A Cocktail-Based Formula for the Design of Nanosized Cosmeceuticals as Skincare and Anti-Age Products
Abstract
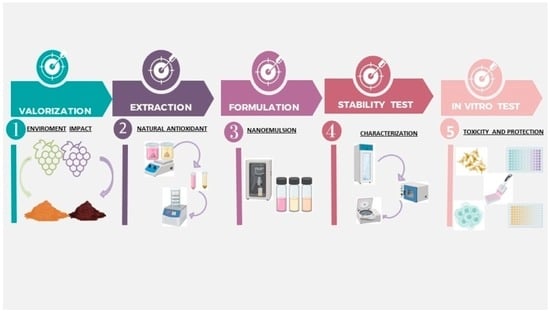
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Grape Pomace Extracts
2.3. Characterization of the Extracts
2.3.1. Folin–Ciocalteu and DPPH Colorimetric Assays
2.3.2. Identification and Quantification of Phenolic Components by HPLC
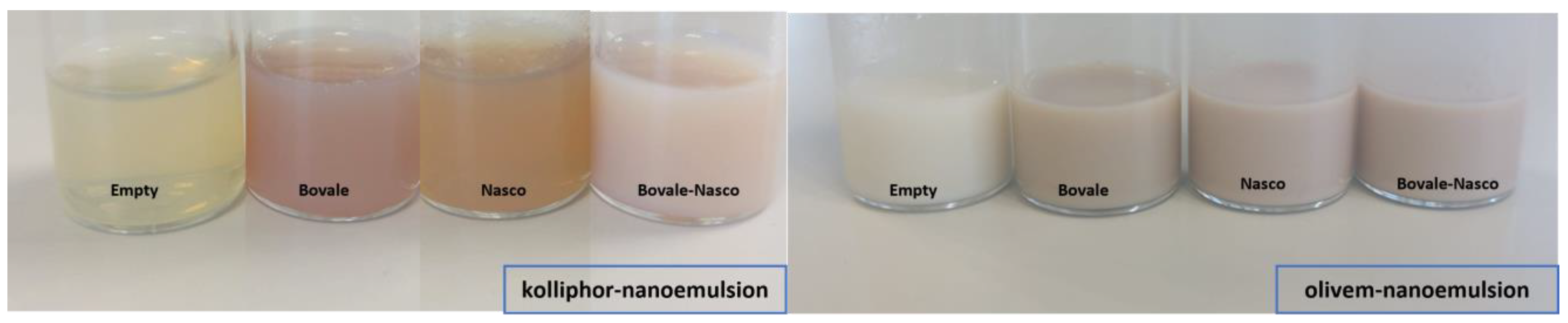
2.4. Preparation of Nanoemulsions
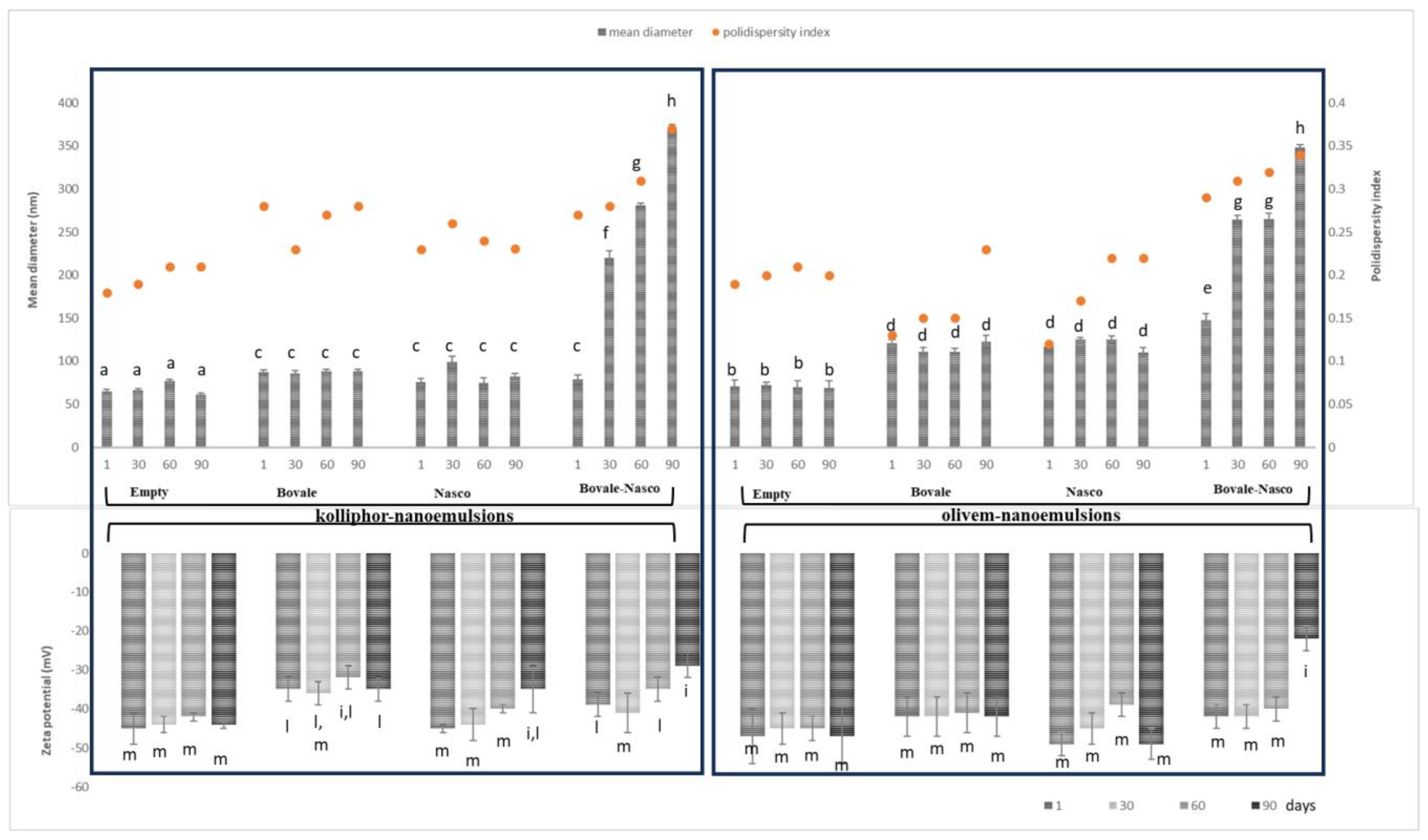
2.5. Characterization of Nanoemulsions
2.6. Stability of Nanoemulsions under Stress Conditions
2.7. UV Stability Testing
2.8. In Vitro Cytotoxicity of Formulations
2.9. In Vitro Protective Effect of Formulations against Oxidative Damage in Keratinocytes
2.10. Inhibitory Effect of Formulations in Nitric Oxide Generation by Macrophages
2.11. Statistical Analysis of the Data
3. Results
3.1. Characterization of Extracts
3.2. Characterization of Nanoemulsions
3.3. Stability of Nanoemulsions under Stress Conditions
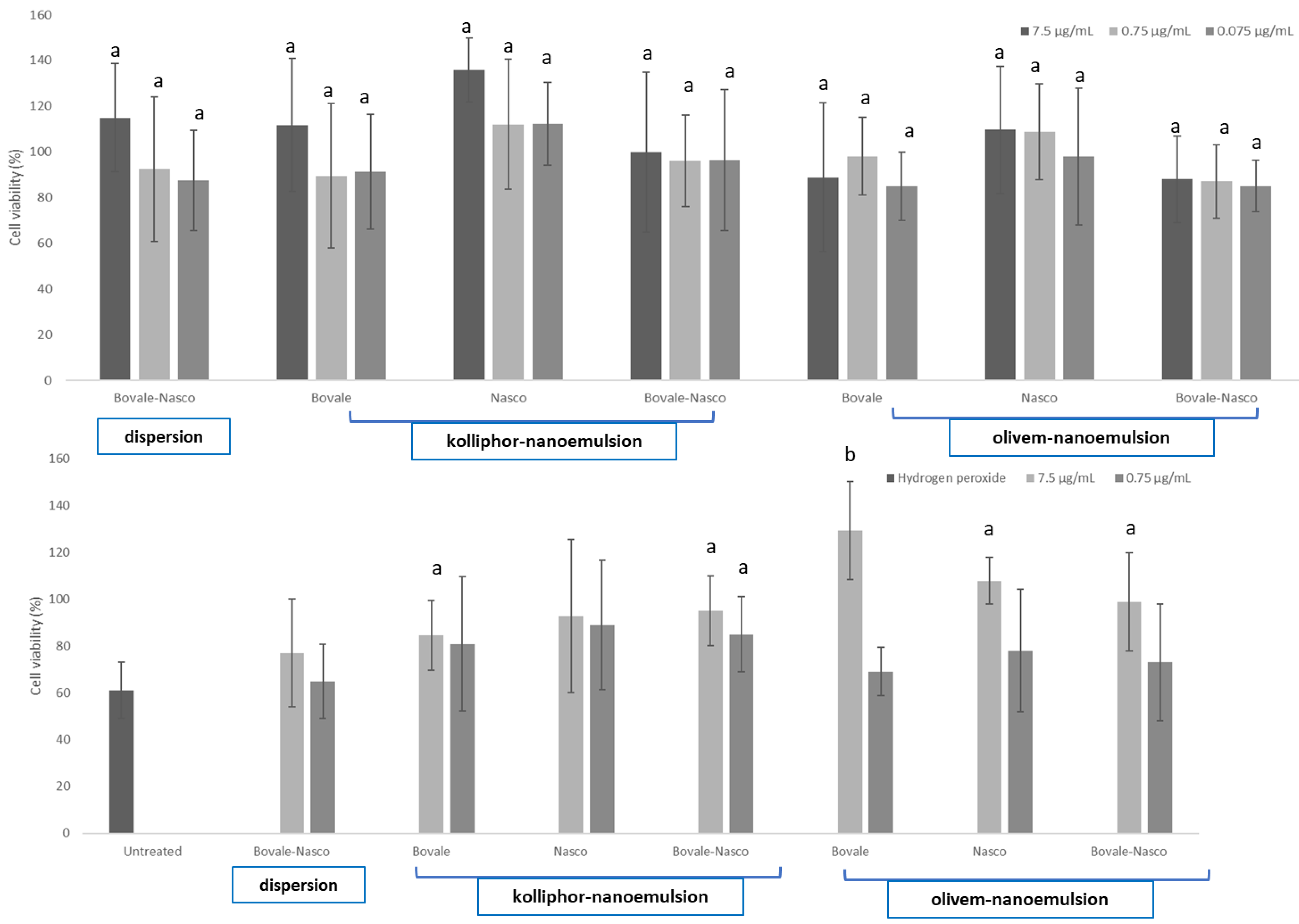
3.4. In Vitro Cytotoxicity of Nanoemulsions and Protection of Cells against Oxidative Damage
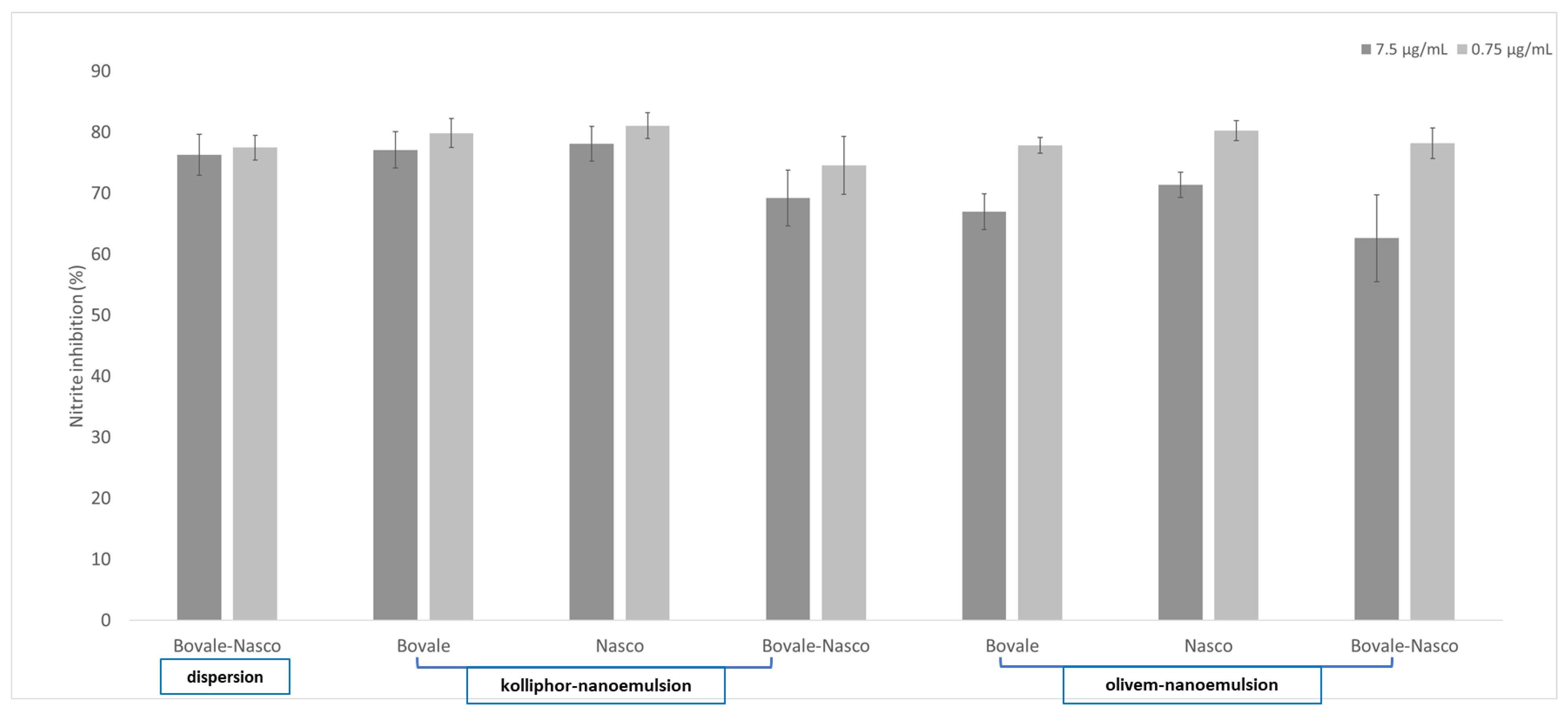
3.5. Inhibition of Nitric Oxide Generation in Macrophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, I.A.; Mikail, M.A.; Zamakshshari, N.; Abdullah, A.S.H. Natural Anti-Aging Skincare: Role and Potential. Biogerontology 2020, 21, 293–310. [Google Scholar] [CrossRef]
- Kohl, E.; Steinbauer, J.; Landthaler, M.; Szeimies, R.M. Skin Ageing. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Csekes, E.; Račková, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and Extrinsic Factors in Skin Ageing: A Review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Lin, P.C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for Physicochemical Characterization of Nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef]
