Effect of Water on CO2 Adsorption on CaNaY Zeolite: Formation of Ca2+(H2O)(CO2), Ca2+(H2O)(CO2)2 and Ca2+(H2O)2(CO2) Complexes
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Basic Characterization of the Samples
3.1.1. Background IR Spectrum
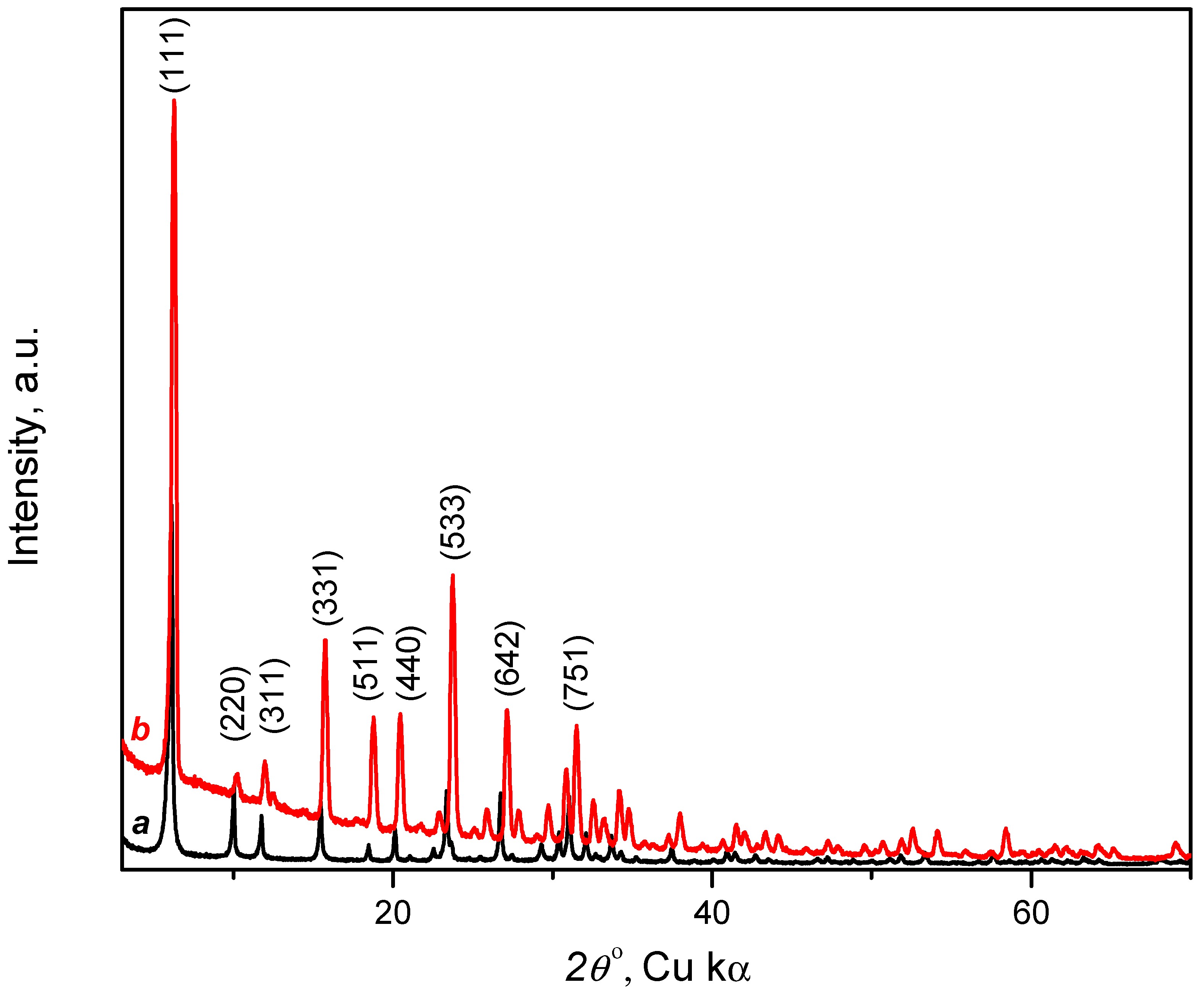
3.1.2. X-ray Diffraction
3.1.3. Electron Microscopy and EDX
