Preparation and Characterization of Spray-Dried Hybrid Nanocrystal–Amorphous Solid Dispersions (HyNASDs) for Supersaturation Enhancement of a Slowly Crystallizing Drug
Abstract
1. Introduction
| Drug | Material Type | Process | Formulation a | % Relative Supersaturation b | Ref. |
|---|---|---|---|---|---|
| Celocoxibe | Nanocomposite | Spray drying | Tween 80, dextrin | NR | [24] |
| Fenofibrate | Nanocomposite | Spray drying | HPMC–SDS | <50 | [27] |
| Glyburide | Nanocomposite | Spray drying | HPMC–SDS | NR | [11] |
| Griseofulvin | Nanocomposite | Nanoextrusion | HPC–SDS | 55 | [28] |
| Griseofulvin | ASD | Nanoextrusion | Sol–SDS | 330 | [28] |
| Griseofulvin | Nanocomposite | Fluid bed coating | HPC–SDS | NR | [21] |
| Griseofulvin | Nanocomposite | Spray drying | Sol–SDS | 30 | [31] |
| Griseofulvin | HyNASD | Spray drying | Sol–SDS | 250 | [31] |
| Griseofulvin | ASD | Spray drying | Sol–SDS | 360 | [33] |
| Itraconazole | Nanocomposite | Spray drying | HPC/HPMC–SDS | NR | [23] |
| Itraconazole | ASD | Hot melt extrusion | HPMC | 4400 | [34] |
| Miconazole | Nanocomposite | Spray drying | HPC/HPMC–SDS | NR | [23] |
| Nimodipine | Nanocomposite | Lyophilization | HPMC–PF 127 | <100 | [16] |
2. Materials and Methods
2.1. Materials
2.2. Preparation Methods
2.2.1. Preparation of Feeds for Spray Drying Process
2.2.2. Production of Spray-Dried Powders
2.2.3. Particle Sizing and Solid-State Characterization
2.2.4. Assessment of Actual Drug Content and Drug Release
2.2.5. ITZ Wettability Enhancement by Polymer Solutions with or without SDS
2.2.6. Precipitation Behavior of ITZ and Potential Impact of Polymers and SDS
3. Results
3.1. Properties of ITZ Nanosuspensions Prepared via Wet Stirred Media Milling
3.2. Residual Moisture, Drug Content, and Particle Sizes of the Spray-Dried Powders
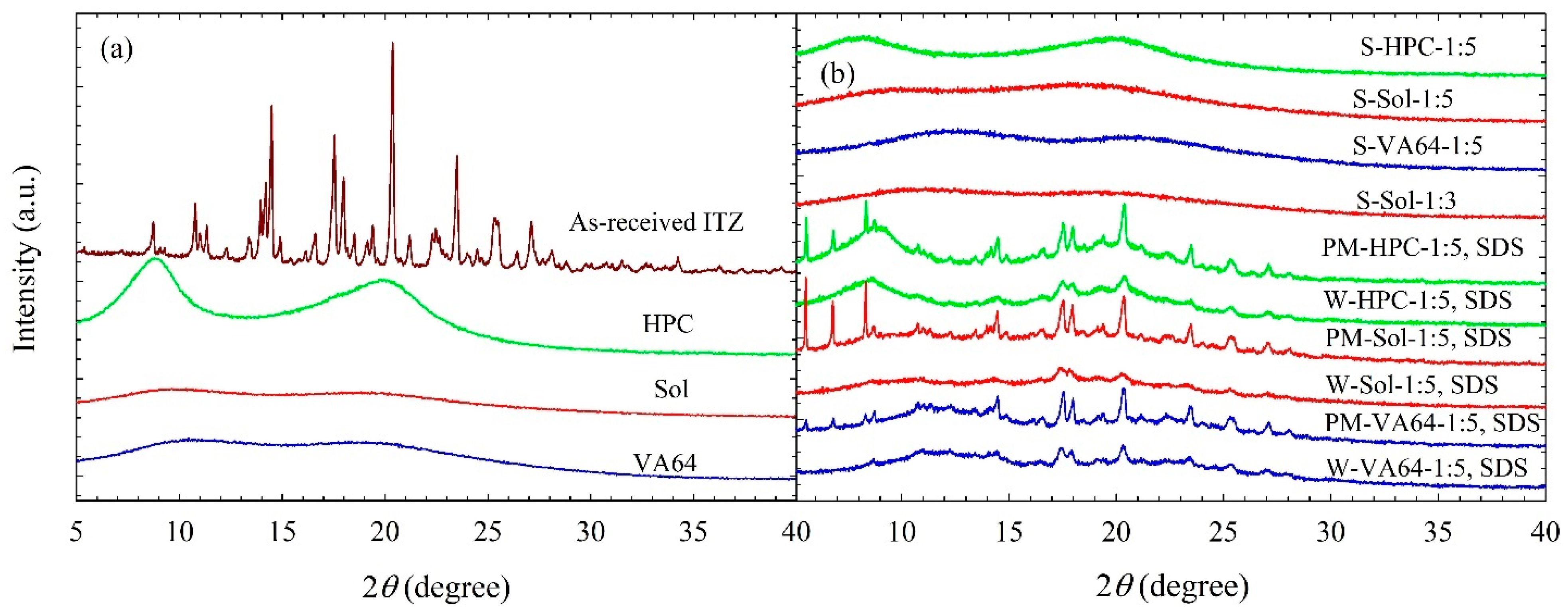
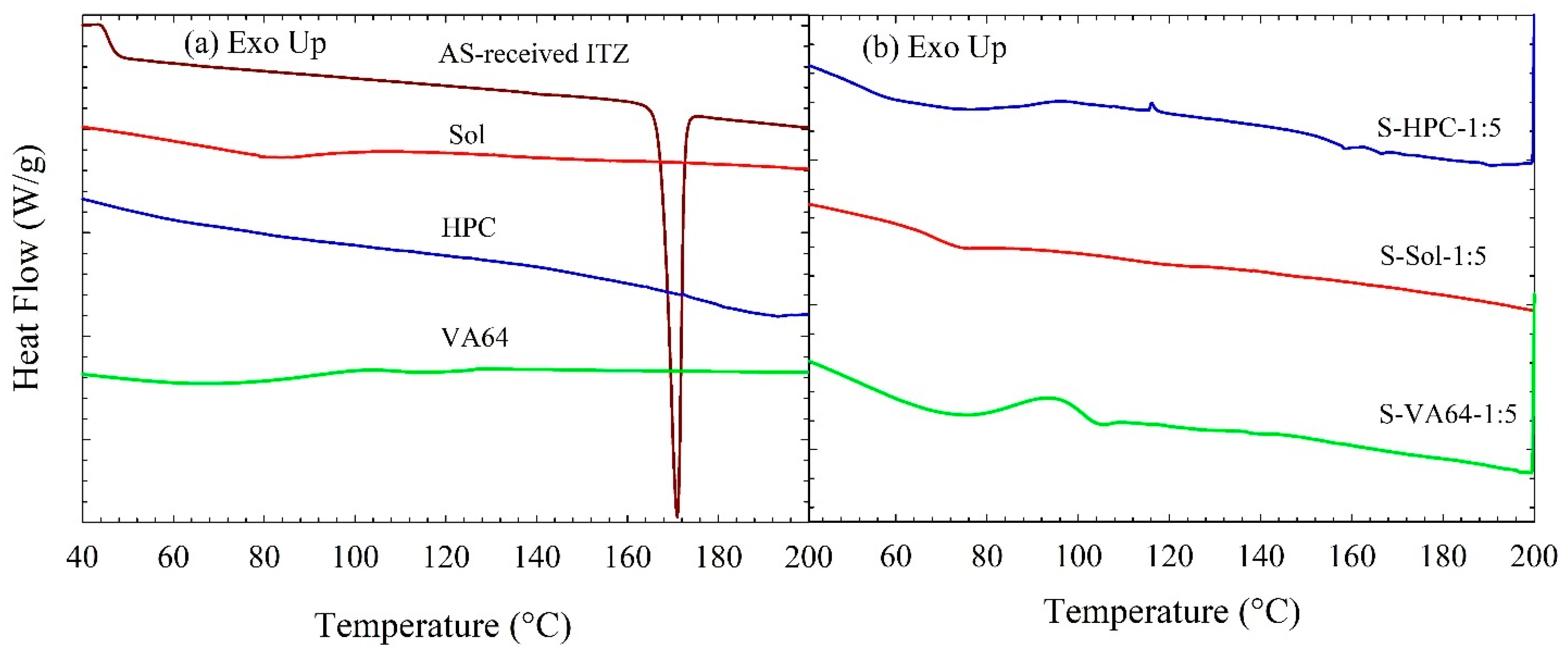
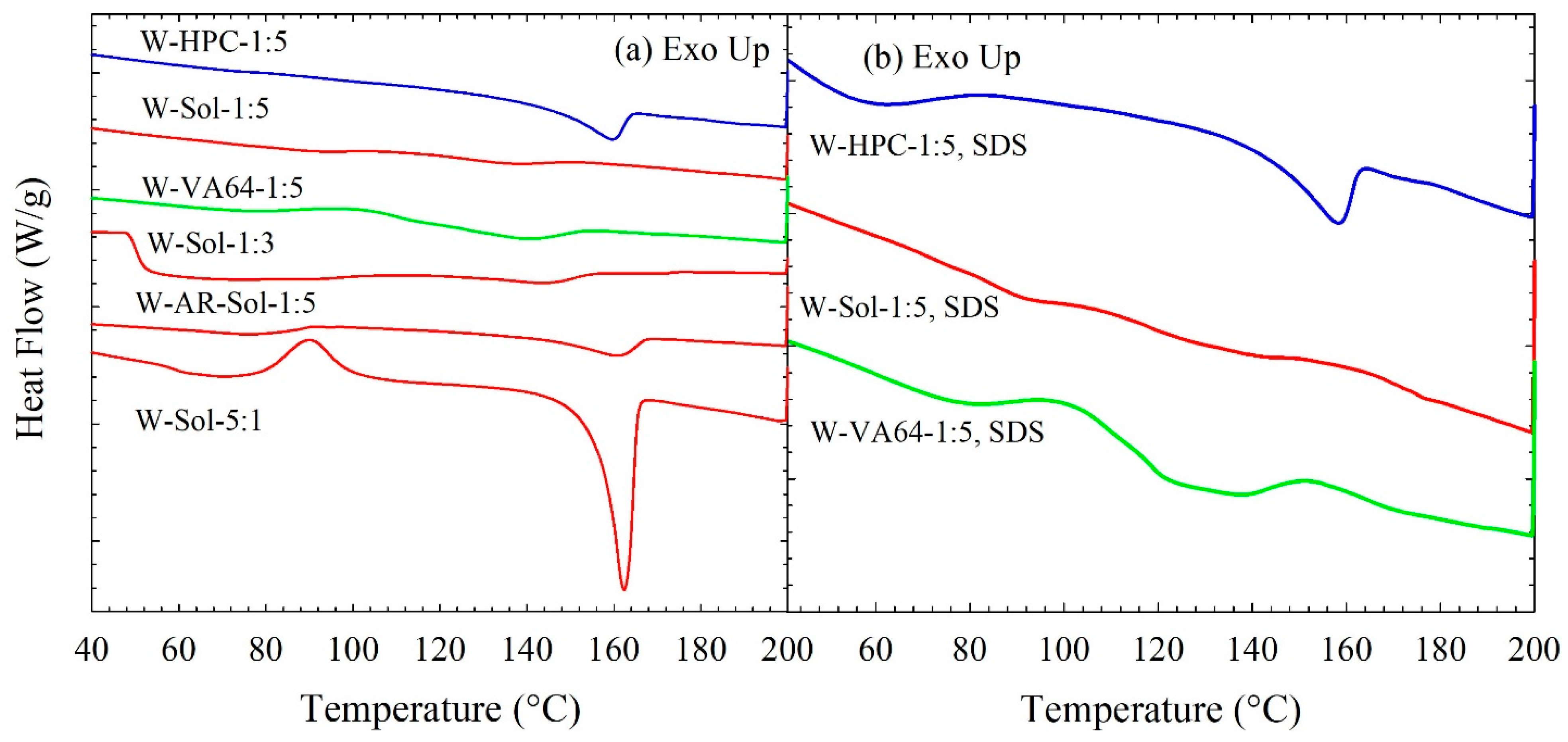
3.3. Solid State Characterization of the Spray-Dried Powders
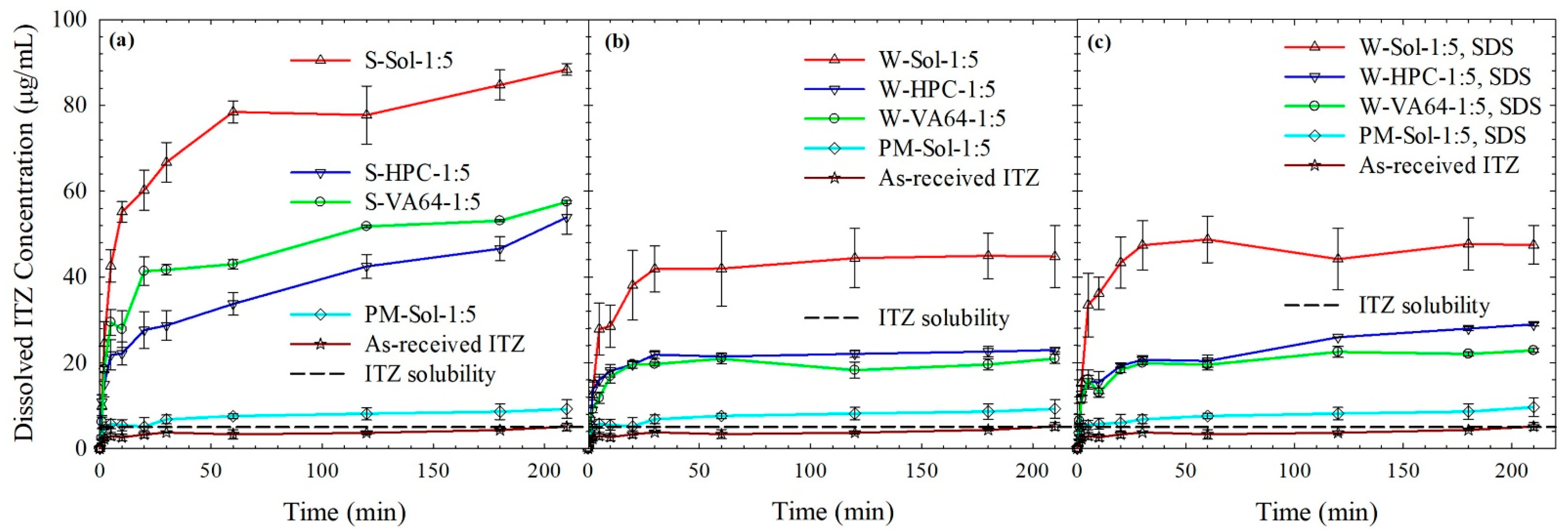
3.4. ITZ Release from the Spray-Dried Powders
3.4.1. Spray-Dried Powders vs. as-Received Drug and Physical Mixtures
3.4.2. ITZ Release from HyNASDs and ASDs
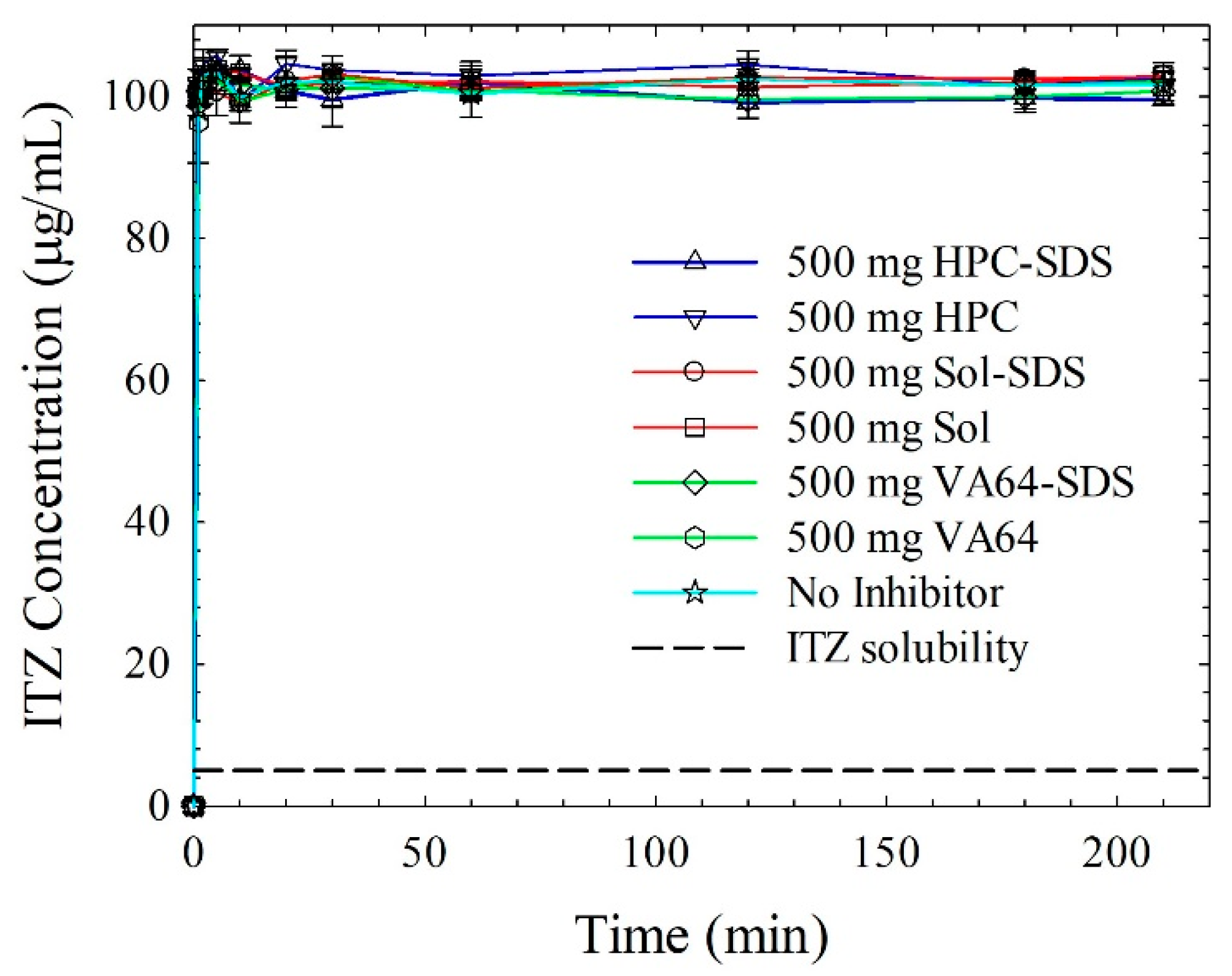
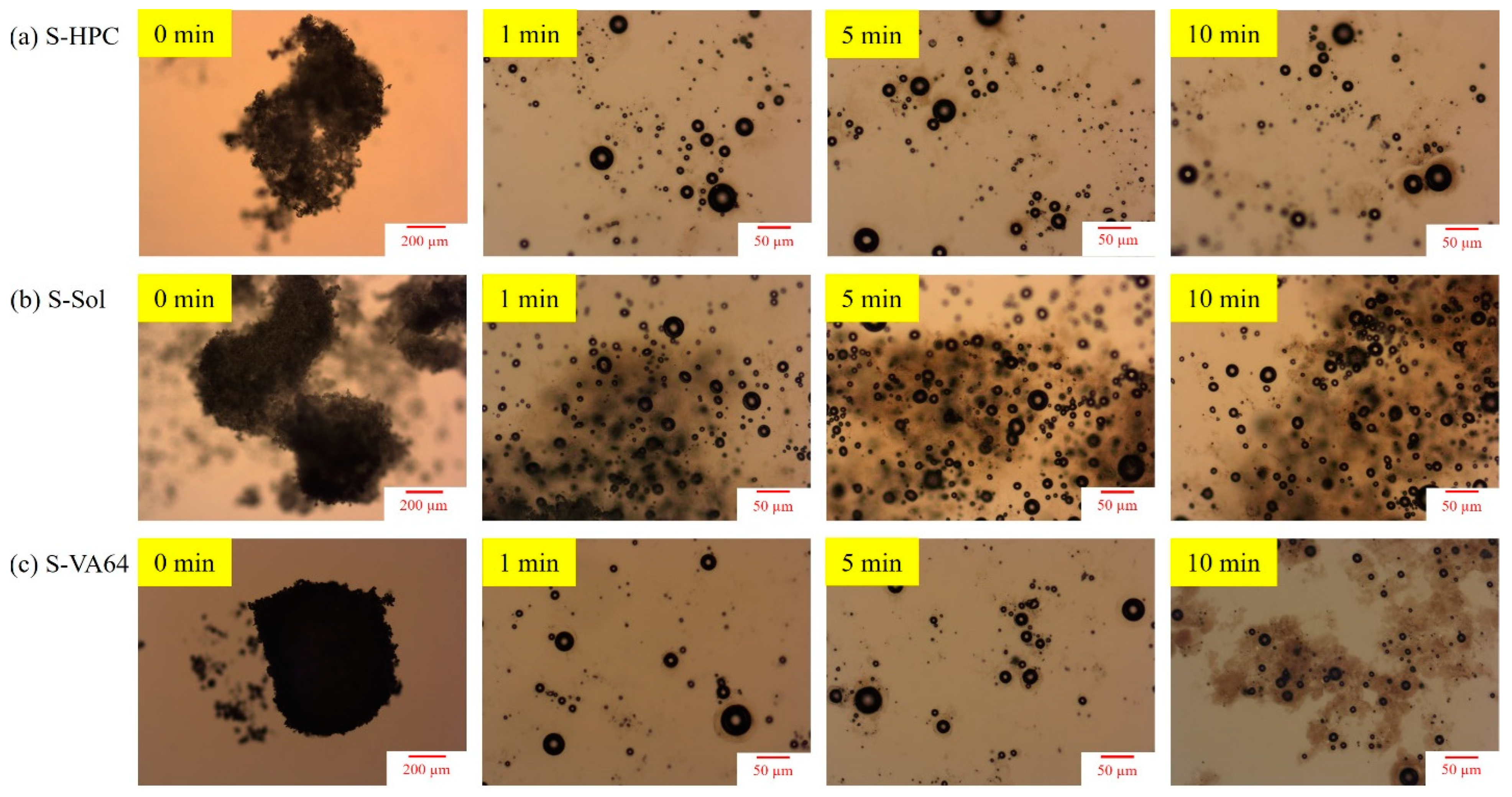
3.4.3. Insights from Wettability, Solvent-Shift, and Water-Imbibition Tests
3.4.4. Dissolution of HyNASDs: Polymer Loading and ITZ Particle Size Effect
3.5. Future Improvement Strategies for HyNASDs and Outlook