- Ozogul, Y.; Karsli, G.T.; Durmuş, M.; Yazgan, H.; Oztop, H.M.; McClements, D.J.; Ozogul, F. Recent Developments in Industrial Applications of Nanoemulsions. Adv. Colloid Interface Sci. 2022, 304, 102685. [Google Scholar] [CrossRef] [PubMed]
- Datta, H.S.; Paramesh, R. Trends in Aging and Skin Care: Ayurvedic Concepts. J. Ayurveda Integr. Med. 2010, 1, 110–113. [Google Scholar] [CrossRef]
- Kusumawati, I.; Indrayanto, G. Natural Antioxidants in Cosmetics. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; Volume 40, pp. 485–505. [Google Scholar] [CrossRef]
- Moccia, F.; Agustin-Salazar, S.; Verotta, L.; Caneva, E.; Giovando, S.; D’Errico, G.; Panzella, L.; D’Ischia, M.; Napolitano, A. Antioxidant Properties of Agri-Food Byproducts and Specific Boosting Effects of Hydrolytic Treatments. Antioxidants 2020, 9, 438. [Google Scholar] [CrossRef]
- Sathya, R.; Valan Arasu, M.; Ilavenil, S.; Rejiniemon, T.S.; Vijayaraghavan, P. Cosmeceutical Potentials of Litchi Fruit and Its By-Products for a Sustainable Revalorization. Biocatal. Agric. Biotechnol. 2023, 50, 102683. [Google Scholar] [CrossRef]
- Perra, M.; Manca, M.L.; Tuberoso, C.I.G.; Caddeo, C.; Marongiu, F.; Peris, J.E.; Orrù, G.; Ibba, A.; Fernàndez-Busquets, X.; Fattouch, S.; et al. A Green and Cost-Effective Approach for the Efficient Conversion of Grape Byproducts into Innovative Delivery Systems Tailored to Ensure Intestinal Protection and Gut Microbiota Fortification. Innov. Food Sci. Emerg. Technol. 2022, 80, 103103. [Google Scholar] [CrossRef]
- Hübner, A.d.A.; de Aguiar, M.M.G.B.; Demarque, D.P.; Rosado, C.; Velasco, M.V.R.; Kikuchi, I.S.; Baby, A.R.; Pessoa, F.V.L.S. Technological Aspects and Potential Cutaneous Application of Wine Industry By-Products. Appl. Sci. 2023, 13, 9068. [Google Scholar] [CrossRef]