3.2. Adsorption of CO2 in Absence of Water
3.2.1. FTIR Spectra of CO2 Adsorbed at Ambient Temperature
- (i)
- (ii)
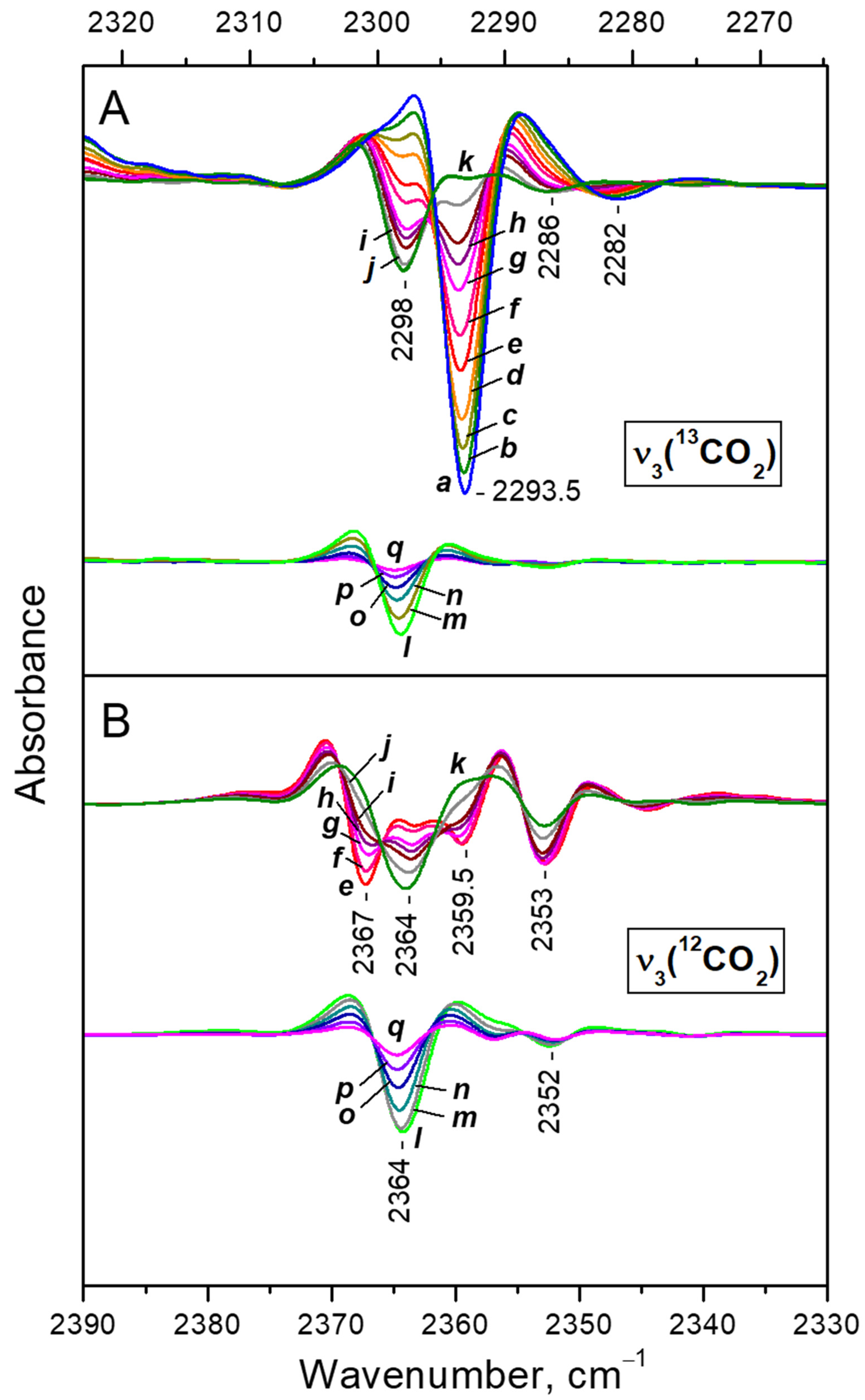
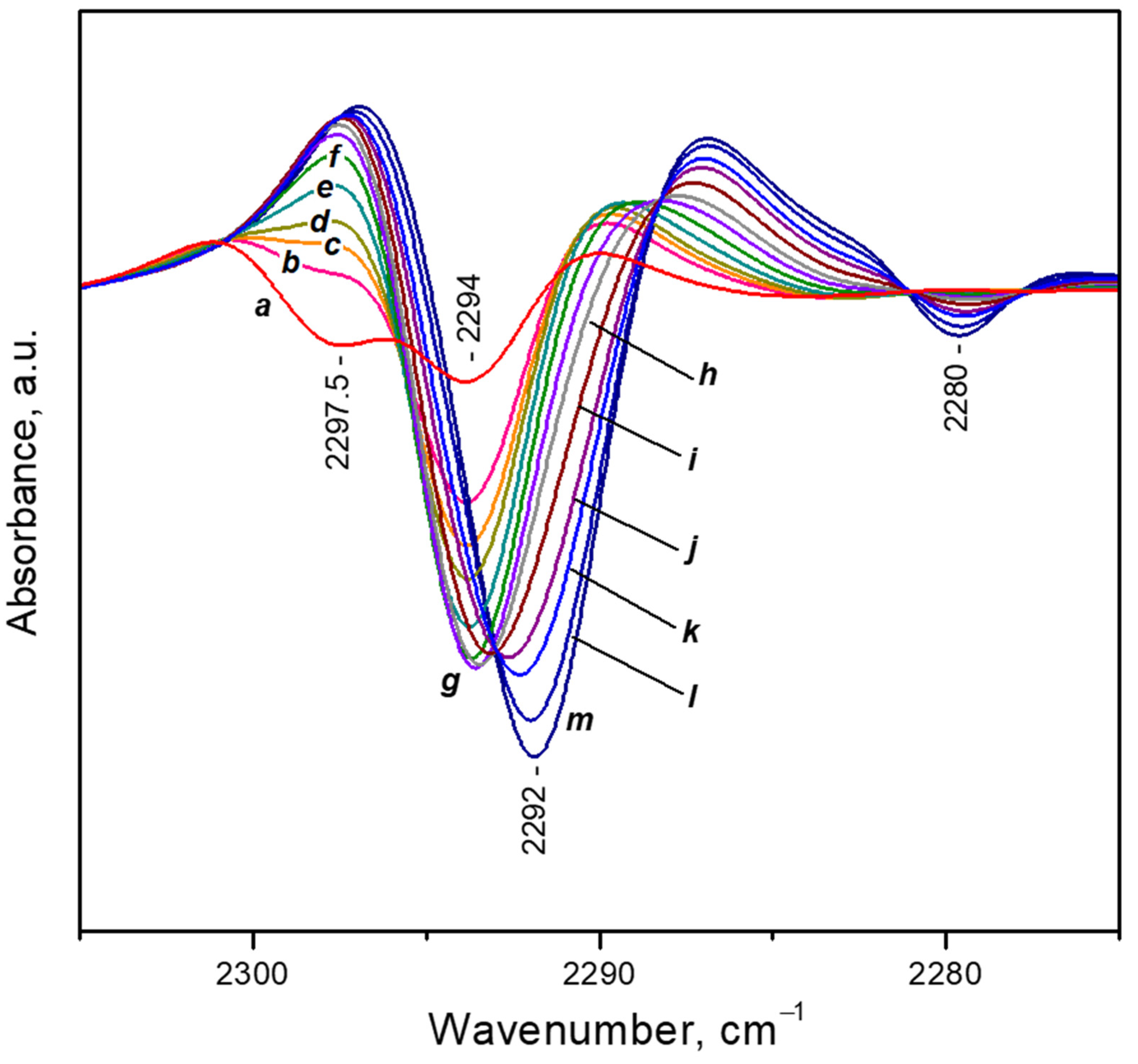
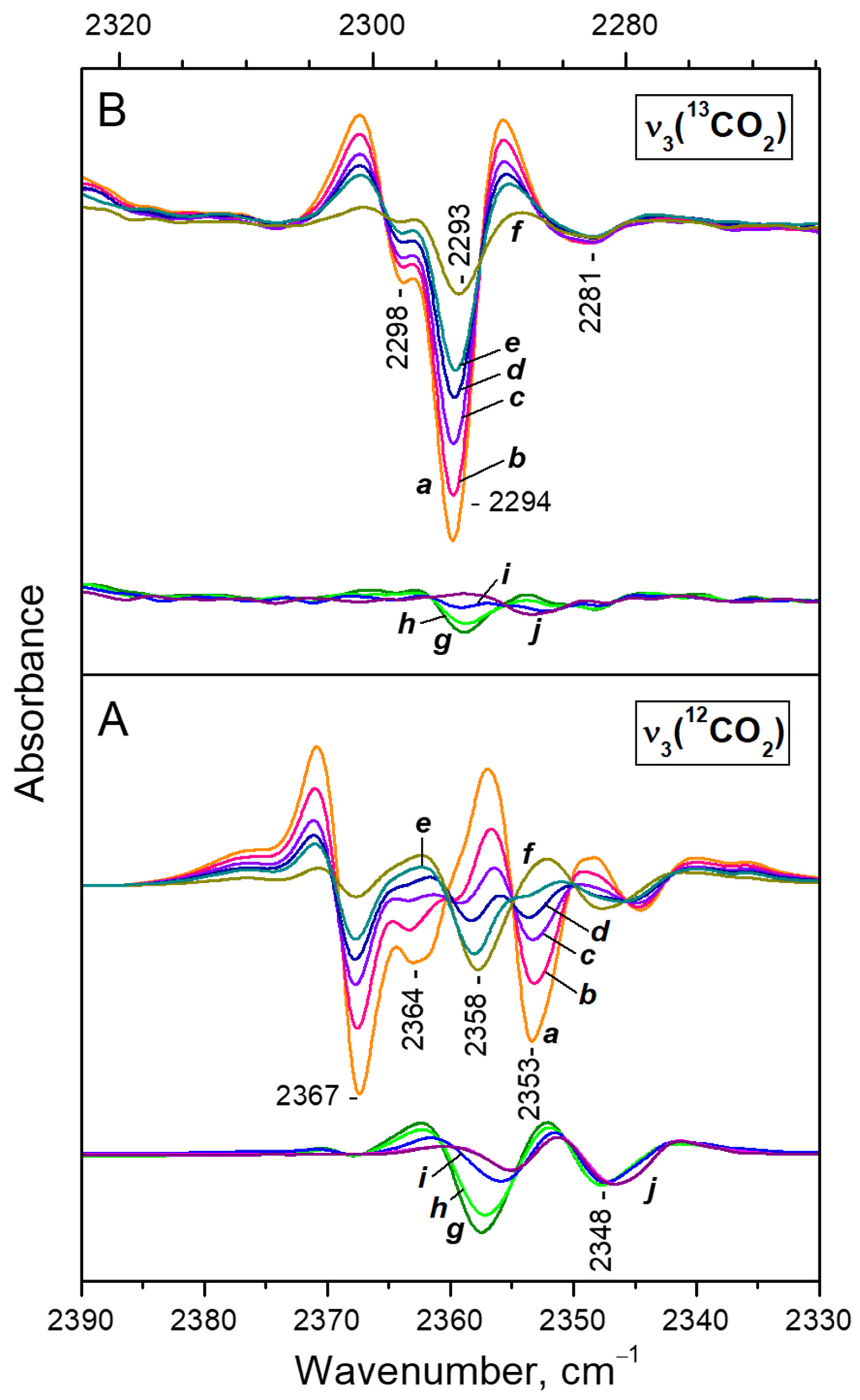
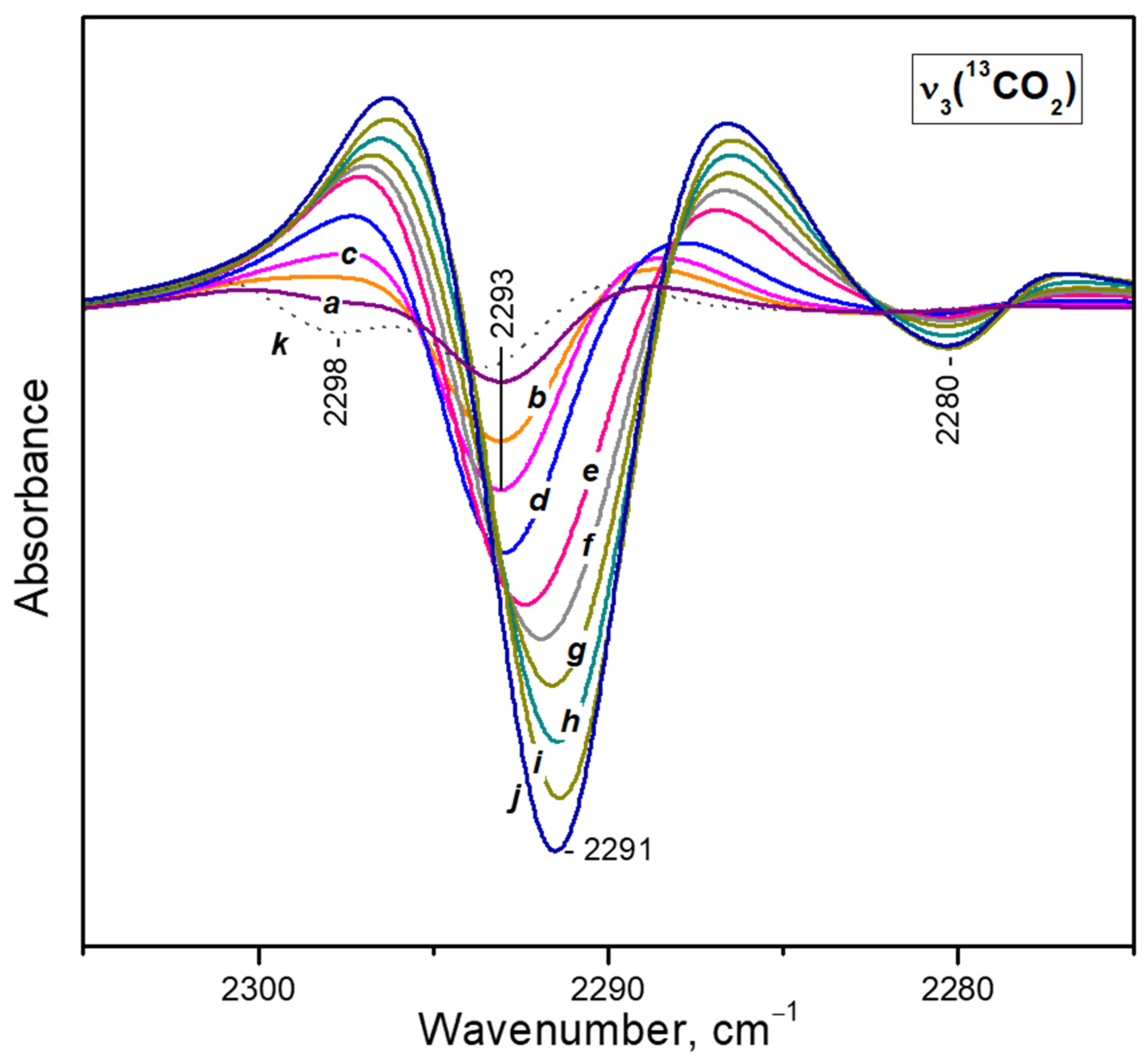
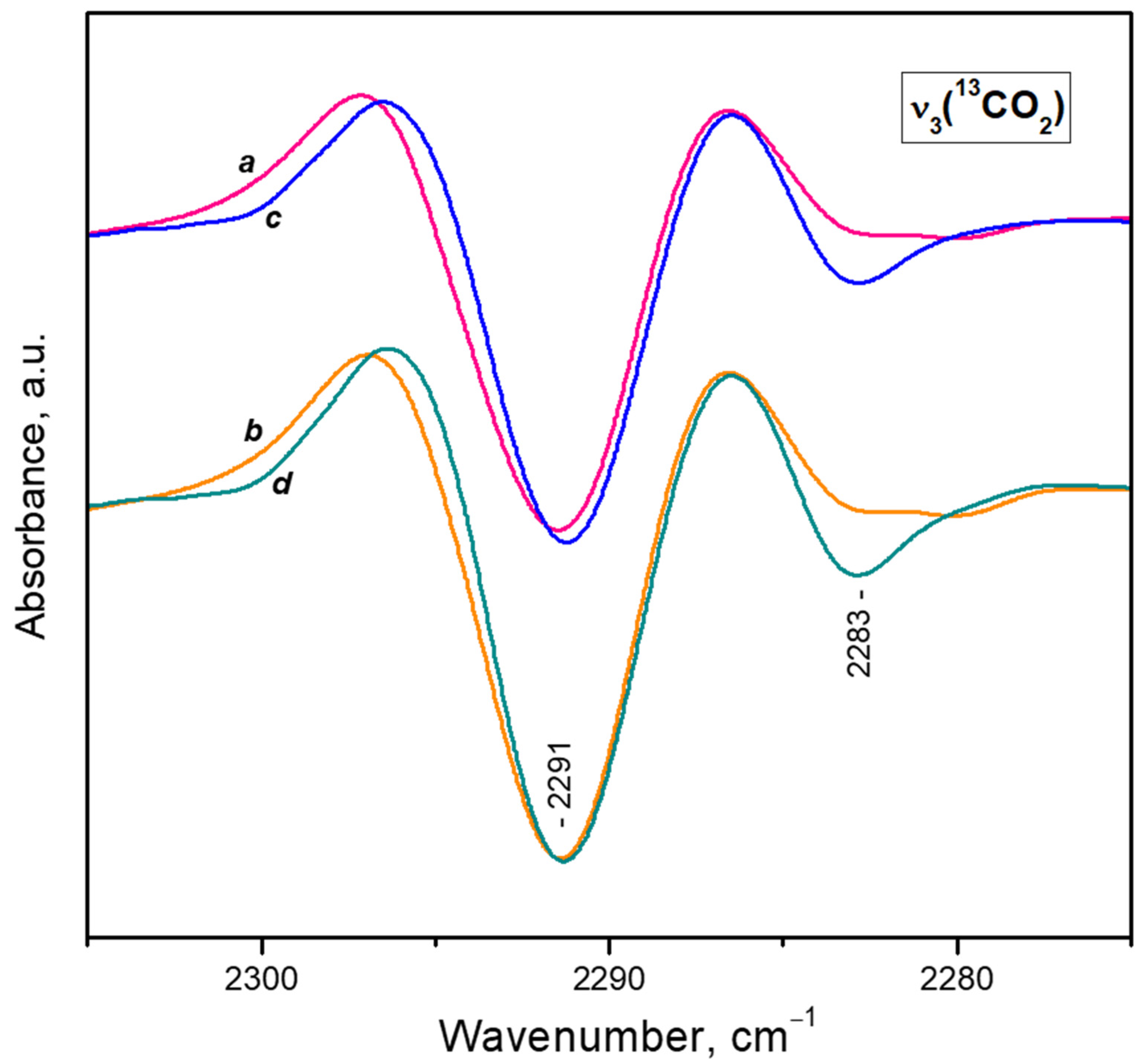
- After reaching a maximum, the 2298 cm−1 band starts to decline and another band at 2193 cm−1 develops at its expense (Figure 4A, upper set of spectra). The latter is assigned to geminal species with one labelled ligand, namely Ca2+(12CO2)(13CO2) [23]. Thus, the adsorption-induced shift decreases because the two CO2 molecules adsorbed simultaneously at the same site compete and their interaction with the cation weakens. Note that the probability of formation of Ca2+(13CO2)2 species is almost zero. Weak bands below 2290 cm−1 also develop. These bands are less intense as compared with a sample having higher sodium content [23] and are associated with residual Na+ sites.
