Corrosion Resistance in Artificial Perspiration of Cr-Based Decorative Coatings
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Chemical Composition (EDS)
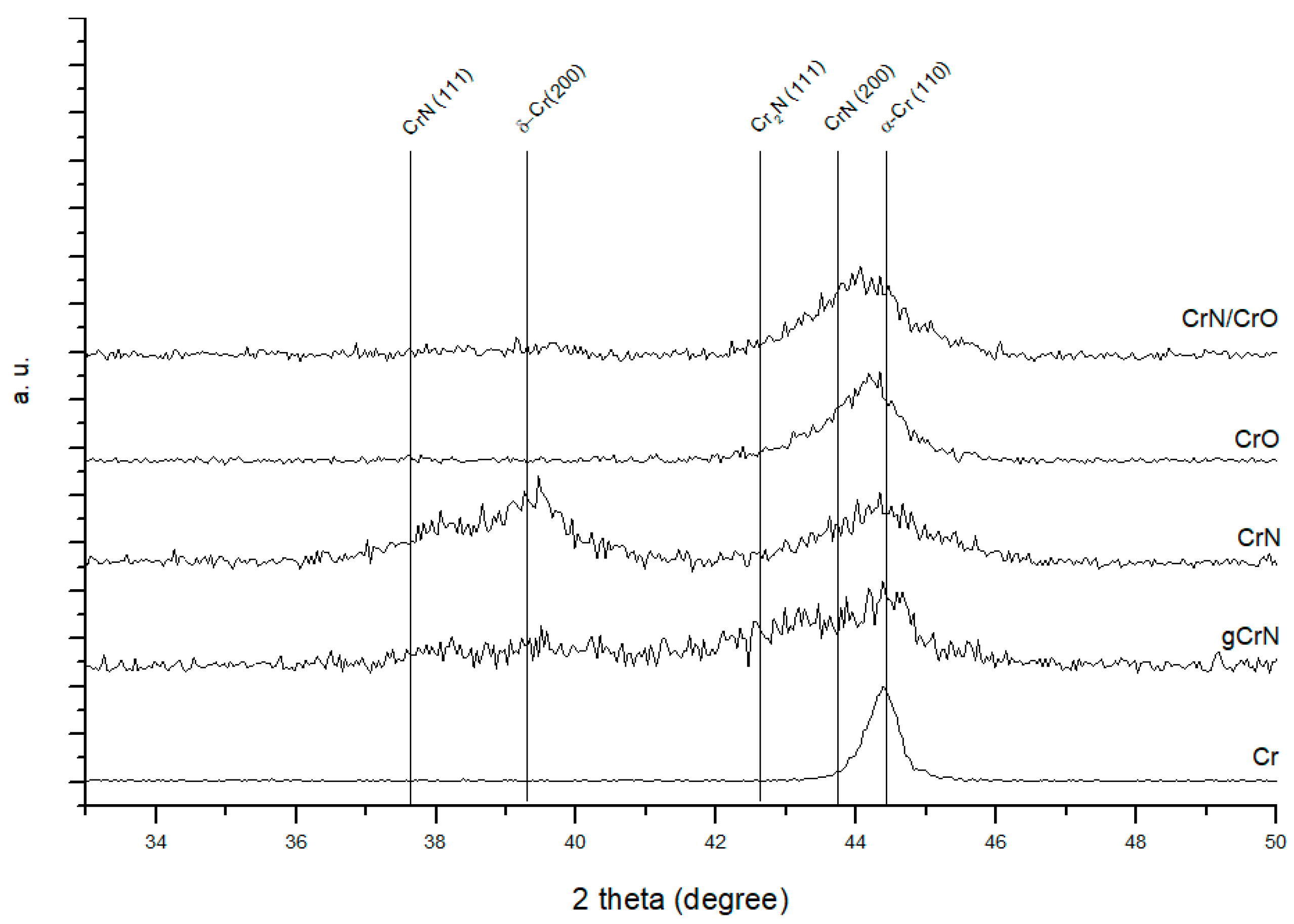
3.2. Structural Characterization (XRD)
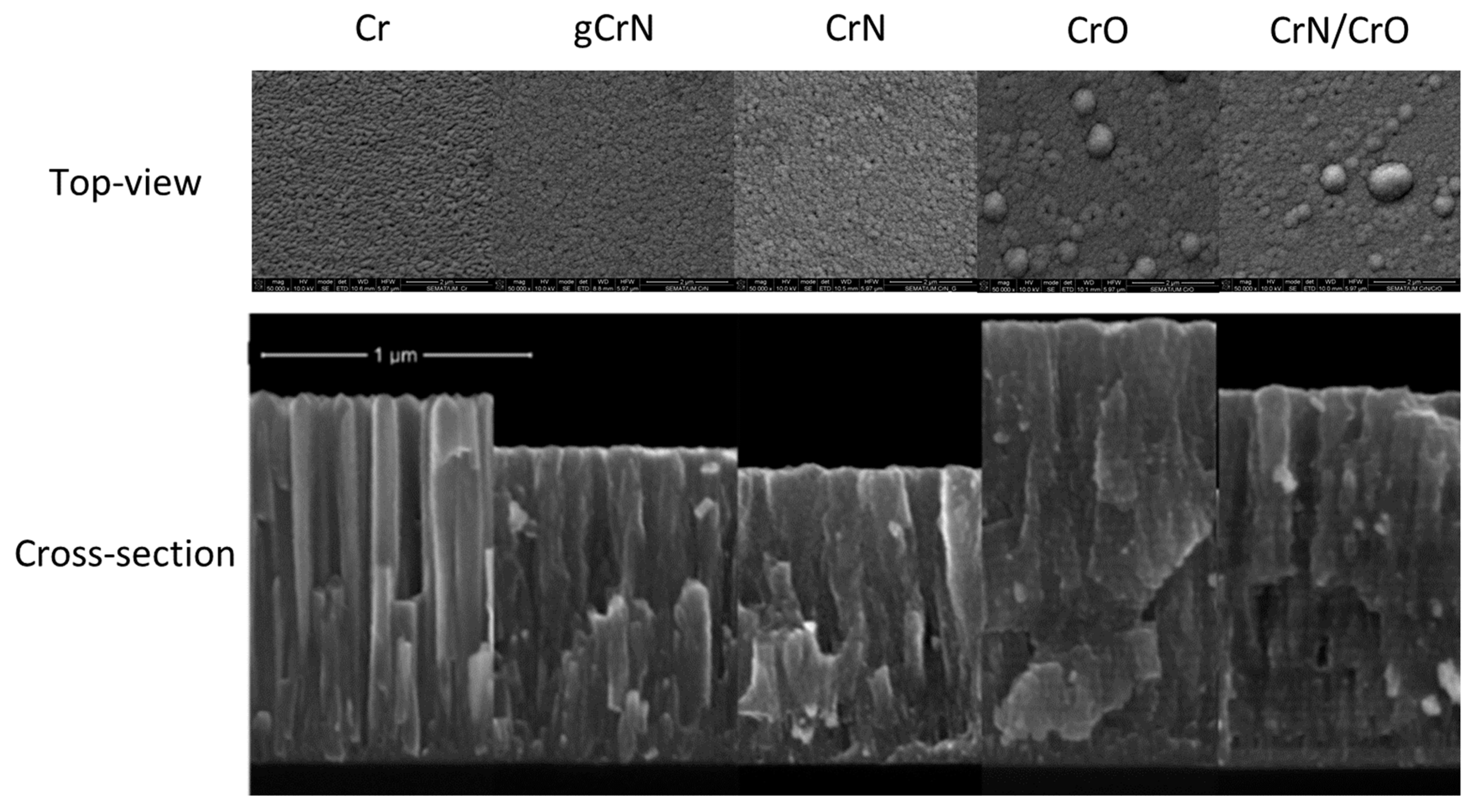
3.3. Morphology (SEM)
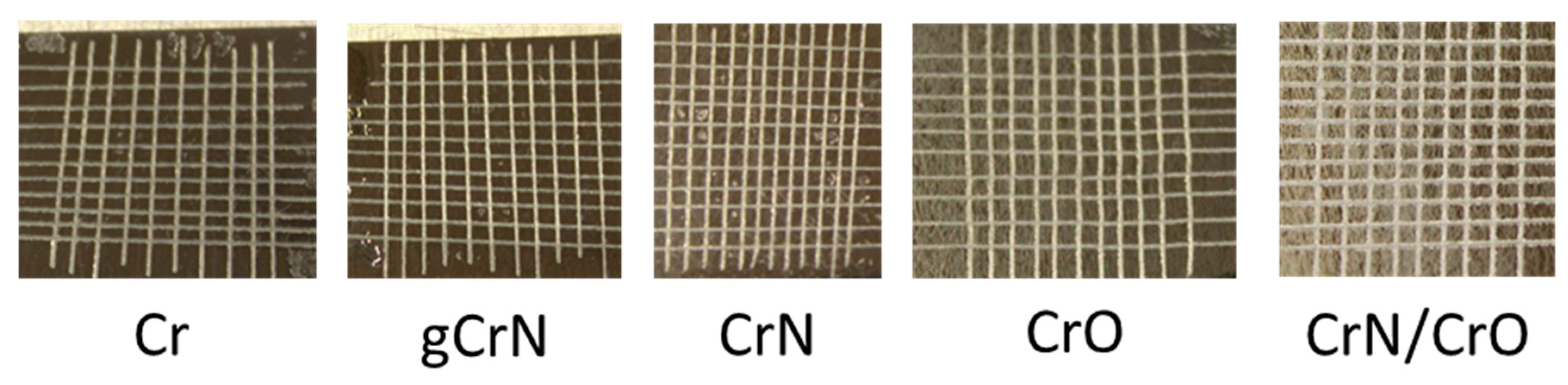
3.4. Adhesion (Tape Test)
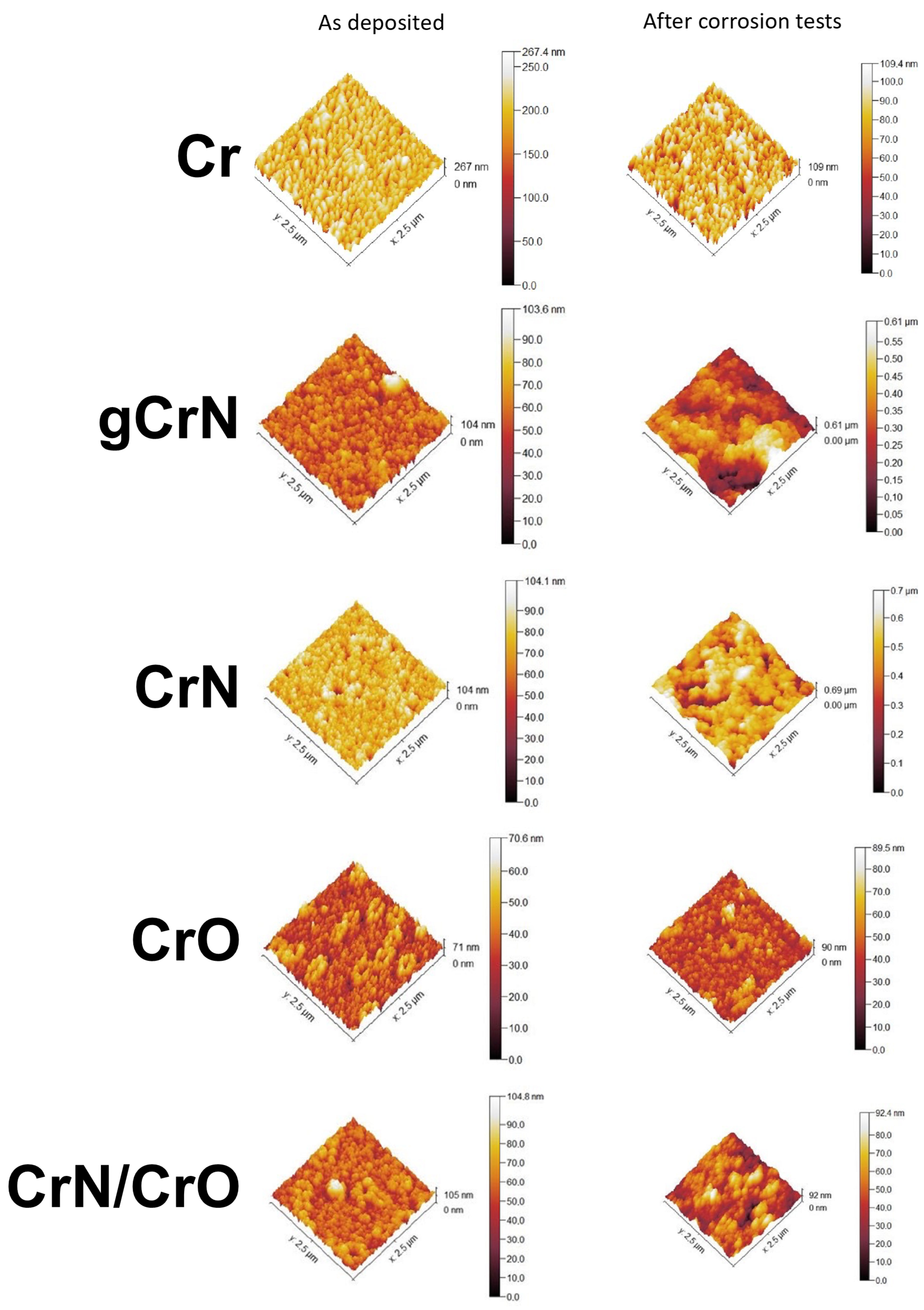
3.5. Roughness and Wettability
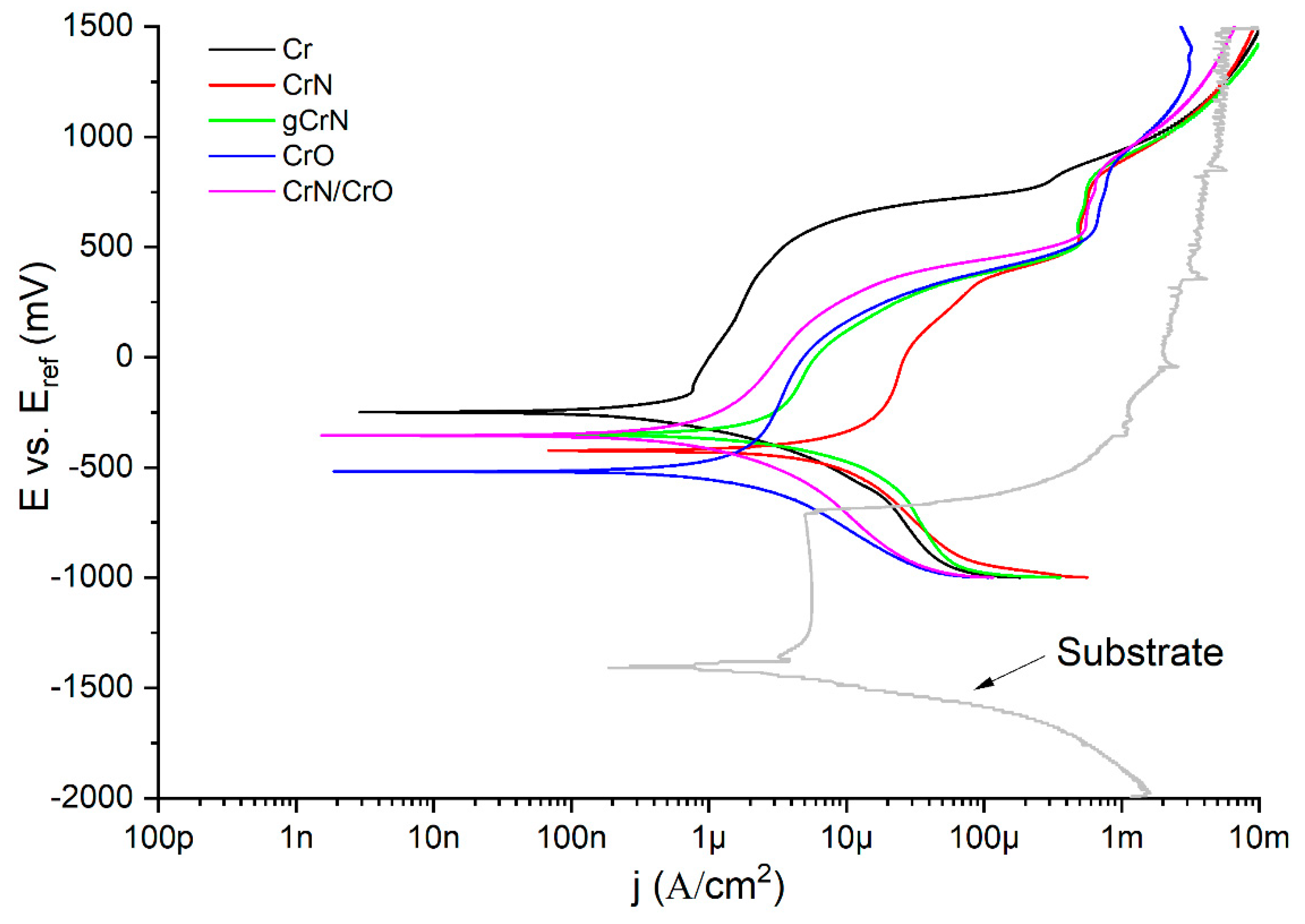
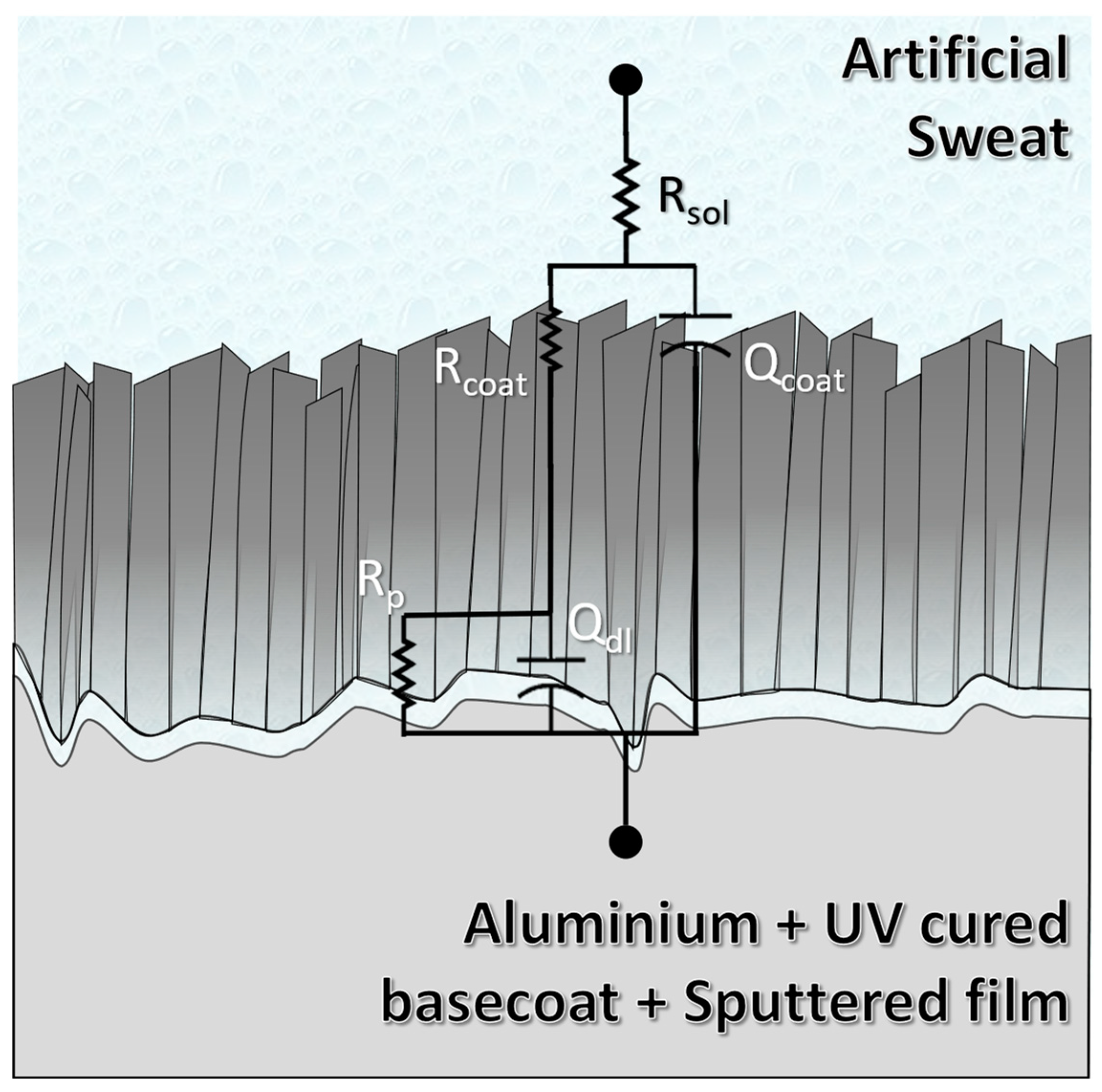
3.6. Corrosion Resistance against Artificial Sweat
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, R.; Miyakoshi, T. Lacquer Chemistry and Applications; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Chodun, R.; Skowronski, L.; Okrasa, S.; Wicher, B.; Nowakowska-Langier, K.; Zdunek, K. Optical TiO 2 layers deposited on polymer substrates by the Gas Injection Magnetron Sputtering technique. Appl. Surf. Sci. 2019, 466, 12–18. [Google Scholar] [CrossRef]
- Skowronski, L.; Chodun, R.; Zdunek, K. TiO2-based decorative interference coatings produced at industrial conditions. Thin Solid Film. 2020, 711, 138294. [Google Scholar] [CrossRef]
- Ota, Y.; Masuda, T.; Kimura, S. Technical trends in aluminum alloy sheets for automotive body panels. Res. Dev. Res. Dev. Kobe Steel Eng. Rep. 2019, 69, 15–18. [Google Scholar]
- Farfan-Cabrera, L.I.; Tapia-Gaspar, M.; Pérez-González, J. Tribology of Polymer Matrix Composites Within the Automotive Industry. In Encyclopedia of Materials: Composites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 970–982. [Google Scholar] [CrossRef]
