Synthesis of Zinc Oxide Nanorods from Zinc Borate Precursor and Characterization of Supercapacitor Properties
Abstract
:1. Introduction
2. Experimental
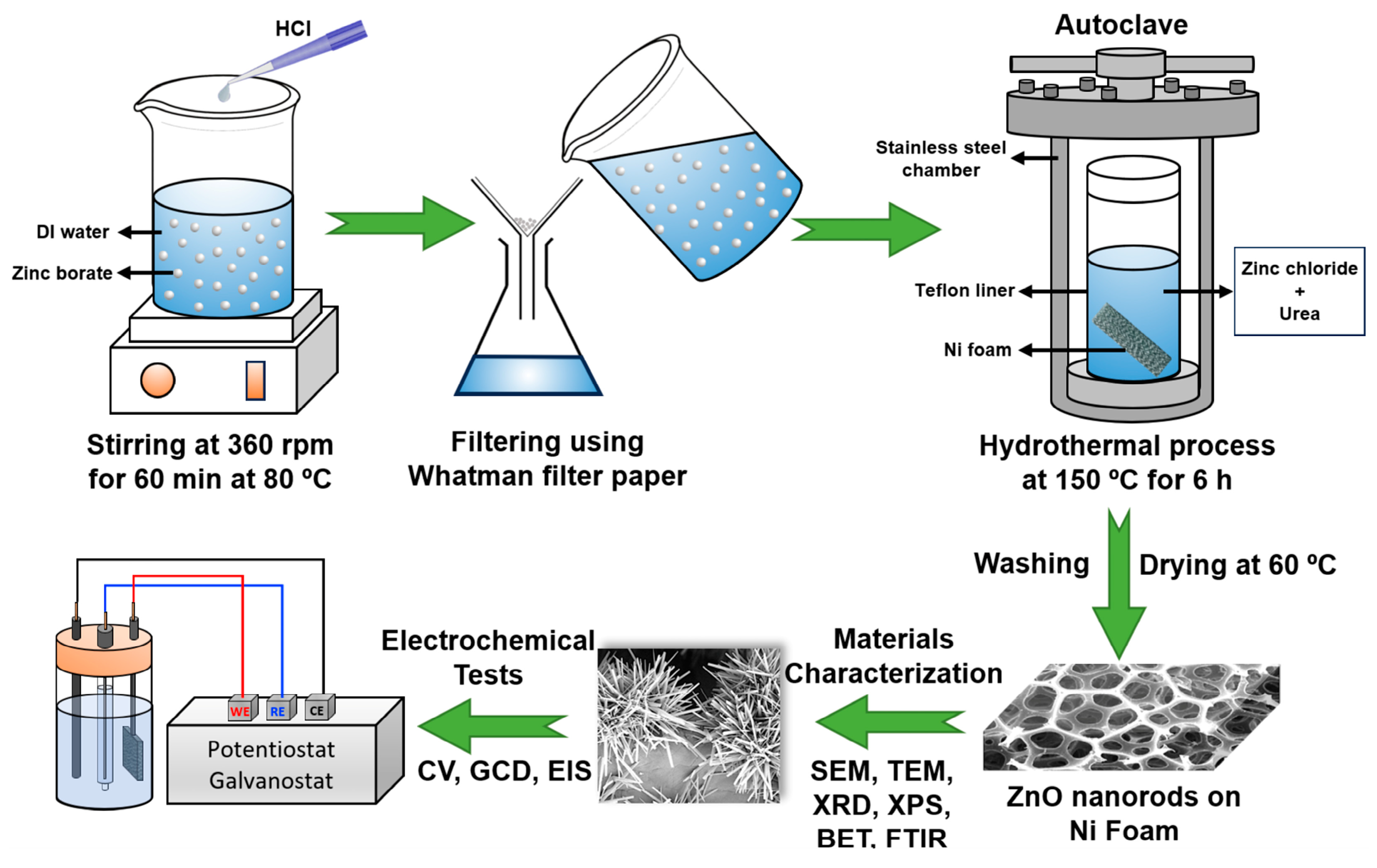
2.1. Materials and Method
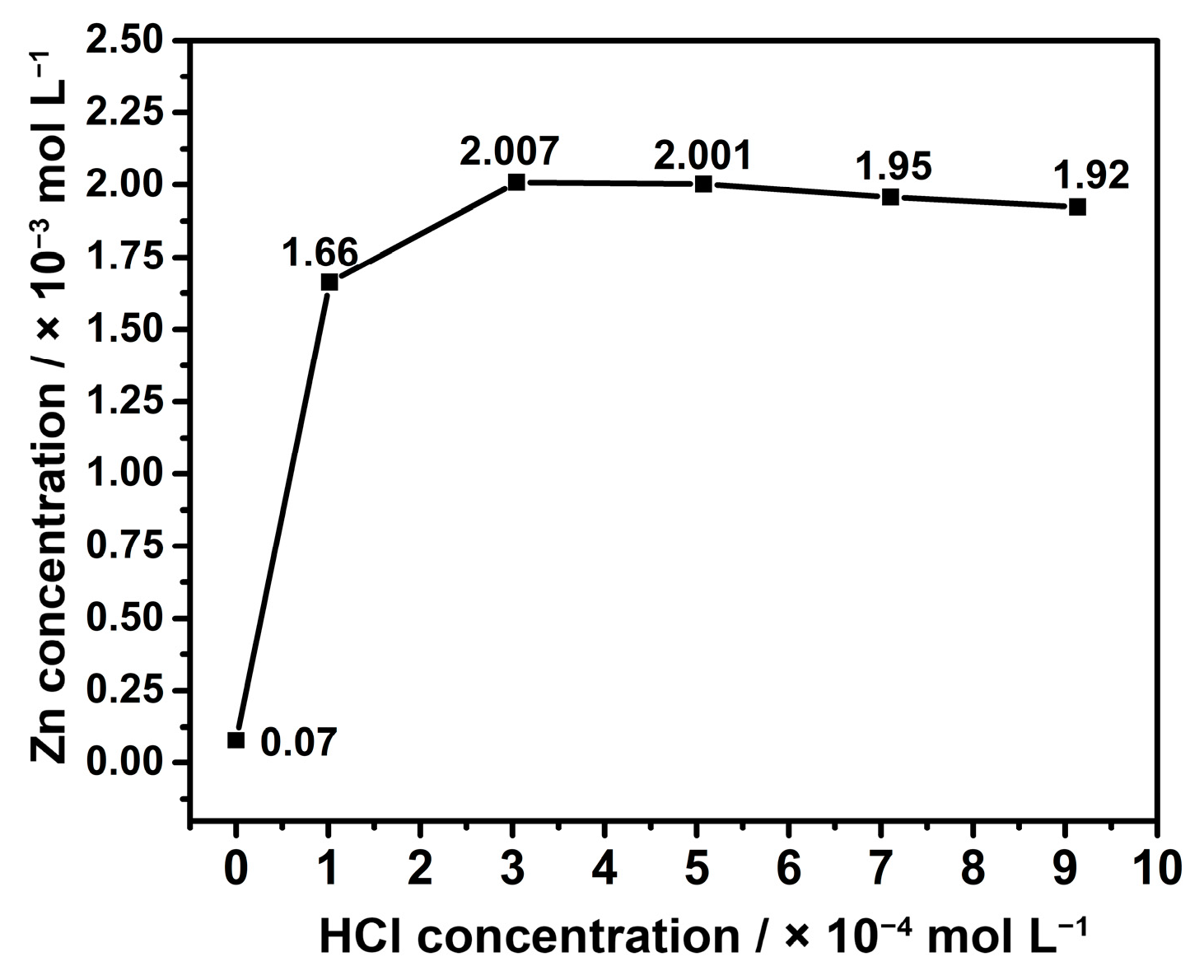
2.2. Leaching of Zinc Borate
2.3. Production of Electrode by Hydrothermal Technique
2.4. Materials Characterization
2.5. Electrochemical Tests
3. Results
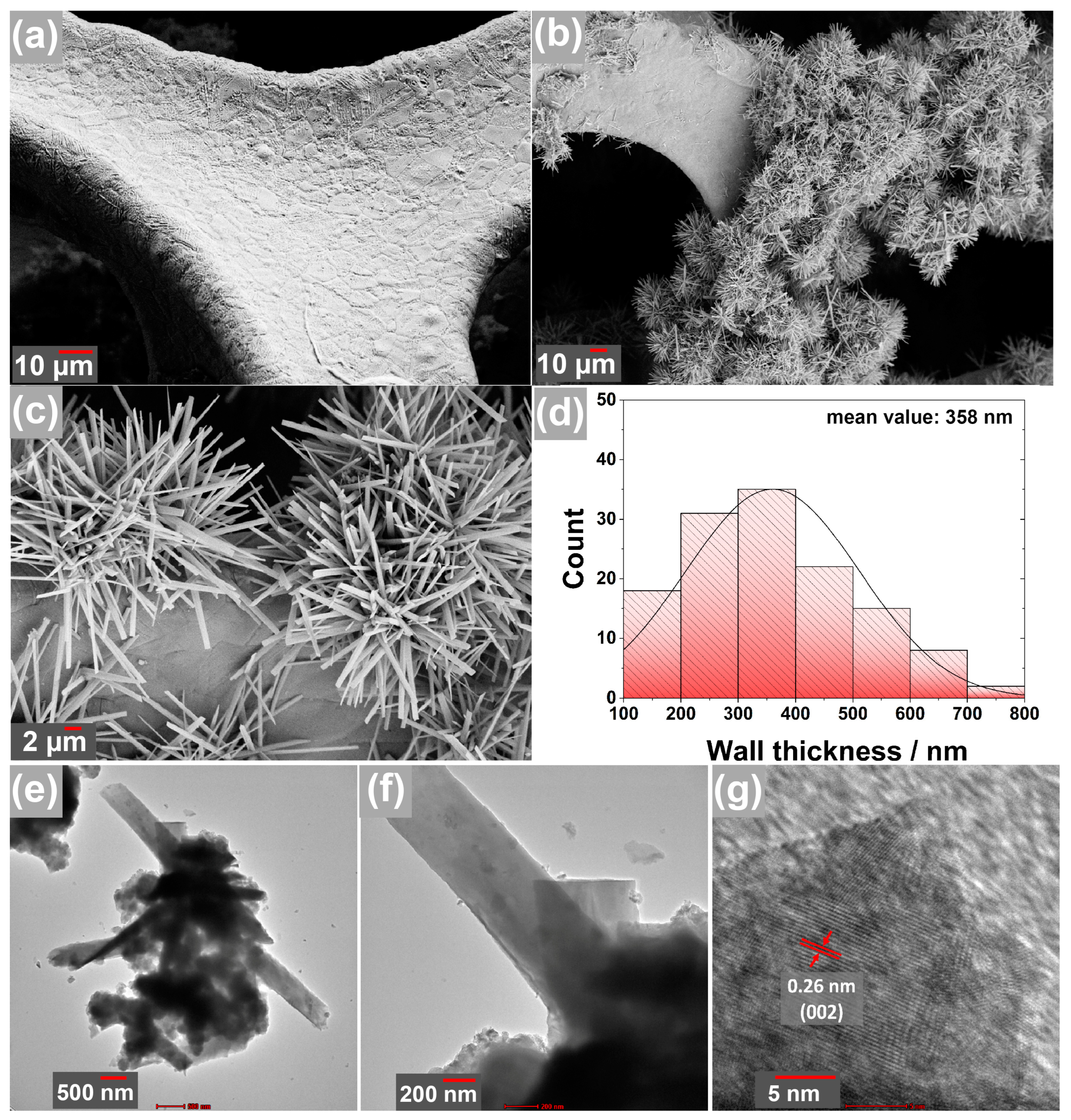
3.1. Material Characterization
3.2. Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of Supercapacitors: Materials and Devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Su, H.; Huang, W.; Dong, X. Binary Metal Oxide: Advanced Energy Storage Materials in Supercapacitors. J. Mater. Chem. A 2015, 3, 43–59. [Google Scholar] [CrossRef]
- Polat, S. Production of ZnFe2O4 Doped Carbon Cloth-Based Flexible Composite Electrodes for Supercapacitors. Türk Doğa ve Fen Dergisi 2021, 10, 199–205. [Google Scholar] [CrossRef]
- Breitenbach, S.; Duchoslav, J.; Mardare, A.I.; Unterweger, C.; Stifter, D.; Hassel, A.W.; Fürst, C. Comparative Behavior of Viscose-Based Supercapacitor Electrodes Activated by KOH, H2O, and CO2. Nanomaterials 2022, 12, 677. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Aziz, U. Supercapattery: Merging of Battery-Supercapacitor Electrodes for Hybrid Energy Storage Devices. J. Energy Storage 2022, 46, 103823. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abbas, Q.; Al Makky, A.; Abdelkareem, M.A. Supercapacitors as next Generation Energy Storage Devices: Properties and Applications. Energy 2022, 248, 123617. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Ansari, S.A.; Kim, S.-H. Fundamentals and Recent Progress of Sn-Based Electrode Materials for Supercapacitors: A Comprehensive Review. J. Energy Storage 2022, 53, 105187. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, H.; Wu, X.; Wang, L.; Zhang, A.; Xia, T.; Dong, H.; Li, X.; Zhang, L. Progress of Electrochemical Capacitor Electrode Materials: A Review. Int. J. Hydrogen Energy 2009, 34, 4889–4899. [Google Scholar] [CrossRef]