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Löbenberg, R.; Amidon, G.L. Modern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. Eur. J. Pharm. Biopharm. 2000, 50, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Bhakay, A.; Rahman, M.; Dave, R.N.; Bilgili, E. Bioavailability enhancement of poorly water-soluble drugs via nanocomposites: Formulation processing aspects and challenges. Pharmaceutics 2018, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Brough, C.; Williams Iii, R. Amorphous solid dispersions and nano-crystal technologies for poorly water-soluble drug delivery. Int. J. Pharm. 2013, 453, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Gohla, S.; Keck, C.M. State of the art of nanocrystals–special features, production, nanotoxicology aspects and intracellular delivery. Eur. J. Pharm. Biopharm. 2011, 78, 1–9. [Google Scholar] [CrossRef]
- Li, M.; Azad, M.; Davé, R.; Bilgili, E. Nanomilling of drugs for bioavailability enhancement: A holistic formulation–process perspective. Pharmaceutics 2016, 8, 17. [Google Scholar] [CrossRef]
- Peltonen, L.; Hirvonen, J. Pharmaceutical nanocrystals by nanomilling: Critical process parameters, particle fracturing and stabilization methods. J. Pharm. Pharmacol. 2010, 62, 1569–1579. [Google Scholar] [CrossRef]
- Kesisoglou, F.; Panmai, S.; Wu, Y. Nanosizing—Oral formulation development and biopharmaceutical evaluation. Adv. Drug Delivery Rev. 2007, 59, 631–644. [Google Scholar] [CrossRef]
- Junghanns, J.-U.A.; Müller, R.H. Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008, 3, 295–309. [Google Scholar]
- Müller, R.; Jacobs, C.; Kayser, O. Nanosuspensions as particulate drug formulations in therapy: Rationale for development and what we can expect for the future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Hancock, B.C.; Parks, M. What is the true solubility advantage for amorphous pharmaceuticals? Pharm. Res. 2000, 17, 397–404. [Google Scholar] [CrossRef]
- Singh, S.K.; Srinivasan, K.; Gowthamarajan, K.; Singare, D.S.; Prakash, D.; Gaikwad, N.B. Investigation of preparation parameters of nanosuspension by top-down media milling to improve the dissolution of poorly water-soluble glyburide. Eur. J. Pharm. Biopharm. 2011, 78, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Inkyo, M.; Yumoto, R.; Nagai, J.; Takano, M.; Nagata, S. Nanoparticulation of probucol, a poorly water-soluble drug, using a novel wet-milling process to improve in vitro dissolution and in vivo oral absorption. Drug Dev. Ind. Pharm. 2012, 38, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Benita, S.; Böhm, B.H. Nanosuspensions. In Emulsions and Nanosuspensions for the Formulation of Poorly Soluble Drugs; Benita, S., Böhm, B.H., Eds.; Medpharm Scientific: Stuttgart, Germany, 1998; pp. 149–173. [Google Scholar]
