Rapid SERS Detection of Botulinum Neurotoxin Type A
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Oligonucleotides
2.3. Affinity Assay
2.4. Circular Dichroism and UV Spectroscopy
2.5. SERS Substrates
2.6. Aptamer Immobilization
2.7. Reaction with the Antigen
2.8. SERS Measurements
2.9. Study of Surface Topology
3. Results
3.1. Selection of the Aptamer for the Biosensor
3.2. Determination of the Optimal Aptamer Concentration
- Narrow distribution of SERS intensity, providing good reproducibility of experimental results;
- The average SERS intensity value that provides the lowest possible BoNT A detection limit.
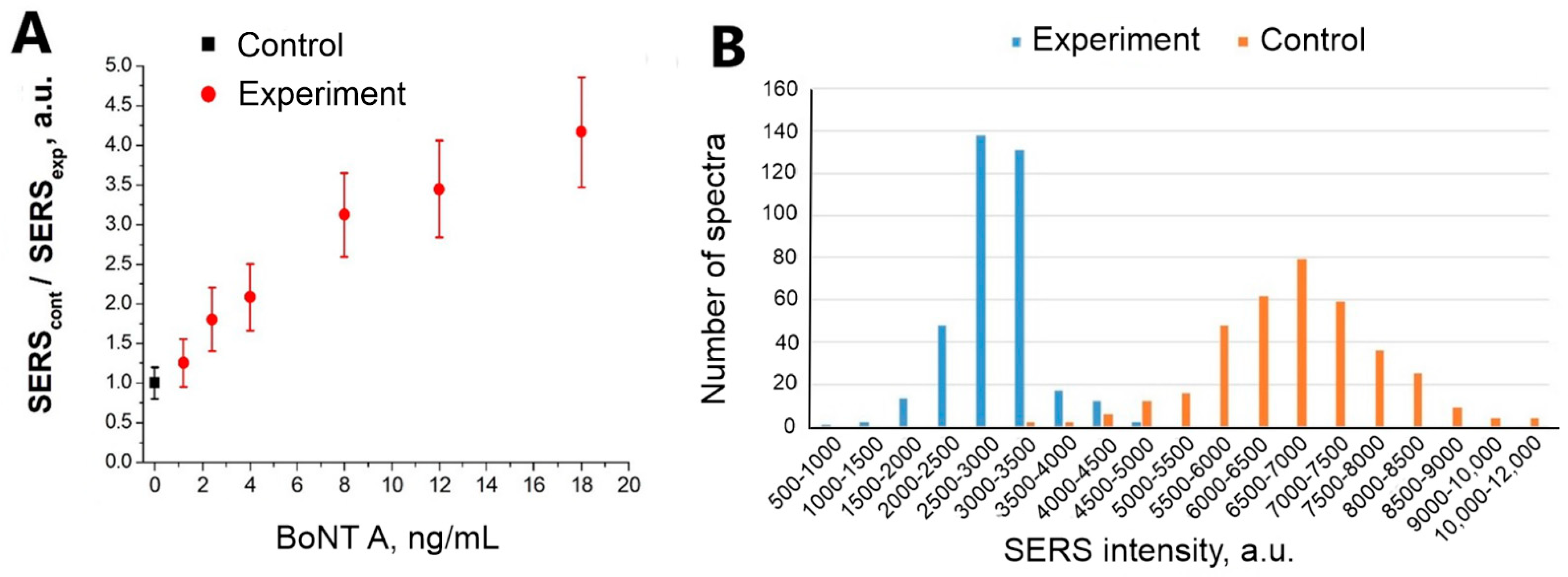
3.3. Detection of BoNT A
3.4. Choosing the Optimal Analysis Time
3.5. Defining the Specificity of BoNT A Binding on SERS Substrate
3.6. The Influence of the Matrix on BoNT A Detection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecuccco, C. Botulinum neurotoxins: Biology, pharmacology and toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef]
- Smith, T.J.; Hill, K.K.; Raphael, B.H. Historical and current perspectives on clostridium botulinum diversity. Res. Microbiol. 2015, 166, 290–302. [Google Scholar] [CrossRef]
- Rummel, A. The long journey of botulinum neurotoxins into the synapse. Toxicon 2015, 107, 9–24. [Google Scholar] [CrossRef]
- Tian, D.; Zheng, T. Comparison and analysis of biological agent category lists based on biosafety and biodefense. PLoS ONE 2014, 9, e101163. [Google Scholar] [CrossRef]
- Hill, K.K.; Smith, T.J.; Helma, C.H.; Ticknor, L.O.; Foley, B.T.; Svensson, R.T.; Brown, J.L.; Johnson, E.A.; Smith, L.A.; Okinaka, R.T.; et al. Genetic diversity among botulinum neurotoxin-producing clostridial strains. J. Bacteriol. 2007, 189, 818–832. [Google Scholar] [CrossRef] [PubMed]
- Bahman, J. Botulinum Toxin Treatment: What Everyone Should Know; Springer: Cham, Switzerland, 2018; ISBN 9783319999449. [Google Scholar]
- Lacy, D.; Tepp, W.; Cohen, A.; DasGupta, B.R.; Stevens, R.C. Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nat. Struct. Mol. Biol. 1998, 5, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Strotmeier, J.; Willjes, G.; Binz, T.; Rummel, A. Human synaptotagmin-II is not a high affinity receptor for botulinum neurotoxin B and G: Increased therapeutic dosage and immunogenicity. FEBS Lett. 2012, 586, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Kavalali, E.T. The mechanisms and functions of spontaneous neurotransmitter release. Nat. Rev. Neurosci. 2015, 16, 5–16. [Google Scholar] [CrossRef]
- Kamińska, A.; Winkler, K.; Kowalska, A.; Witkowska, E.; Szymborski, T.; Janeczek, A.; Waluk, J. SERS-based immunoassay in a microfluidic system for the multiplexed recognition of interleukins from blood plasma: Towards picogram detection. Sci. Rep. 2017, 7, 10656. [Google Scholar] [CrossRef]
