New Eco-Friendly and Low-Energy Synthesis to Produce ZnO Nanoparticles for Real-World Scale Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ZnO Nanoparticles Synthesis
2.3. Characterization of the Produced Zinc Oxide Nanoparticles
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borysiewicz, M.A. ZnO as a functional material, a review. Crystals 2019, 9, 505. [Google Scholar] [CrossRef]
- Gujel, A.A.; Bandeira, M.; Menti, C.; Perondi, D.; Guégan, R.; Roesch-Ely, M.; Giovanela, M.; Crespo, J.S. Evaluation of vulcanization nanoactivators with low zinc content: Characterization of zinc oxides, cure, physico-mechanical properties, Zn2+ release in water and cytotoxic effect of EPDM compositions. Polym. Eng. Sci. 2018, 58, 1800–1809. [Google Scholar] [CrossRef]
- Grasland, F.; Chazeau, L.; Chenal, J.M.; Schach, R. About thermo-oxidative ageing at moderate temperature of conventionally vulcanized natural rubber. Polym. Degrad. Stab. 2019, 161, 74–84. [Google Scholar] [CrossRef]
- Xie, Z.T.; Luo, M.C.; Huang, C.; Wei, L.Y.; Liu, Y.H.; Fu, X.; Huang, G.; Wu, J. Effects of graphene oxide on the strain-induced crystallization and mechanical properties of natural rubber crosslinked by different vulcanization systems. Polymer 2018, 151, 279–286. [Google Scholar] [CrossRef]
- Ahmoum, H.; Boughrara, M.; Su’ait, M.S.; Chopra, S.; Kerouad, M. Impact of position and concentration of sodium on the photovoltaic properties of zinc oxide solar cells. Phys. B Condens. Matter 2019, 560, 28–36. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Chanda, D.K.; Das, P.S.; Ghosh, J.; Mukhopadhyay, A.K.; Dey, A. Synthesis of Nano Calcium Hydroxide in Aqueous Medium. J. Am. Ceram. Soc. 2016, 99, 787–795. [Google Scholar] [CrossRef]
- Kathalewar, M.; Sabnis, A.; Waghoo, G. Effect of incorporation of surface treated zinc oxide on non-isocyanate polyurethane based nano-composite coatings. Prog. Org. Coat. 2013, 76, 1215–1229. [Google Scholar] [CrossRef]