- Hattori, C.S.; Almeida, G.F.C.; Gonçalves, R.L.P.; Santos, R.G.; Souza, R.C.; da Silva, W.C., Jr.; Cunali, J.R.C., Jr.; Couto, A.A. Microstructure and fatigue properties of extruded aluminum alloys 7046 and 7108 for automotive applications. J. Mater. Res. Technol. 2021, 14, 2970–2981. [Google Scholar] [CrossRef]
- Hirsch, J. Automotive trends in aluminium—The European perspective. Mater. Forum 2004, 28, 15–23. [Google Scholar]
- Sivanur, K.; Umananda, K.V.; Pai, D. Advanced materials used in automotive industry-a review. AIP Conf. Proc. 2021, 2317, 020032. [Google Scholar] [CrossRef]
- Liu, M.; Guo, Y.; Wang, J.; Yergin, M. Corrosion avoidance in lightweight materials for automotive applications. Npj Mater. Degrad. 2018, 2, 24. [Google Scholar] [CrossRef]
- Mihora, D.J.; Ramamurthy, A.C. Friction induced damage: Preliminary numerical analysis of stresses within painted automotive plastics induced by large curvature counterfaces. Wear 1997, 203–204, 362–374. [Google Scholar] [CrossRef]
- Ramamurthy, A.C.; Lorenzen, W.I.; Bless, S.J. Stone impact damage to automotive paint finishes: An introduction to impact physics and impact induced corrosion. Prog. Org. Coat. 1994, 25, 43–71. [Google Scholar] [CrossRef]
- Ryntz, R.A.; Everson, M.; Pollano, G. Friction induced paint damage as affected by clearcoat chemistry. Prog. Org. Coat. 1997, 31, 281–288. [Google Scholar] [CrossRef]
- Ramamurthy, A.C.; Charest, J.A.; Lilly, M.D.; Mihora, D.J.; Freese, J.W. Friction induced paint damage—A novel method for objective assessment of painted engineering plastics. Wear 1997, 203–204, 350–361. [Google Scholar] [CrossRef]