- Lim, C.Y.; Granger, J.H.; Porter, M.D. SERS detection of Clostridium botulinum neurotoxin serotypes A and B in buffer and serum: Towards the development of a biodefense test platform. Anal. Chim. Acta X 2019, 1, 100002. [Google Scholar] [CrossRef]
- Kim, K.; Choi, N.; Jeon, J.H.; Rhie, G.-E.; Choo, J. SERS-based immunoassays for the detection of botulinum toxins A and B using magnetic beads. Sensors 2019, 19, 4081. [Google Scholar] [CrossRef]
- Arabi, M.; Ostovan, A.; Zhang, Z.; Wang, Y.; Mei, R.; Fu, L.; Wang, X.; Ma, J.; Chen, L. Label-free SERS detection of Raman-inactive protein biomarkers by Raman reporter indicator: Toward ultrasensitivity and universality. Biosens. Bioelectron. 2021, 174, 112825. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Jo, H.; Oh, S.S. Detection and beyond: Challenges and advances in aptamer-based biosensors. Mater. Adv. 2020, 1, 2663–2687. [Google Scholar] [CrossRef]
- Lou, X.; Qian, J.; Xiao, Y.; Viel, L.; Gerdon, A.E.; Lagally, E.T.; Atzberger, P.; Tarasow, T.M.; Heeger, A.J.; Soh, H.T. Micromagnetic selection of aptamers in microfluidic channels. Proc. Nat. Acad. Sci. USA 2009, 106, 2989–2994. [Google Scholar] [CrossRef] [PubMed]
- Tok, J.B.; Fischer, N.O. Single microbead SELEX for efficient ssDNA aptamer generation against botulinum neurotoxin. Chem. Commun. 2008, 16, 1883–1885. [Google Scholar]
- Oh, I.-H.; Park, D.-Y.; Cha, J.-M.; Shin, W.-R.; Kim, J.H.; Kim, S.C.; Cho, B.-K.; Ahn, J.-Y.; Kim, Y.-H. Docking simulation and sandwich assay for aptamer-based botulinum neurotoxin type C detection. Biosensors 2020, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.W.; Blank, M.; Janardhanan, P.; Singh, B.R.; Mello, C.; Blind, M.; Cai, S. In vitro selection of RNA aptamers that inhibit the activity of type A botulinum neurotoxin. Biochem. Biophys. Res. Commun. 2010, 396, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Ho, C.M. Aptamer-based electrochemical biosensor for Botulinum neurotoxin. Anal. Bioanal. Chem. 2009, 393, 1943–1948. [Google Scholar] [CrossRef]
- Kukushkin, V.; Ambartsumyan, O.; Subekin, A.; Astrakhantseva, A.; Gushchin, V.; Nikonova, A.; Dorofeeva, A.; Zverev, V.; Keshek, A.; Meshcheryakova, N.; et al. Multiplex lithographic SERS aptasensor for detection of several respiratory viruses in one pot. Int. J. Mol. Sci. 2023, 24, 8081. [Google Scholar]
- Frevert, J. Content of botulinum neurotoxin in Botox®/Vistabel®, Dysport®/Azzalure®, and Xeomin®/Bocouture®. Drugs R D 2010, 10, 67–73. [Google Scholar] [CrossRef]
- Oh, S.S.; Qian, J.; Lou, X.; Zhang, Y.; Xiao, Y.; Soh, H.T. Generation of highly specific aptamers via micromagnetic selection. Anal. Chem. 2009, 81, 5490–5495. [Google Scholar] [CrossRef] [PubMed]
- RNAfold. Available online: http://rna.tbi.univie.ac.at/ (accessed on 15 August 2023).