- Castro, M.L.; Ferreira, J.P.; Pintado, M.; Ramos, O.L.; Borges, S.; Baptista-Silva, S. Grape By-Products in Sustainable Cosmetics: Nanoencapsulation and Market Trends. Appl. Sci. 2023, 13, 9168. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural Antioxidants from Some Fruits, Seeds, Foods, Natural Products, and Associated Health Benefits: An Update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The Role of Plant-derived Natural Antioxidants in Reduction of Oxidative Stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Firoznezhad, M.; Caddeo, C.; Marongiu, F.; Escribano-Ferrer, E.; Sarais, G.; Peris, J.E.; Usach, I.; Zaru, M.; Manconi, M.; et al. Phytocomplexes Extracted from Grape Seeds and Stalks Delivered in Phospholipid Vesicles Tailored for the Treatment of Skin Damages. Ind. Crops Prod. 2019, 128, 471–478. [Google Scholar] [CrossRef]
- Vivarelli, S.; Costa, C.; Teodoro, M.; Giambò, F.; Tsatsakis, A.M.; Fenga, C. Polyphenols: A Route from Bioavailability to Bioactivity Addressing Potential Health Benefits to Tackle Human Chronic Diseases. Arch. Toxicol. 2023, 97, 3–38. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Tsagkaris, C.; Matthews, L.; Gautam, R.K.; Kamal, M.A. Novel Approaches for the Application of Herbs for Skin Care. Curr. Pharm. Biotechnol. 2022, 24, 164–187. [Google Scholar] [CrossRef]
- Sharma, A.; Kuhad, A.; Bhandari, R. Novel Nanotechnological Approaches for Treatment of Skin-Aging. J. Tissue Viability 2022, 31, 374–386. [Google Scholar] [CrossRef]
- Eskens, O.; Amin, S. Challenges and Effective Routes for Formulating and Delivery of Epidermal Growth Factors in Skin Care. Int. J. Cosmet. Sci. 2021, 43, 123–130. [Google Scholar] [CrossRef]
- Mihranyan, A.; Ferraz, N.; Strømme, M. Current Status and Future Prospects of Nanotechnology in Cosmetics. Prog. Mater. Sci. 2012, 57, 875–910. [Google Scholar] [CrossRef]
- Perra, M.; Lozano-Sánchez, J.; Leyva-Jiménez, F.J.; Segura-Carretero, A.; Pedraz, J.L.; Bacchetta, G.; Muntoni, A.; De Gioannis, G.; Manca, M.L.; Manconi, M. Extraction of the Antioxidant Phytocomplex from Wine-Making by-Products and Sustainable Loading in Phospholipid Vesicles Specifically Tailored for Skin Protection. Biomed. Pharmacother. 2021, 142, 111959. [Google Scholar] [CrossRef] [PubMed]
