid-State Z-Scheme g-C3N4/TiO2 Thin Film for the Efficient Visible-Light Degradation of Organic Pollutant. Nanomaterials 2020, 10, 600. [Google Scholar] [CrossRef]
- Zhang, G.P.; Chen, D.Y.; Li, N.J.; Xu, Q.F.; Li, H.; He, J.H.; Lu, J.M. Fabrication of Bi2MoO6/ZnO hierarchical heterostructures with enhanced visible-light photocatalytic activity. App. Catal. B 2019, 250, 313–324. [Google Scholar] [CrossRef]
- Wang, S.Y.; Yang, H.; Yi, Z.; Wang, X.X. Enhanced photocatalytic performance by hybridization of Bi2WO6 nanoparticles with honeycomb-like porous carbon skeleton. J. Environ. Manag. 2019, 248, 109341. [Google Scholar] [CrossRef]
- Zhu, D.D.; Zhou, Q.X. Novel Bi2WO6 modified by N-doped graphitic carbon nitride photocatalyst for efficient photocatalytic degradation of phenol under visible light. Appl. Catal. B 2020, 268, 118426. [Google Scholar] [CrossRef]
- Xie, Z.K.; Xu, Y.Y.; Li, D.; Meng, S.C.; Chen, M.; Jiang, D.L. Covalently Bonded Bi2O3 Nanosheet/Bi2WO6 Network Heterostructures for Efficient Photocatalytic CO2 Reduction. ACS Appl. Energy Mater. 2020, 3, 12194–12203. [Google Scholar] [CrossRef]
- Ni, Z.W.; Shen, Y.; Xu, L.H.; Xiang, G.H.; Chen, M.Y.; Shen, N.; Li, K.; Ni, K. Facile construction of 3D hierarchical flower-like Ag2WO4/Bi2WO6 Z-scheme heterojunction photocatalyst with enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2022, 576, 151868. [Google Scholar] [CrossRef]
- Zhang, L.-W.; Wang, Y.-J.; Cheng, H.-Y.; Yao, W.-Q.; Zhu, Y.-F. Synthesis of Porous Bi2WO6 Thin Films as Efficient Visible-Light-Active Photocatalysts. Adv. Mater. 2009, 21, 1286–1290. [Google Scholar] [CrossRef]
- Belousov, A.S.; Parkhacheva, A.A.; Suleimanov, E.V.; Fukina, D.G.; Koryagin, A.V.; Shafiq, I.; Krasheninnikova, O.V.; Kuzmichev, V.V. Doping vs. heterojunction: A comparative study of the approaches for improving the photocatalytic activity of flower-like Bi2WO6 for water treatment with domestic LED light. Catal. Commun. 2023, 180, 106705. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, Y.L.; Xu, G.Z.; Wang, X.Y.; Liang, Y.; Li, A.M.; Xie, X.C.; Wu, D.S. A novel Bi2WO6/DMPBP[5] composite for cyclic adsorption and photocatalytic degradation of organic pollutant in water. Proc. Saf. Environ. Prot. 2023, 171, 763–772. [Google Scholar] [CrossRef]
