Development and Upscaling of SiO2@TiO2 Core-Shell Nanoparticles for Methylene Blue Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of SiO2 and SiO2@TiO2 Core-Shell Nanoparticles
2.3. Physical Chemical Characterization of SiO2 and SiO2@TiO2 Core-Shell Nanoparticles
2.4. Reactive Species Generation
2.5. Photocatalytic Removal of Dye
3. Results and Discussion
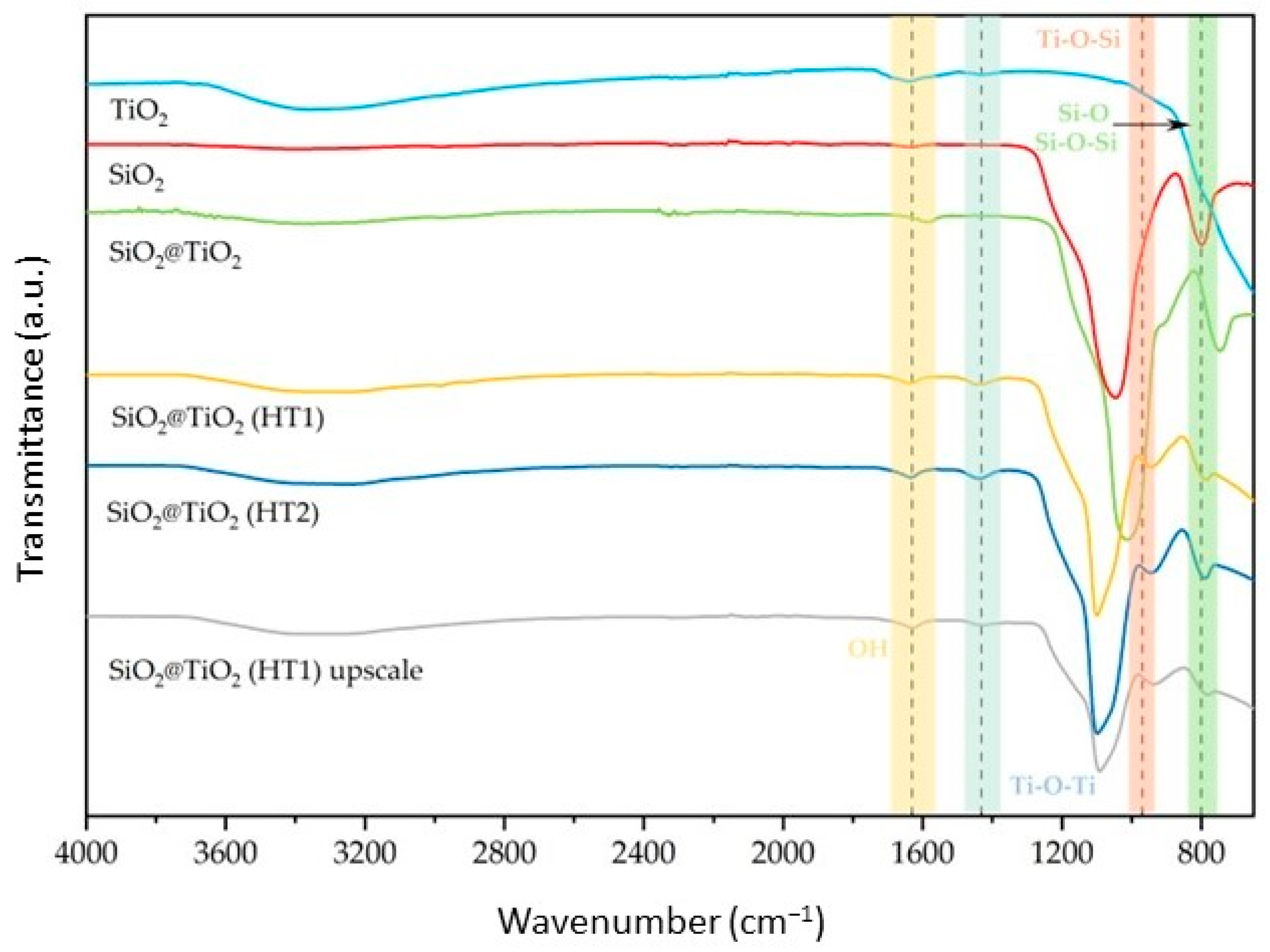
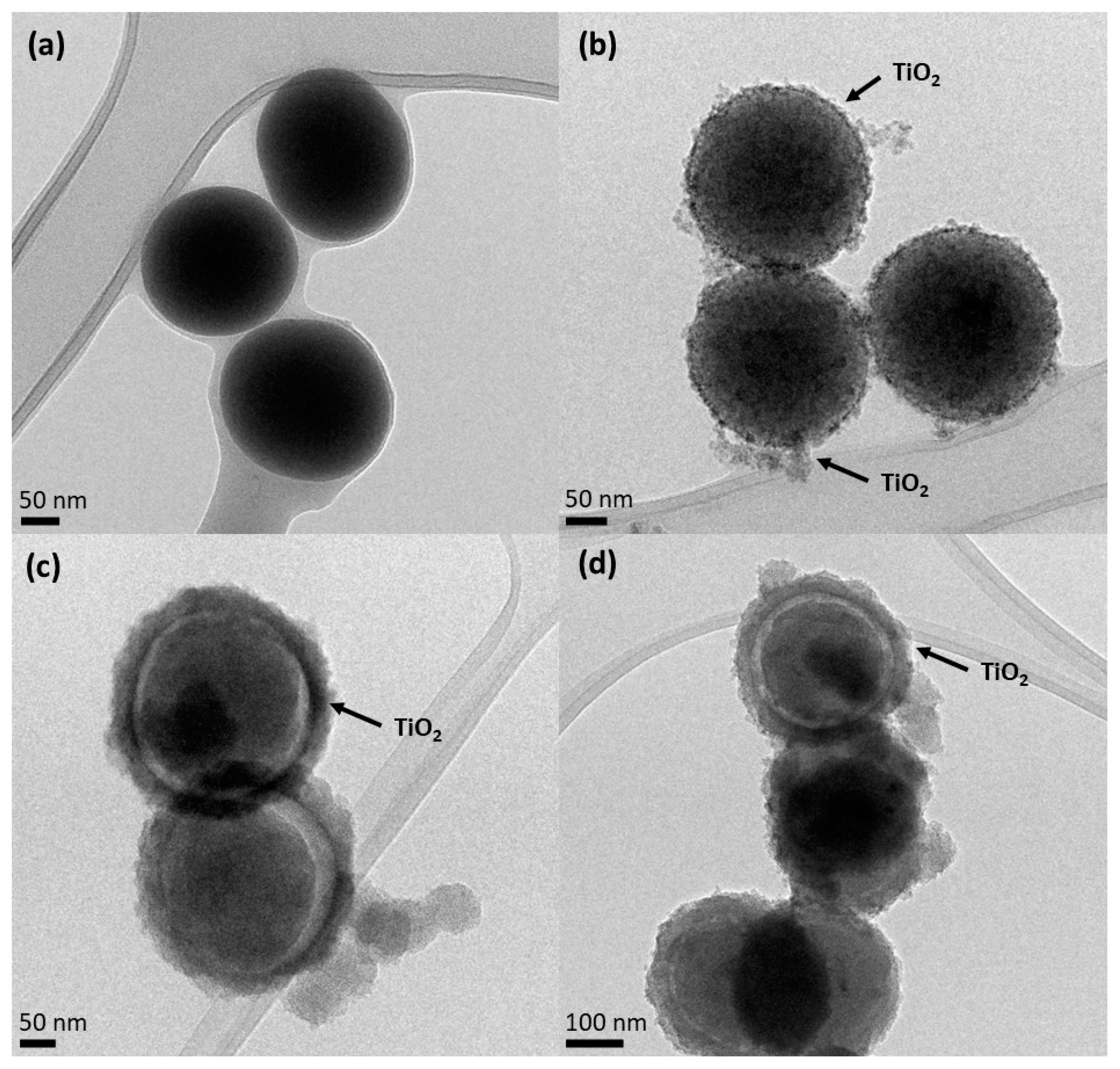
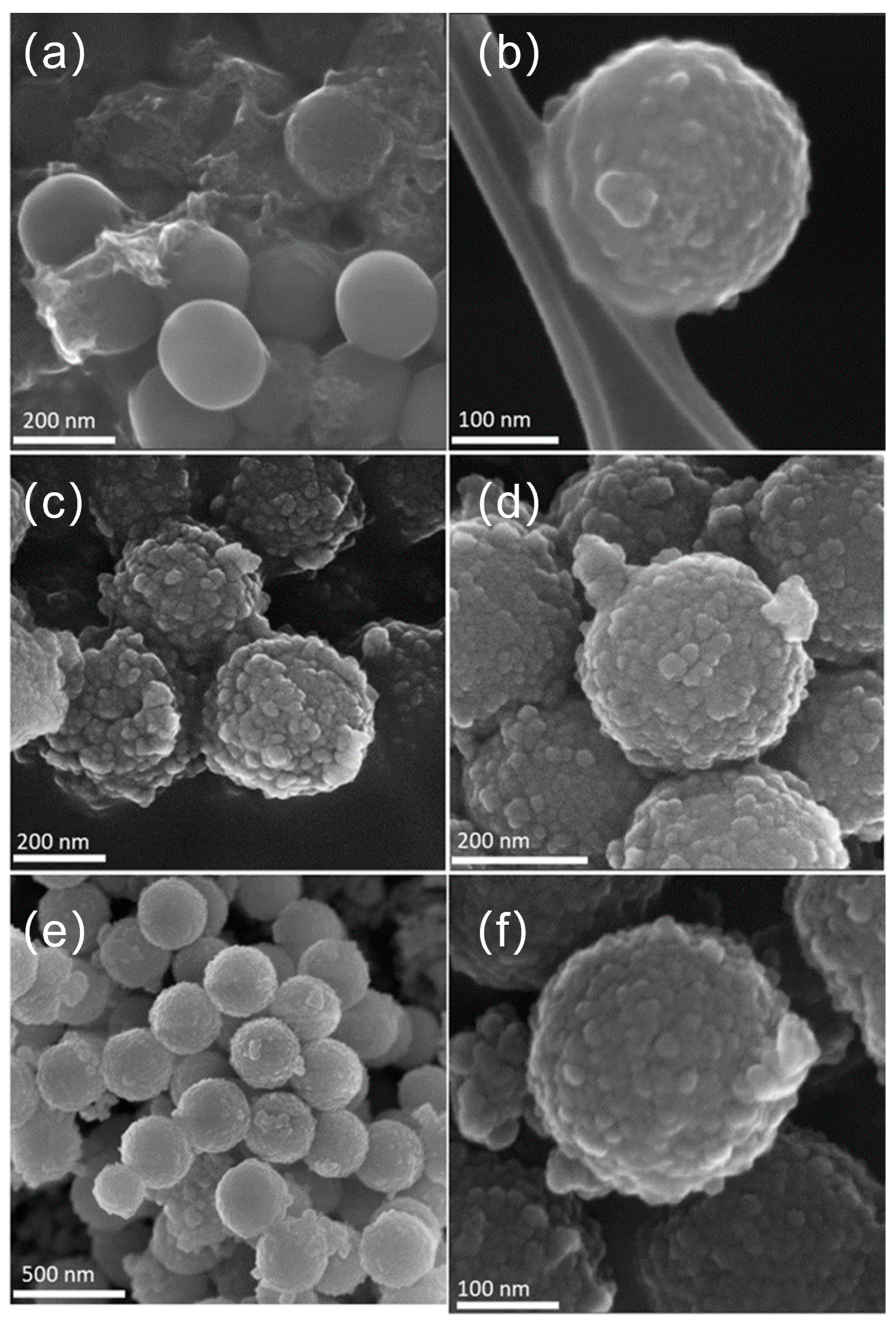