3.2.2. VTIR Experiments
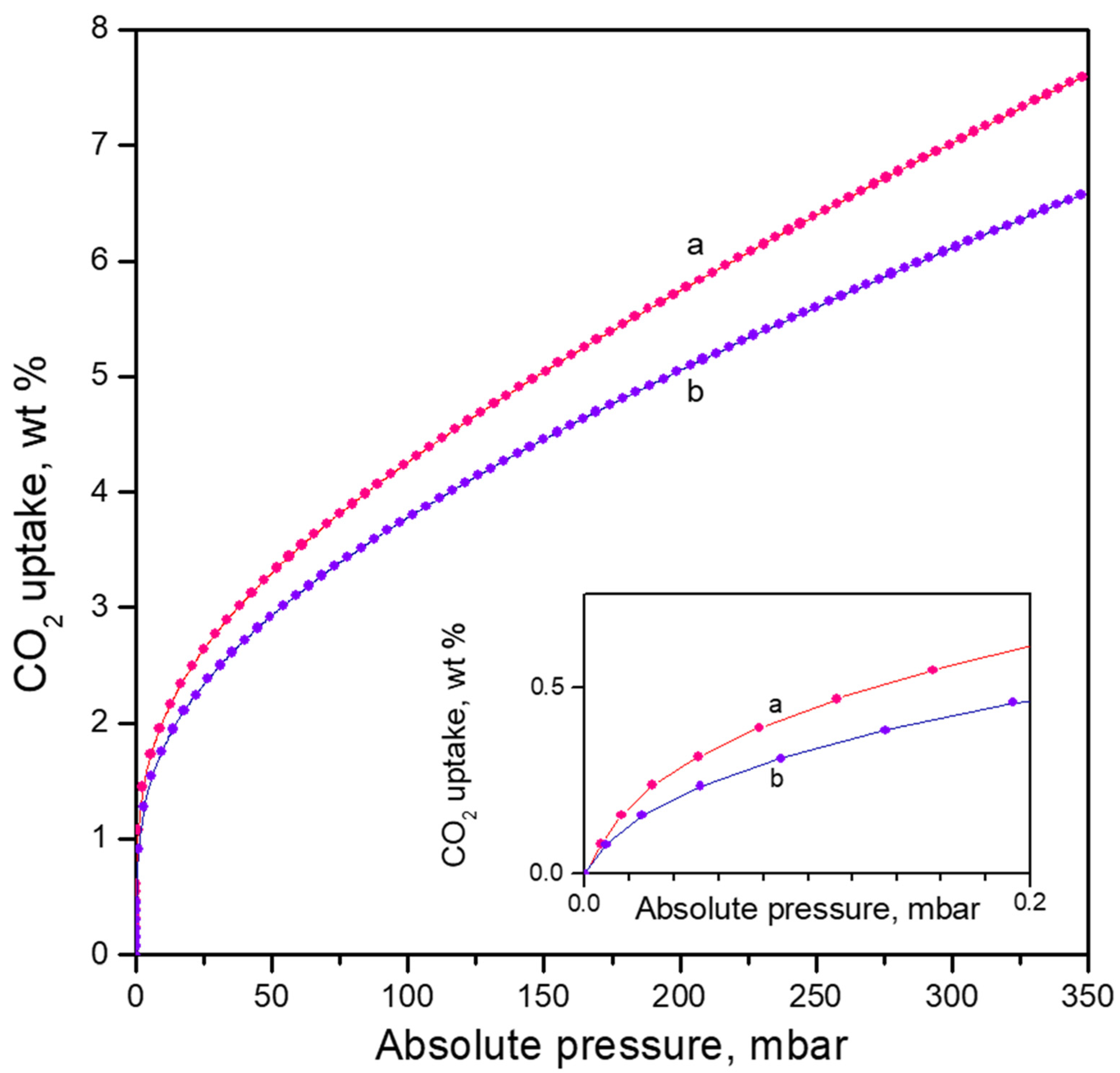
3.2.3. Adsorption Isotherms
3.2.4. XRD Patterns
3.3. Adsorption of H2O on CaNaX
3.3.1. Adsorption of Water Followed by FTIR
- Non-specifically adsorbed water with two ν(OH) bands, at 3687 cm−1 and around 3560 cm−1. This adsorption form also contributes to the broad absorbance between 3600 and 3000 cm−1 because of the formation of H-bonds [32].
- Water adsorbed on hydroxyl groups; gives rise to a band at 3708 cm−1 together with an ill-defined lower frequency band [42].
- A small band at 3746 cm−1 due to silanol groups [32].
- Unresolved bands due to water adsorbed on cationic sites. These bands will be discussed below.
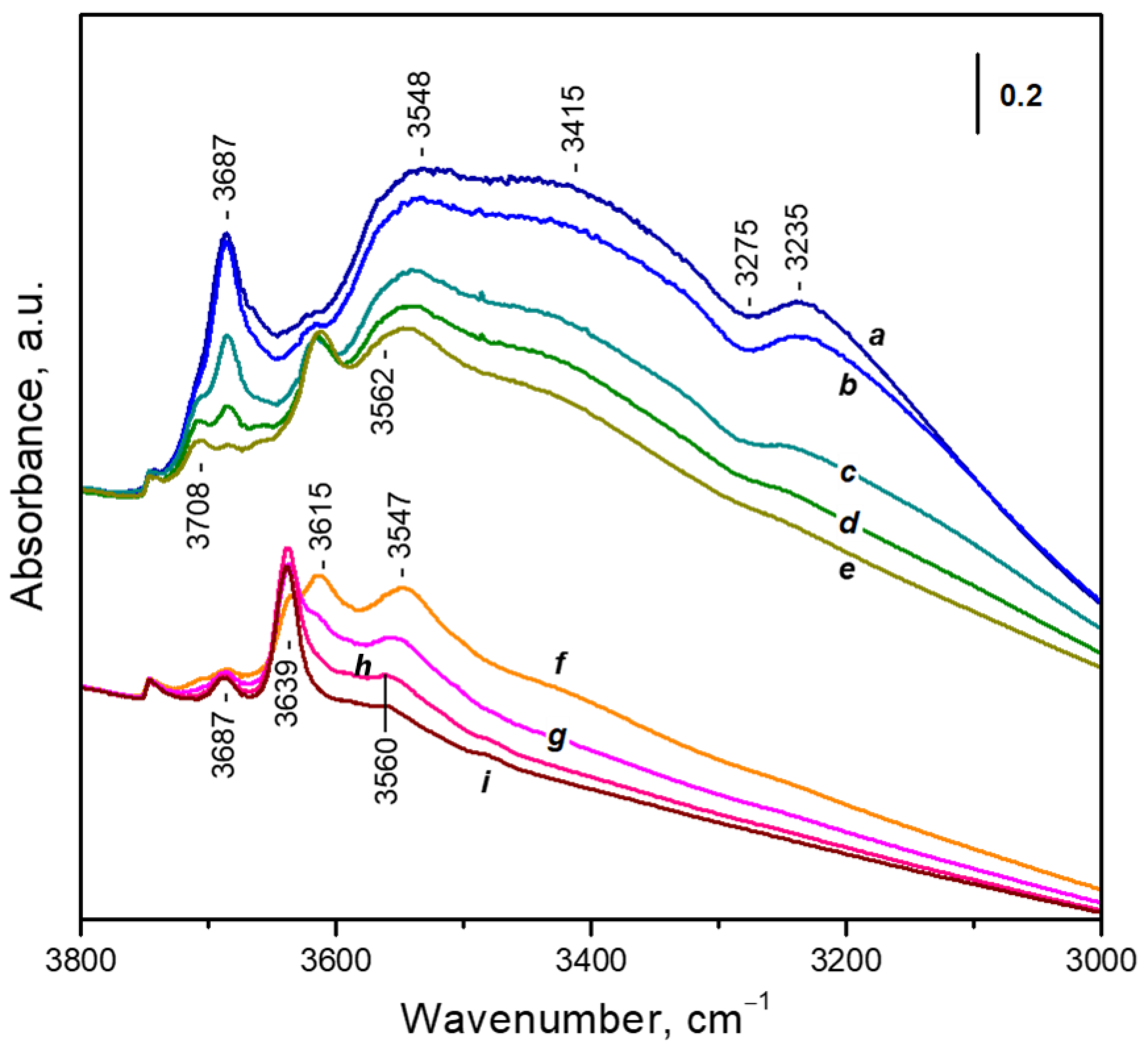
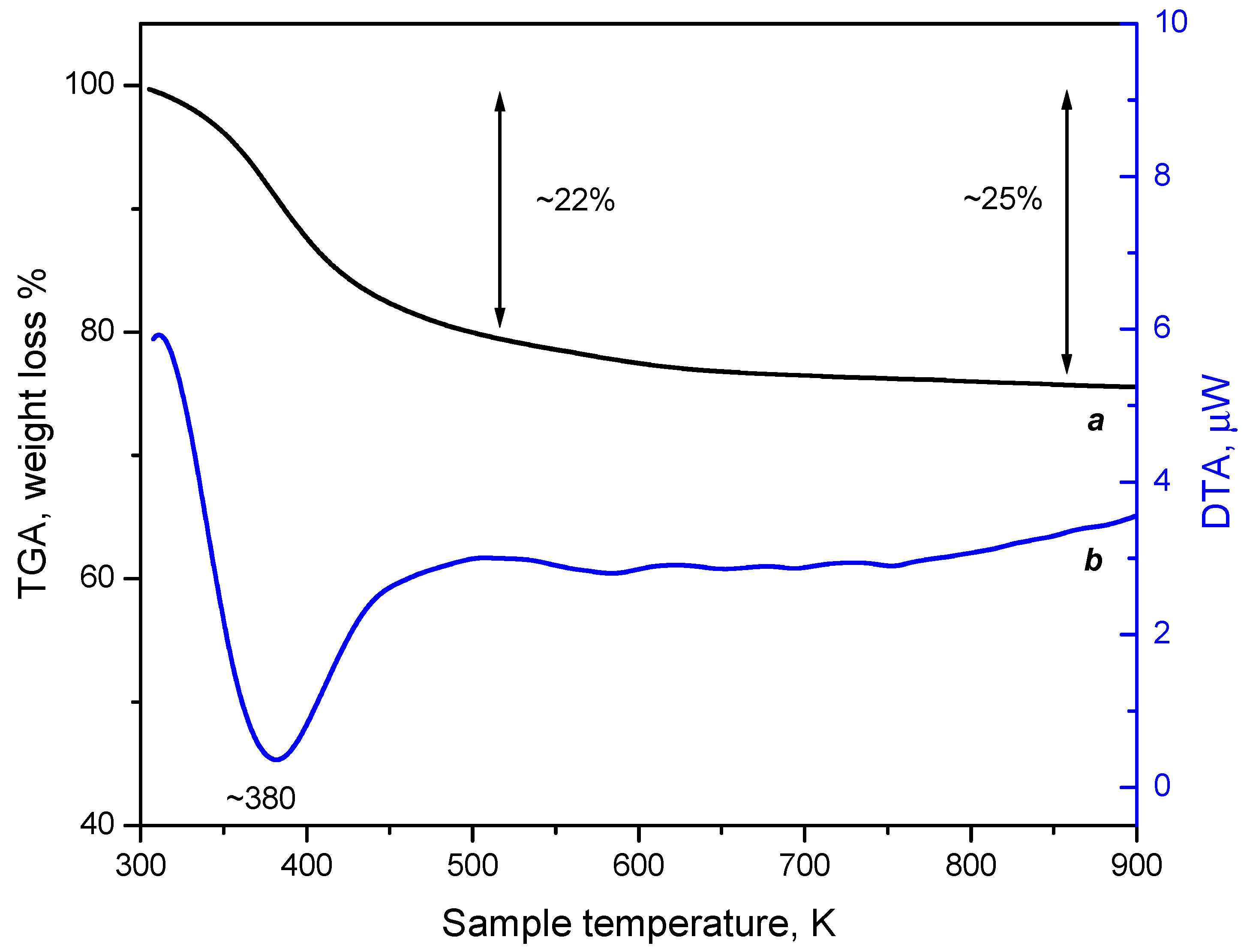
3.3.2. Thermogravimetric Analysis
3.4. Co-Adsorption of CO2 and H2O
3.4.1. Dosing Water on Sample with Pre-Adsorbed CO2 at Ambient Temperature
3.4.2. VTIR Experiments
3.4.3. Adsorption Isotherms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Ghussain, L. Global warming: Review on driving forces and mitigation. Environ. Prog. Sustain. Energy 2019, 38, 13–21. [Google Scholar] [CrossRef]
- Wilberforce, T.; Olabi, A.G.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Progress in carbon capture technologies. Sci. Total Environ. 2021, 761, 143203. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Hefny, M.; Abdel Maksoud, M.I.A.; Elgarahy, A.M.; Rooney, D.W. Recent advances in carbon capture storage and utilisation technologies: A review. Environ. Chem. Lett. 2021, 19, 797–849. [Google Scholar] [CrossRef]
- Montanari, T.; Finocchio, E.; Salvatore, E.; Garuti, G.; Giordano, A.; Pistarino, C.; Busca, G. CO2 separation and landfill biogas upgrading: A comparison of 4A and 13X zeolite adsorbents. Energy 2011, 36, 314–319. [Google Scholar] [CrossRef]
- Dindi, A.; Quang, D.V.; Vega, L.F.; Nashef, E.; Abu-Zahra, M.R.M. Applications of fly ash for CO2 capture, utilization, and storage. J. CO2 Util. 2019, 29, 82–102. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, Y.; Wang, B.; Sun, Q.; Liu, H. Waste is a misplayed resource: Synthesis of zeolites from fly ash for CO2 capture. Energy Procedia 2017, 114, 2537–2544. [Google Scholar] [CrossRef]
- Soe, J.T.; Kim, S.-S.; Lee, Y.-R.; Ahn, J.-W.; Ahn, W.-S. CO2 capture and Ca2+ exchange using zeolite A and 13X prepared from power plant fly ash. Bull. Korean Chem. Soc. 2016, 37, 490–493. [Google Scholar] [CrossRef]
- Zgureva, D. Carbon dioxide adsorption studies on fly ash zeolites. Coal Combust. Gasificat. Prod. 2016, 8, 54–59. [Google Scholar] [CrossRef]
- Boycheva, S.; Zgureva, D.; Lazarova, H.; Popova, M. Comparative studies of carbon capture onto coal fly ash zeolites Na-X and Na-Ca-X. Chemosphere 2021, 271, 129505. [Google Scholar] [CrossRef]
- Boycheva, S.; Chakarova, K.; Mihaylov, M.; Hadjiivanov, K.; Popova, M. Effect of calcium on enhanced carbon capture potential of coal fly ash zeolites. Part II: A study on the adsorption mechanisms. Environ. Sci. Process. Impacts 2022, 24, 1934–1944. [Google Scholar] [CrossRef]