- Sun, S.M.; Wang, W.Z.; Zhang, L. Facile preparation of three-dimensionally ordered macroporous Bi2WO6 with high photocatalytic activity. J. Mater. Chem. 2012, 22, 19244–19249. [Google Scholar] [CrossRef]
- Shivani, V.; Harish, S.; Archana, J.; Navaneethan, M.; Ponnusamy, S.; Hayakawa, Y. Highly efficient 3-D hierarchical Bi2WO6 catalyst for environmental remediation. Appl. Surf. Sci. 2019, 488, 696–706. [Google Scholar] [CrossRef]
- Li, G.S.; Zhang, D.Q.; Yu, J.C.; Leung, M.K.H. An Efficient Bismuth Tungstate Visible-Light-Driven Photocatalyst for Breaking Down Nitric Oxide. Environ. Sci. Technol. 2010, 44, 4276–4281. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, L.Z.; Hu, C.; Huang, H.W. Recent advances on Bi2WO6-based photocatalysts for environmental and energy applications. Chin. J. Catal. 2021, 42, 1413–1438. [Google Scholar] [CrossRef]
- Li, C.M.; Chen, G.; Sun, J.X.; Rao, J.C.; Han, Z.H.; Hu, Y.D.; Xing, W.N.; Zhang, C.M. Doping effect of phosphate in Bi2WO6 and universal improved photocatalytic activity for removing various pollutants in water. Appl. Catal. B. 2016, 188, 39–47. [Google Scholar] [CrossRef]
- Tong, M.Q.; Zhang, N.; Tan, Z.X.; Chi, L.L.; Zhang, K.W.; Chen, B.; Hu, F.X.; Guo, C.X. Oxygen vacancy-rich Bi2WO6 nanocrystals for fast and wide-range photoelectrochemical sensing of hydrogen peroxide. Microchem. J. 2023, 190, 108618. [Google Scholar] [CrossRef]
- Yang, C.Y.; Zhang, Z.H.; Wang, P.; Xu, P.; Shen, T.Y.; Xing, Y.J.; Zhang, G.S. A novel vertical immobilized photocatalytic membrane reactor based on Bi2WO6-g-C3N4/PVDF for enhanced removal of atrazine, anti-fouling performance and long-term stability. Chem. Eng. J. 2023, 471, 144672. [Google Scholar] [CrossRef]
- Ma, H.Q.; Yang, W.Y.; Gao, S.A.; Geng, W.R.; Lu, Y.L.; Zhou, C.L.; Shang, J.K.; Shi, T.; Li, Q. Superior photopiezocatalytic performance by enhancing spontaneous polarization through post-synthesis structure distortion in ultrathin Bi2WO6 nanosheet polar photocatalyst. Chem. Eng. J. 2023, 455, 140471. [Google Scholar] [CrossRef]