- Bacaksiz, E.; Parlak, M.; Tomakin, M.; Özçelik, A.; Karakiz, M.; Altunbaş, M. The effects of zinc nitrate, zinc acetate and zinc chloride precursors on investigation of structural and optical properties of ZnO thin films. J. Alloys Compd. 2008, 466, 447–450. [Google Scholar] [CrossRef]
- Chaari, M.; Matoussi, A. Electrical conduction and dielectric studies of ZnO pellets. Phys. B Condens. Matter 2012, 407, 3441–3447. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Barbosa, H.P.; Araújo, D.A.G.; Pradela-Filho, L.A.; Takeuchi, R.M.; de Lima, R.G.; Ferrari, J.L.; Sousa Góes, M.; dos Santos, A.L. Zinc Oxide as a Multifunctional Material: From Biomedical Applications to Energy Conversion and Electrochemical Sensing. In Metal and Metal Oxides for Energy and Electronics; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Vaseeharan, B.; Malaikozhundan, B.; Shobiya, M. Laurus nobilis leaf extract mediated green synthesis of ZnO nanoparticles: Characterization and biomedical applications. Biomed. Pharmacother. 2016, 84, 1213–1222. [Google Scholar] [CrossRef]
- Gomez, J.L.; Tigli, O. Zinc oxide nanostructures: From growth to application. J. Mater. Sci. 2013, 48, 612–624. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Tang, F.; Ma, J.; Zhou, X. A Facile Approach for the Preparation of Nano-size Zinc Oxide in Water/Glycerol with Extremely Concentrated Zinc Sources. Nanoscale Res. Lett. 2018, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, A.; Kumar, N.; Hahm, J.I. Highly sensitive biomolecular fluorescence detection using nanoscale ZnO platforms. Langmuir 2006, 22, 4890–4895. [Google Scholar] [CrossRef]
- Umar, A.; Kim, S.H.; Lee, H.; Lee, N.; Hahn, Y.B. Optical and field emission properties of single-crystalline aligned ZnO nanorods grown on aluminium substrate. J. Phys. D Appl. Phys. 2008, 41, 065412. [Google Scholar] [CrossRef]
- Zikalala, N.E.; Azizi, S.; Zikalala, S.A.; Kamika, I.; Maaza, M.; Zinatizadeh, A.A.; Mokrani, T.; Kaviyarasu, K. An Evaluation of the Biocatalyst for the Synthesis and Application of Zinc Oxide Nanoparticles for Water Remediation—A Review. Catalysts 2022, 12, 1442. [Google Scholar] [CrossRef]
