Abstract
Even though transition metals can activate Oxone to degrade toxic contaminants, bimetallic materials possess higher catalytic activities because of synergistic effects, making them more attractive for Oxone activation. Herein, nanoscale CuCo-bearing N-doped carbon (CuCoNC) can be designed to afford a hollow structure as well as CuCo species by adopting cobaltic metal organic frameworks as a template. In contrast to Co-bearing N-doped carbon (CoNC), which lacks the Cu dopant, CuCo alloy nanoparticles (NPs) are contained by the Cu dopant within the carbonaceous matrix, giving CuCoNC more prominent electrochemical properties and larger porous structures and highly nitrogen moieties. CuCoNC, as a result, has a significantly higher capability compared to CoNC and Co3O4 NPs, for Oxone activation to degrade a toxic contaminant, Rhodamine B (RDMB). Furthermore, CuCoNC+Oxone has a smaller activation energy for RDMB elimination and maintains its superior effectiveness for removing RDMB in various water conditions. The computational chemistry insights have revealed the RDMB degradation mechanism. This study reveals that CuCoNC is a useful activator for Oxone to eliminate RDMB.
1. Introduction
Wet oxidation technologies (WOTs) would be practical for eliminating organic pollutants, particularly contaminants from textile industries. Conventionally, WOTs are associated with production of radicals that exhibit strong redox powers to decompose contaminants. As the Fenton’s reaction is commonly employed for •OH to degrade pollutants [1], SO4•−-related WOTs would receive increasing attention since SO4•− exhibits a notably higher potential [2,3,4], making SO4•−-related WOTs appealing to eliminate organic pollutants.
To produce SO4•−, a few reagents would be accessible, such as Oxone®, which, however, requires activation for quickly releasing SO4•− from Oxone. Amid several activating strategies [5,6,7,8], heterogeneous catalysis is considered to be a practical approach [3,4]. For instance, Hu et al. studied Oxone activation using CoMg/SBA-15 for eliminating Rhodamine B (RDMB) [9]. CoxFe3−xO4 nanocatalysts were also reported to activate Oxone for the removal of RDMB [10]. Likewise, several metallic materials had been also reported for activating Oxone to degrade RDMB [11,12].
Even though Co has been validated as the most efficient metal and a few cobaltic materials (e.g., Co3O4) have been fabricated for activating Oxone [13,14], recent studies reported that dual-metal substances, comprising Co with other metals, would possess additional benefits that could enhance capabilities [15,16]. Particularly, dual-metal Co-bearing alloys would exhibit enhanced performances because of more active sites [17], and the combination of Cu and Co species seem to be useful dual-metal catalysts for activating Oxone [17,18,19] as Cu is abundant and possesses superior redox properties.
However, the employment of CuCo alloy nanoparticles (NPs) might encounter challenges, including the aggregation of NPs, inconvenient recovery of NPs, and vulnerable instability [20,21]. Thus, immobilizing CuCo NPs into supports is a useful technique.
On the other hand, as carbon is the most Earth-abundant, highly accessible, and stable, carbon materials would be favorable media for supporting/embedding CuCo NPs [22,23]. Carbonaceous media are also modified by nitrogen to increase active sites [24,25]. Thus, fabricating a material by immobilizing CuCo on N-doped carbon (NC) media seems promising for activating Oxone.
Even though embedding NPs onto carbon media is feasible by additional fabrication, such an additional fabrication strategy unfortunately faces many issues, including uneven distribution, agglomeration, and insufficient loading of active species [26,27]. Adopting templates that consist of desired materials (e.g., metal and carbon precursors) appears to be a convenient method to obtain such a composite after the carbonization of such templates. Specifically, zeolitic imidazolate frameworks (ZIFs) would be a useful precursor because of cobaltic constituents and nitrogenic ligands [28] that would be transformed to NC. Doping Cu into ZIF could further lead to the formation of CuCo.
While Co-ZIF has a cubic geometry and sub-micron sizes, the derived Co/carbon hybrids are quite challenging to access by Oxone [11,29]. Thus, here, we present a convenient strategy to afford so-called hollow-structured Co-ZIF using an etching technique to remove its interior, forming a hollow configuration. Following doping Cu on such a hollow material and then carbonization, a nanoscale CuCo species-bearing NC (CuCoNC) is then created. This CuCoNC comprising CuCo bimetallic NP embedded in NC appears to be a useful catalyst for activating Oxone to eliminate toxic contaminants. In particular, Rhodamine B (RDMB) is then selected as a model toxic organic pollutant as RDMB is a carcinogenic pollutant [30,31].
To further examine the catalytic activities of CuCoNC, a Co-bearing N-doped carbon (CoNC), which is an analogous to CuCoNC without the Cu dopant, was also prepared.
2. Experimental
The fabrication of CuCoNC was implemented as depicted in Figure 1, and the detailed synthesis process, RDMB degradation by Oxone, analytical procedures, and computational chemistry are provided in the supplementary material.
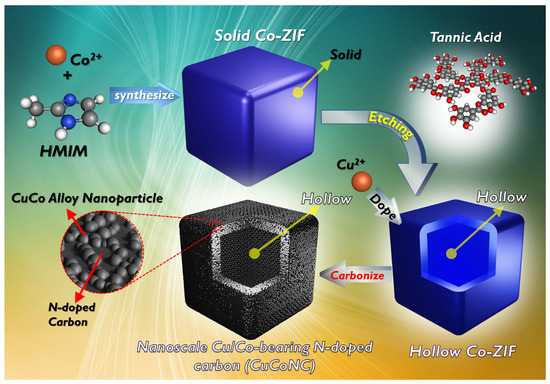
Figure 1.
Preparation scheme for CuCoNC.
For calculating the elimination rate constant, the following equation was adopted for the estimatiation of k:
Ln(Ct/C0) = −kt
3. Results and Discussion
3.1. Properties of CuCoNC
Morphologies and Compositional analyses
Since CuCoNC was derived from Co-ZIF, the appearance of Co-ZIF was firstly characterized as displayed in Figure 2a, revealing the cubic morphology with flat faces and clearly defined edges. The transmission electron microscopic image of Co-ZIF (Figure 2b) demonstrates that the pristine Co-ZIF was solid without any holes/voids. Figure 3a shows that the XRD result of pristine Co-ZIF was consistent with the literature [11,32,33], confirming that Co-ZIF was successfully formed.
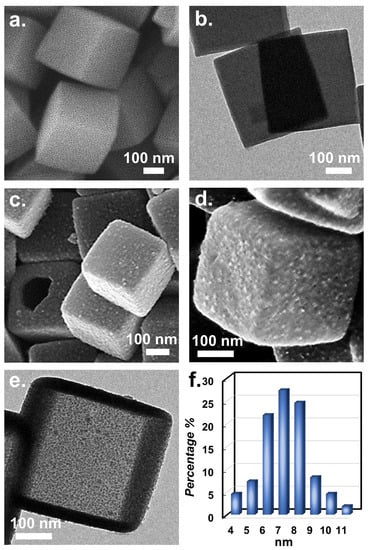
Figure 2.