- Tomou, E.-M.; Papakyriakopoulou, P.; Saitani, E.-M.; Valsami, G.; Pippa, N.; Skaltsa, H. Recent Advances in Nanoformulations for Quercetin Delivery. Pharmaceutics 2023, 15, 1656. [Google Scholar] [CrossRef]
- Graván, P.; Aguilera-Garrido, A.; Marchal, J.A.; Navarro-Marchal, S.A.; Galisteo-González, F. Lipid-Core Nanoparticles: Classification, Preparation Methods, Routes of Administration and Recent Advances in Cancer Treatment. Adv. Colloid Interface Sci. 2023, 314, 102871. [Google Scholar] [CrossRef]
- Pereira, T.A.; Guerreiro, C.M.; Maruno, M.; Ferrari, M.; Rocha-Filho, P.A. Exotic Vegetable Oils for Cosmetic O/W Nanoemulsions: In Vivo Evaluation. Molecules 2016, 21, 248. [Google Scholar] [CrossRef]
- Fuentes, K.; Matamala, C.; Martínez, N.; Zúñiga, R.N.; Troncoso, E. Comparative Study of Physicochemical Properties of Nanoemulsions Fabricated with Natural and Synthetic Surfactants. Processes 2021, 9, 2002. [Google Scholar] [CrossRef]
- Allaw, M.; Manca, M.L.; Gómez-Fernández, J.C.; Pedraz, J.L.; Terencio, M.C.; Sales, O.D.; Nacher, A.; Manconi, M. Oleuropein Multicompartment Nanovesicles Enriched with Collagen as a Natural Strategy for the Treatment of Skin Wounds Connected with Oxidative Stress. Nanomedicine 2021, 16, 2363–2376. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Fulgheri, F.; Leyva-Jimenez, F.J.; Alañón, M.E.; Cádiz-Gurrea, M.d.l.L.; Marongiu, F.; Meloni, M.C.; Aroffu, M.; Perra, M.; Allaw, M.; et al. From Grape By-Products to Enriched Yogurt Containing Pomace Extract Loaded in Nanotechnological Nutriosomes Tailored for Promoting Gastro-Intestinal Wellness. Antioxidants 2023, 12, 1285. [Google Scholar] [CrossRef] [PubMed]
- Lerche, D.; Sobisch, T. Direct and Accelerated Characterization of Formulation Stability. J. Dispers. Sci. Technol. 2011, 32, 1799–1811. [Google Scholar] [CrossRef]