- Han, L.; Wang, X.; Wu, B.; Zhu, S.; Wang, J.; Zhang, Y. In-situ synthesis of zeolite X in foam geopolymer as a CO2 adsorbent. J. Clean. Prod. 2022, 372, 133591. [Google Scholar] [CrossRef]
- Bonelli, B.; Civalleri, B.; Fubini, B.; Ugliengo, P.; Otero Areán, C.; Garrone, E. Experimental and quantum chemical studies on the adsorption of carbon dioxide on alkali-metal-exchanged ZSM-5 zeolites. J. Phys. Chem. B 2000, 104, 10978–10988. [Google Scholar] [CrossRef]
- Yang, K.; Yang, G.; Wu, J. Quantitatively understanding the insights into CO2 adsorption on faujasite from the heterogeneity and occupancy sequence of adsorption sites. J. Phys. Chem. C 2021, 125, 15676–15686. [Google Scholar] [CrossRef]
- Chakarova, K.; Mihaylov, M.; Hadjiivanov, K. Can two CO2 molecules be simultaneously bound to one Na+ site in NaY zeolite? A detailed FTIR investigation. Microporous Mesoporous Mater. 2022, 345, 112270. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Knözinger, H. FTIR study of the low-temperature adsorption and co-adsorption of CO and N2 on NaY zeolite: Evidence of simultaneous coordination of two molecules to one Na+ site. Chem. Phys. Lett. 1999, 303, 513–520. [Google Scholar] [CrossRef]
- Bonelli, B.; Onida, B.; Fubini, B.; Otero Areán, C.; Garrone, E. Vibrational and thermodynamic study of the adsorption of carbon dioxide on the zeolite Na-ZSM-5. Langmuir 2000, 16, 4976–4983. [Google Scholar] [CrossRef]
- Thang, H.V.; Grajciar, L.; Nachtigall, P.; Bludsky, O.; Otero Areán, C.; Frydová, E.; Bulánek, R. Adsorption of CO2 in FAU zeolites: Effect of zeolite composition. Catal. Today 2014, 227, 50–56. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Ivanova, E.; Kantcheva, M.; Cifitikli, E.; Klissurski, D.; Dimitrov, L.; Knözinger, H. FTIR study of low-temperature co adsorption on Mn-ZSM-5 and MnY zeolites. Effect of the zeolite matrix on the formation of Mn2+(CO)2 geminal species. Catal. Commun. 2002, 3, 313–319. [Google Scholar] [CrossRef][Green Version]
- Bae, M.N.; Song, M.K.; Kim, Y.; Seff, K. Crystal structure of Mn46Si100Al92O384.89H2S, a hydrogen sulfide sorption complex of fully dehydrated Mn2+-exchanged zeolite X. Microporous Mesoporous Mater. 2003, 63, 21–31. [Google Scholar] [CrossRef]
- Available online: https://en.wikipedia.org/wiki/Ionic_radius (accessed on 11 July 2023).
- Hadjiivanov, K.; Knözinger, H.; Ivanova, E.; Dimitrov, L. FTIR Study of low-temperature CO and 15N2 adsorption on a CaNaY zeolite: Formation of site-specified Ca2+(CO)3 and Ca2+(15N2)3 complexes. Phys. Chem. Chem. Phys. 2001, 3, 2531–2536. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Knözinger, H. Formation of Ca2+(CO)3 complexes during low-temperature CO adsorption on CaNaY zeolite. J. Phys. Chem. B 2001, 105, 4531–4534. [Google Scholar] [CrossRef]