Images of (a,b) solid Co-ZIF; (c–e) hollow Co-ZIF; and (f) size distribution of NPs in CuCoNC.
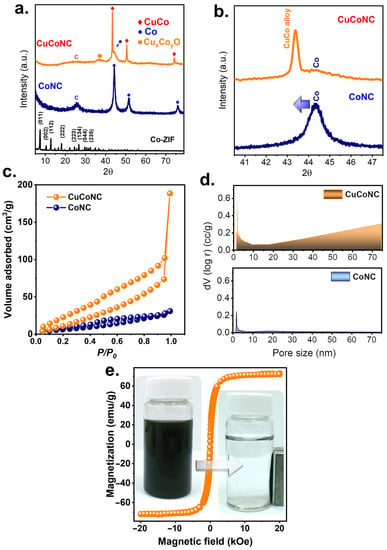
Figure 3.
(a,b). Crystalline structures; (c). adsorption, (d) pores of CuCoNC and CoNC; (e) magnetism of CuCoNC.
Via modification by tannic acid as well as Cu2+, and then pyrolysis, the resulting material (Figure 2e) maintained the cubic shape; nevertheless, the planes of the resultant material became noticeably coarse and decorated by many NPs. Moreover, the solid structure changed to a configuration with pores, as observed by the rupture. Its transmission electron microscopic image (Figure 2f) further validated that the solid texture of pristine Co-ZIF disappeared and was substituted by a hollow structure. The formation of a hollow structure could be attributed to the addition of tannic acid which releases free proton (H+), permeating into Co-ZIF, eventually affording a hollow structure. Moreover, the transmission electron microscopic image also indicated that the NPs decorated ranged from 4 to 11 nm, demonstrating that these NPs were extremely small and the sizes were relatively similar.
Moreover, Figure 2e shows that the resulting material was comprised of numerous NPs. Therefore, the solid Co-ZIF was successfully changed, exhibiting a different XRD result (Figure 3a).
In particular, a few peaks were observed at 25.6°, 37.0°, 43.4°, 44.3°, 50.5°, and 74.1°. While the broad peak at 25.6° could be attributed to carbon, the noticeable peaks at 43.4, 50.5, and 74.1° would be ascribed to the formation of CuCo [34] as a result of the spinel Co structure being substituted by Cu, leading to the peak shift towards the lower value of 2θ as shown in Figure 3b [34]. Nevertheless, a peak of Co (111) could still be noted at 44.3°, suggesting that a fraction of Co remained in the resulting material. Moreover, the minor existence of Cu/Co oxide was also observed at 37.0°, possibly due to the surficial oxidation of CuCo species upon exposure to air. Figure 4 further displays the elemental analysis of this resulting product, in which notable signals of C, N, Co, and Cu were observed. While most elements can be well-distributed over the carbon matrix, in the case of the distribution of Cu, some localization in the intercubic spaces can also be observed. This was possibly because Cu was doped onto Co-ZIF through mixing Cu and Co-ZIF, and such a phenomenon of localization possibly occurred.
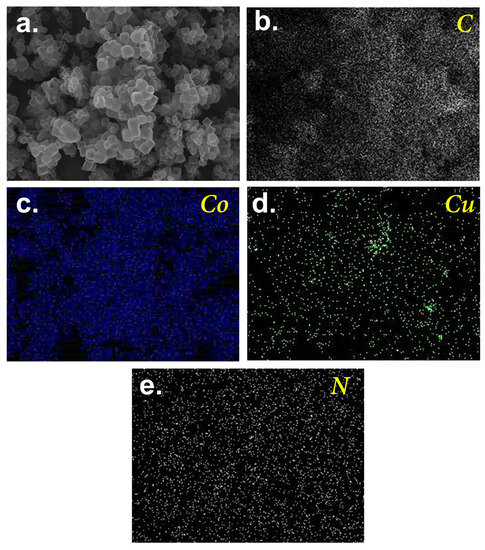
Figure 4.
Mapping analysis of CuCoNC: (a) scanned region, (b) C, (c) Co, (d) Cu, and (e) N.
These results suggest that the resultant NPs adorned on the hollow cubic product were comprised of Cu/Co species, formulating the nanoscale Cu/Co-bearing N-doped carbon (CuCoNC).
A similarity to CuCoNC was also fabricated in the absence of Cu2+ dopant in Figure S1a,b with only noticeable signals of Co and C in the absence of Cu (Figure S1c). Figure 3a also displays the XRD pattern of this resulting product from Co-ZIF without Cu2+, which contained peaks at 25.6°, 44.3°, 51.6°, and 75.9°. While the peak at 25.6° could be ascribed to carbon, the rest of the peaks can be well-indexed to Co0. Thus, the ZIF without Cu2+ dopant became Co-bearing N-doped carbon (CoNC) after the etching and carbonization treatments.
Textural properties
Figure 3c further shows its adsorption result which could be categorized as an IUPAC IV sorption with a notable hysteresis loop, indicating that CuCoNC exhibited mesopores. Simultaneously, Figure 3c also reveals the N2 sorption of CoNC, which was actually similar to that of CuCoNC but with a much smaller N2 sorption volume. the surface area of CuCoNC was 70 m2/g, while CoNC merely showed a surface area of 26 m2/g. Figure 3d also reveals the pore size distribution of CuCoNC, in which a large fraction of pores in the mesoscale range could be observed, and the total pore volume was 0.29 cc/g, whereas the total pore volume of CoNC volume was merely 0.045 cc/g. The relatively superior and complicated textural properties in CuCoNC were possibly owing to the fact that the faces of CuCoNC exhibited noticeable small holes as seen in Figure 3d and the extremely small spherical NPs were decorated on the surface, leading to much more roughened surfaces. In contrast, the faces of CoNC were smooth and flattened without noticeable bumps and holes on the surface as seen in Figure S1. Such a difference also suggests that the Cu dopant into the Co-ZIF seemed to alter the texture by introducing hetero-atoms, thereby causing the greater surface area.
It would be interesting to examine the magnetism of CuCoNC. In Figure 3e, CuCoNC exhibited a significantly high magnetism approaching 72 emu/g, which enabled CuCoNC to be easily controllable for uniform dispersion and quick collection as shown in the inset.