- Kesisoglou, F.; Wu, Y. Understanding the effect of API properties on bioavailability through absorption modeling. AAPS J. 2008, 10, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Peters, K. Nanosuspensions for the formulation of poorly soluble drugs: I. Preparation by a size-reduction technique. Int. J. Pharm. 1998, 160, 229–237. [Google Scholar] [CrossRef]
- Fu, Q.; Sun, J.; Zhang, D.; Li, M.; Wang, Y.; Ling, G.; Liu, X.; Sun, Y.; Sui, X.; Luo, C. Nimodipine nanocrystals for oral bioavailability improvement: Preparation, characterization and pharmacokinetic studies. Colloids Surf. B Biointerfaces 2013, 109, 161–166. [Google Scholar] [CrossRef]
- Oktay, A.N.; Ilbasmis-Tamer, S.; Celebi, N. The effect of critical process parameters of the high pressure homogenization technique on the critical quality attributes of flurbiprofen nanosuspensions. Pharm. Dev. Technol. 2019, 24, 1278–1286. [Google Scholar] [CrossRef]
- Karakucuk, A.; Celebi, N.; Teksin, Z.S. Preparation of ritonavir nanosuspensions by microfluidization using polymeric stabilizers: I. A Design of Experiment approach. Eur. J. Pharm. Sci. 2016, 95, 111–121. [Google Scholar] [CrossRef]
- Kim, C.-J. Advanced Pharmaceutics: Physicochemical Principles; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Bilgili, E.; Afolabi, A. A combined microhydrodynamics–polymer adsorption analysis for elucidation of the roles of stabilizers in wet stirred media milling. Int. J. Pharm. 2012, 439, 193–206. [Google Scholar] [CrossRef]
- Li, M.; Lopez, N.; Bilgili, E. A study of the impact of polymer–surfactant in drug nanoparticle coated pharmatose composites on dissolution performance. Adv. Powder Technol. 2016, 27, 1625–1636. [Google Scholar] [CrossRef]
- Poozesh, S.; Bilgili, E. Scale-up of pharmaceutical spray drying using scale-up rules: A review. Int. J. Pharm. 2019, 562, 271–292. [Google Scholar] [CrossRef]
- Cerdeira, A.M.; Mazzotti, M.; Gander, B. Formulation and drying of miconazole and itraconazole nanosuspensions. Int. J. Pharm. 2013, 443, 209–220. [Google Scholar] [CrossRef]
- Kim, H.-I.; Park, S.Y.; Park, S.J.; Lee, J.; Cho, K.H.; Jee, J.-P.; Kim, H.-C.; Maeng, H.-J.; Jang, D.-J. Development and evaluation of a reconstitutable dry suspension to improve the dissolution and oral absorption of poorly water-soluble celecoxib. Pharmaceutics 2018, 10, 140. [Google Scholar] [CrossRef]
- Kumar, S.; Gokhale, R.; Burgess, D.J. Quality by design approach to spray drying processing of crystalline nanosuspensions. Int. J. Pharm. 2014, 464, 234–242. [Google Scholar] [CrossRef]