- Chami, A.; Benabbou, R.; Taleb, M.; Rais, Z.; El Haji, M. Analysis of survey data on corrosion in the automotive industry. Mater. Today Proc. 2021, 45, 7636–7642. [Google Scholar] [CrossRef]
- Bing, L.; Zhou, X.; Zhang, X. Filiform corrosion behaviour on machined AA7150 aluminium alloy. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2020, 30, 2056–2066. [Google Scholar] [CrossRef]
- Rathish, R.J.; Rajendran, S.; Christy, J.L.; Devi, B.S.; Johnmary, S.; Manivannan, M.; Rajam, K.; Rengan, P. Corrosion Behaviour of Metals in Artificial Sweat. Open Corros. J. 2010, 3, 38–44. [Google Scholar] [CrossRef]
- Shimpi, K.C.; Ravindranath, K.; Jani, A.K.; Kothari, D.C.; Harindranath, C.S. Decorative coatings produced using combination of reactive arc evaporation and magnetron sputtering. Surf. Coat. Technol. 1997, 90, 115–122. [Google Scholar] [CrossRef]
- Suo, X.; Guo, C.; Kong, D.; Wang, L. Corrosion behaviour of TiN and CrN coatings produced by magnetron sputtering process on aluminium alloy. Int. J. Electrochem. Sci. 2019, 14, 826–837. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, K.; Ambat, R. Statistical analysis of corrosion failures in hearing aid devices from tropical regions. Eng. Fail. Anal. 2021, 130, 105758. [Google Scholar] [CrossRef]
- Fenker, M.; Jackson, N.; Spolding, M.; Nicole, P.; Schönhut, K.; Gregory, G.; Hovsepian, P.E.; Münz, W.D. Corrosion performance of PVD-coated and anodised materials for the decorative market. Surf. Coat. Technol. 2004, 188–189, 466–472. [Google Scholar] [CrossRef]