- Gunasekaran, A.; Rajamani, A.K.; Masilamani, C.; Chinnappan, I.; Ramamoorthy, U.; Kaviyarasu, K. Synthesis and Characterization of ZnO Doped TiO2 Nanocomposites for Their Potential Photocatalytic and Antimicrobial Applications. Catalysts 2023, 13, 215. [Google Scholar] [CrossRef]
- Condello, M.; De Berardis, B.; Ammendolia, M.G.; Barone, F.; Condello, G.; Degan, P.; Meschini, S. ZnO nanoparticle tracking from uptake to genotoxic damage in human colon carcinoma cells. Toxicol. Vitr. 2016, 35, 169–179. [Google Scholar] [CrossRef]
- Chauhan, I.; Aggrawal, S.; Mohanty, P. ZnO nanowire-immobilized paper matrices for visible light-induced antibacterial activity against Escherichia coli. Environ. Sci. Nano 2015, 2, 273–279. [Google Scholar] [CrossRef]
- Mirhosseini, M.; Firouzabadi, F.B. Antibacterial activity of zinc oxide nanoparticle suspensions on food-borne pathogens. Int. J. Dairy Technol. 2013, 66, 291–295. [Google Scholar] [CrossRef]
- Darshitha, M.N.; Sood, R. Review on synthesis and applications of zinc oxide nanoparticles. Preprints 2021, 2021050688. [Google Scholar]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Salah, N.; Habib, S.S.; Khan, Z.H.; Memic, A.; Azam, A.; Alarfaj, E.; Zahed, N.; Al-Hamedi, S. High-energy ball milling technique for ZnO nanoparticles as antibacterial material. Int. J. Nanomed. 2011, 6, 863–869. [Google Scholar] [CrossRef]
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles—An eco-friendly approach. Resour. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Dejene, F.B.; Ali, A.G.; Swart, H.C.; Botha, R.J.; Roro, K.; Coetsee, L.; Biggs, M.M. Optical properties of ZnO nanoparticles synthesized by varying the sodium hydroxide to zinc acetate molar ratios using a Sol-Gel process. Cent. Eur. J. Phys. 2011, 9, 1321–1326. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Liang, J.; Gao, F.; Yin, K.; Dai, P. Hierarchical Heterostructure of ZnO@TiO2 Hollow Spheres for Highly Efficient Photocatalytic Hydrogen Evolution. Nanoscale Res. Lett. 2017, 12, 531. [Google Scholar] [CrossRef]