- Šimková, K.; Joost, B.; Imanidis, G. Production of fast-dissolving low-density powders for improved lung deposition by spray drying of a nanosuspension. Eur. J. Pharm. Biopharm. 2020, 146, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Zuo, B.; Sun, Y.; Li, H.; Liu, X.; Zhai, Y.; Sun, J.; He, Z. Preparation and in vitro/in vivo evaluation of fenofibrate nanocrystals. Int. J. Pharm. 2013, 455, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ioannidis, N.; Gogos, C.; Bilgili, E. A comparative assessment of nanocomposites vs. amorphous solid dispersions prepared via nanoextrusion for drug dissolution enhancement. Eur. J. Pharm. Biopharm. 2017, 119, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric amorphous solid dispersions: A review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef] [PubMed]
- Van Eerdenbrugh, B.; Van den Mooter, G.; Augustijns, P. Top-down production of drug nanocrystals: Nanosuspension stabilization, miniaturization and transformation into solid products. Int. J. Pharm. 2008, 364, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Ahmad, S.; Tarabokija, J.; Bilgili, E. Roles of surfactant and polymer in drug release from spray-dried hybrid nanocrystal-amorphous solid dispersions (HyNASDs). Powder Technol. 2020, 361, 663–678. [Google Scholar] [CrossRef]
- Baird, J.A.; Van Eerdenbrugh, B.; Taylor, L.S. A classification system to assess the crystallization tendency of organic molecules from undercooled melts. J. Pharm. Sci. 2010, 99, 3787–3806. [Google Scholar] [CrossRef]
- Rahman, M.; Arevalo, F.; Coelho, A.; Bilgili, E. Hybrid nanocrystal–amorphous solid dispersions (HyNASDs) as alternative to ASDs for enhanced release of BCS Class II drugs. Eur. J. Pharm. Biopharm. 2019, 145, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Verreck, G.; Six, K.; Van den Mooter, G.; Baert, L.; Peeters, J.; Brewster, M.E. Characterization of solid dispersions of itraconazole and hydroxypropylmethylcellulose prepared by melt extrusion—Part I. Int. J. Pharm. 2003, 251, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Ghazal, H.S.; Dyas, A.M.; Ford, J.L.; Hutcheon, G.A. In vitro evaluation of the dissolution behaviour of itraconazole in bio-relevant media. Int. J. Pharm. 2009, 366, 117–123. [Google Scholar] [CrossRef]
- Bilgili, E.; Rahman, M.; Palacios, D.; Arevalo, F. Impact of polymers on the aggregation of wet-milled itraconazole particles and their dissolution from spray-dried nanocomposites. Adv. Powder Technol. 2018, 9, 2941–2956. [Google Scholar] [CrossRef]
- Bilgili, E.; Li, M.; Afolabi, A. Is the combination of cellulosic polymers and anionic surfactants a good strategy for ensuring physical stability of BCS Class II drug nanosuspensions? Pharm. Dev. Technol. 2016, 21, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Bilgili, E.; Hamey, R.; Scarlett, B. Production of pigment nanoparticles using a wet stirred mill with polymeric media. China Particuol. 2004, 2, 93–100. [Google Scholar] [CrossRef]