- Carneiro, E.; Parreira, N.M.G.; Vuchkov, T.; Cavaleiro, A.; Ferreira, J.; Andritschky, M.; Carvalho, S. Cr-Based Sputtered Decorative Coatings for Automotive Industry. Materials 2021, 14, 5527. [Google Scholar] [CrossRef]
- Hodgman, C.D.; Lange, N.A. Handbook of chemistry and physics. J. Frankl. Inst. 1930, 209, 847. [Google Scholar] [CrossRef]
- Ando, E.; Suzuki, S. Optical and mechanical properties of Cr and CrNx films by dc magnetron sputtering. J. Non Cryst. Solids 1997, 218, 68–73. [Google Scholar] [CrossRef]
- Rothhaar, U.; Oechsner, H.R.f. magnetron sputter deposition of Cr2O3 layers on ceramic Al2O3 substrates. Surf. Coat. Technol. 1993, 59, 183–186. [Google Scholar] [CrossRef]
- Ponte, F.; Sharma, P.; Figueiredo, N.M.; Ferreira, J.; Carvalho, S. Decorative Chromium Coatings on Polycarbonate Substrate for the Automotive Industry. Materials 2023, 16, 2315. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Ponte, F.; Lima, M.J.; Figueiredo, N.M.; Ferreira, J.; Carvalho, S. Plasma etching of polycarbonate surfaces for improved adhesion of Cr coatings. Appl. Surf. Sci. 2023, 637, 157903. [Google Scholar] [CrossRef]
- Nezhad, A.N.; Davoodi, A.; Zahrani, E.M.; Arefinia, R. The effects of an inorganic corrosion inhibitor on the electrochemical behavior of superhydrophobic micro-nano structured Ni films in 3.5% NaCl solution. Surf. Coat. Technol. 2020, 395, 125946. [Google Scholar] [CrossRef]
- Józwik, J.; Ostrowski, D.; Milczarczyk, R.; Krolczyk, G.M. Analysis of relation between the 3D printer laser beam power and the surface morphology properties in Ti-6Al-4V titanium alloy parts. J. Braz. Soc. Mech. Sci. Eng. 2018, 40, 215. [Google Scholar] [CrossRef]