- Casula, E.; Manconi, M.; Vázquez, J.; Lopez-Mendez, T.; Pedraz, J.; Calvo, E.; Lozano, A.; Zaru, M.; Ascenso, A.; Manca, M. Design of a Nasal Spray Based on Cardiospermum Halicacabum Extract Loaded in Phospholipid Vesicles Enriched with Gelatin or Chondroitin Sulfate. Molecules 2021, 26, 6670. [Google Scholar] [CrossRef]
- Abruzzo, A.; Cappadone, C.; Farruggia, G.; Luppi, B.; Bigucci, F.; Cerchiara, T. Glycyrrhetinic Acid Liposomes and Hyalurosomes on Spanish Broom, Flax, and Hemp Dressings to Heal Skin Wounds. Molecules 2020, 25, 2558. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Casula, E.; Marongiu, F.; Bacchetta, G.; Sarais, G.; Zaru, M.; Escribano-Ferrer, E.; Peris, J.E.; Usach, I.; Fais, S.; et al. From Waste to Health: Sustainable Exploitation of Grape Pomace Seed Extract to Manufacture Antioxidant, Regenerative and Prebiotic Nanovesicles within Circular Economy. Sci. Rep. 2020, 10, 14184. [Google Scholar] [CrossRef] [PubMed]
- Salem, Y.; Rajha, H.N.; Sunoqrot, S.; Hammad, A.M.; Castangia, I.; Manconi, M.; Manca, M.L.; Al Lababidi, D.; Touma, J.A.; Maroun, R.G.; et al. Exhausted Grape Seed Residues as a Valuable Source of Antioxidant Molecules for the Formulation of Biocompatible Cosmetic Scrubs. Molecules 2023, 28, 5049. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Voss, G.B.; Coelho, M.C.; Pintado, M.E. Food Waste and By-Product Valorization as an Integrated Approach with Zero Waste: Future Challenges. In Future Foods; Elsevier: Amsterdam, The Netherlands, 2022; pp. 569–596. [Google Scholar]
- Paini, J.; Benedetti, V.; Ail, S.S.; Castaldi, M.J.; Baratieri, M.; Patuzzi, F. Valorization of Wastes from the Food Production Industry: A Review Towards an Integrated Agri-Food Processing Biorefinery. Waste Biomass Valorization 2022, 13, 31–50. [Google Scholar] [CrossRef]