- Park, W.I.; Lee, C.H.; Chae, J.H.; Lee, D.H.; Yi, G.C. Ultrafine ZnO nanowire electronic device arrays fabricated by selective metal-organic chemical vapor deposition. Small 2009, 5, 181–184. [Google Scholar] [CrossRef]
- Sarkar, D.; Tikku, S.; Thapar, V.; Srinivasa, R.S.; Khilar, K.C. Formation of zinc oxide nanoparticles of different shapes in water-in-oil microemulsion. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 381, 123–129. [Google Scholar] [CrossRef]
- Amarilio-Burshtein, I.; Tamir, S.; Lifshitz, Y. Growth modes of ZnO nanostructures from laser ablation. Appl. Phys. Lett. 2010, 96, 103104. [Google Scholar] [CrossRef]
- Fakhari, S.; Jamzad, M.; Kabiri Fard, H. Green synthesis of zinc oxide nanoparticles: A comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–304. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metrics of Green Chemistry and Sustainability: Past, Present, and Future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef]
- Osuntokun, J.; Onwudiwe, D.C.; Ebenso, E.E. Green synthesis of ZnO nanoparticles using aqueous Brassica oleracea L. var. italica and the photocatalytic activity. Green Chem. Lett. Rev. 2019, 12, 444–457. [Google Scholar] [CrossRef]
- Suresh, D.; Nethravathi, P.C.; Udayabhanu; Rajanaika, H.; Nagabhushana, H.; Sharma, S.C. Green synthesis of multifunctional zinc oxide (ZnO) nanoparticles using Cassia fistula plant extract and their photodegradative, antioxidant and antibacterial activities. Mater. Sci. Semicond. Process. 2015, 31, 446–456. [Google Scholar] [CrossRef]
- Qu, J.; Yuan, X.; Wang, X.; Shao, P. Zinc accumulation and synthesis of ZnO nanoparticles using Physalis alkekengi L. Environ. Pollut. 2011, 159, 1783–1788. [Google Scholar] [CrossRef]
- Bitenc, M.; Crnjak Orel, Z. Synthesis and characterization of crystalline hexagonal bipods of zinc oxide. Mater. Res. Bull. 2009, 44, 381–387. [Google Scholar] [CrossRef]