- Duboust, N.; Ghadbeigi, H.; Pinna, C.; Ayvar-Soberanis, S.; Collis, A.; Scaife, R.; Kerrigan, K. An optical method for measuring surface roughness of machined carbon fibre-reinforced plastic composites. J. Compos. Mater. 2017, 51, 289–302. [Google Scholar] [CrossRef]
- Chipatecua, Y.L.; Olaya, J.J.; Arias, D.F. Corrosion behaviour of CrN/Cr multilayers on stainless steel deposited by unbalanced magnetron sputtering. Vacuum 2012, 86, 1393–1401. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Jin, J.; Liu, H.; Zheng, D.; Zhu, Z. Effects of Mo content on the interfacial contact resistance and corrosion properties of CrN coatings on SS316L as bipolar plates in simulated PEMFCs environment. Int. J. Hydrogen Energy 2018, 43, 10048–10060. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Selvakumar, N.; Deepthi, B.; Rajam, K.S. A comparative study of reactive direct current magnetron sputtered CrAlN and CrN coatings. Surf. Coat. Technol. 2006, 201, 2193–2201. [Google Scholar] [CrossRef]
- Creus, J.; Idrissi, H.; Mazille, H.; Sanchette, F.; Jacquot, P. Improvement of the corrosion resistance of CrN coated steel by an interlayer. Surf. Coat. Technol. 1998, 107, 183–190. [Google Scholar] [CrossRef]
- Ibrahim, M.A.M.; Korablov, S.F.; Yoshimura, M. Corrosion of stainless steel coated with TiN, (TiAl)N and CrN in aqueous environments. Corros. Sci. 2002, 44, 815–828. [Google Scholar] [CrossRef]
- ASTM G3-14(2019); ASTM G3—Conventions Applicable to Electrochemical Measurements in Corrosion Testing. ASTM international: West Conshohocken, PA, USA, 2014; pp. 1–9. [CrossRef]