3.2. Degradation of RDMB by CuCoNC+Oxone
The degradation of RDMB by Oxone using CuCoNC was then measured in Figure 5. Because RDMB might be removed by sorption, the sorption of RDMB on CuCoNC was then studied. However, RDMB barely decreased using CuCoNC, and RDMB was not eliminated by sorption. On the other hand, by using Oxone alone, RDMB was only slightly degraded in 30 min. Nevertheless, when CuCoNC and Oxone were adopted simultaneously, RDMB dropped quickly in 30 min, indicating that CuCoNC activated Oxone to eliminate RDMB. The commercial Co3O4 (Figure S2) and CoNC were also employed to activate Oxone to eliminate RDMB in Figure 5a. Co3O4+Oxone enabled Ct/C0 to reach 0.32 after 30 min, while CoNC+Oxone led to Ct/C0 = 0.07.
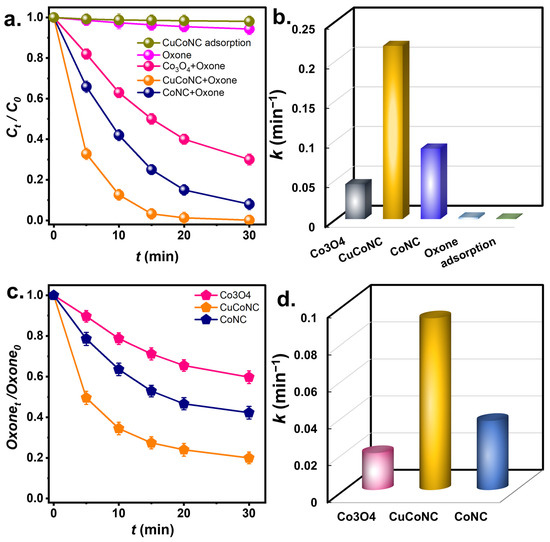
Figure 5.
(a) RDMB elimination (initial concentration of RDMB = 10 mg/L) and (b) kinetics; (c) Oxone decomposition and (d) kinetics of Oxone consumption (Catalyst = 50 mg/L, Oxone = 100 mg/L, T = 30 °C).
In addition, the rate constants for RDMB degradation are then summarized in Figure 5b. The rate constant (k) for CuCoNC+Oxone would be 0.219 min−1, which was greater than the rate constants for Co3O4 NP+Oxone (i.e., 0.043 min−1) and CoNC+Oxone (0.089 min−1), indicating that CuCoNC was certainly superior to the commercial Co3O4 NP and CoNC.
As CuCoNC led to the much faster degradation of RDMB, it was interesting to study Oxone decomposition by CuCoNC. In Figure 5c, the consumption of Oxone during RDMB degradation was analyzed. In 30 min, >80% of Oxone was consumed by CuCoNC, whereas Oxone consumptions by CoNC and Co3O4 NP were significantly lower (40% and 55%, respectively). K values of Oxone decomposition are also summarized in Figure 5d. The k using CuCoNC was 0.092 min−1, the k by CoNC was 0.037 min−1, and that by Co3O4 NP was 0.020 min−1, confirming that the Oxone decomposition by CuCoNC was significantly faster and more efficient than CoNC and Co3O4 NP, thereby causing faster RDMB degradation.
Oxone activation correlates to surficial chemistry between Oxone and catalysts [35]. Since CuCoNC showed a significantly superior capability for activating Oxone, it was intriguing to investigate these catalysts. In Figure 6, X-ray photoelectron spectroscopy (XPS) results of CuCoNC and CoNC are displayed. In particular, the C1s result of CuCoNC (Figure 6a) is decomposed to several peaks at 283.9, 286.5, and 288.6 eV, corresponding to the C-C, C-N, and C=O groups [36]. In addition, the Co2p result of CuCoNC would be deconvoluted to a series of peaks (Figure 6b). In particular, the peak at 778.7 eV was Co0, whereas the peaks at 780.3 and 796.2 eV were Co2+ [37]. Moreover, the bands at 933.0 and 953.2 eV correspond to Cu0 and Cu+ in CuCo alloy [38]; the bands at 935.2 and 954.4 eV can be ascribed to Cu2+ species, as the oxidation of CuCo can occur in contact with O2 as seen in the XRD pattern. Furthermore, Figure 6d shows many peaks at 398.2, 399.3, and 400.6 eV, attributed to the pyridinic N, pyrrolic N, and quaternary N group [39].
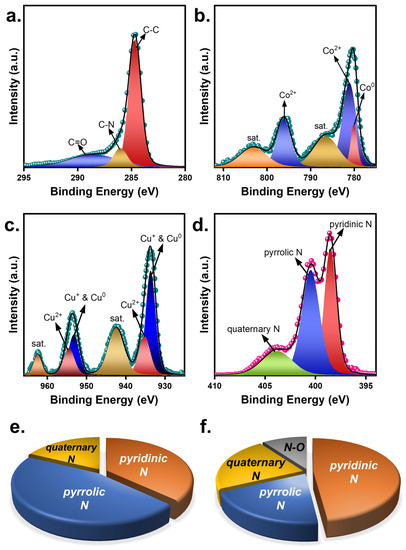
Figure 6.
(a–d) XPS results of CuCoNC; nitrogen groups in (e) CuCoNC; (f) CoNC.
Similarly, the N1s in CoNC (Figure S3) also had pyridinic N, pyrrolic N, quaternary N, and the N-O group, respectively [39]. Despite the fact that both CuCoNC and CoNC consisted of Co species, CuCoNC additionally contained Cu0, Cu+, and Cu2+, that act as additional mediating groups for Oxone activation [40,41].
In addition, CuCoNC also showed a different result for nitrogen components, with pyridinic N, pyrrolic N, and quaternary N of 37.0%, 45.3%, and 17.7%, respectively (Table S1), while CoNC showed pyridinic N, pyrrolic N, quaternary N, and N-O of 48.1%, 19.9%, 22.1%, and 9.9%, respectively. As pyridinic N and pyrrolic N are confirmed as highly effective for Oxone activation [42], the total quantity of these N species in CuCoNC (i.e., 82.3%) was much larger than in CoNC (68.0%), thereby promoting catalytic activities of CuCoNC for activating Oxone in RDMB decomposition.
Figure 7a displays the Raman result of CuCoNC and CoNC; both showed similar bands at around 190, 470, and 670 cm−1, corresponding to F2g, Eg, and A1g [43]. As CuCoNC and CoNC were composed of similar patterns, the zoomed-in images of these bands unraveled differences. Specifically, the F2g peak of CoNC was located at 190 cm−1 (Figure 7b), but the peak of CuCoNC changed to 184 cm−1. The peak variance could also be seen in the case of A1g (Figure 7c). The A1g peak of CoNC was located at 672 cm−1, and the peak in CuCoNC changed from 672 to 662 cm−1. As A1g and F2g are relevant to the coordination of Co species, these changes implied that the CuCoNC seemed to comprise more defects or oxygen vacancies [44]. The higher fraction of D band in CuCoNC (Figure 7d) (Table S2) might also be the reason for CoNC exhibiting higher catalytic activities [45].