- Sabbagh, F.; Kiarostami, K.; Khatir, N.M.; Rezania, S.; Muhamad, I.I. Green synthesis of Mg0.99 Zn0.01O nanoparticles for the fabrication of κ-Carrageenan/NaCMC hydrogel in order to deliver catechin. Polymers 2020, 12, 861. [Google Scholar] [CrossRef]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; da Silva Crespo, J. Green synthesis of zinc oxide nanoparticles: A review of the synthesis methodology and mechanism of formation. Sustain. Chem. Pharm. 2020, 15, 100223. [Google Scholar] [CrossRef]
- Taglieri, G.; Macera, L.; Daniele, V. Procedimento per la Sintesi di Nanoparticelle di Ferridrite o di Magnetite Mediante Resine a Scambio Ionico. Italian Patent 102019000017981, 4 October 2019. [Google Scholar]
- Volpe, R.; Taglieri, G.; Daniele, V.; Del Re, G. A Process for the Synthesis of Ca(OH)2 Nanoparticles by Means of Ionic Exchange Resins. European Patent EP13773400.0A, 21 December 2016. [Google Scholar]
- Macera, L.; Daniele, V.; Mondelli, C.; Capron, M.; Taglieri, G. New sustainable, scalable and one-step synthesis of iron oxide nanoparticles by ion exchange process. Nanomaterials 2021, 11, 798. [Google Scholar] [CrossRef]
- Fiala, J. D. L. Bish, J. E. Post (eds). Modern Powder Diffraction. Mineralogical Society of America: Washington, 1989, Volume XI + 369 p, 167 figures, $ 20.00, ISBN 0-939950-24-3. Cryst. Res. Technol. 1990, 25, 1358. [Google Scholar] [CrossRef]
- Klug, H.; Alexander, L. X-ray Diffraction Procedures: For Polycrystalline and Amorphous Materials, 2nd ed.; Willey: New York, NY, USA, 1974. [Google Scholar]
- Wang, M.; Zhou, Y.; Zhang, Y.; Hahn, S.H.; Kim, E.J. From Zn(OH)2 to ZnO: A study on the mechanism of phase transformation. CrystEngComm 2011, 13, 6024–6026. [Google Scholar] [CrossRef]
- Qazi, S.J.S.; Rennie, A.R.; Cockcroft, J.K.; Vickers, M. Use of wide-angle X-ray diffraction to measure shape and size of dispersed colloidal particles. J. Colloid Interface Sci. 2009, 338, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Taglieri, G.; Daniele, V.; Macera, L.; Mondelli, C. Nano Ca(OH)2 synthesis using a cost-effective and innovative method: Reactivity study. J. Am. Ceram. Soc. 2017, 100, 5766–5778. [Google Scholar] [CrossRef]
- Taglieri, G.; Daniele, V.; Macera, L. Synthesizing alkaline earth metal hydroxides nanoparticles through an innovative, single-step and eco-friendly method. Solid State Phenom. 2019, 286, 3–14. [Google Scholar] [CrossRef]