- Drenchev, N.L.; Ivanova, E.Z.; Mihaylov, M.Y.; Aleksandrov, H.A.; Vayssilov, G.N.; Hadjiivanov, K.I. One Ca2+ Site in CaNaY Zeolite Can Attach Three CO2 Molecules. J. Phys. Chem. Lett. 2023, 14, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-H.; Huang, C.-H.; Tan, C.S. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Drenchev, N.L.; Chakarova, K.K.; Lagunov, O.V.; Mihaylov, M.Y.; Ivanova, E.Z.; Strauss, I.; Hadjiivanov, K.I. In situ FTIR spectroscopy as a tool for investigation of gas/solid interaction: Water-enhanced CO2 adsorption in UiO-66 metal-organic framework. J. Vis. Exp. 2020, 156, e60285. [Google Scholar] [CrossRef]
- Xu, X.; Song, C.; Miller, B.G.; Scaroni, A.W. Influence of moisture on CO2 separation from gas mixture by a nanoporous adsorbent based on polyethylenimine-modified molecular sieve MCM-41. Ind. Eng. Chem. Res. 2005, 44, 8113–8119. [Google Scholar] [CrossRef]
- Guo, H.; Yan, S.; Zhao, Y.; Ma, X.; Wang, S. Influence of water vapor on cyclic CO2 capture performance in both carbonation and decarbonation stages for Ca-Al mixed oxide. Chem. Eng. J. 2019, 359, 542–551. [Google Scholar] [CrossRef]
- Panayotov, D.; Zdravkova, V.; Lagunov, O.; Andonova, S.; Spassova, I.; Nihtianova, D.; Atanasova, G.; Drenchev, N.; Ivanova, E.; Mihaylov, M.; et al. Capturing CO2 by ceria and ceria–zirconia nanomaterials of different origin. Phys. Chem. Chem. Phys. 2023, 25, 17154–17175. [Google Scholar] [CrossRef]
- Karimi, M.; Rodrigues, A.E.; Silva, J.A.C. Biomass as a source of adsorbents for CO2 capture. In Advances in Bioenergy and Microfluidic Applications; Rahimpour, M.R., Kamali, R., Makarem, M.A., Manshadi, M.K.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 255–274. [Google Scholar] [CrossRef]
- Kolle, J.M.; Fayaz, M.; Sayari, A. Understanding the effect of water on CO2 adsorption. Chem. Rev. 2021, 121, 7280–7345. [Google Scholar] [CrossRef]
- Legodi, M.A.; De Waal, D.; Potgieter, J.H.; Potgieter, S.S. Rapid determination of CaCO3 in mixtures utilising FT-IR spectroscopy. Miner. Eng. 2001, 14, 1107–1111. [Google Scholar] [CrossRef]
- Hadjiivanov, K. Identification and characterization of surface hydroxyl groups by infrared spectroscopy. Adv. Catal. 2014, 57, 99–318. [Google Scholar] [CrossRef]
- Lim, W.T.; Seo, S.M.; Kim, G.H.; Lee, H.S.; Seff, K. Six single-crystal structures showing the dehydration, deamination, dealumination, and decomposition of NH4+-exchanged zeolite Y (FAU) with increasing evacuation temperature. Identification of a Lewis acid site. J. Phys. Chem. C 2007, 111, 18294–18306. [Google Scholar] [CrossRef]
- Novikov, R.G.; Konopnitskii, R.; Tsyganenko, A.A. Distortions in IR spectra related to registration conditions: II. The influence of scattering. Opt. Spectrosc. 2018, 124, 655–659. [Google Scholar] [CrossRef]
- Gallei, E.; Stumpf, G. Studies of the adsorption of carbon dioxide and the coadsorption of carbon dioxide and water on CaY- and NiY-zeolites. J. Colloid Interface Sci. 1976, 55, 415–420. [Google Scholar] [CrossRef]
- Davarpanah, E.; Armandi, M.; Hernandez, S.; Fino, D.; Arletti, R.; Bensaid, S.; Piumetti, M. CO2 capture on natural zeolite clinoptilolite: Effect of temperature and role of the adsorption sites. J. Environ. Manag. 2020, 275, 111229. [Google Scholar] [CrossRef]