- Bahaa, A.; Abdelkareem, M.A.; Al Naqbi, H.; Mohamed, A.Y.; Yousef, B.A.A.; Sayed, E.T.; Chae, K.-J.; Al-Asheh, S.; Olabi, A.G. Structural Engineering and Surface Modification of Nickel Double Hydroxide Nanosheets for All-Solid-State Asymmetric Supercapacitors. J. Energy Storage 2022, 45, 103720. [Google Scholar] [CrossRef]
- Bahaa, A.; Abdelkareem, M.A.; Al Naqbi, H.; Yousef Mohamed, A.; Shinde, P.A.; Yousef, B.A.A.; Sayed, E.T.; Alawadhi, H.; Chae, K.-J.; Al-Asheh, S.; et al. High Energy Storage Quasi-Solid-State Supercapacitor Enabled by Metal Chalcogenide Nanowires and Iron-Based Nitrogen-Doped Graphene Nanostructures. J. Colloid Interface Sci. 2022, 608, 711–719. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, S.-C.; Sun, G.-T.; Kang, K.; Zhu, M.-Q.; Geng, Z.-C. Comparison of Activated Carbons Prepared by One-Step and Two-Step Chemical Activation Process Based on Cotton Stalk for Supercapacitors Application. Energy 2021, 215, 119144. [Google Scholar] [CrossRef]
- Wang, J.; Chen, R.; Xiang, L.; Komarneni, S. Synthesis, Properties and Applications of ZnO Nanomaterials with Oxygen Vacancies: A Review. Ceram. Int. 2018, 44, 7357–7377. [Google Scholar] [CrossRef]
- Polat, S. Dielectric Properties of BN-ZnO-GNP Doped PU-EG Composites. IJERAD 2021, 13, 635–644. [Google Scholar] [CrossRef]
- Kumar Panda, P.; Grigoriev, A.; Kumar Mishra, Y.; Ahuja, R. Progress in Supercapacitors: Roles of Two Dimensional Nanotubular Materials. Nanoscale Adv. 2020, 2, 70–108. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Mia, M.M.; Shah, S.S.; Naher, S.; Shaikh, M.N.; Aziz, M.A.; Ahammad, A.J.S. Recent Advancements in Electrochemical Deposition of Metal-Based Electrode Materials for Electrochemical Supercapacitors. Chem. Rec. 2022, 22, e202200013. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.; Parida, S.; Badrayyana, S.; Singh, B.K. High Performance Flexible Asymmetric CNO-ZnO//ZnO Supercapacitor with an Operating Voltage of 1.8V in Aqueous Medium. Appl. Mater. Today 2017, 7, 212–221. [Google Scholar] [CrossRef]