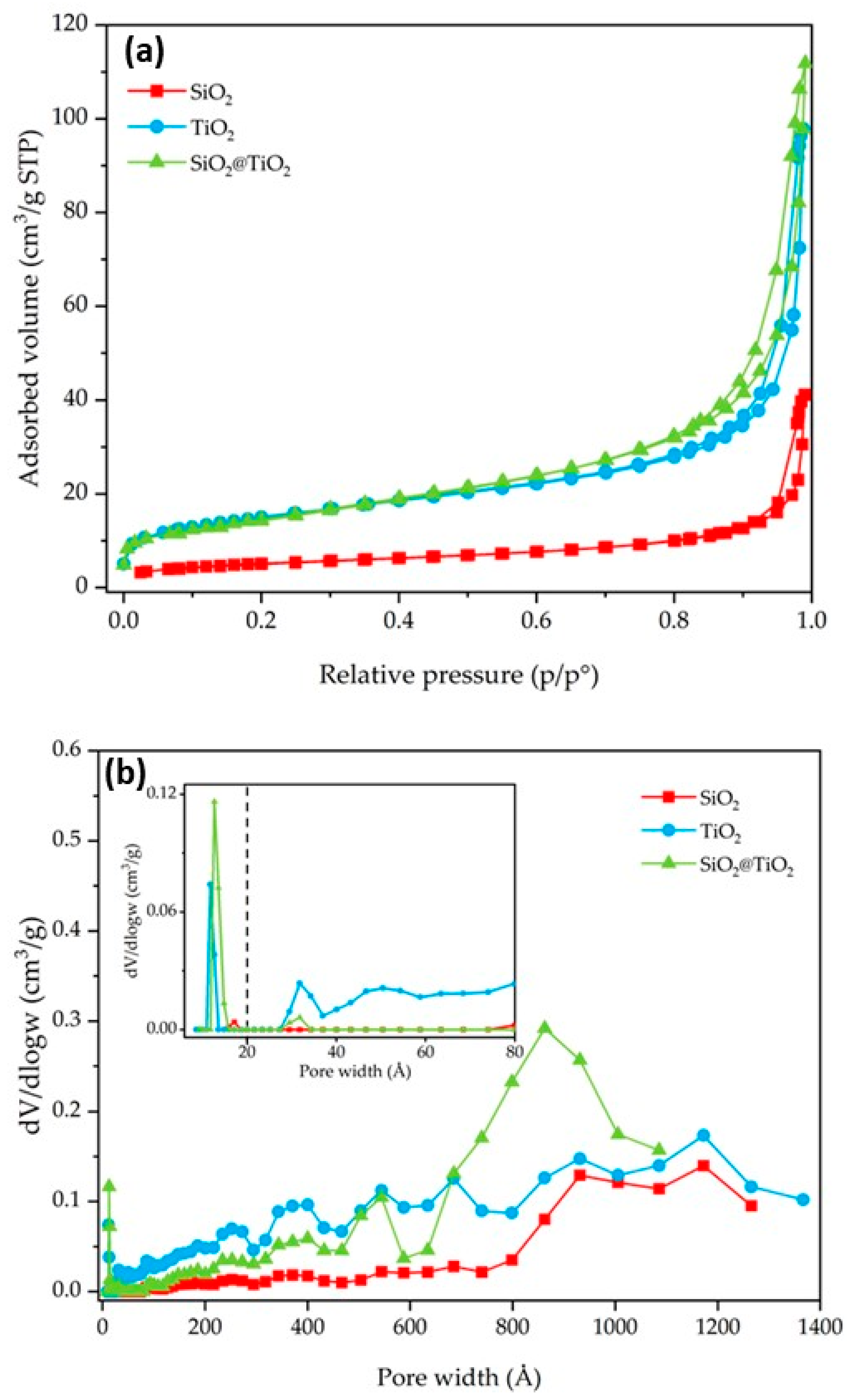
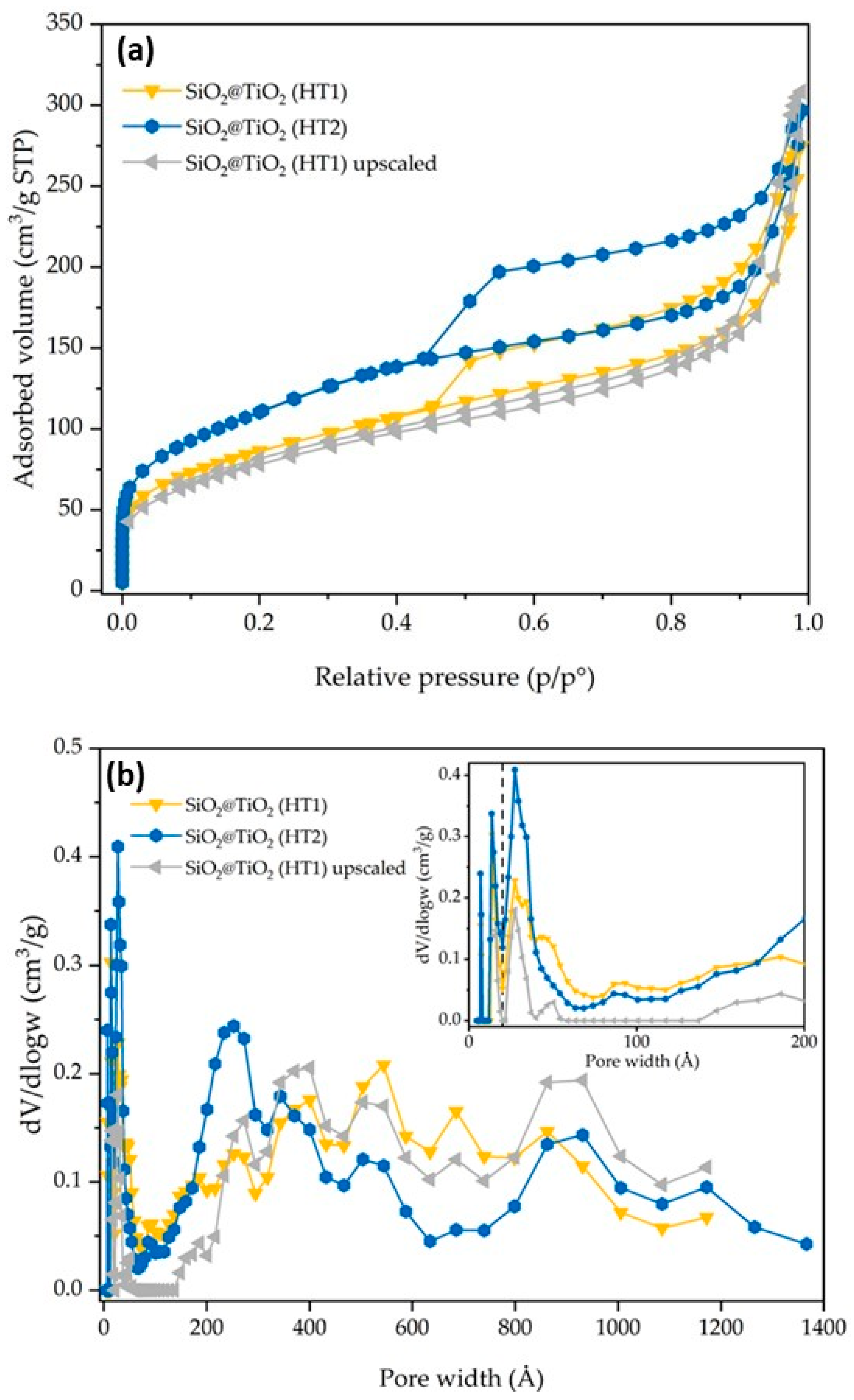
3.1. Physical Chemical Characterization of SiO2 and SiO2@TiO2 Core-Shell Nanoparticles