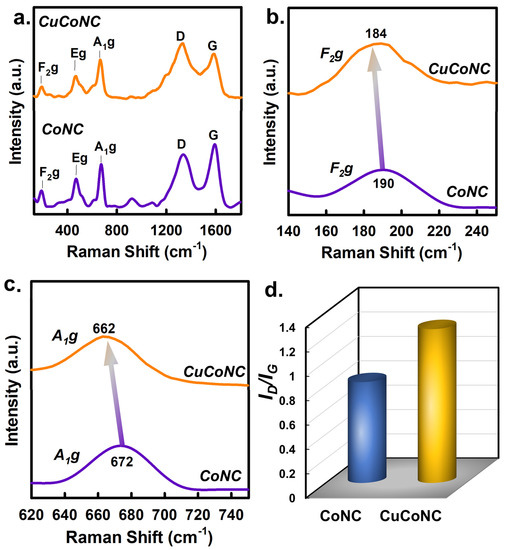
Figure 7.
(a) Raman analysis of CuCoNC and CoNC; (b,c) regional result of CuCoNC and CoNC; and (d) G and D peaks.
These differences might also allow CuCoNC to exhibit more reactive sites [44], thereby enhancing the activation of Oxone. Moreover, Oxone activation would also be associated with redox properties [35]. Figure 8a displays the cyclic voltammetry (CV) curves of CuCoNC and CoNC. CoNC showed a smaller CV loop, while CuCoNC revealed a larger CV loop. CuCoNC possessed 14.0 F/g, which was larger than that of CoNC as 3.3 F/g, demonstrating that CuCoNC showed superior redox properties.
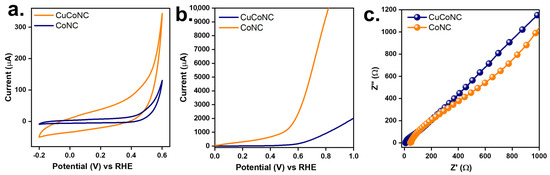
Figure 8.
Electrochemical properties of CuCoNC and CoNC: (a) CV curves, (b) LSV curves, (c) EIS Nyquist plots.
Moreover, the linear sweep voltammogram (LSV) result of CuCoNC is shown in Figure 8b. CuCoNC required an initial potential of 0.50 V but CoNC required a slightly higher initial potential of 0.82 V to reach 0.1 mA. This result demonstrated that CuCoNC was superior to CoNC for electrical transport. Figure 8c further shows that CuCoNC possessed a slightly narrower semi-circle in the high-frequency region than CoNC, revealing that CuCoNC possessed a larger extent of charge transfer [46].
3.3. RDMB by CuCoNC+Oxone: Other Effects
As CuCoNC+Oxone eliminated RDMB effectively, temperature and pH would also impact RDMB degradation. Figure 9a shows RDMB eradication by CuCoNC+Oxone at 25 to 50 °C. As the temperature rose from 25 to 38 °C, the k value for RDMB degradation rose from 0.219 min−1 to 0.329 min−1. When it further rose to 50 °C, the rate of RDMB degradation increased to 0.520 min−1, indicating the favorable effect of elevated temperatures on RDMB elimination. Furthermore, these rate constants at different temperatures were correlated using the Arrhenius equation [47]:
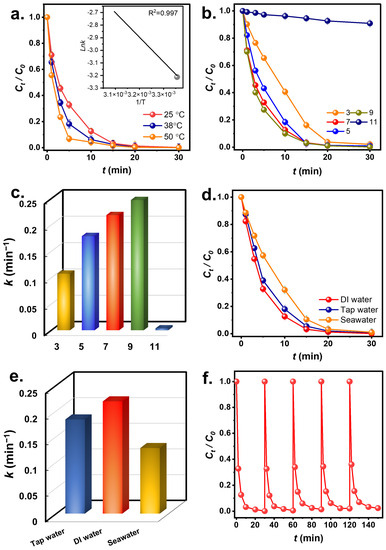
Figure 9.
(a) Temperature effect, (b,c) pH effect and corresponding k, (d,e) scavenger effect; (f) reusability.
The corresponding Ea was 27.7 kJ/mol, much lower than the reported Ea values of RDMB elimination in the literature (Table S3). Additionally, Table S4 also comprehensively compares CuCoNC with other reported catalysts for RDMB degradation. These comparisons further demonstrate that CuCoNC was a superior activator for Oxone to degrade RDMB.
In Figure 9b, the pH effect was then studied. At pH = 7, RDMB was degraded quickly; nevertheless, at pH = 5, the k dropped to 0.178 min−1. At pH = 3, RDMB degradation was even slower with k = 0.107 min−1. H+ under these acidic conditions might react with radicals to “destroy” them as follows: [48]:
H+ + SO4•− + e− → HSO4−
H+ + •OH + e− → H2O
Furthermore, Oxone tends to become sluggish in acidic environments [49], thus resulting in less effective RDMB degradation. As the solution was alkaline at pH = 9, RDMB elimination seemed to remain the same with a slightly increased rate constant of 0.247 min−1. This might be because RDMB is a cationic molecule, the surface charge of CuCoNC became relatively more negative as shown in Figure S4. Therefore, the electrostatic attraction between RDMB and CuCoNC might grow stronger, facilitating RDMB degradation. At pH = 11, the k considerably dropped to k = 0.004 min−1. This result also demonstrates that the strongly alkaline condition would cause unfavorable effects on RDMB degradation [49].
In addition to DI water, tap water and seawater would be also employed as water matrices in Figure 9d. With tap water, the beginning of RDMB elimination with k = 0.183 min−1 was still very efficient. Tap water may contain impurities but the RDMB elimination was quite effective, as RDMB was still eliminated. In the case of seawater, the lowered k value of 0.123 min−1 was obtained as seawater contains a large amounts of substances and impurities, such as Cl-, which has been validated to interfere with Oxone [50].
3.4. Reusability Test
Since CuCoNC can efficiently eliminate RDMB, the reusability of CuCoNC to eliminate RDMB over multiple rounds was examined. Figure 9f shows that CuCoNC could eliminate RDMB over 5 rounds. This result validates that CuCoNC retained its effectiveness for Oxone activation in RDMB elimination. The XRD analysis of spent CuCoNC (Figure S5) shows that it remained almost comparable, demonstrating that CuCoNC would be a durable activator.