- Ban, T.; Asano, K.; Takai-Yamashita, C.; Ohya, Y. Bottom-up synthesis of titanophosphate nanosheets by the aqueous solution process. Nanoscale Adv. 2020, 2, 3542–3549. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, Y.; Imai, H. Bottom-Up Synthesis of Titanate Nanosheets with Hierarchical Structures and a High Specific Surface Area. Small 2006, 2, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.G.; Ding, X.; Yang, N.; Zhan, G.M.; Zhang, X.H.; Dong, G.H.; Zhang, L.Z.; Chen, H. Insight into the effect of bromine on facet-dependent surface oxygen vacancies construction and stabilization of Bi2MoO6 for efficient photocatalytic NO removal. Appl. Catal. B. 2020, 265, 118585. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Zhang, Y.F.; Lin, M.S.; Long, J.L.; Zhang, Z.Z.; Lin, H.X.; Wu, J.C.S.; Wang, X.X. Monolayered Bi2WO6 nanosheets mimicking heterojunction interface with open surfaces for photocatalysis. Nat. Commun. 2015, 6, 8340. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Bøjesen, E.D.; Mamakhel, A.H.; Iversen, B.B. Why Does Bi2WO6 Visible-Light Photocatalyst Always Form as Nanoplatelets? Inorg. Chem. 2020, 59, 9364–9373. [Google Scholar] [CrossRef]
- Xu, S.L.; Zhang, Y.-J.; Tang, R.; Zhang, X.Y.; Hu, Z.-H.; Yu, H.-Q. Enhancing Fenton-like catalytic efficiency of Bi2WO6 by iodine doping for pollutant degradation. Sep. Pur. Technol. 2021, 277, 119447. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Wang, Y.X.; Di, Y.; Liu, J.Q.; Li, D.Y.; Wang, Y.M.; Zhu, Z.; Liu, H.L.; Wei, M.B. Withered magnolia-derived BCDs onto 3D flower-like Bi2WO6 for efficient photocatalytic TC degradation and CO2 reduction. J. Alloys Compd. 2023, 965, 171520. [Google Scholar] [CrossRef]
- Maczka, M.; Hanuza, J.; Paraguassu, W.; Gomes Souza Filho, A.; Tarso Cavalcante Freire, P.; Mendes Filho, J. Phonons in Ferroelectric Bi2WO6: Raman and Infrared Spectra and Lattice Dynamics. Appl. Phys. Lett. 2008, 92, 112911. [Google Scholar] [CrossRef]