- Dinu, M.; Mouele, E.S.M.; Parau, A.C.; Vladescu, A.; Petrik, L.F.; Braic, M. Enhancement of the corrosion resistance of 304 stainless steel by Cr-N and Cr(N,O) coatings. Coatings 2018, 8, 132. [Google Scholar] [CrossRef]
- Yuan, X.; Song, C.; Wang, H.; Zhang, J. Electrochemical Impedance Spectroscopy in PEM Fuel Cells Fundamentals and Applications; Springer: London, UK, 2010. [Google Scholar] [CrossRef]
- Castro, J.D.; Lima, M.J.; Carvalho, S. Corrosion resistance of Cu-Zr(O) N films in a simulated seawater environment. Surf. Coat. Technol. 2022, 451, 129050. [Google Scholar] [CrossRef]
- Castro, J.D.; Pinto, B.; Ferreira, F.; Serra, R.; Carvalho, S. Wettability and corrosion resistance of zirconium nitride films obtained via reactive high-power impulse magnetron sputtering. J. Vac. Sci. Technol. A 2023, 41, 023106. [Google Scholar] [CrossRef]
- Gao, S.; Dong, C.; Luo, H.; Xiao, K.; Pan, X.; Li, X. Scanning electrochemical microscopy study on the electrochemical behavior of CrN film formed on 304 stainless steel by magnetron sputtering. Electrochim. Acta 2013, 114, 233–241. [Google Scholar] [CrossRef]
- ASM International. Alloy Phase Diagrams, 1st ed.; ASM International: West Conshohocken, PA, USA, 1992; Volume 3. [Google Scholar]
- Chase, M.W., Jr. NIST-JANAF Thermochemical Tables, Part I, A1-Co—Monograph No. 9, 4th ed.; The American Chemical Society; The American Institute of Physics: New York, NY, USA, 1998. [Google Scholar]
- Nielsen, H.P.; Frandsen, F.J.; Dam-Johansen, K.; Baxter, L.L. The implications of chlorine-associated corrosion on the operation of biomass-fired boilers. Prog. Energy Combust. Sci. 2000, 26, 283–298. [Google Scholar] [CrossRef]