- Vorlíčková, M.; Kejnovská, I.; Bednářová, K.; Renčiuk, D.; Kypr, J. Circular dichroism spectroscopy of DNA: From duplexes to quadruplexes. Chirality 2012, 24, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Kukushkin, V.I.; Astrakhantseva, A.S.; Morozova, E.N. Influence of the morphology of metal nanoparticles deposited on surfaces of silicon oxide on the optical properties of SERS substrates. Bull. Russ. Acad. Sci. Phys. 2021, 85, 133–140. [Google Scholar] [CrossRef]
- Percze, K.; Szakács, Z.; Scholz, É.; András, J.; Szeitner, Z.; van den Kieboom, C.H.; Ferwerda, G.; de Jonge, M.I.; Gyurcsányi, R.E.; Mészáros, T. Aptamers for respiratory syncytial virus detection. Sci. Rep. 2017, 7, 42794. [Google Scholar] [CrossRef]
- Zengin, A.; Tamer, U.; Caykara, U. Fabrication of a SERS based aptasensor for detection of ricin B toxin. J. Mater. Chem. B 2015, 3, 306–315. [Google Scholar] [CrossRef]
- Pang, Y.; Wan, N.; Shi, L.; Wang, C.; Sun, Z.; Xiao, R.; Wang, S. Dual-recognition surface-enhanced Raman scattering (SERS) biosensor for pathogenic bacteria detection by using vancomycin-SERS tags and aptamer-Fe3O4@Au. Anal. Chim. Acta 2019, 1077, 288–296. [Google Scholar] [CrossRef]
- Zavyalova, E.; Ambartsumyan, O.; Zhdanov, G.; Gribanyov, D.; Gushchin, V.; Tkachuk, A.; Rudakova, E.; Nikiforova, M.; Kuznetsova, N.; Popova, L.; et al. SERS-based aptasensor for rapid quantitative detection of SARS-CoV-2. Nanomaterials 2021, 11, 1394. [Google Scholar] [CrossRef]
- Lu, X.; Hou, X.; Tang, H.; Yi, X.; Wang, J. A high-quality CdSe/CdS/ZnS quantum-dot-based FRET aptasensor for the simultaneous detection of two different Alzheimer’s disease core biomarkers. Nanomaterials 2022, 12, 4031. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, K.; Dhakal, S. FRET-based aptasensor for the selective and sensitive detection of lysozyme. Sensors 2020, 20, 914. [Google Scholar] [CrossRef]

| Code | Sequence | Length, Nucleotides | KD, nM | Reference |
|---|---|---|---|---|
| Apt_2.22 | 5′-ATACCAGCTTATTCAATTGACATGACTGGGATTTTTGGCG AAATCGAAGGAAGCGGAGAGATAGTAAGTGCAATCT-3′ | 76 | 3 | Tok and Fischer (2008) [16] |
| Apt_C | 5′-AGCAGCACAGAGGTCAGATGCTTGAGTGTCATGGACGTT CCGGTCTTGGGCGGGATATTTGTTTGTTTTCTGCCTATGTTC CTATGCGTGCTACCGTGAA-3′ | 100 | 34 | Lou et al. (2009) [15] |
| Apt_2 | 5′-AGCAGCACAGAGGTCAGATGAGGTTTAGTGAATATCTTC GATGATCCGAGGCAGGCTAGATTCCGAAACATCGCTGAGC GCCTATGCGTGCTACCGTGAA-3′ | 100 | No | Oh et al. (2009) [22] |