- Shi, S.; Zhuang, X.; Cheng, B.; Wang, X. Solution Blowing of ZnO Nanoflake-Encapsulated Carbon Nanofibers as Electrodes for Supercapacitors. J. Mater. Chem. A 2013, 1, 13779–13788. [Google Scholar] [CrossRef]
- Song, A.; Kim, J.H.; Yong, H.; Rho, Y.; Song, D.-S.; Cho, E.; Kim, M.J.; Chung, K.-B.; Kim, H.-S.; Lee, S.-J. ZnO–Plasma Polymer Fluorocarbon Thin Films for Stable Battery Anodes and High-Output Triboelectric Nanogenerators. ACS Appl. Nano Mater. 2022, 5, 14540–14550. [Google Scholar] [CrossRef]
- Dhiman, P. ZnO: A Potential Candidate as a Host Material for Diluted Magnetic Semiconductors. In Materials Research Foundations; Sharma, G., Ed.; Materials Research Forum LLC: Millersville, PA, USA, 2023; Volume 146, pp. 67–85. ISBN 978-1-64490-239-4. [Google Scholar]
- Zhang, S.; Yin, B.; Jiang, H.; Qu, F.; Umar, A.; Wu, X. Hybrid ZnO/ZnS Nanoforests as the Electrode Materials for High Performance Supercapacitor Application. Dalton Trans. 2015, 44, 2409–2415. [Google Scholar] [CrossRef]
- Kalpana, D.; Omkumar, K.S.; Kumar, S.S.; Renganathan, N.G. A Novel High Power Symmetric ZnO/Carbon Aerogel Composite Electrode for Electrochemical Supercapacitor. Electrochim. Acta 2006, 52, 1309–1315. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Palaniappa, M.; Balasubramanian, K. Single Step Solution Combustion Synthesis of ZnO/Carbon Composite and Its Electrochemical Characterization for Supercapacitor Application. Int. J. Electrochem. Sci. 2008, 3, 96–103. [Google Scholar] [CrossRef]
- Selvakumar, M.; Krishna Bhat, D.; Manish Aggarwal, A.; Prahladh Iyer, S.; Sravani, G. Nano ZnO-Activated Carbon Composite Electrodes for Supercapacitors. Phys. B Condens. Matter 2010, 405, 2286–2289. [Google Scholar] [CrossRef]