- Sommer, M.; Stenger, F.; Peukert, W.; Wagner, N. Agglomeration and breakage of nanoparticles in stirred media mills—A comparison of different methods and models. Chem. Eng. Sci. 2006, 61, 135–148. [Google Scholar] [CrossRef]
- Kayaert, P.; Van den Mooter, G. Is the amorphous fraction of a dried nanosuspension caused by milling or by drying? A case study with Naproxen and Cinnarizine. Eur. J. Pharm. Biopharm. 2012, 81, 650–656. [Google Scholar] [CrossRef]
- Forster, A.; Hempenstall, J.; Rades, T. Characterization of glass solutions of poorly water-soluble drugs produced by melt extrusion with hydrophilic amorphous polymers. J. Pharm. Pharmacol. 2001, 53, 303–315. [Google Scholar] [CrossRef]
- Greenhalgh, D.J.; Williams, A.C.; Timmins, P.; York, P. Solubility parameters as predictors of miscibility in solid dispersions. J. Pharm. Sci. 1999, 88, 1182–1190. [Google Scholar] [CrossRef]
- Kolter, K.; Karl, M.; Gryczke, A.; Ludwigshafen am Rhein, B. Hot-Melt Extrusion with BASF Pharma Polymers: Extrusion Compendium, 2nd ed.; BASF: Ludwigshafen am Rhein, Germany, 2012. [Google Scholar]
- Choi, P.; Kavassalis, T.A.; Rudin, A. Estimation of Hansen solubility parameters for (hydroxyethyl) and (hydroxypropyl) cellulose through molecular simulation. Ind. Eng. Chem. Res. 1994, 33, 3154–3159. [Google Scholar] [CrossRef]
- Sarode, A.; Wang, P.; Cote, C.; Worthen, D.R. Low-viscosity hydroxypropylcellulose (HPC) grades SL and SSL: Versatile pharmaceutical polymers for dissolution enhancement, controlled release, and pharmaceutical processing. AAPS Pharm. Sci. Technol. 2013, 14, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Luebbert, C.; Stoyanov, E.; Sadowski, G. Phase behavior of ASDs based on hydroxypropyl cellulose. Int. J. Pharm. X 2021, 3, 100070. [Google Scholar] [CrossRef] [PubMed]
- Wlodarski, K.; Sawicki, W.; Kozyra, A.; Tajber, L. Physical stability of solid dispersions with respect to thermodynamic solubility of tadalafil in PVP-VA. Eur. J. Pharm. Biopharm. 2015, 96, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.A.; Taylor, L.S. Evaluation of amorphous solid dispersion properties using thermal analysis techniques. Adv. Drug Delivery Rev. 2012, 64, 396–421. [Google Scholar] [CrossRef] [PubMed]
- França, M.T.; Pereira, R.N.; Riekes, M.K.; Pinto, J.M.O.; Stulzer, H.K. Investigation of novel supersaturating drug delivery systems of chlorthalidone: The use of polymer-surfactant complex as an effective carrier in solid dispersions. Eur. J. Pharm. Sci. 2018, 111, 142–152. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.; Chen, Z.; Su, C.; Hageman, M.; Hussain, M.; Haskell, R.; Stefanski, K.; Qian, F. Drug–polymer–water interaction and its implication for the dissolution performance of amorphous solid dispersions. Mol. Pharm. 2015, 12, 576–589. [Google Scholar] [CrossRef]