- Macera, L.; Taglieri, G.; Daniele, V.; Passacantando, M.; D’Orazio, F. Nano-Sized Fe(III) Oxide Particles Starting from an Innovative and Eco-Friendly Synthesis Method. Nanomaterials 2020, 10, 323. [Google Scholar] [CrossRef]
- Taglieri, G.; Felice, B.; Daniele, V.; Ferrante, F. Mg(OH)2 nanoparticles produced at room temperature by an innovative, facile, and scalable synthesis route. J. Nanopart. Res. 2015, 17, 411. [Google Scholar] [CrossRef]
- Cousy, S.; Gorodylova, N.; Svoboda, L.; Zelenka, J. Influence of synthesis conditions over simonkolleite/ZnO precipitation. Chem. Pap. 2017, 71, 2325–2334. [Google Scholar] [CrossRef]
- McMahon, M.E.; Santucci, R.J.; Scully, J.R. Advanced chemical stability diagrams to predict the formation of complex zinc compounds in a chloride environment. RSC Adv. 2019, 9, 19905–19916. [Google Scholar] [CrossRef]
- Xie, J.; Li, P.; Li, Y.; Wang, Y.; Wei, Y. Morphology control of ZnO particles via aqueous solution route at low temperature. Mater. Chem. Phys. 2009, 114, 943–947. [Google Scholar] [CrossRef]
- Rasines, I.; Morales de Setién, J.I. Thermal analysis of β-Co2(OH)3Cl and Zn5(OH)5Cl2·H2O. Thermochim. Acta 1980, 37, 239–246. [Google Scholar] [CrossRef]
- Garcia-Martinez, O.; Vila, E.; Martin de Vidales, J.L.; Rojas, R.M.; Petrov, K. On the thermal decomposition of the Zinc(II) hydroxide chlorides Zn5(OH)8Cl2·H2O and Β-Zn(OH)Cl. J. Mater. Sci. 1994, 29, 5429–5434. [Google Scholar] [CrossRef]
- Carp, O.; Tirsoaga, A.; Ene, R.; Ianculescu, A.; Negrea, R.F.; Chesler, P.; Ionita, G.; Birjega, R. Facile, high yield ultrasound mediated protocol for ZnO hierarchical structures synthesis: Formation mechanism, optical and photocatalytic properties. Ultrason. Sonochem. 2017, 36, 326–335. [Google Scholar] [CrossRef]
- Lizandara-Pueyo, C.; Siroky, S.; Wagner, M.R.; Hoffmann, A.; Reparaz, J.S.; Lehmann, M.; Polarz, S. Shape anisotropy influencing functional properties: Trigonal prismatic ZnO nanoparticles as an example. Adv. Funct. Mater. 2011, 21, 295–304. [Google Scholar] [CrossRef]