- Xiao, X.; Han, B.; Chen, G.; Wang, L.; Wang, Y. Preparation and Electrochemical Performances of Carbon Sphere@ZnO Core-Shell Nanocomposites for Supercapacitor Applications. Sci. Rep. 2017, 7, 40167. [Google Scholar] [CrossRef]
- Zhang, Z.; Ren, L.; Han, W.; Meng, L.; Wei, X.; Qi, X.; Zhong, J. One-Pot Electrodeposition Synthesis of ZnO/Graphene Composite and Its Use as Binder-Free Electrode for Supercapacitor. Ceram. Int. 2015, 41, 4374–4380. [Google Scholar] [CrossRef]
- Jayachandiran, J.; Yesuraj, J.; Arivanandhan, M.; Raja, A.; Suthanthiraraj, S.A.; Jayavel, R.; Nedumaran, D. Synthesis and Electrochemical Studies of RGO/ZnO Nanocomposite for Supercapacitor Application. J. Inorg. Organomet. Polym. 2018, 28, 2046–2055. [Google Scholar] [CrossRef]
- Gürhan, D. Zinc Borate Production in a Batch Reactor. Master’s Thesis, Middle East Technical University, Ankara, Turkey, 2005. [Google Scholar]
- Xu, H.; Wang, H.; Zhang, Y.; He, W.; Zhu, M.; Wang, B.; Yan, H. Hydrothermal Synthesis of Zinc Oxide Powders with Controllable Morphology. Ceram. Int. 2004, 30, 93–97. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Hydrothermal Growth of ZnO Nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 013001. [Google Scholar] [CrossRef]
- Mbebou, M.; Polat, S.; Zengin, H. Sustainable Cauliflower-Patterned CuFe2O4 Electrode Production from Chalcopyrite for Supercapacitor Applications. Nanomaterials 2023, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Polat, S.; Mashrah, M. Synthesis and Electrochemical Performance of MgFe2O4 with G-C3N4 on Ni-Foam as Composite Anode Material in Supercapacitors. J. Mater. Sci. Mater. Electron. 2022, 33, 23427–23436. [Google Scholar] [CrossRef]
- Shin, Y.; Jung, Y.; Cho, C.P.; Pyo, Y.D.; Jang, J.; Kim, G.; Kim, T.M. NOx Abatement and N2O Formation over Urea-SCR Systems with Zeolite Supported Fe and Cu Catalysts in a Nonroad Diesel Engine. Chem. Eng. J. 2020, 381, 122751. [Google Scholar] [CrossRef]
- Su, M.; He, C.; Shih, K. Facile Synthesis of Morphology and Size-Controlled α-Fe2O3 and Fe3O4 Nano-and Microstructures by Hydrothermal/Solvothermal Process: The Roles of Reaction Medium and Urea Dose. Ceram. Int. 2016, 42, 14793–14804. [Google Scholar] [CrossRef]