- Mistry, P.; Mohapatra, S.; Gopinath, T.; Vogt, F.G.; Suryanarayanan, R. Role of the strength of drug–polymer interactions on the molecular mobility and crystallization inhibition in ketoconazole solid dispersions. Mol. Pharm. 2015, 12, 3339–3350. [Google Scholar] [CrossRef]
- Dian, L.; Yu, E.; Chen, X.; Wen, X.; Zhang, Z.; Qin, L.; Wang, Q.; Li, G.; Wu, C. Enhancing oral bioavailability of quercetin using novel soluplus polymeric micelles. Nanoscale Res. Lett. 2014, 9, 684. [Google Scholar] [CrossRef]
- Shi, N.-Q.; Lai, H.-W.; Zhang, Y.; Feng, B.; Xiao, X.; Zhang, H.-M.; Li, Z.-Q.; Qi, X.-R. On the inherent properties of Soluplus and its application in ibuprofen solid dispersions generated by microwave-quench cooling technology. Pharm. Dev. Technol. 2018, 23, 573–586. [Google Scholar] [CrossRef]
- Thiry, J.; Broze, G.; Pestieau, A.; Tatton, A.S.; Baumans, F.; Damblon, C.; Krier, F.; Evrard, B. Investigation of a suitable in vitro dissolution test for itraconazole-based solid dispersions. Eur. J. Pharm. Sci. 2016, 85, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Coelho, A.; Tarabokija, J.; Ahmad, S.; Radgman, K.; Bilgili, E. Synergistic and antagonistic effects of various amphiphilic polymer combinations in enhancing griseofulvin release from ternary amorphous solid dispersions. Eur. J. Pharm. Sci. 2020, 150, 105354. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M. Roles of Surfactant and Binary Polymers on Dissolution Enhancement of BCS II Drugs from Nanocomposites and Amorphous Solid Dispersions. Ph.D. Thesis, New Jersey Institute of Technology, Newark, NJ, USA, 2019. [Google Scholar]
- Zhong, Y.; Jing, G.; Tian, B.; Huang, H.; Zhang, Y.; Gou, J.; Tang, X.; He, H.; Wang, Y. Supersaturation induced by Itraconazole/Soluplus® micelles provided high GI absorption in vivo. Asian J. Pharm. Sci. 2016, 11, 255–264. [Google Scholar] [CrossRef][Green Version]
- Li, M.; Yaragudi, N.; Afolabi, A.; Dave, R.; Bilgili, E. Sub-100 nm drug particle suspensions prepared via wet milling with low bead contamination through novel process intensification. Chem. Eng. Sci. 2015, 130, 207–220. [Google Scholar] [CrossRef]
- Washburn, E.W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Hołownia, D.; Kwiatkowska, I.; Hupka, J. An investigation on wetting of porous materials. Physicochem. Prob. Miner. Process. 2008, 42, 251–262. [Google Scholar]
- Korson, L.; Drost-Hansen, W.; Millero, F.J. Viscosity of water at various temperatures. J. Phys. Chem. 1969, 73, 34–39. [Google Scholar] [CrossRef]


| ID | Formulation a | ITZ (% w/v) b | SDS (% w/v) b | Polymers (% w/v) b | Water (mL) | DCM (mL) | ||
|---|---|---|---|---|---|---|---|---|
| Sol | HPC | VA64 | ||||||
| W1 | W-AR-Sol-1:5 | 2.5 | 0 | 12.5 | - | - | 240 | - |