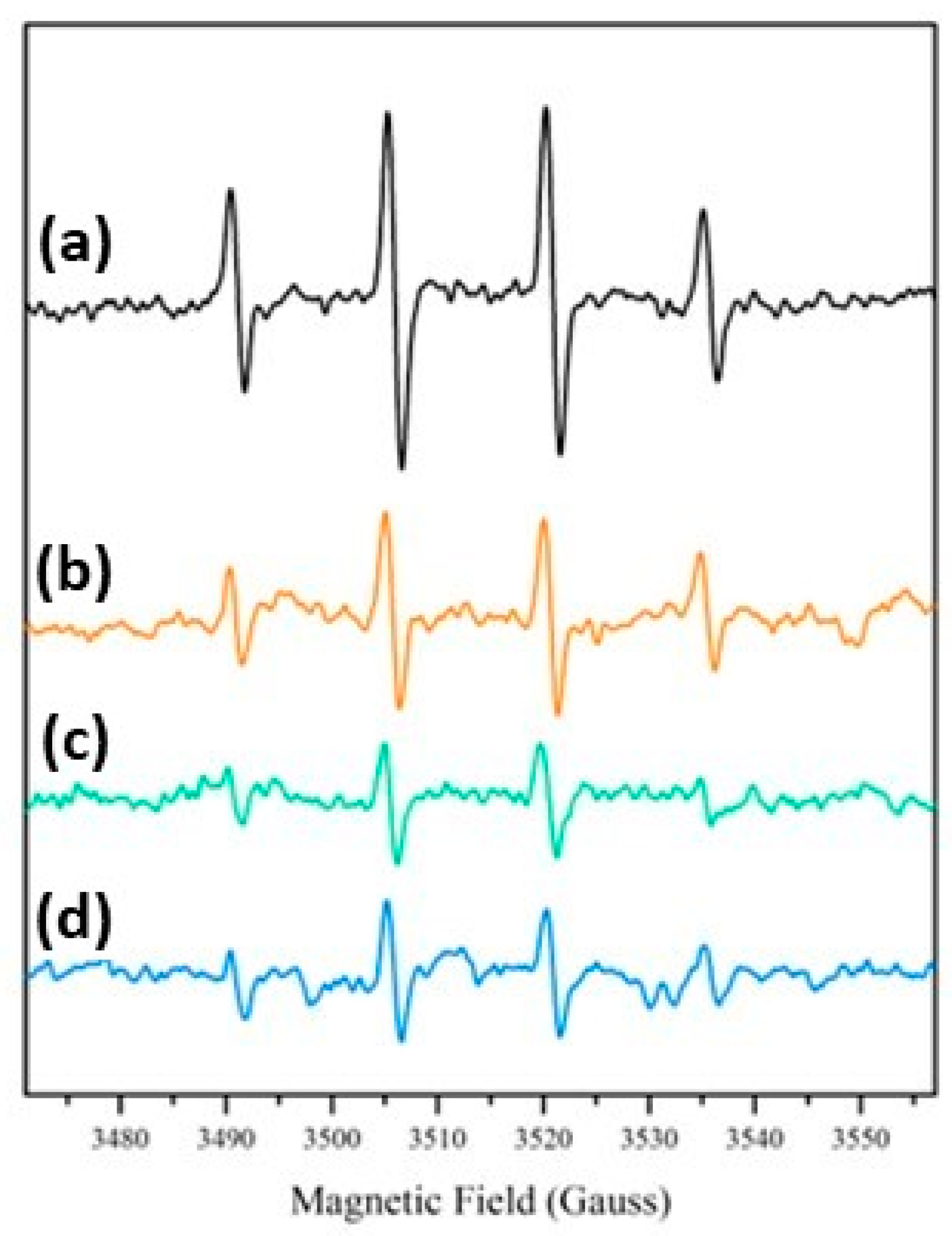
3.2. Reactive Species Generation
3.3. Photocatalytic Removal of Dye
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- What Are the Main Sources of Water Pollution?—European Environment Agency. Available online: https://www.eea.europa.eu/help/faq/what-are-the-main-sources (accessed on 22 July 2023).