- Mashrah, M.; Polat, S. Hydrothermal Synthesis and Electrochemical Performance of GNPs-Doped MgFe2O4 Electrodes for Supercapacitors. Solid State Ion. 2023, 391, 116107. [Google Scholar] [CrossRef]
- Polat, S.; Faris, D. Fabrication of CuFe2O4@g-C3N4@GNPs Nanocomposites as Anode Material for Supercapacitor Applications. Ceram. Int. 2022, 48, 24609–24618. [Google Scholar] [CrossRef]
- Tugrul, N.; Bardakci, M.; Ozturk, E. Synthesis of Hydrophobic Nanostructured Zinc Borate from Zinc Carbonate, and Characterization of the Product. Res. Chem. Intermed. 2015, 41, 4395–4403. [Google Scholar] [CrossRef]
- Bi, Z.; Lai, B.; Zhao, Y.; Yan, L. Fast Disassembly of Lignocellulosic Biomass to Lignin and Sugars by Molten Salt Hydrate at Low Temperature for Overall Biorefinery. ACS Omega 2018, 3, 2984–2993. [Google Scholar] [CrossRef]
- Shaheen, I.; Ahmad, K.S.; Zequine, C.; Gupta, R.K.; Thomas, A.G.; Azad Malik, M. Sustainable Synthesis of Organic Framework-Derived ZnO Nanoparticles for Fabrication of Supercapacitor Electrode. Environ. Technol. 2022, 43, 605–616. [Google Scholar] [CrossRef]
- Sahai, A.; Goswami, N. Structural and Optical Investigations of Oxygen Defects in Zinc Oxide Nanoparticles. AIP Conf. Proc. 2015, 1665, 050023. [Google Scholar] [CrossRef]
- Ilyas, U.; Rawat, R.S.; Tan, T.L.; Lee, P.; Chen, R.; Sun, H.D.; Fengji, L.; Zhang, S. Oxygen Rich P-Type ZnO Thin Films Using Wet Chemical Route with Enhanced Carrier Concentration by Temperature-Dependent Tuning of Acceptor Defects. J. Appl. Phys. 2011, 110, 093522. [Google Scholar] [CrossRef]
- Li, J.Y.; Chen, X.L.; Li, H.; He, M.; Qiao, Z.Y. Fabrication of Zinc Oxide Nanorods. J. Cryst. Growth 2001, 233, 5–7. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, Y.; Li, Q.K.; Nan, C.W. Synthesis and Optical Properties of Tetrapod-like Zinc Oxide Nanorods. Chem. Phys. Lett. 2002, 358, 83–86. [Google Scholar] [CrossRef]