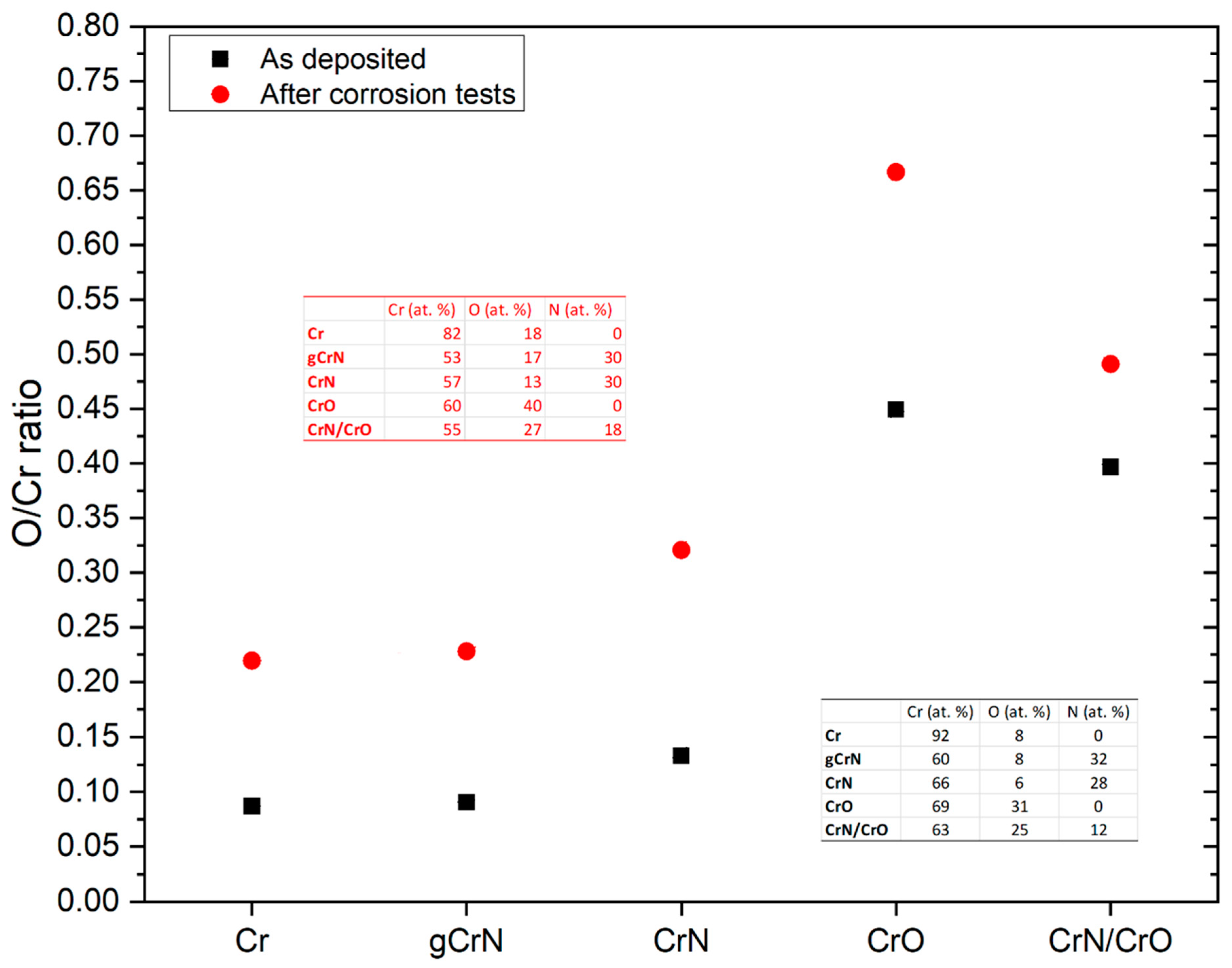
| Coating | Gas Flow (sccm) | Deposition Time (s) | Thickness | Deposition Rate | Chemical Composition (at. %) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ar | N2 | O2 | Interlayer | Coating | (nm) | (nm/s) | Cr | O | N | |
| Cr | 100 | 720 | 1390 | 1.9 | 91 | 9 | - | |||
| gCrN | 70 | 5→25 | 720 | 1180 | 1.6 | 66 | 6 | 28 | ||
| CrN | 70 | 25 | 50 | 670 | 1110 | 1.5 | 60 | 8 | 32 | |
| CrO | 100 | 15 | 50 | 670 | 1660 | 2.3 | 69 | 31 | - | |
| CrN/CrO | 70 | 25 | 15 | 50 | 720 | 1420 | 1.8 | 63 | 25 | 12 |
| Sample | As Deposited | After Corrosion Tests | Contact Angle (°) | ||||
|---|---|---|---|---|---|---|---|
| Sq (nm) | Ssk | Sku | Sq (nm) | Ssk | Sku | ||
| Cr | 14.4 ± 0.1 | −0.7 | 0.4 | 27.0 ± 0.3 | −1.2 | 2.3 | 122.6 ± 1 |
| gCrN | 9.5 ± 0.2 | 0.1 | 2.1 | 93.7 ± 4.2 | −1.1 | 2.3 | 105.2 ± 1 |
| CrN | 8.6 ± 0.1 | −0.6 | 1.9 | 92.8 ± 3.8 | −1.1 | 1.5 | 94.6 ± 1 |
| CrO | 8.3 ± 0.1 | 0.2 | 0.1 | 8.4 ± 0.2 | 0.7 | 1.4 | 108.5 ± 2 |
| CrN/CrO | 9.8 ± 0.1 | 0.2 | 1.0 | 12.9 ± 0.5 | 0.1 | −0.2 | 108.1 ± 4 |
| βA (mV/decade) | |βC|(mV/decade) | Jcorr (nA/cm2) | Ecorr (mV) | Rp (KΩ*cm2) * | Corrosion Rate (×10−3 mm/yr) | |
|---|---|---|---|---|---|---|
| Cr | 170 ± 58 | 193 ± 18 | 476 ± 67 | −238 ± 11 | 82 | 5 |
| gCrN | 124 ± 7 | 309 ± 63 | 3471 ± 129 | −258 ± 92 | 11 | 27 |
| CrN | 203 ± 38 | 139 ± 14 | 5075 ± 1335 | −409 ± 15 | 7 | 35 |
| CrO | 127 ± 26 | 74 ± 15 | 321 ± 23 | −505 ± 14 | 63 | 3 |
| CrN/CrO | 195 ± 30 | 94 ± 13 | 486 ± 19 | −364 ± 7 | 57 | - |