- Opoku, F.; Kiarii, E.M.; Govender, P.P. Nanotechnology for Water and Wastewater Treatment Using Graphene Semiconductor Composite Materials; Springer: Cham, Switzerland, 2020; pp. 1–34. [Google Scholar] [CrossRef]
- Hussein, U.A.-R.; Mahmoud, Z.H.; Alaziz, K.M.A.; Alid, M.L.; Yasin, Y.; Ali, F.K.; Faisal, A.N.; Abd, A.N.; Kianfar, E. Antimicrobial finishing of textiles using nanomaterials. Braz. J. Biol. 2023, 84, e264947. [Google Scholar] [CrossRef] [PubMed]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N. Titanium dioxide (TiO2)-based photocatalyst materials activity enhancement for contaminants of emerging concern (CECs) degradation: In the light of modification strategies. Chem. Eng. J. Adv. 2022, 10, 100262. [Google Scholar] [CrossRef]
- Al Jitan, S.; Palmisano, G.; Garlisi, C. Synthesis and Surface Modification of TiO2-Based Photocatalysts for the Conversion of CO2. Catalysts 2020, 10, 227. [Google Scholar] [CrossRef]
- Vasiljevic, Z.Z.; Dojcinovic, M.P.; Vujancevic, J.D.; Jankovic-Castvan, I.; Ognjanovic, M.; Tadic, N.B.; Stojadinovic, S.; Brankovic, G.O.; Nikolic, M.V. Photocatalytic degradation of methylene blue under natural sunlight using iron titanate nanoparticles prepared by a modified sol–gel method. R. Soc. Open Sci. 2020, 7, 200708. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, U.; Khandelwal, M.; Deshpande, A.S. TiO2@SiO2 nanoparticles for methylene blue removal and photocatalytic degradation under natural sunlight and low-power UV light. Appl. Surf. Sci. 2022, 576, 151745. [Google Scholar] [CrossRef]
- Ferreira-Neto, E.P.; Ullah, S.; Martinez, V.P.; Yabarrena, J.M.S.C.; Simões, M.B.; Perissinotto, A.P.; Wender, H.; de Vicente, F.S.; Noeske, P.-L.M.; Ribeiro, S.J.L.; et al. Thermally stable SiO2@TiO2 core@shell nanoparticles for application in photocatalytic self-cleaning ceramic tiles. Mater. Adv. 2021, 2, 2085–2096. [Google Scholar] [CrossRef]
- Ren, J.; Lai, Y.; Gao, J. Exploring the influence of SiO2 and TiO2 nanoparticles on the mechanical properties of concrete. Constr. Build. Mater. 2018, 175, 277–285. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Assadi, M.H.N.; Li, S.; Yu, A.; Sorrell, C.C. Ab Initio Study of Phase Stability in Doped TiO2. Comput. Mech. 2012, 50, 185–194. [Google Scholar] [CrossRef]
- Ullah, S.; Ferreira-Neto, E.P.; Pasa, A.A.; Alcântara, C.C.; Acuña, J.J.; Bilmes, S.A.; Ricci, M.L.M.; Landers, R.; Fermino, T.Z.; Rodrigues-Filho, U.P. Enhanced photocatalytic properties of core@shell SiO2@TiO2 nanoparticles. Appl. Catal. B 2015, 179, 333–343. [Google Scholar] [CrossRef]
- Gupta, S.; Tripathi, M. A review on the synthesis of TiO2 nanoparticles by solution route. Open Chem. 2012, 10, 279–294. [Google Scholar] [CrossRef]
- Hofman-Caris, C.H.M.; Bäuerlein, P.S.; Siegers, W.G.; Mintenig, S.M.; Messina, R.; Dekker, S.C.; Bertelkamp, C.; Cornelissen, E.R.; van Wezel, A.P. Removal of nanoparticles (both inorganic nanoparticles and nanoplastics) in drinking water treatment—Coagulation/flocculation/sedimentation, and sand/granular activated carbon filtration. Environ. Sci. Water Res. Technol. 2022, 8, 1675–1686. [Google Scholar] [CrossRef]