- Thiagarajan, K.; Bavani, T.; Arunachalam, P.; Lee, S.J.; Theerthagiri, J.; Madhavan, J.; Pollet, B.G.; Choi, M.Y. Nanofiber NiMoO4/g-C3N4 Composite Electrode Materials for Redox Supercapacitor Applications. Nanomaterials 2020, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Kavil, J.; Anjana, P.M.; Periyat, P.; Rakhi, R.B. One-Pot Synthesis of g-C3N4/MnO2 and g-C3N4/SnO2 Hybrid Nanocomposites for Supercapacitor Applications. Sustain. Energy Fuels 2018, 2, 2244–2251. [Google Scholar] [CrossRef]
- Pradeeswari, K.; Venkatesan, A.; Pandi, P.; Karthik, K.; Krishna, K.V.H.; Kumar, R.M. Study on the Electrochemical Performance of ZnO Nanoparticles Synthesized via Non-Aqueous Sol-Gel Route for Supercapacitor Applications. Mater. Res. Express 2019, 6, 105525. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, W.; Xu, S.; Li, Y.; Yang, Y. Microspherical ZnO Synthesized from a Metal-Organic Precursor for Supercapacitors. Ionics 2016, 22, 2169–2174. [Google Scholar] [CrossRef]
- Chandel, M.; Moitra, D.; Makkar, P.; Sinha, H.; Singh Hora, H.; Nath Ghosh, N. Synthesis of Multifunctional CuFe2O4–Reduced Graphene Oxide Nanocomposite: An Efficient Magnetically Separable Catalyst as Well as High Performance Supercapacitor and First-Principles Calculations of Its Electronic Structures. RSC Adv. 2018, 8, 27725–27739. [Google Scholar] [CrossRef]
- Guan, B.Y.; Kushima, A.; Yu, L.; Li, S.; Li, J.; Lou, X.W. (David) Coordination Polymers Derived General Synthesis of Multishelled Mixed Metal-Oxide Particles for Hybrid Supercapacitors. Adv. Mater. 2017, 29, 1605902. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-W.; Feng, Y.-Q.; Yan, X.-B.; Chen, J.-T.; Xue, Q.-J. Superior Micro-Supercapacitors Based on Graphene Quantum Dots. Adv. Funct. Mater. 2013, 23, 4111–4122. [Google Scholar] [CrossRef]
- Heydari, H.; Moosavifard, S.E.; Shahraki, M.; Elyasi, S. Facile Synthesis of Nanoporous CuS Nanospheres for High-Performance Supercapacitor Electrodes. J. Energy Chem. 2017, 26, 762–767. [Google Scholar] [CrossRef]