3.5. Mechanistic Studies by CuCoNC+Oxone
For further unraveling the degradation mechanism, a few probe agents were then employed for elucidating ROS during RDMB degradation. At first, t-butyl alcohol (TBA) was used to be a probe agent for •OH, while MeOH would be used to identify presence of both SO4•− and •OH. After TBA was added to the solution, RDMB degradation proceeded noticeably slower (Figure S6a), and the k decreased from 0.219 to 0.050 min−1 (Figure S6b), signifying that •OH shall occur during RDMB elimination. Next, as MeOH was used, the RDMB elimination was substantially inhibited with k = 0.006 min−1, signifying that SO4•− and •OH co-exist in RDMB elimination. Moreover, benzoquinone (BQ) was adopted to examine superoxide (O2•−). Figure S6a reveals that BQ inhibited RDMB elimination with k = 0.034 min−1, demonstrating that O2•− might be present in RDMB elimination. Moreover, NaN3 was used to study singlet oxygen (1O2), and NaN3 also inhibited RDMB elimination with k = 0.024 min−1, revealing that 1O2 is also produced from CuCoNC+Oxone.
The occurrence of these radicals at different reaction times were then determined [51,52]. In particular, Figure S6c shows the variations of SO4•− and •OH as a function of the reaction time. Nevertheless, the concentration of SO4•− seemed much higher than that of •OH, which agrees with the above-mentioned discussions.
Further, the occurrence of 1O2 was associated with furfuryl alcohol (FFA) consumption [53]. Figure S6c reveals the FFA oxidation by using Oxone alone and CuCoNC+Oxone. Oxone alone seemed to barely lead to FFA consumption, whereas CuCoNC+Oxone led to significant oxidation of FFA, suggesting that CuCoNC would cause the consumption of Oxone and promote production of 1O2.
Additionally, electron spin resonance (ESR) was employed here to measure radicals species in RDMB degradation. Firstly, when 5,5-Dimethyl-1-pyrroline N-oxide (DMPO) was adopted, no distinct feature was observed in the case of Oxone alone (Figure S6d). When DMPO, Oxone, and CuCoNC were added simultaneously, a noticeable result was produced and attributed to DMPO-SO4 and DMPO-OH, as displayed in Figure S6e, validating the existence of SO4•− and •OH. Moreover, the same test was then implemented in MeOH to examine whether O2•− was present (Figure S6e), and a notable signal was observed and ascribed to DMPO-O2.
On the other hand, as another trapping agent 2,2,6,6-tetramethylpiperidine (TEMP) was used, no notable signal was seen for Oxone alone. Once TEMP, Oxone, and CuCoNC were all present, a strong signal was observed and ascribed to TEMP-1O2 (Figure S6e), confirming that 1O2 existed.
3.6. Decomposition Process of RDMB
As CuCoNC can promote Oxone activation to eliminate RDMB, it was essential to study the RDMB elimination process. To this end, the First-Principle-based calculation was employed here [54,55]. In Figure 10a, the optimal configuration of RDMB is calculated and afforded. Specifically, Figure 10b,c reveals the HOMO and LUMO of RDMB. Generally, HOMO regions are relatively susceptible to electrophilic degradation. As SO4•−, •OH, and 1O2 would be electrophilic species, the HOMO at the xanthene group of RDMB would be the most susceptible to electrophilic degradation by these species. Figure 10d depicts the ESP distribution of RDMB, indicating that the electron-rich area of RDMB is also relatively accessible to electrophilic degradation.
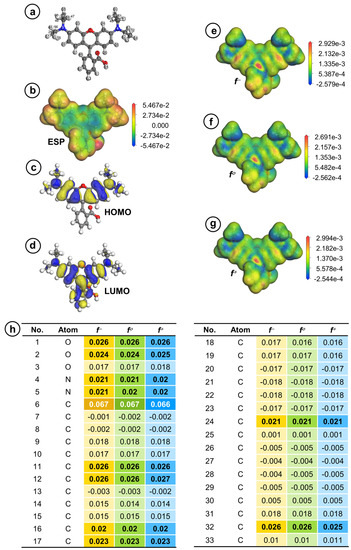
Figure 10.
(a) Optimized structure; (b) ESP; (c) HOMO; (d) LUMO; (e–g) electron density mapped by (e) f−, (f) fο, and (g) f+; and (h) Fukui indexes of RDMB molecule (isosurface value = 0.02 electrons/Å3).
The Fukui values of RDMB are also generated and summarized in Figure 10e–h for determining potential sites of RDMB for receiving attacks. In general, f –, f 0, and f + indicate the electrophilic, radical, and nucleophilic attacks, separately. As 1O2 is an electrophilic molecule, fractions of RDMB with larger f-values would be more susceptible to electrophilic degradation. Thus, 6C exhibits the highest f-values, indicating that 6C might be the initial site for receiving electrophilic degradation (as seen in Figure 10e). In addition, fractions of RDMB with high f 0 values are also more susceptible to radical degradation; similarly, 6C might be more susceptible to radical degradation during RDMB elimination (as seen in Figure 10f).
To further examine the degradation process of RDMB using CuCoNC+Oxone, the mass spectrometry of RDMB elimination (Figure S7 and Table S5) was then analyzed and studied with the computational insights to deduce the elimination route in Figure 11. Initially, the bonding between the xanthene ring and benzoic acid group of RDMB received initial attacks that caused the breakage of RDMB, forming P1 and P2 intermediates. P2 would then undergo further oxidation to produce a series of intermediates, such as P3, P4, and P5. Next, P2 was degraded to afford P6, which would then evolve upon oxidation to become P7. P5 and P7, subsequently, would be further broken down to afford a few intermediates, including P8 and P9, finally to CO2 and H2O.
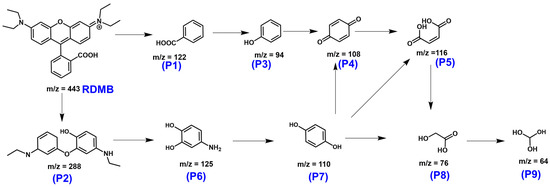
Figure 11.
A potential degradation pathway of RDMB degradation by CuCoNC-activated Oxone.
4. Conclusions
In this study, CuCoNC was fabricated to reveal a special hollow-configured morphology and Cu/Co using the template of Co-ZIF followed by the straightforward etching and Cu dopant to afford a practical activator for Oxone. This Cu doping enabled the encapsulation of CuCo into NC, enabling CuCoNC to possess enhanced porous structures, more active sites, and strengthened electrochemistry. Thus, CuCoNC showed much higher catalytic activities than CoNC for Oxone activation to eliminate RDMB. In addition, CuCoNC+Oxone exhibited a lower activation energy for RDMB elimination and retained its effectiveness in eliminating RDMB. These results render CuCoNC a promising activator for Oxone to degrade RDMB.