- Wu, S.J.; Sun, J.G.; Li, Q.; Hood, Z.D.; Yang, S.Z.; Su, T.M.; Peng, R.; Wu, Z.L.; Sun, W.W.; Kent, P.R.C.; et al. Effects of Surface Terminations of 2D Bi2WO6 on Photocatalytic Hydrogen Evolution from Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 20067–20074. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.R.; Liu, X.J.; Wang, Z.X.; Wu, Y.; Dou, M.G.; Yang Hua Zhu, H.J.; Li, D.C.; Dou, J.M. Functionalized 2D defect g-C3N4 for artificial photosynthesis of H2O2 and synchronizing tetracycline fluorescence detection and degradation. Environ. Res. 2023, 232, 116345. [Google Scholar] [CrossRef]
- Ferrer, M.M.; Rodrigues, J.E.F.S.; Almeida, M.A.P.; Moura, F.; Longo, E.; Pizani, P.S.; Sambrano, J.R. Theoretical Methods for Calculations of Optical Phonons in BiOBr: Analysis and Correction of Propagated Errors. J. Raman Spectrosc. 2018, 49, 1356–1363. [Google Scholar] [CrossRef]
- Cui, Z.; Song, H.; Ge, S.; He, W.; Liu, Y. Fabrication of BiOCl/BiOBr Hybrid Nanosheets with Enhanced Superoxide Radical Dominating Visible Light-Driven Photocatalytic Activity. Appl. Surf. Sci. 2019, 467–468, 505–513. [Google Scholar] [CrossRef]
- Cheng, T.T.; Gao, H.J.; Wang, S.F.; Yi, Z.; Liu, G.R.; Pu, Z.S.; Wang, X.X.; Yang, H. Surface doping of Bi4Ti3O12 with S: Enhanced photocatalytic activity, mechansim and potential photodegradation application. Mater. Res. Bull. 2022, 149, 111711. [Google Scholar] [CrossRef]
- Rauf, A.; Sher Shah, M.S.A.; Choi, G.H.; Humayoun, U.B.; Yoon, D.H.; Bae, J.W.; Park, J.; Kim, W.J.; Yoo, P.J. Facile Synthesis of Hierarchically Structured Bi2S3/Bi2WO6 Photocatalysts for Highly Efficient Reduction of Cr(VI). ACS Sustain. Chem. Eng. 2015, 3, 2847–2855. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X.F.; Ma, J.Y.; Yi, Z.; Xian, T.; Wang, S.F.; Liu, G.R.; Wang, X.X.; Yang, H. Development of highly-effiecnt 0D/1D/0D dual Z-scheme CdS/ZnWO4/ZnS heteojunction photocatalysts in pollutant removal and involved mechanism. Appl. Surf. Sci. 2023, 611, 155681. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Zhang, L.; Hu, C. Enhanced photoactivity of Bi2WO6 by iodide insertion into the interlayer for water purification under visible light. Chem. Eng. J. 2018, 352, 664–672. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Chen, P.; Lv, W.Y.; Xiao, Z.J.; Zhang, J.F.; Wu, J.Q.; Lin, Z.L.; Zhang, G.Z.; Yu, Z.S.; Liu, H.J.; et al. Key role of Fe(VI)-activated Bi2WO6 in the photocatalytic oxidation of sulfonamides: Mediated electron transfer mechanism. J. Haz. Mater. 2023, 458, 132009. [Google Scholar] [CrossRef]