- An, C.; Wang, Y.; Huang, Y.; Xu, Y.; Xu, C.; Jiao, L.; Yuan, H. Novel Three-Dimensional NiCo 2 O 4 Hierarchitectures: Solvothermal Synthesis and Electrochemical Properties. CrystEngComm 2014, 16, 385–392. [Google Scholar] [CrossRef]
- Bessekhouad, Y.; Brahimi, R.; Hamdini, F.; Trari, M. Cu2S/TiO2 Heterojunction Applied to Visible Light Orange II Degradation. J. Photochem. Photobiol. A Chem. 2012, 248, 15–23. [Google Scholar] [CrossRef]
- Scully, J.R. Electrochemical Impedance: Analysis and Interpretation; ASTM International: Philadelphia, PA, USA, 1993; ISBN 978-0-8031-1861-4. [Google Scholar]
- Wang, H.; Zhang, C.; Liu, Z.; Wang, L.; Han, P.; Xu, H.; Zhang, K.; Dong, S.; Yao, J.; Cui, G. Nitrogen-Doped Graphene Nanosheets with Excellent Lithium Storage Properties. J. Mater. Chem. 2011, 21, 5430–5434. [Google Scholar] [CrossRef]
- Zhao, W.; Ai, Z.; Dai, J.; Zhang, M. Enhanced Photocatalytic Activity for H2 Evolution under Irradiation of UV–Vis Light by Au-Modified Nitrogen-Doped TiO2. PLoS ONE 2014, 9, e103671. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, G.; Ma, J. CuMn2O4/Graphene Nanosheets as Excellent Anode for Lithium-Ion Battery. Mater. Res. Bull. 2018, 104, 53–59. [Google Scholar] [CrossRef]
- Gopi, C.V.V.M.; Vinodh, R.; Sambasivam, S.; Obaidat, I.M.; Singh, S.; Kim, H.-J. Co9S8-Ni3S2/CuMn2O4-NiMn2O4 and MnFe2O4-ZnFe2O4/Graphene as Binder-Free Cathode and Anode Materials for High Energy Density Supercapacitors. Chem. Eng. J. 2020, 381, 122640. [Google Scholar] [CrossRef]
- Zhu, M.; Meng, D.; Wang, C.; Diao, G. Facile Fabrication of Hierarchically Porous CuFe2O4 Nanospheres with Enhanced Capacitance Property. ACS Appl. Mater. Interfaces 2013, 5, 6030–6037. [Google Scholar] [CrossRef]
- Bandgar, S.B.; Vadiyar, M.M.; Suryawanshi, U.P.; Jambhale, C.L.; Kim, J.-H.; Kolekar, S.S. Rotational Reflux Chemistry Approach Derived Flat Holey CuFe2O4 Nanosheets for Supercapacitors Application. Mater. Lett. 2020, 279, 128514. [Google Scholar] [CrossRef]