- Becheri, A.; Dürr, M.; Lo Nostro, P.; Baglioni, P. Synthesis and characterization of zinc oxide nanoparticles: Application to textiles as UV-absorbers. J. Nanopart. Res. 2008, 10, 679–689. [Google Scholar] [CrossRef]
- Alothman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Yu, J.; Liu, W.; Yu, H. A one-pot approach to hierarchically nanoporous titania hollow microspheres with high photocatalytic activity. Cryst. Growth Des. 2008, 8, 930–934. [Google Scholar] [CrossRef]
- Rigby, S.P.; Fletcher, R.S. Experimental Evidence for Pore Blocking as the Mechanism for Nitrogen Sorption Hysteresis in a Mesoporous Material. J. Phys. Chem. B 2004, 108, 4690–4695. [Google Scholar] [CrossRef]
- Jesionowski, T.; Kołodziejczak-Radzimska, A.; Ciesielczyk, F.; Sójka-Ledakowicz, J.; Olczyk, J.; Sielski, J. Synthesis of zinc oxide in an emulsion system and its deposition on PES nonwoven fabrics. Fibres Text. East. Eur. 2011, 85, 70–75. [Google Scholar]
- Benhebal, H.; Chaib, M.; Salmon, T.; Geens, J.; Leonard, A.; Lambert, S.D.; Crine, M.; Heinrichs, B. Photocatalytic degradation of phenol and benzoic acid using zinc oxide powders prepared by the sol-gel process. Alex. Eng. J. 2013, 52, 517–523. [Google Scholar] [CrossRef]
- Koodziejczak-Radzimska, A.; Markiewicz, E.; Jesionowski, T. Structural characterisation of ZnO particles obtained by the emulsion precipitation method. J. Nanomater. 2012, 2012, 656353. [Google Scholar] [CrossRef]
- Sabura Begum, P.M.; Mohammed Yusuff, K.K.; Joseph, R. Preparation and use of nano zinc oxide in neoprene rubber. Int. J. Polym. Mater. Polym. Biomater. 2008, 57, 1083–1094. [Google Scholar] [CrossRef]
- Aghababazadeh, R.; Mazinani, B.; Mirhabibi, A.; Tamizifar, M. ZnO Nanoparticles Synthesised by mechanochemical processing. J. Phys. Conf. Ser. 2006, 26, 312. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z. Facile Synthesis of Quasi Spherical ZnO Nanoparticles with Excellent Photocatalytic Activity. J. Clust. Sci. 2015, 26, 1187–1201. [Google Scholar] [CrossRef]