- Pancielejko, A.; Luczak, J.; Lisowski, W.; Zaleska-Medynska, A.; Mazierski, P. Novel two-step synthesis method of thin film heterojunction of BiOBr/Bi2WO6 with improved visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2021, 569, 151082. [Google Scholar] [CrossRef]
- Zhao, B.X.; Shao, N.; Chen, X.L.; Ma, J.X.; Gao, Y.J.; Chen, X.Q. Construction of novel type II heterojunction WO3/Bi2WO6 and Z-scheme heterojunction CdS/Bi2WO6 photocatalysts with significantly enhanced photocatalytic activity for the degradation of rhodamine B and reduction of Cr(VI). Colloids Surf. A 2023, 663, 131072. [Google Scholar] [CrossRef]
- Wang, G.W.; Cheng, H.F. Recyclable MXene-bridged Z-scheme NiFe2O4/MXene/Bi2WO6 heterojunction with enhanced charge separation for efficient sonocatalytic removal of ciprofloxacin. Sci. Total. Environ. 2023, 901, 165833. [Google Scholar] [CrossRef]
- Zhao, C.R.; Cai, L.Y.; Wang, K.Q.; Li, B.X.; Yuan, S.D.; Zeng, Z.H.; Zhao, L.H.; Wu, Y.; He, Y.M. Novel Bi2WO6/ZnSnO3 heterojunction for the ultrasonic-vibration-driven piezocatalytic degradation of RhB. Env. Pol. 2023, 319, 120982. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, S.A.; Zhang, X.R.; Qu, L.N.; Qi, H.J.; Wang, B.; Xu, B.B.; Huang, Z.H. Carbon-doped flower-like Bi2WO6 decorated carbon nanosphere nanocomposites with enhanced visible light photocatalytic degradation of tetracycline. Adv. Compos. Hybrid Mater. 2023, 6, 47. [Google Scholar] [CrossRef]
- Yang, C.Y.; Zhang, Z.H.; Wang, P.; Xu, P.; Shen, T.Y.; Wang, M.Q.; Zheng, Q.Z.; Zhang, G.S. Ultrathin g-C3N4 composite Bi2WO6 embedded in PVDF UF membrane with enhanced permeability, anti-fouling performance and durability for efficient removal of atrazine. J. Hazard. Mater. 2023, 451, 131154. [Google Scholar] [CrossRef]
- Meng, L.J.; How, Z.T.; Chelme-Ayala, P.; Benally, C.; El-Din, M.G. Z-scheme plasmonic Ag decorated Bi2WO6/NiO hybrids for enhanced photocatalytic treatment of naphthenic acids in real oil sands process water under simulated solar irradiation. J. Hazard. Mater. 2023, 454, 131441. [Google Scholar] [CrossRef]