- Sasirekha, C.; Arumugam, S.; Muralidharan, G. Green Synthesis of ZnO/Carbon (ZnO/C) as an Electrode Material for Symmetric Supercapacitor Devices. Appl. Surf. Sci. 2018, 449, 521–527. [Google Scholar] [CrossRef]



| Electrode Materials | Electrolyte | Potential (V) | Scan Rate/ Current Density | Specific Capacity (Cs) | % Retention | Ref. |
|---|---|---|---|---|---|---|
| ZnO nanostrips | 6 M KOH | 0 to 0.4 | 2 A·g−1 | 867 mF·cm−2 | ~66% after 1000 cycles at 10 mA | This study |
| ZnO/C | 1 M Na2SO4 | 0 to 0.8 | 1 A·g−1 | 820 F·g−1 | ~92% after 400 cycles at 50 mV·s−1 | [60] |
| ZnO/C Core-shell | 6 M KOH | 0 to 0.4 | 2 A·g−1 | 630 F·g−1 | ~70% after 5000 cycles | [24] |
| ZnO/GO | 1 M Na2SO4 | 0 to 1 | 5 mV·s−1 | 312 F·g−1 | ~95% after 100 cycles at 10 mV·s−1 | [26] |
| ZnO/Graphene | 1 M KOH | 0 to 0.5 | 3 A·g−1 | 291 F·g−1 | ~88% after 1000 cycles at 4 A·g−1 | [25] |
| ZnO/ZnS | 1 M KOH | −0.6 to −1.3 | 1 mA·cm−2 | 217 mF·cm−2 | ~81% after 2000 cycles at 2 mA·cm−2 | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lefdhil, C.; Polat, S.; Zengin, H. Synthesis of Zinc Oxide Nanorods from Zinc Borate Precursor and Characterization of Supercapacitor Properties. Nanomaterials 2023, 13, 2423. https://doi.org/10.3390/nano13172423
Lefdhil C, Polat S, Zengin H. Synthesis of Zinc Oxide Nanorods from Zinc Borate Precursor and Characterization of Supercapacitor Properties. Nanomaterials. 2023; 13(17):2423. https://doi.org/10.3390/nano13172423
Chicago/Turabian StyleLefdhil, Chikh, Safa Polat, and Hüseyin Zengin. 2023. "Synthesis of Zinc Oxide Nanorods from Zinc Borate Precursor and Characterization of Supercapacitor Properties" Nanomaterials 13, no. 17: 2423. https://doi.org/10.3390/nano13172423
APA StyleLefdhil, C., Polat, S., & Zengin, H. (2023). Synthesis of Zinc Oxide Nanorods from Zinc Borate Precursor and Characterization of Supercapacitor Properties. Nanomaterials, 13(17), 2423. https://doi.org/10.3390/nano13172423