| Apt_2.22_mod | (SH-(CH2)6)-5′-ATACCAGCTTATTCAATTGACATGACTGGGATTT TTGGCGAAATCGAAGGAAGCGGAGAGATAGTAAGTGCAA TCT-3′-(TAMRA) | 76 | n.d. | This work |
| SH-H8-TAMRA | (SH-(CH2)6)-5′-AGTGCGGTGAGCCGTCGGACATACAAATAC-3′-(TAMRA) | 30 | No | Kukushkin et al. (2023) [20] |
| Aptamer Concentration, nM | Average Value of Signal Intensity (μ), a. u. | Variation Coefficient (V) |
|---|---|---|
| 140 | 30,000 ± 12,000 | 0.39 |
| 70 | 23,000 ± 7000 | 0.32 |
| 35 | 19,300 ± 1200 | 0.06 |
| 17.5 | 7600 ± 900 | 0.12 |
| 8.8 | 3300 ± 500 | 0.16 |
| Concentration of Apt_2.22_mod Aptamer, nM | Intensity Value (Experiment), a.u. | Intensity Value (Control), a.u. | Probability of Determining BoNT A (Concentration 2.4 ng/mL), % (p < 0.001) |
|---|---|---|---|
| 35 | 16,000 ± 2000 | 16,000 ± 2000 | 0 |
| 17.5 | 2900 ± 600 | 6500 ± 1200 | 95 |
| 8.8 | 2700 ± 1200 | 2000 ± 600 | 0 |
| Concentration of Apt_2.22_mod Aptamer, nM | Intensity Value (Experiment), a.u. | Intensity Value (Control), a.u. | Probability of Non-Specific Binding IgGHum (Concentration 2.4 ng/mL), % (p < 0.001) |
|---|---|---|---|
| 17.5 | 3500 ± 600 | 3500 ± 200 | No more than 2.5 |
| Concentration of SH-H8-TAMRA Aptamer, nM | Intensity Value (Experiment), a.u. | Intensity Value (Control), a.u. | Probability of Non-Specific Binding BoNT A (Concentration 2.4 ng/mL), % |
|---|---|---|---|
| 17.5 | 6400 ± 400 | 6100 ± 600 | No more than 2.5 |
| Concentration of Apt_2.22_mod Aptamer, nM | Intensity Value (Experiment), a.u. | Intensity Value (Control), a.u. | Probability of Determining BoNT A (Concentration 2.4 ng/mL), % (p < 0.001) |
|---|---|---|---|
| 17.5 | 1700 ± 100 | 2200 ± 200 | 95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subekin, A.; Alieva, R.; Kukushkin, V.; Oleynikov, I.; Zavyalova, E. Rapid SERS Detection of Botulinum Neurotoxin Type A. Nanomaterials 2023, 13, 2531. https://doi.org/10.3390/nano13182531
Subekin A, Alieva R, Kukushkin V, Oleynikov I, Zavyalova E. Rapid SERS Detection of Botulinum Neurotoxin Type A. Nanomaterials. 2023; 13(18):2531. https://doi.org/10.3390/nano13182531
Chicago/Turabian StyleSubekin, Alexei, Rugiya Alieva, Vladimir Kukushkin, Ilya Oleynikov, and Elena Zavyalova. 2023. "Rapid SERS Detection of Botulinum Neurotoxin Type A" Nanomaterials 13, no. 18: 2531. https://doi.org/10.3390/nano13182531
APA StyleSubekin, A., Alieva, R., Kukushkin, V., Oleynikov, I., & Zavyalova, E. (2023). Rapid SERS Detection of Botulinum Neurotoxin Type A. Nanomaterials, 13(18), 2531. https://doi.org/10.3390/nano13182531