- Kang, Z.H.; Chen, M.S.; Wu, J.A.; Qin, N.; Bao, D.H. Insights on enhancing piezocatalytic performance of Bi2WO6@PDA homojunction from phase coexistence and electron transfer mediators. J. Colloids Interface Sci. 2023, 650, 169–181. [Google Scholar] [CrossRef]
- Ming, J.; Sun, X.A.; Ma, Q.S.; Liu, N.; Zhang, C.; Kawazoe, N.; Cen, G.P.; Yang, Y.N. Advanced photocatalytic sterilization for recalcitrant Enterococcus sp. contaminated water by newly developed Z-scheme Bi2WO6 based composites under solar light. Chemosphere 2023, 310, 136912. [Google Scholar] [CrossRef]
- Hu, S.J.; Fei, Q.R.; Li, Y.J.; Wang, B.L.; Yu, Y.J. Br-terminated 2D Bi2WO6 nanosheets as a sensitive light-regenerated electrochemical sensor for detecting sulfamethoxazole antibiotic. Surf. Interfaces 2021, 25, 101302. [Google Scholar] [CrossRef]
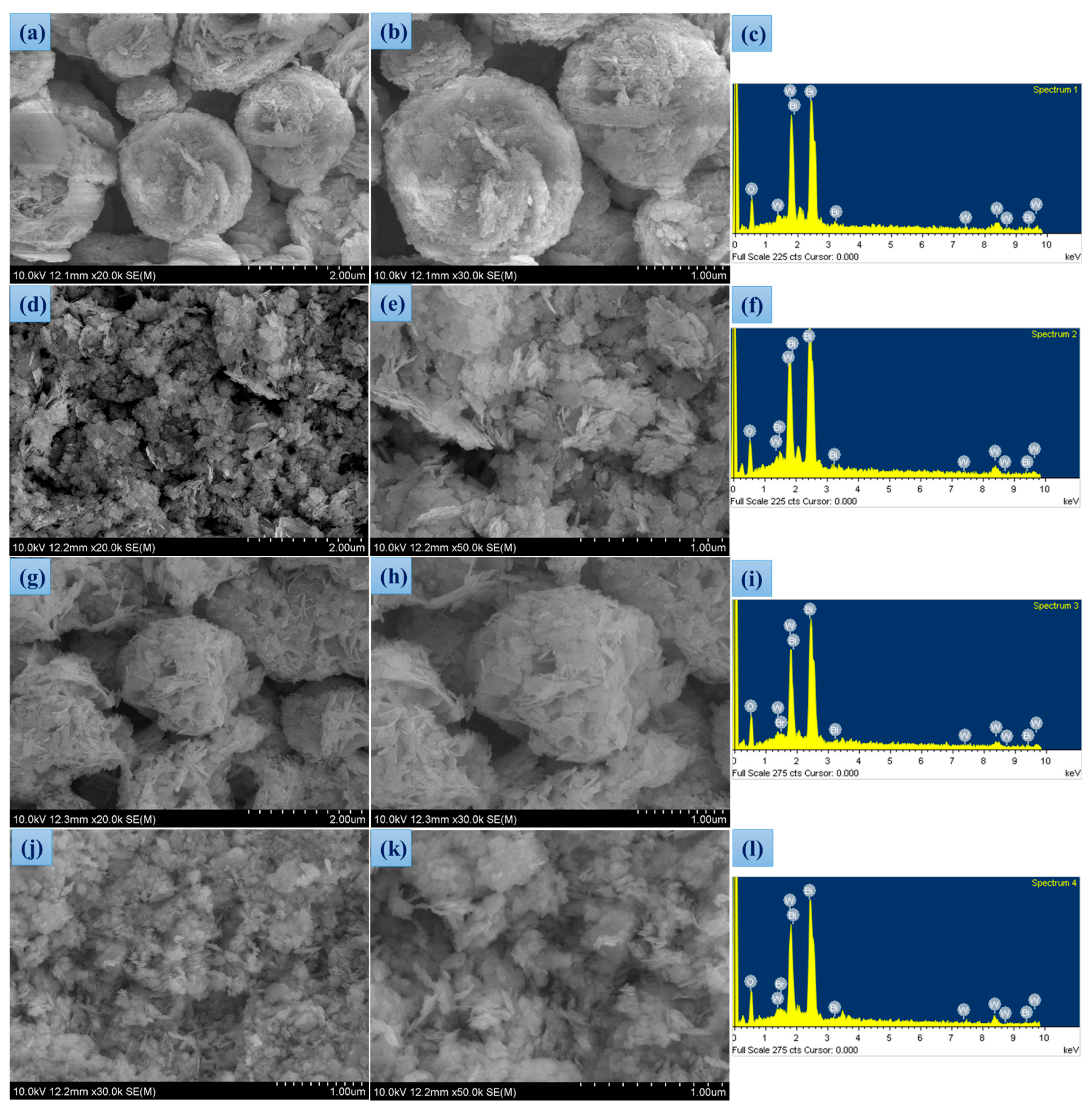



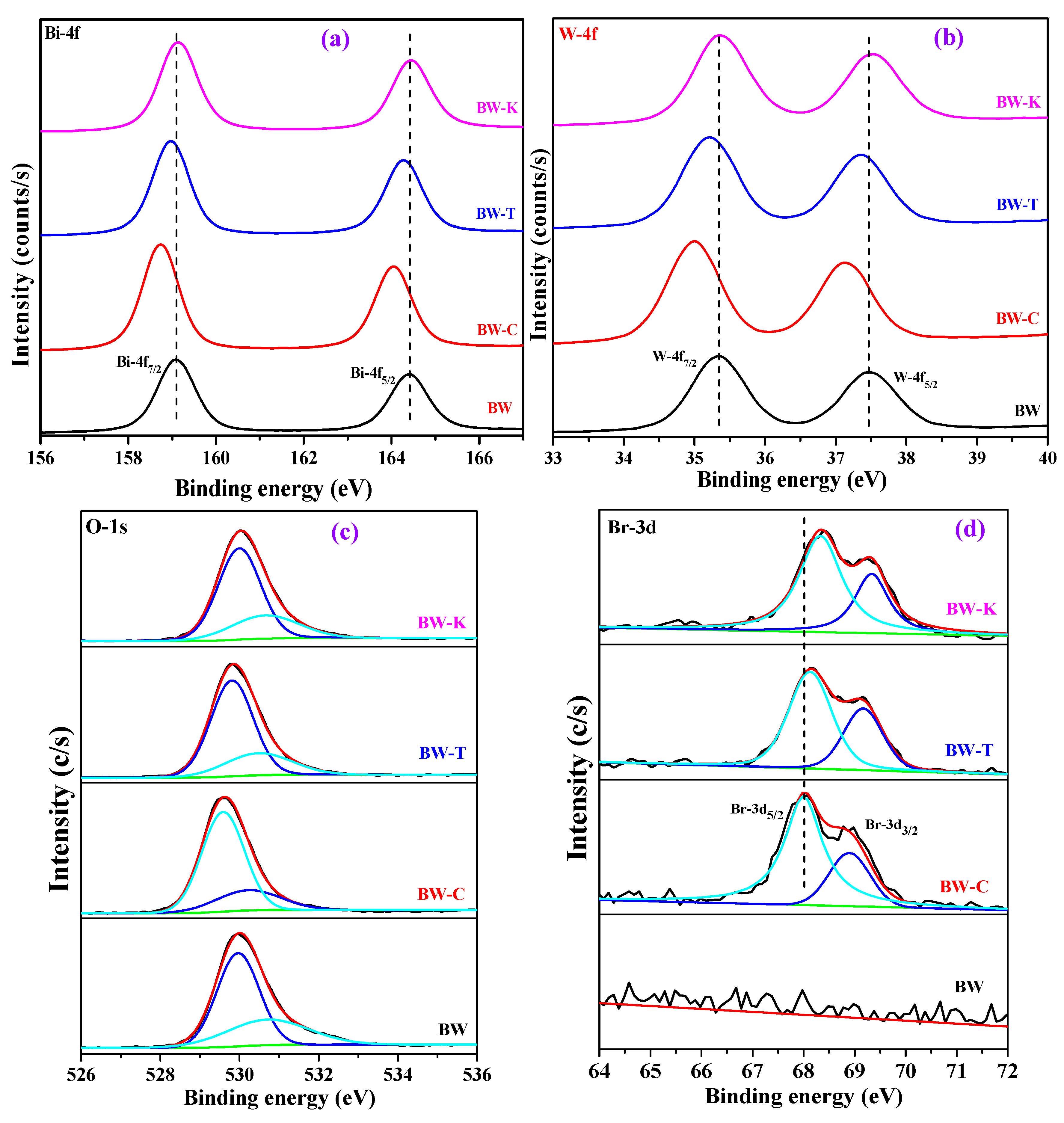





| Sample | Bi (wt %) | W (wt %) | O (wt %) | Br (wt %) |
|---|---|---|---|---|
| BW | 59.18 | 28.84 | 11.98 | - |
| BW-C | 60.94 | 26.98 | 10.94 | 1.14 |
| BW-T | 59.58 | 26.16 | 12.97 | 1.30 |
| BW-K | 59.70 | 26.80 | 12.30 | 1.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chava, R.K.; Kang, M. Bromine Ion-Intercalated Layered Bi2WO6 as an Efficient Catalyst for Advanced Oxidation Processes in Tetracycline Pollutant Degradation Reaction. Nanomaterials 2023, 13, 2614. https://doi.org/10.3390/nano13182614
Chava RK, Kang M. Bromine Ion-Intercalated Layered Bi2WO6 as an Efficient Catalyst for Advanced Oxidation Processes in Tetracycline Pollutant Degradation Reaction. Nanomaterials. 2023; 13(18):2614. https://doi.org/10.3390/nano13182614
Chicago/Turabian StyleChava, Rama Krishna, and Misook Kang. 2023. "Bromine Ion-Intercalated Layered Bi2WO6 as an Efficient Catalyst for Advanced Oxidation Processes in Tetracycline Pollutant Degradation Reaction" Nanomaterials 13, no. 18: 2614. https://doi.org/10.3390/nano13182614
APA StyleChava, R. K., & Kang, M. (2023). Bromine Ion-Intercalated Layered Bi2WO6 as an Efficient Catalyst for Advanced Oxidation Processes in Tetracycline Pollutant Degradation Reaction. Nanomaterials, 13(18), 2614. https://doi.org/10.3390/nano13182614