- Flamini, R.; De Rosso, M.; Bavaresco, L. Study of Grape Polyphenols by Liquid Chromatography-High-Resolution Mass Spectrometry (UHPLC/QTOF) and Suspect Screening Analysis. J. Anal. Methods Chem. 2015, 2015, 350259. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Raposo, F.; Evtuguin, D.V.; Fonseca, F.; Ferreira-da-Silva, F.; Mateus, N.; Coimbra, M.A.; de Freitas, V. Grape Pectic Polysaccharides Stabilization of Anthocyanins Red Colour: Mechanistic Insights. Carbohydr. Polym. 2021, 255, 117432. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J. Nanoemulsions for Health, Food, and Cosmetics: A Review. Environ. Chem. Lett. 2021, 19, 3381–3395. [Google Scholar] [CrossRef]
- Wojciechowska, K.; Walczak, A.; Rostowska, E.; Poleszak, E. Comparison of Sensory and Rheological Properties of Green Cosmetic Creams Prepared on Different Natural, ECOCERT and BDIH Certificated Self-Emulsifying Bases. Curr. Issues Pharm. Med. Sci. 2021, 34, 218–223. [Google Scholar] [CrossRef]
- Berthelsen, R.; Holm, R.; Jacobsen, J.; Kristensen, J.; Abrahamsson, B.; Müllertz, A. Kolliphor Surfactants Affect Solubilization and Bioavailability of Fenofibrate. Studies of in Vitro Digestion and Absorption in Rats. Mol. Pharm. 2015, 12, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and Nanoemulsions in Skin Drug Delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Davidov-Pardo, G.; McClements, D.J. Nutraceutical Delivery Systems: Resveratrol Encapsulation in Grape Seed Oil Nanoemulsions Formed by Spontaneous Emulsification. Food Chem. 2015, 167, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, G.K.; Caregnato, F.; Moreira, J.C.F.; Teixeira, H.F.; Carvalho, E.L.S. Antioxidant Effect of Nanoemulsions Containing Extract of Achyrocline Satureioides (Lam) D.C.—Asteraceae. AAPS PharmSciTech 2016, 17, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Gadkari, P.V.; Shashidhar, M.G.; Balaraman, M. Delivery of Green Tea Catechins through Oil-in-Water (O/W) Nanoemulsion and Assessment of Storage Stability. J. Food Eng. 2017, 199, 65–76. [Google Scholar] [CrossRef]
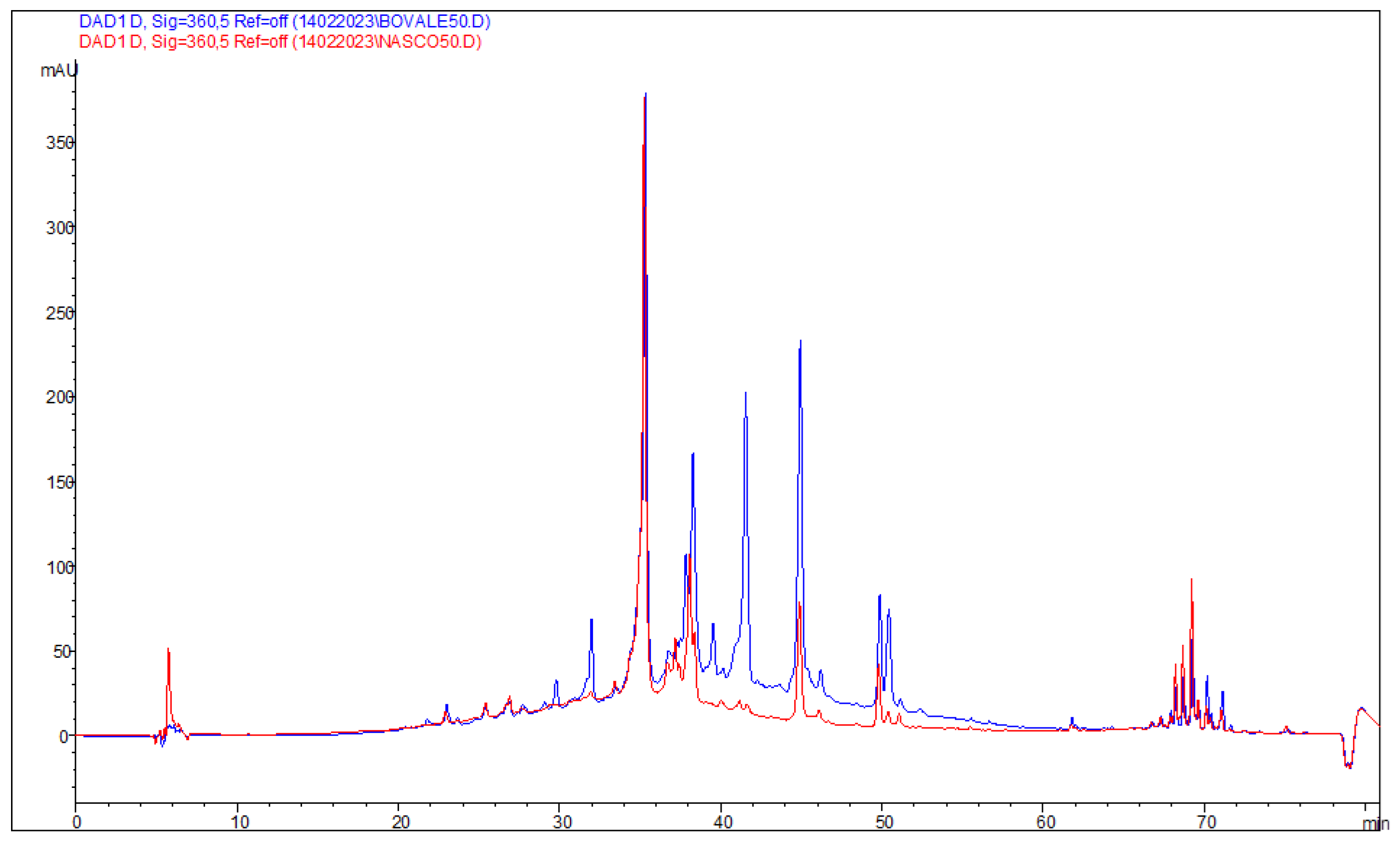