| W2 | W-Sol-5:1 | 2.5 | 0 | 0.5 | - | - | 240 | - |
| W3 | W-Sol-1:3 | 2.5 | 0 | 7.5 | - | - | 240 | - |
| W4 | W-Sol-1:5 | 2.5 | 0 | 12.5 | - | - | 240 | - |
| W5 | W-HPC-1:5 | 2.5 | 0 | - | 12.5 | - | 240 | - |
| W6 | W-VA64-1:5 | 2.5 | 0 | - | - | 12.5 | 240 | - |
| W7 | W-Sol-1:5, SDS | 2.5 | 0.125 | 12.5 | - | - | 240 | - |
| W8 | W-HPC-1:5, SDS | 2.5 | 0.125 | - | 12.5 | - | 240 | - |
| W9 | W-VA64-1:5, SDS | 2.5 | 0.125 | - | - | 12.5 | 240 | - |
| S1 | S-Sol-1:5 | 2.5 | 0 | 12.5 | - | - | - | 240 |
| S2 | S-HPC-1:5 | 2.5 | 0 | - | 12.5 | - | - | 240 |
| S3 | S-VA64-1:5 | 2.5 | 0 | - | - | 12.5 | - | 240 |
| ID | Formulation a | After 65 min Milling (µm) | After 7-Day Storage (µm) | ||||
|---|---|---|---|---|---|---|---|
| d10 ± SD | d50 ± SD | d90 ± SD | d10 ± SD | d50 ± SD | d90 ± SD | ||
| W1 | W-AR-Sol-1:5 | — | — | — | — | — | — |
| W2 | W-Sol-5:1 | 0.18 ± 0.00 | 0.36 ± 0.00 | 2.11 ± 0.02 | 0.19 ± 0.00 | 0.37 ± 0.04 | 2.14 ± 0.00 |
| W3 | W-Sol-1:3 | 0.15 ± 0.01 | 0.28 ± 0.01 | 1.68 ± 0.11 | 0.16 ± 0.00 | 0.26 ± 0.00 | 1.82 ± 0.01 |
| W4 | W-Sol-1:5 | 0.13 ± 0.00 | 0.26 ± 0.01 | 1.85 ± 0.08 | 0.14 ± 0.00 | 0.37 ± 0.00 | 2.16 ± 0.20 |
| W5 | W-HPC-1:5 | 0.13 ± 0.00 | 0.21 ± 0.00 | 0.32 ± 0.01 | 0.15 ± 0.01 | 0.23 ± 0.02 | 0.35 ± 0.02 |
| W6 | W-VA64-1:5 | 0.18 ± 0.00 | 0.44 ± 0.02 | 2.19 ± 0.01 | 0.18 ± 0.00 | 0.53 ± 0.06 | 2.18 ± 0.00 |
| W7 | W-Sol-1:5, SDS | 0.11 ± 0.00 | 0.16 ± 0.00 | 0.25 ± 0.01 | 0.12 ± 0.00 | 0.18 ± 0.00 | 0.25 ± 0.01 |
| W8 | W-HPC-1:5, SDS | 0.12 ± 0.00 | 0.18 ± 0.00 | 0.25 ± 0.01 | 0.12 ± 0.00 | 0.18 ± 0.00 | 0.25 ± 0.01 |
| W9 | W-VA64-1:5, SDS | 0.14 ± 0.00 | 0.24 ± 0.01 | 0.45 ± 0.02 | 0.15 ± 0.00 | 0.27 ± 0.01 | 1.81 ± 0.12 |
| ID | Formulation a | Characteristic Particle Size (µm) | Theoretical Drug Content (% w/w) b | Actual Drug Content, RSD, (% w/w, %) | ||
|---|---|---|---|---|---|---|
| d10 ± SD | d50 ± SD | d90 ± SD | ||||
| W1 | W-Sol-AR-1:5 | 5.41 ± 0.2 | 15.5 ± 0.2 | 31.9 ± 0.3 | 16.7 | 14.9, 1.01 |
| W2 | W-Sol-5:1 | 3.82 ± 0.1 | 8.21 ± 0.2 | 18.4 ± 0.4 | 83.3 | 74.7, 2.41 |
| W3 | W-Sol-1:3 | 5.53 ± 0.1 | 17.4 ± 0.2 | 39.7 ± 0.6 | 25.0 | 25.6, 1.06 |
| W4 | W-Sol-1:5 | 7.32 ± 0.4 | 21.8 ± 0.3 | 47.6 ± 0.3 | 16.7 | 17.1, 0.49 |
| W5 | W-HPC-1:5 | 7.81 ± 0.3 | 23.1 ± 0.1 | 54.7 ± 1.9 | 16.7 | 17.2, 3.56 |
| W6 | W-VA64-1:5 | 2.74 ± 0.6 | 9.98 ± 0.5 | 24.0 ± 0.9 | 16.7 | 17.1, 4.31 |
| W7 | W-Sol-1:5, SDS | 4.95 ± 0.2 | 13.5 ± 0.4 | 27.9 ± 0.2 | 16.5 | 16.8, 5.70 |
| W8 | W-HPC-1:5, SDS | 7.96 ± 0.1 | 25.0 ± 0.9 | 56.4 ± 0.5 | 16.5 | 16.8, 4.37 |
| W9 | W-VA64-1:5, SDS | 3.16 ± 0.1 | 10.0 ± 0.5 | 25.5 ± 0.9 | 16.5 | 16.9, 3.82 |
| S1 | S-Sol-1:5 | 4.58 ± 0.5 | 15.4 ± 0.5 | 38.3 ± 0.4 | 16.7 | 17.1, 2.02 |