Supplementary Materials
The supplementary material can be downloaded at: https://www.mdpi.com/article/10.3390/nano13182565/s1. Table S1. Fractions of N species in catalysts. Table S2. Ratios of ID/IG in catalysts. Table S3. A comparison of activation energy (Ea) between CuCoNC+Oxone and other processes for RDMB degradation. Table S4. A comparison between CuCoNC+Oxone and other processes for RDMB degradation. Table S5. Detected by-products of RDMB degradation by CuCoNC+Oxone. Figure S1. (a) SEM, (b) TEM, and (c) EDS of CoNC. Figure S2. (a) SEM image and (b) textural properties of commercial Co3O4 NP. Figure S3. XPS spectroscopy of CoNC: (a) C1s (b) Co2p, and (c) N1s. Figure S4. zeta potential of CuCoNC. Figure S5. The XRD pattern of used CuCoNC. Figure S6. ESI mass spectra of (a) RDMB and (b) intermediates of RDMB degradation. Figure S7. (a) Effects of various scavengers on RDMB degradation (initial concentration of RDMB = 10 mg/L) and (b) corresponding rate constants; EPR analyses: (c) DMPO, (d) DMPO in methanol, (e) TEMP, (f) Concentrations of ROS; and FFA consumption by CuCoNC+Oxone. (References [56,57,58,59,60,61,62,63] are cited in the supplementary materials).
Author Contributions
Conceptualization, T.D.T. and J.-Y.L.; methodology, H.-C.C.; software, N.N.H.; validation, S.G.; formal analysis, V.S.M.; data curation, Y.F.Y.; writing—original draft preparation, K.-Y.A.L.; writing—review and editing, Y.-F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cui, K.-P.; He, Y.-Y.; Xu, K.-J.; Zhang, Y.; Chen, C.-B.; Xu, Z.-J.; Chen, X. Degradation of Tetracycline Hydrochloride by Cu-Doped MIL-101(Fe) Loaded Diatomite Heterogeneous Fenton Catalyst. Nanomaterials 2022, 12, 811. [Google Scholar] [CrossRef]
- Hassani, A.; Eghbali, P.; Mahdipour, F.; Wacławek, S.; Lin, K.-Y.A.; Ghanbari, F. Insights into the synergistic role of photocatalytic activation of peroxymonosulfate by UVA-LED irradiation over CoFe2O4-rGO nanocomposite towards effective Bisphenol A degradation: Performance, mineralization, and activation mechanism. Chem. Eng. J. 2023, 453, 139556. [Google Scholar] [CrossRef]
- Choong, Z.Y.; Lin, K.Y.A.; Lisak, G.; Lim, T.T.; Oh, W.D. Multi-heteroatom-doped carbocatalyst as peroxymonosulfate and peroxydisulfate activator for water purification: A critical review. J. Hazard. Mater. 2022, 426, 128077. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Yang, S.Y.; Wang, P.; Yang, X.; Shan, L.; Zhang, W.Y.; Shao, X.T.; Niu, R. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide. J. Hazard. Mater. 2010, 179, 552–558. [Google Scholar] [CrossRef]
- Zhang, B.T.; Xiang, W.X.; Jiang, X.M.; Zhang, Y.; Teng, Y.G. Oxidation of Dyes by Alkaline-Activated Peroxymonosulfate. J Environ. Eng. 2016, 142, 04016003. [Google Scholar] [CrossRef]
- Wei, G.L.; Liang, X.L.; He, Z.S.; Liao, Y.S.; Xie, Z.Y.; Liu, P.; Ji, S.C.; He, H.P.; Li, D.Q.; Zhang, J. Heterogeneous activation of Oxone by substituted magnetites Fe3−xMxO4 (Cr, Mn, Co, Ni) for degradation of Acid Orange II at neutral pH. J. Mol. Catal. A Chem. 2015, 398, 86–94. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, H.; Zhong, X.; Hou, L. Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a bimetallic Fe–Co/SBA-15 catalyst for the degradation of Orange II in water. J. Hazard. Mater. 2015, 283, 70–79. [Google Scholar] [CrossRef]
- Hu, L.; Yang, F.; Lu, W.; Hao, Y.; Yuan, H. Heterogeneous activation of oxone with CoMg/SBA-15 for the degradation of dye Rhodamine B in aqueous solution. Appl. Catal. B Environ. 2013, 134, 7–18. [Google Scholar] [CrossRef]
- Su, S.; Guo, W.; Leng, Y.; Yi, C.; Ma, Z. Heterogeneous activation of Oxone by CoxFe3−xO4 nanocatalysts for degradation of rhodamine B. J. Hazard. Mater. 2013, 244, 736–742. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chang, H.-A. Zeolitic Imidazole Framework-67 (ZIF-67) as a heterogeneous catalyst to activate peroxymonosulfate for degradation of Rhodamine B in water. J. Taiwan Inst. Chem. Eng. 2015, 53, 40–45. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chen, Y.-C.; Lin, T.-Y.; Yang, H. Lanthanum cobaltite perovskite supported on zirconia as an efficient heterogeneous catalyst for activating Oxone in water. J. Colloid Interface Sci. 2017, 497, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.-H.; Kwon, E.; Chang, H.-C.; Bui, H.M.; Phattarapattamawong, S.; Tsai, Y.-C.; Lin, K.-Y.A.; Ebrahimi, A.; Yee, Y.F.; Yuan, M.-H. Modulating Direct Growth of Copper Cobaltite Nanostructure on Copper Mesh as a Hierarchical Catalyst of Oxone Activation for Efficient Elimination of Azo Toxicant. Nanomaterials 2022, 12, 4396. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-F.; Lai, Y.-R.; Sung, H.-L.; Chung, T.-W.; Lin, K.-Y.A. Design of Amine-Modified Zr–Mg Mixed Oxide Aerogel Nanoarchitectonics with Dual Lewis Acidic and Basic Sites for CO2/Propylene Oxide Cycloaddition Reactions. Nanomaterials 2022, 12, 3442. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-J.; Park, Y.-K.; Bui, H.M.; Huy, N.N.; Lin, C.-H.; Ghotekar, S.; Wi-Afedzi, T.; Lin, K.-Y.A. Hofmann-MOF-derived CoFeNi nanoalloy@ CNT as a magnetic activator for peroxymonosulfate to degrade benzophenone-1 in water. J. Alloys Compd. 2022, 937, 165189. [Google Scholar] [CrossRef]