| Bovale | Nasco | ||||
|---|---|---|---|---|---|
| Anthocyanins | TR (min) | mg/kg ± RSD% | Anthocyanins | TR (min) | mg/kg ± RSD% |
| Peonidin 3 glucopyranoside | 29.07 | 2010.6 ± 1.3 | Peonidin 3 glucopyranoside | 29.07 | <LOQ |
| Malvidin 3 glucoside a | 29.8 | 1919.1 ± 3.2 | Malvidin 3 glucoside a | 29.79 | 39.1 ± 1.1 |
| Anthocyanins derivative a | 37.28 | 625.9 ± 4.5 | Anthocyanins derivative a | 37.24 | <LOQ |
| Anthocyanins derivative a | 39.56 | 1625.5 ± 1.0 | Anthocyanins derivative a | 39.52 | 29.9 ± 2.5 |
| Anthocyanins derivative a | 41.54 | 24,174.4 ± 5.5 | Anthocyanins derivative a | 41.62 | 585.8 ± 6.6 |
| Flavonoids | TR (min) | mg/kg ± RSD% | Flavonoids | TR (min) | mg/kg ± RSD% |
| Quercetin glucopyranoside | 35.32 | 2075.1 ± 3.4 | Quercetin glucopyranoside | 35.26 | 1977.7 ± 8.1 |
| Luteolin derivative b | 38.28 | 1409.6 ± 8.5 | Luteolin derivative b | 38.37 | 658.0 ± 8.0 |
| Quercetin dihydrate | 44.94 | 1162.8 ± 1.1 | Quercetin dihydrate | 44.89 | 412.0 ± 6.7 |
| Kaempferol | 49.87 | 103.5 ± 2.4 | Kaempferol | 49.8 | 61.4 ± 1.9 |
| Quercetin derivative c | 50.44 | 181.7 ± 4.1 | Quercetin derivative c | 50.39 | 19.8 ± 2.6 |
| Hydroxycinnamic acids | TR (min) | mg/kg ± RSD% | Hydroxycinnamic acids | TR (min) | mg/kg ± RSD% |
| Cumaric acid derivative d | 25.41 | 273.0 ± 7.6 | Cumaric acid derivative d | 25.38 | 34.2 ± 5.7 |
| Hydroxybenzoic acids | TR (min) | mg/kg ± RSD% | Hydroxybenzoic acids | TR (min) | mg/kg ± RSD% |
| Gallic acid | 13.08 | 1291.9 ± 3.3 | Gallic acid | 12.99 | 1462.2 ± 5.8 |
| Syringic acid | 24.93 | 255.4 ± 4.2 | |||
| Flavan-3-ols | TR (min) | mg/kg ± RSD% | Flavan-3-ols | TR (min) | mg/kg ± RSD% |
| Epicatechin | 23.06 | 5289.1 ± 4.3 | Unknown e | 23.19 | 6004.9 ± 7.1 |
| Unknown e | 26.81 | 4138.6 ± 5.6 | Unknown e | 26.66 | 4302.4 ± 3.4 |
| Total polyphenols | 46,280.8 ± 4.8 | Total polyphenols | 15,842.8 ± 6.1 | ||
| MD (nm) | PDI | ZP (mV) | AA (%) | pH | |
|---|---|---|---|---|---|