- Montanari, T.; Busca, G. On the mechanism of adsorption and separation of CO2 on LTA zeolites: An IR investigation. Vibr. Spectrosc. 2008, 46, 45–51. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Mihaylov, M.; Panayotov, D.; Ivanova, E.; Chakarova, K. Isotopes in the FTIR investigations of solid surfaces. Spectrosc. Prop. Inorg. Organomet. Compd. 2014, 45, 43–78. [Google Scholar] [CrossRef]
- Jang, S.L.; Jeong, M.S.; Kim, Y.; Jan, Y.; Seff, K. Crystal structure of a hydrogen sulfide sorption complex of fully Ca2+-exchanged zeolite X. Microporous Mesoporous Mater. 1999, 23, 33–44. [Google Scholar] [CrossRef]
- Jang, S.L.; Jeong, M.S.; Kim, Y.; Song, S.H.; Seff, K. Crystal structure of an ammonia sorption complex of dehydrated fully Ca2+-exchanged zeolite X. Microporous Mesoporous Mater. 1999, 28, 173–183. [Google Scholar] [CrossRef]
- Paze, C.; Bordiga, S.; Lamberti, C.; Salvalaggio, M.; Zecchina, A.; Bellussi, G. Acidic properties of H–β zeolite as probed by bases with proton affinity in the 118–204 kcal mol−1 range: a FTIR investigation. J. Phys. Chem. B 1997, 101, 4740–4751. [Google Scholar] [CrossRef]
- Wakabayashi, F.; Kondo, J.N.; Domen, K.; Hirose, C. FT-IR study of H218O adsorption on H-ZSM-5: Direct evidence for the hydrogen-bonded adsorption of water. J. Phys. Chem. 1996, 100, 1442–1444. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Vayssilov, G. Characterization of oxide surfaces and zeolites by carbon monoxide as an IR probe molecule. Adv. Catal. 2002, 47, 307–511. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Kantcheva, M.; Klissurski, D. IR study of CO adsorption on Cu-ZSM-5 and CuO/SiO2 Catalysts: σ- and π-components of the Cu+-CO bond. J. Chem. Soc. Faraday Trans. 1996, 92, 4595–4600. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drenchev, N.L.; Shivachev, B.L.; Dimitrov, L.D.; Hadjiivanov, K.I. Effect of Water on CO2 Adsorption on CaNaY Zeolite: Formation of Ca2+(H2O)(CO2), Ca2+(H2O)(CO2)2 and Ca2+(H2O)2(CO2) Complexes. Nanomaterials 2023, 13, 2278. https://doi.org/10.3390/nano13162278
Drenchev NL, Shivachev BL, Dimitrov LD, Hadjiivanov KI. Effect of Water on CO2 Adsorption on CaNaY Zeolite: Formation of Ca2+(H2O)(CO2), Ca2+(H2O)(CO2)2 and Ca2+(H2O)2(CO2) Complexes. Nanomaterials. 2023; 13(16):2278. https://doi.org/10.3390/nano13162278
Chicago/Turabian StyleDrenchev, Nikola L., Boris L. Shivachev, Lubomir D. Dimitrov, and Konstantin I. Hadjiivanov. 2023. "Effect of Water on CO2 Adsorption on CaNaY Zeolite: Formation of Ca2+(H2O)(CO2), Ca2+(H2O)(CO2)2 and Ca2+(H2O)2(CO2) Complexes" Nanomaterials 13, no. 16: 2278. https://doi.org/10.3390/nano13162278
APA StyleDrenchev, N. L., Shivachev, B. L., Dimitrov, L. D., & Hadjiivanov, K. I. (2023). Effect of Water on CO2 Adsorption on CaNaY Zeolite: Formation of Ca2+(H2O)(CO2), Ca2+(H2O)(CO2)2 and Ca2+(H2O)2(CO2) Complexes. Nanomaterials, 13(16), 2278. https://doi.org/10.3390/nano13162278