- Wu, J.; Yan, X.; Wang, W.; Jin, M.; Xie, Y.; Wang, C. Highly Dispersed CoNi Alloy Embedded in N-doped Graphitic Carbon for Catalytic Transfer Hydrogenation of Biomass-derived Furfural. Chem. Asian J. 2021, 16, 3194–3201. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lu, S.; Zheng, J.; Li, N.; Lou, Y.; Tang, J.; Zhou, J.; Zhang, H.; Huang, M.; Wang, D. MOFs-derived hollow FeCo@C as peroxymonosulfate activator for degradation of organic pollutants: Insight into the catalytic sites by experimental and theoretical study. Sep. Purif. Technol. 2022, 299, 121779. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, R.; Wei, L.; Zhang, F.; Chen, Q. Synthesis of FeCo nanocrystals encapsulated in nitrogen-doped graphene layers for use as highly efficient catalysts for reduction reactions. Nanoscale 2015, 7, 450–454. [Google Scholar] [CrossRef]
- Deng, Y.; Zou, X.; Liu, Z.; Wang, J.; Wang, Z.; Tang, J. Co7Fe3/CoFe2O4@C Lamellar composites derived from Co–Fe LDH/PVA as an effective heterogeneous activator of peroxymonosulfate. J. Alloys Compd. 2021, 854, 157244. [Google Scholar] [CrossRef]
- Shukla, P.; Sun, H.; Wang, S.; Ang, H.M.; Tadé, M.O. Nanosized Co3O4/SiO2 for heterogeneous oxidation of phenolic contaminants in waste water. Sep. Purif. Technol. 2011, 77, 230–236. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, Z.; Sun, H.; Wang, S. Hydrothermal Synthesis of Co3O4–Graphene for Heterogeneous Activation of Peroxymonosulfate for Decomposition of Phenol. Ind. Eng. Chem. Res. 2012, 51, 14958–14965. [Google Scholar] [CrossRef]
- Ma, W.J.; Wang, N.; Du, Y.C.; Tong, T.Z.; Zhang, L.J.; Lin, K.Y.A.; Han, X.J. One-step synthesis of novel Fe3C@nitrogen-doped carbon nanotubes/graphene nanosheets for catalytic degradation of Bisphenol A in the presence of peroxymonosulfate. Chem. Eng. J. 2019, 356, 1022–1031. [Google Scholar] [CrossRef]
- Wang, N.; Ma, W.J.; Ren, Z.Q.; Du, Y.C.; Xu, P.; Han, X.J. Prussian blue analogues derived porous nitrogen-doped carbon microspheres as high-performance metal-free peroxymonosulfate activators for non-radical-dominated degradation of organic pollutants. J. Mater. Chem. A 2018, 6, 884–895. [Google Scholar] [CrossRef]
- Nguyen, H.; Lee, J.; Kwon, E.; Lisak, G.; Thanh, B.X.; Ghanbari, F.; Lin, K.Y.A. Bamboo-like N-doped carbon nanotube-confined cobalt as an efficient and robust catalyst for activating monopersulfate to degrade bisphenol A. Chemosphere 2021, 279, 130569. [Google Scholar] [CrossRef]
- Duan, X.G.; Sun, H.Q.; Wang, Y.X.; Kang, J.; Wang, S.B. N-Doping-Induced Nonradical Reaction on Single-Walled Carbon Nanotubes for Catalytic Phenol Oxidation. Acs Catal. 2015, 5, 553–559. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, S.; Zhou, Y.; Yang, Y.; Zhang, J.; Luo, L.; Duan, X.; Wang, S.; Wang, L.; Tsang, D.C.W. Insights into the oxidation of organic contaminants by iron nanoparticles encapsulated within boron and nitrogen co-doped carbon nanoshell: Catalyzed Fenton-like reaction at natural pH. Environ. Int. 2019, 128, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zhou, L.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. Carbon microspheres supported cobalt catalysts for phenol oxidation with peroxymonosulfate. Chem. Eng. Res. Des. 2015, 101, 15–21. [Google Scholar] [CrossRef]
- Wu, S.; Liu, J.; Wang, H.; Yan, H. A review of performance optimization of MOF-derived metal oxide as electrode materials for supercapacitors. Int. J. Energy Res. 2019, 43, 697–716. [Google Scholar] [CrossRef]
- Lin, J.Y.; Lee, J.; Oh, W.D.; Kwon, E.; Tsai, Y.C.; Lisak, G.; Phattarapattamawong, S.; Hu, C.; Lin, K.Y.A. Hierarchical ZIF-decorated nanoflower-covered 3-dimensional foam for enhanced catalytic reduction of nitrogen-containing contaminants. J. Colloid Interface Sci. 2021, 602, 95–104. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Lops, C.; Ancona, A.; Di Cesare, K.; Dumontel, B.; Garino, N.; Canavese, G.; Hérnandez, S.; Cauda, V. Sonophotocatalytic degradation mechanisms of Rhodamine B dye via radicals generation by micro- and nano-particles of ZnO. Appl. Catal. B 2019, 243, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-Y.A.; Chang, H.-A. Ultra-high adsorption capacity of zeolitic imidazole framework-67 (ZIF-67) for removal of malachite green from water. Chemosphere 2015, 139, 624–631. [Google Scholar] [CrossRef]
- Tuan, D.D.; Lin, K.-Y.A. Ruthenium supported on ZIF-67 as an enhanced catalyst for hydrogen generation from hydrolysis of sodium borohydride. Chem. Eng. J. 2018, 351, 48–55. [Google Scholar] [CrossRef]
- Christensen, D.B.; Mortensen, R.L.; Kramer, S.; Kegnæs, S. Study of CoCu Alloy Nanoparticles Supported on MOF-Derived Carbon for Hydrosilylation of Ketones. Catal. Lett. 2020, 150, 1537–1545. [Google Scholar] [CrossRef]
- Liu, W.-J.; Kwon, E.; Huy, N.N.; Khiem, T.C.; Lisak, G.; Wi-Afedzi, T.; Wu, C.-C.; Ghanbari, F.; Lin, K.-Y.A. Facilely-prepared sulfide-doped Co3O4 nanocomposite as a boosted catalyst for activating Oxone to degrade a sunscreen agent. J. Taiwan Inst. Chem. Eng. 2022, 133, 104253. [Google Scholar] [CrossRef]
- Li, G.; Sun, J.H.; Hou, W.P.; Jiang, S.D.; Huang, Y.; Geng, J.X. Three-dimensional porous carbon composites containing high sulfur nanoparticle content for high-performance lithium-sulfur batteries. Nat. Commun. 2016, 7, 10601. [Google Scholar] [CrossRef]
- Hasan, Z.; Cho, D.W.; Chon, C.M.; Yoon, K.; Song, H. Reduction of p-nitrophenol by magnetic Co-carbon composites derived from metal organic frameworks. Chem. Eng. J. 2016, 298, 183–190. [Google Scholar] [CrossRef]
- Sun, H.; Zelekew, O.A.; Chen, X.; Guo, Y.; Kuo, D.-H.; Lu, Q.; Lin, J. A noble bimetal oxysulfide CuVOS catalyst for highly efficient catalytic reduction of 4-nitrophenol and organic dyes. RSC Adv. 2019, 9, 31828–31839. [Google Scholar] [CrossRef]