| Empty kolliphor nanoemulsions | 65 ± 2 | 0.18 | −45 e ± 1 | - | 6.1 |
| Bovale kolliphor nanoemulsions | 87 a ± 4 | 0.18 | −35 d ± 4 | 70 h ± 2 | 5.1 |
| Nasco kolliphor nanoemulsions | 76 a ± 3 | 0.21 | −45 d ± 1 | 53 ± 4 | 5.4 |
| Bovale–Nasco kolliphor nanoemulsions | 79 a ± 7 | 0.27 | −39 e ± 7 | 79 i ± 3 | 4.8 |
| Empty olivem nanoemulsions | 71 ± 3 | 0.19 | −42 f ± 3 | - | 6.0 |
| Bovale olivem nanoemulsions | 121 b ± 4 | 0.13 | −47 g ± 3 | 71 h ± 3 | 5.0 |
| Nasco olivem nanoemulsions | 117 b ± 4 | 0.12 | −49 g ± 3 | 62 ± 5 | 5.7 |
| Bovale–Nasco olivem nanoemulsions | 148 c ± 7 | 0.29 | −42 f ± 1 | 82 i ± 2 | 4.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castangia, I.; Fulgheri, F.; Perra, M.; Bacchetta, G.; Fancello, L.; Corrias, F.; Usach, I.; Peris, J.E.; Manca, M.L.; Manconi, M. A Cocktail-Based Formula for the Design of Nanosized Cosmeceuticals as Skincare and Anti-Age Products. Nanomaterials 2023, 13, 2485. https://doi.org/10.3390/nano13172485
Castangia I, Fulgheri F, Perra M, Bacchetta G, Fancello L, Corrias F, Usach I, Peris JE, Manca ML, Manconi M. A Cocktail-Based Formula for the Design of Nanosized Cosmeceuticals as Skincare and Anti-Age Products. Nanomaterials. 2023; 13(17):2485. https://doi.org/10.3390/nano13172485
Chicago/Turabian StyleCastangia, Ines, Federica Fulgheri, Matteo Perra, Gianluigi Bacchetta, Laura Fancello, Francesco Corrias, Iris Usach, Josè Esteban Peris, Maria Letizia Manca, and Maria Manconi. 2023. "A Cocktail-Based Formula for the Design of Nanosized Cosmeceuticals as Skincare and Anti-Age Products" Nanomaterials 13, no. 17: 2485. https://doi.org/10.3390/nano13172485
APA StyleCastangia, I., Fulgheri, F., Perra, M., Bacchetta, G., Fancello, L., Corrias, F., Usach, I., Peris, J. E., Manca, M. L., & Manconi, M. (2023). A Cocktail-Based Formula for the Design of Nanosized Cosmeceuticals as Skincare and Anti-Age Products. Nanomaterials, 13(17), 2485. https://doi.org/10.3390/nano13172485