| S2 | S-HPC-1:5 | 5.31 ± 0.4 | 25.7 ± 0.6 | 66.8 ± 0.6 | 16.7 | 16.9, 5.23 |
| S3 | S-VA64-1:5 | 3.74 ± 0.7 | 18.7 ± 0.6 | 36.9 ± 1.0 | 16.7 | 17.0, 3.41 |
| ID | Formulation | Tg (°C) | Tm (°C) | ΔHf (J/g) | % Crystallinity |
|---|---|---|---|---|---|
| AR | As-received ITZ | — | 171 | 70.9 | 100 |
| W1 | W-AR-Sol-1:5 | — | 161 | 5.79 | 87.1 |
| W2 | W-Sol-5:1 | — | 162 | 34.4 | 83.6 |
| W3 | W-Sol-1:3 | — | 145 | 3.32 | 81.7 |
| W4 | W-Sol-1:5 | — | 136 | 1.64 | 79.8 |
| W5 | W-HPC-1:5 | — | 160 | 6.32 | 95.6 |
| W6 | W-VA64-1:5 | — | 141 | 7.46 | 92.7 |
| W7 | W-Sol-1:5, SDS | — | 130 | 1.35 | 69.7 |
| W8 | W-HPC-1:5, SDS | — | 159 | 4.91 | 84.9 |
| W9 | W-VA64-1:5, SDS | — | 131 | 7.21 | 82.7 |
| S1 | S-Sol-1:5 | 72.0 | — | — | — |
| S2 | S-HPC-1:5 | 58.1 | 158 | 0.14 | — |
| S3 | S-VA64-1:5 | 100.9 | — | — | — |
| Liquid | η, (cP) | ρ, (g/mL) | γ, (mN/m) | Slope, (g2/s) a | R2 | cos θss/cos θw | log(cos θss/cos θw) |
|---|---|---|---|---|---|---|---|
| 0.1 N HCl | 0.89 | 1.00 | 72.0 | 3.5 × 10−5 | 0.988 | 1 | 0 |
| Sol | 8.85 | 1.01 | 41.1 | 2.3 × 10−3 | 0.998 | 1120 | 3.05 |
| HPC | 22.3 | 1.01 | 37.9 | 1.1 × 10−3 | 0.998 | 1470 | 3.17 |
| VA64 | 5.16 | 1.01 | 39.9 | 4.9 × 10−3 | 0.998 | 1440 | 3.16 |
| Sol–SDS | 13.3 | 1.01 | 39.3 | 2.3 × 10−3 | 0.995 | 1760 | 3.25 |
| HPC–SDS | 27.5 | 1.01 | 35.5 | 1.5 × 10−3 | 0.999 | 2630 | 3.42 |
| VA64–SDS | 6.29 | 1.01 | 37.6 | 5.3 × 10−3 | 0.999 | 2010 | 3.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.; Radgman, K.; Tarabokija, J.; Ahmad, S.; Bilgili, E. Preparation and Characterization of Spray-Dried Hybrid Nanocrystal–Amorphous Solid Dispersions (HyNASDs) for Supersaturation Enhancement of a Slowly Crystallizing Drug. Nanomaterials 2023, 13, 2419. https://doi.org/10.3390/nano13172419
Rahman M, Radgman K, Tarabokija J, Ahmad S, Bilgili E. Preparation and Characterization of Spray-Dried Hybrid Nanocrystal–Amorphous Solid Dispersions (HyNASDs) for Supersaturation Enhancement of a Slowly Crystallizing Drug. Nanomaterials. 2023; 13(17):2419. https://doi.org/10.3390/nano13172419
Chicago/Turabian StyleRahman, Mahbubur, Keanu Radgman, James Tarabokija, Stephanie Ahmad, and Ecevit Bilgili. 2023. "Preparation and Characterization of Spray-Dried Hybrid Nanocrystal–Amorphous Solid Dispersions (HyNASDs) for Supersaturation Enhancement of a Slowly Crystallizing Drug" Nanomaterials 13, no. 17: 2419. https://doi.org/10.3390/nano13172419
APA StyleRahman, M., Radgman, K., Tarabokija, J., Ahmad, S., & Bilgili, E. (2023). Preparation and Characterization of Spray-Dried Hybrid Nanocrystal–Amorphous Solid Dispersions (HyNASDs) for Supersaturation Enhancement of a Slowly Crystallizing Drug. Nanomaterials, 13(17), 2419. https://doi.org/10.3390/nano13172419