- Kadam, A.N.; Bhopate, D.P.; Kondalkar, V.V.; Majhi, S.M.; Bathula, C.D.; Tran, A.V.; Lee, S.W. Facile synthesis of Ag-ZnO core–shell nanostructures with enhanced photocatalytic activity. J. Ind. Eng. Chem. 2018, 61, 78–86. [Google Scholar] [CrossRef]
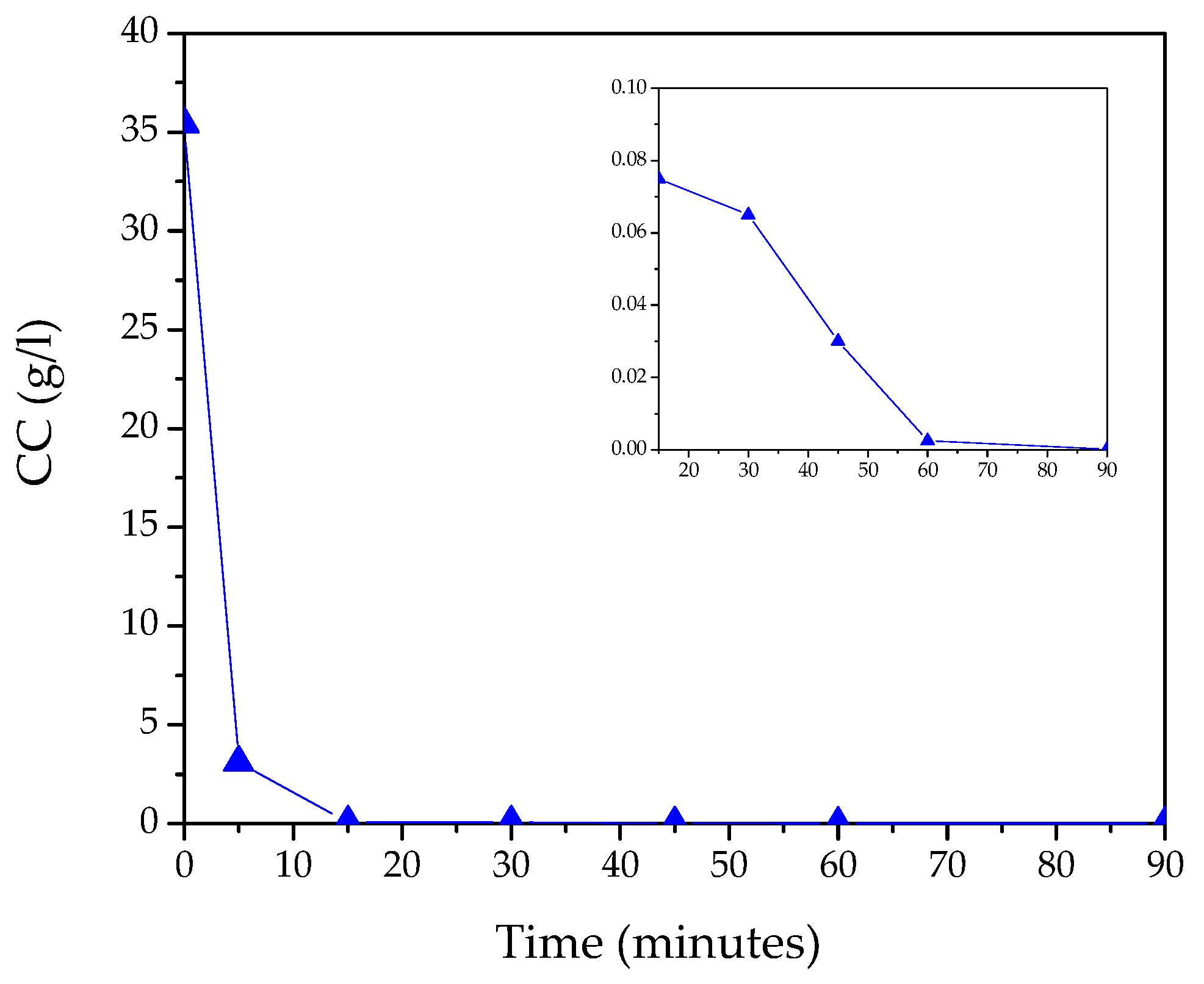
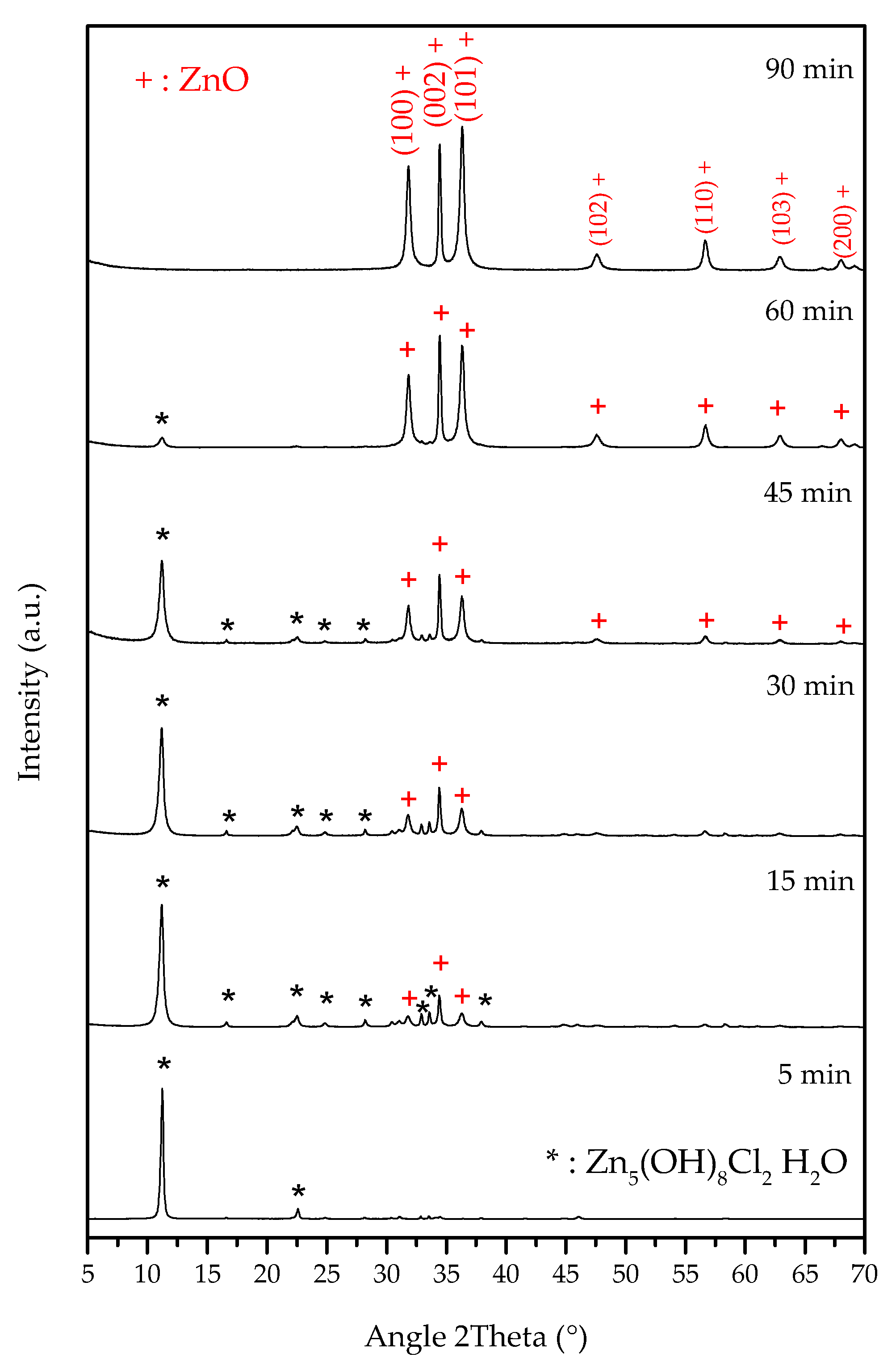


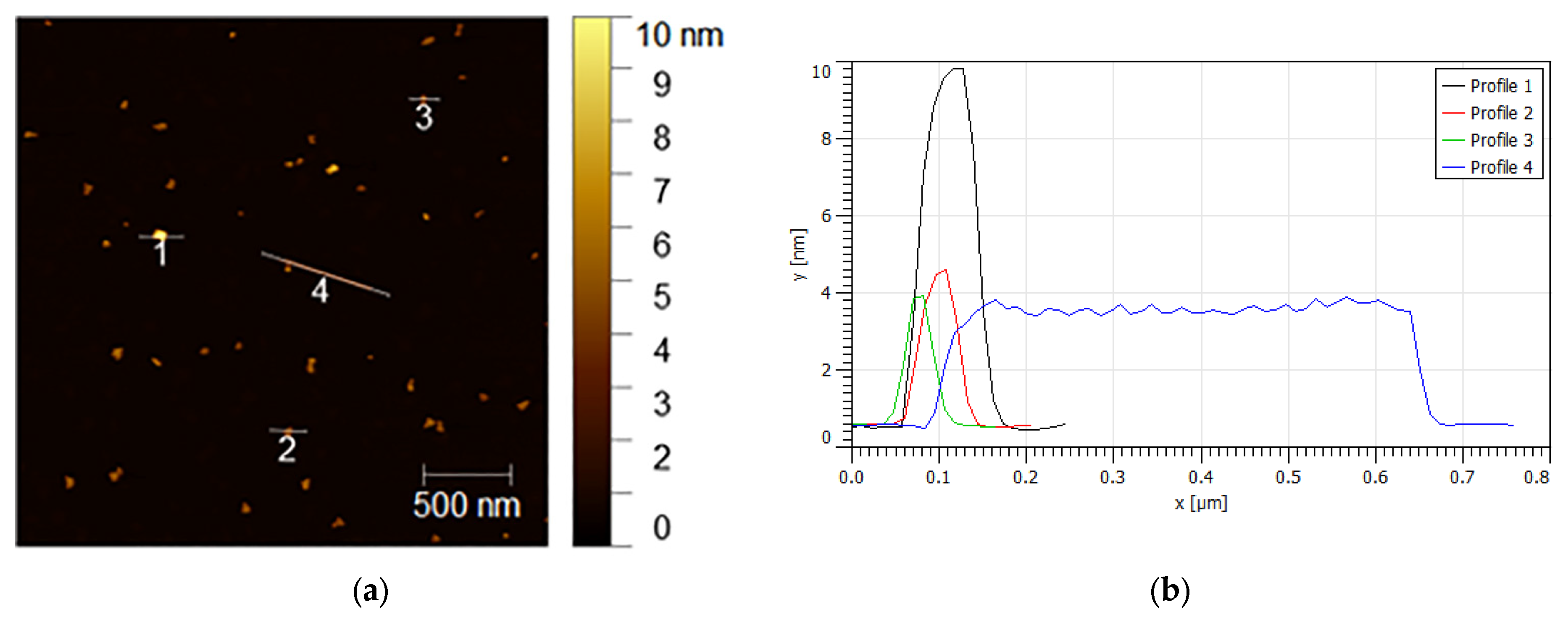
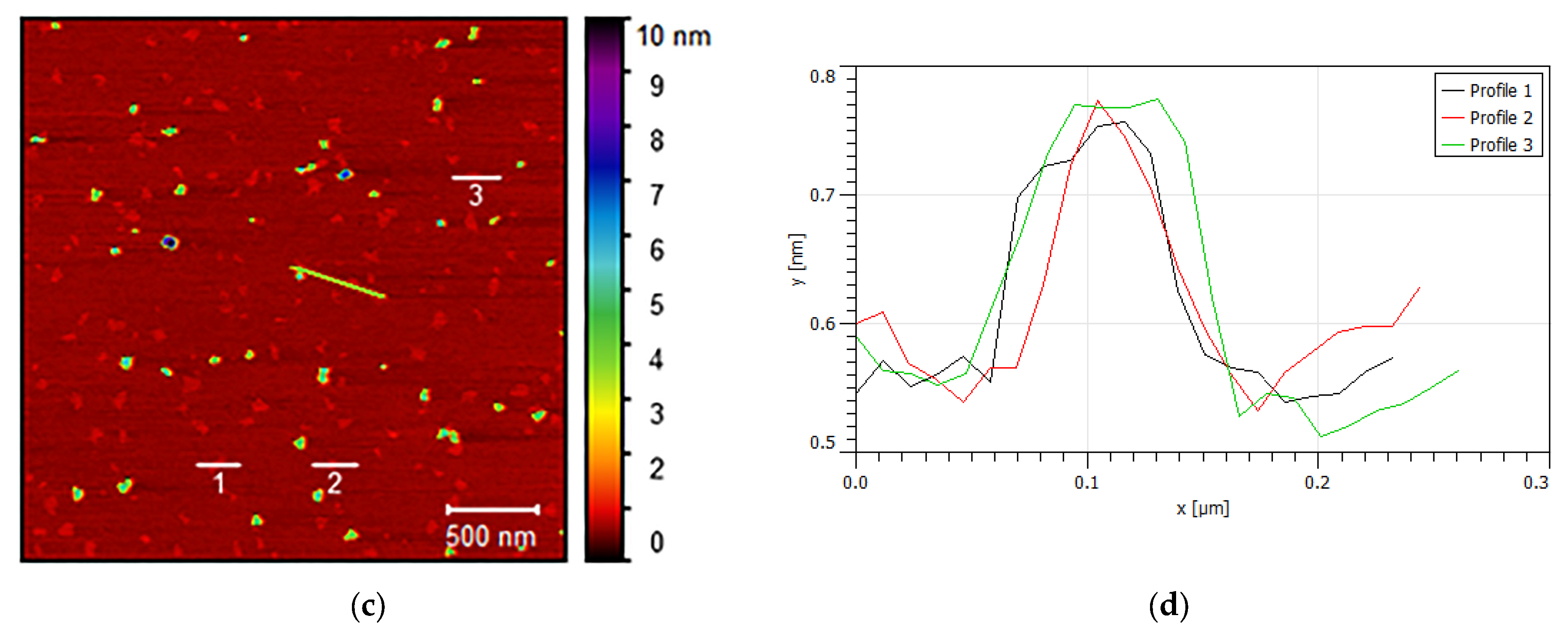
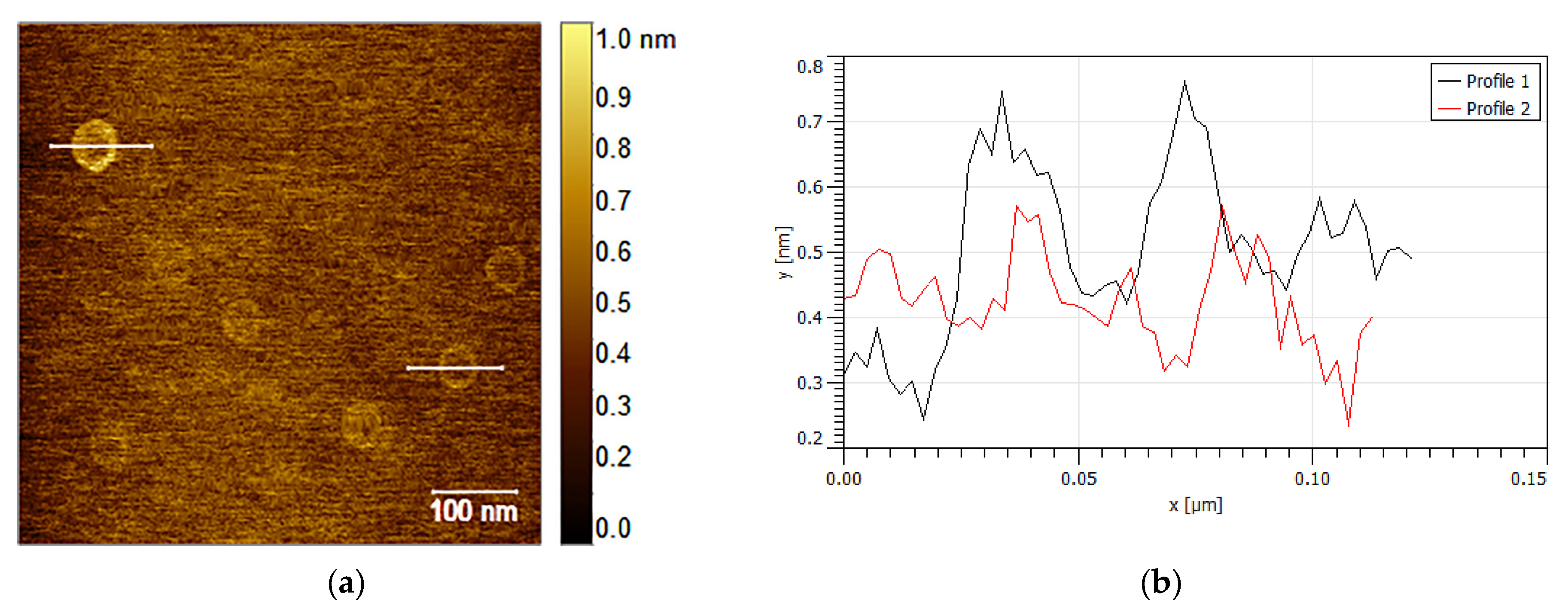

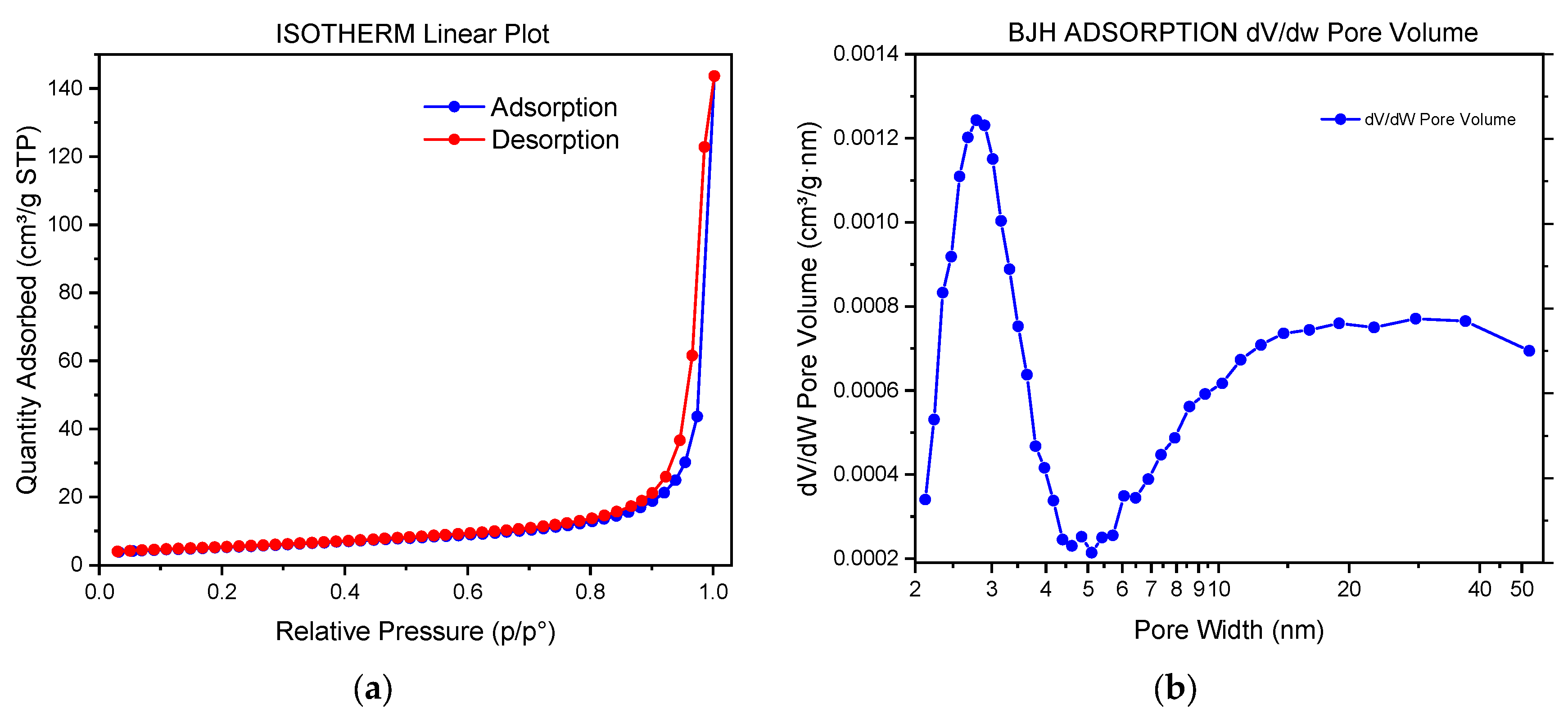
| t (Minutes) | CC (g/L) | Y (%) | R (g/L·min) | % of Crystalline Phases Using Rietveld Analysis |
|---|---|---|---|---|
| 0 | 35.4 | - | ||
| 5 | 3.1 | 91.2 | 6.5 | 100% S |
| 15 | 0.075 | 99.8 | 0.2 | 61.4% S–38.6% Z |
| 30 | 0.065 | 99.8 | 0.0003 | 42.3% S–57.7% Z |
| 45 | 0.030 | 99.9 | 0.0008 | 28.2% S–71.8% Z |
| 60 | 0.0025 | 100.0 | 0.0005 | 7.3% S–92.7% Z |
| 90 | 0.0001 | 100.0 | 0.00003 | 100% Z |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taglieri, G.; Daniele, V.; Maurizio, V.; Merlin, G.; Siligardi, C.; Capron, M.; Mondelli, C. New Eco-Friendly and Low-Energy Synthesis to Produce ZnO Nanoparticles for Real-World Scale Applications. Nanomaterials 2023, 13, 2458. https://doi.org/10.3390/nano13172458
Taglieri G, Daniele V, Maurizio V, Merlin G, Siligardi C, Capron M, Mondelli C. New Eco-Friendly and Low-Energy Synthesis to Produce ZnO Nanoparticles for Real-World Scale Applications. Nanomaterials. 2023; 13(17):2458. https://doi.org/10.3390/nano13172458
Chicago/Turabian StyleTaglieri, Giuliana, Valeria Daniele, Valentina Maurizio, Gabriel Merlin, Cristina Siligardi, Marie Capron, and Claudia Mondelli. 2023. "New Eco-Friendly and Low-Energy Synthesis to Produce ZnO Nanoparticles for Real-World Scale Applications" Nanomaterials 13, no. 17: 2458. https://doi.org/10.3390/nano13172458
APA StyleTaglieri, G., Daniele, V., Maurizio, V., Merlin, G., Siligardi, C., Capron, M., & Mondelli, C. (2023). New Eco-Friendly and Low-Energy Synthesis to Produce ZnO Nanoparticles for Real-World Scale Applications. Nanomaterials, 13(17), 2458. https://doi.org/10.3390/nano13172458