| Rsol (Ω) | Rcoat (Ω) | CPEcoat (µS*Sα) | αcoat | Rp (kΩ) | CPEdl (µS*Sα) | αdl | χ2 (×10−4) | |
|---|---|---|---|---|---|---|---|---|
| Cr | 173 + 2 | 671 + 65 | 46 + 12 | 0.82 + 0.02 | 2205 + 1441 | 124 + 20 | 0.69 + 0.03 | 2.5 |
| gCrN | 167 + 1 | 4899 + 2450 | 267 + 55 | 0.73 + 0.02 | 190 + 77 | 169 + 41 | 0.72 + 0.06 | 1.8 |
| CrN | 198 + 2 | 3286 + 1092 | 314 + 94 | 0.66 + 0.04 | 44 + 17 | 322 + 36 | 0.75 + 0.03 | 23.8 |
| CrO | 195 + 3 | 3844 + 1166 | 23 + 7 | 0.54 + 0.05 | 7829 + 2660 | 9 + 2 | 0.79 + 0.02 | 25.6 |
| CrN/CrO | 167 + 5 | 20685 + 3535 | 31 + 4 | 0.84 + 0.03 | 275 + 195 | 24 + 11 | 0.62 + 0.02 | 7.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carneiro, E.; Castro, J.D.; Lima, M.J.; Ferreira, J.; Carvalho, S. Corrosion Resistance in Artificial Perspiration of Cr-Based Decorative Coatings. Nanomaterials 2023, 13, 2346. https://doi.org/10.3390/nano13162346
Carneiro E, Castro JD, Lima MJ, Ferreira J, Carvalho S. Corrosion Resistance in Artificial Perspiration of Cr-Based Decorative Coatings. Nanomaterials. 2023; 13(16):2346. https://doi.org/10.3390/nano13162346
Chicago/Turabian StyleCarneiro, Edgar, José David Castro, Maria José Lima, Jorge Ferreira, and Sandra Carvalho. 2023. "Corrosion Resistance in Artificial Perspiration of Cr-Based Decorative Coatings" Nanomaterials 13, no. 16: 2346. https://doi.org/10.3390/nano13162346
APA StyleCarneiro, E., Castro, J. D., Lima, M. J., Ferreira, J., & Carvalho, S. (2023). Corrosion Resistance in Artificial Perspiration of Cr-Based Decorative Coatings. Nanomaterials, 13(16), 2346. https://doi.org/10.3390/nano13162346