- Osadchii, D.Y.; Olivos-Suarez, A.I.; Bavykina, A.V.; Gascon, J. Revisiting Nitrogen Species in Covalent Triazine Frameworks. Langmuir 2017, 33, 14278–14285. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Sun, Z. CoNi alloy anchored onto N-doped porous carbon for the removal of sulfamethoxazole: Catalyst, mechanism, toxicity analysis, and application. Chemosphere 2022, 308, 136291. [Google Scholar] [CrossRef]
- Sun, X.; Qi, H.; Mao, S.; Sun, Z. Atrazine removal by peroxymonosulfate activated with magnetic CoFe alloy@N-doped graphitic carbon encapsulated in chitosan carbonized microspheres. Chem. Eng. J. 2021, 423, 130169. [Google Scholar] [CrossRef]
- Miao, J.; Geng, W.; Alvarez, P.J.J.; Long, M. 2D N-Doped Porous Carbon Derived from Polydopamine-Coated Graphitic Carbon Nitride for Efficient Nonradical Activation of Peroxymonosulfate. Environ. Sci. Technol. 2020, 54, 8473–8481. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, X.; Hu, X.; Zhou, W.; Zhao, Y. Effect of Formic Acid Treatment on the Structure and Catalytic Activity of Co3O4 for N2O Decomposition. Catal. Lett. 2019, 149, 1026–1036. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Zhang, L.; Jiang, D. Surface oxygen vacancies on Co3O4 mediated catalytic formaldehyde oxidation at room temperature. Catal. Sci. Technol. 2016, 6, 3845–3853. [Google Scholar] [CrossRef]
- Qu, S.; Yuan, Y.; Yang, X.; Xu, H.; Mohamed, A.K.; Zhang, J.; Zhao, C.; Liu, L.; Wang, B.; Wang, X.; et al. Carbon defects in biochar facilitated nitrogen doping: The significant role of pyridinic nitrogen in peroxymonosulfate activation and ciprofloxacin degradation. Chem. Eng. J. 2022, 441, 135864. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.-M.; Meng, X.-Y.; Li, S.-N.; Zeng, J.-H.; Chen, Y. Ultrathin Co3O4 Nanomeshes for the Oxygen Evolution Reaction. ACS Catal. 2018, 8, 1913–1920. [Google Scholar] [CrossRef]
- Othman, I.; Hisham Zain, J.; Abu Haija, M.; Banat, F. Catalytic activation of peroxymonosulfate using CeVO4 for phenol degradation: An insight into the reaction pathway. Appl. Catal. B 2020, 266, 118601. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Lee, J.; Kwon, E.; Lisak, G.; Thanh, B.X.; Oh, W.D.; Lin, K.-Y.A. Metal-complexed covalent organic frameworks derived N-doped carbon nanobubble–embedded cobalt nanoparticle as a magnetic and efficient catalyst for oxone activation. J. Colloid Interface Sci. 2021, 591, 161–172. [Google Scholar] [CrossRef]
- Guo, W.; Su, S.; Yi, C.; Ma, Z. Degradation of antibiotics amoxicillin by Co3O4-catalyzed peroxymonosulfate system. Environ. Prog. Sustain. Energy 2013, 32, 193–197. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Wang, Y.; Le Roux, J.; Yang, Y.; Croué, J.-P. Efficient Peroxydisulfate Activation Process Not Relying on Sulfate Radical Generation for Water Pollutant Degradation. Environ. Sci. Technol. 2014, 48, 5868–5875. [Google Scholar] [CrossRef]
- Yin, J.-Y.; Wang, H.; Yu, K.-P.; Lee, J.; Lin, K.-Y.A. Degradation of sunscreen agent 2-phenylbenzimidazole-5-sulfonic acid using monopersulfate activated by MOF-derived cobalt sulfide nanoplates. J. Water Process Eng. 2021, 44, 102282. [Google Scholar] [CrossRef]
- Khiem, T.C.; Tuan, D.D.; Kwon, E.; Huy, N.N.; Oh, W.-D.; Chen, W.-H.; Lin, K.-Y.A. Degradation of dihydroxybenzophenone through monopersulfate activation over nanostructured cobalt ferrites with various morphologies: A comparative study. Chem. Eng. J. 2022, 450, 137798. [Google Scholar] [CrossRef]
- Mostafa, S.; Rosario-Ortiz, F.L. Singlet oxygen formation from wastewater organic matter. Environ. Sci. Technol. 2013, 47, 8179–8186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Gong, M.; Wang, W.; Mu, Y.; Hu, Z.-H. α-MnO2/Palygorskite composite as an effective catalyst for heterogeneous activation of peroxymonosulfate (PMS) for the degradation of Rhodamine B. Sep. Purif. Technol. 2020, 230. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, Z.; Fang, L.; Guo, Y.; Feng, Y.; Yang, M. Kinetic and Mechanistic Study of Rhodamine B Degradation by H2O2 and Cu/Al2O3/g-C3N4 Composite. Catalysts 2020, 10, 317. [Google Scholar] [CrossRef]
- Hira, S.A.; Yusuf, M.; Annas, D.; Hui, H.S.; Park, K.H. Biomass-Derived Activated Carbon as a Catalyst for the Effective Degradation of Rhodamine B dye. Processes 2020, 8, 926. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, C.; Wei, H.; Wang, Q.; Li, K. In situ synthesis and immobilization of a Cu(ii)–pyridyl complex on silica microspheres as a novel Fenton-like catalyst for RhB degradation at near-neutral pH. RSC Adv. 2017, 7, 22825–22835. [Google Scholar] [CrossRef]
- Gan, P.P.; Li, S.F.Y. Efficient removal of Rhodamine B using a rice hull-based silica supported iron catalyst by Fenton-like process. Chem. Eng. J. 2013, 229, 351–363. [Google Scholar] [CrossRef]
- Du, Y.; Ma, W.; Liu, P.; Zou, B.; Ma, J. Magnetic CoFe2O4 nanoparticles supported on titanate nanotubes (CoFe2O4/TNTs) as a novel heterogeneous catalyst for peroxymonosulfate activation and degradation of organic pollutants. J. Hazard Mater. 2016, 308, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, Y.; Chen, D.; Wang, C.; Guan, W. Activation of peroxymonosulfate by Co-Mg-Fe layered doubled hydroxide for efficient degradation of Rhodamine B. Environ. Sci. Pollut. Res. 2023, 30, 37634–37645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-J.; Chai, B.-L.; Xu, H.; Zheng, T.-F.; Zhu, Z.-H.; Peng, Y.; Chen, J.-L.; Liu, S.-J.; Wen, H.-R. Enhanced Heterogeneous Catalytic Activity of Peroxymonosulfate for Rhodamine B Degradation via a CoII-Based Metal–Organic Framework. Inorg. Chem. 2023, 62, 2760–2768. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).