- Park, C.M.; Chu, K.H.; Her, N.; Jang, M.; Baalousha, M.; Heo, J.; Yoon, Y. Occurrence and Removal of Engineered Nanoparticles in Drinking Water Treatment and Wastewater Treatment Processes. Sep. Purif. Rev. 2016, 46, 255–272. [Google Scholar] [CrossRef]
- Honda, R.J.; Keene, V.; Daniels, L.; Walker, S.L. Removal of TiO2 Nanoparticles During Primary Water Treatment: Role of Coagulant Type, Dose, and Nanoparticle Concentration. Environ. Eng. Sci. 2014, 31, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Salih, H.H.M.; El Badawy, A.M.; Tolaymat, T.M.; Patterson, C.L. Removal of Stabilized Silver Nanoparticles from Surface Water by Conventional Treatment Processes. Adv. Nanoparticles 2019, 8, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Habib, Z.; Khan, S.J.; Ahmad, N.M.; Shahzad, H.M.A.; Jamal, Y.; Hashmi, I. Antibacterial behaviour of surface modified composite polyamide nanofiltration (NF) membrane by immobilizing Ag-doped TiO2 nanoparticles. Environ. Technol. 2019, 41, 3657–3669. [Google Scholar] [CrossRef] [PubMed]
- Atmianlu, P.A.; Badpa, R.; Aghabalaei, V.; Baghdadi, M. A review on the various beds used for immobilization of nanoparticles: Overcoming the barrier to nanoparticle applications in water and wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 106514. [Google Scholar] [CrossRef]
- Bode-Aluko, C.A.; Pereao, O.; Kyaw, H.H.; Al-Naamani, L.; Al-Abri, M.Z.; Myint, M.T.Z.; Rossouw, A.; Fatoba, O.; Petrik, L.; Dobretsov, S. Photocatalytic and antifouling properties of electrospun TiO2 polyacrylonitrile composite nanofibers under visible light. Mater. Sci. Eng. B 2021, 264, 114913. [Google Scholar] [CrossRef]
- Babyszko, A.; Wanag, A.; Kusiak-Nejman, E.; Morawski, A.W. Effect of Calcination Temperature of SiO2/TiO2 Photocatalysts on UV-VIS and VIS Removal Efficiency of Color Contaminants. Catalysts 2023, 13, 186. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, B.; Chen, Z.; Xu, Y.; Liu, Y.; Li, L.; Jiang, Q.; Newton, M.A.A. Enhanced Degradation of Methylene Blue Dye Using Flexible SiO2–TiO2 Nanofiber Membranes; ICE Publishing: London, UK, 2023; pp. 1–11. [Google Scholar] [CrossRef]
- De Jesús Acosta-Silva, Y.; Toledano-Ayala, M.; Gallardo-Hernández, S.; Godínez, L.A.; Méndez-López, A. Investigation of TiO2 Deposit on SiO2 Films: Synthesis, Characterization, and Efficiency for the Photocatalytic Discoloration of Methylene Blue in Aqueous Solution. Nanomaterials 2023, 13, 1403. [Google Scholar] [CrossRef]
- Al Baroot, A.; Haladu, S.A.; Magami, S.M.; Akhtar, S.; Drmosh, Q.; Elsayed, K.A.; Manda, A.A. Photocatalytic performance of Ag/TiO2/SiO2 nanocomposite synthesized by eco-friendly pulsed laser ablation technique. J. Phys. Chem. Solids 2023, 180, 111489. [Google Scholar] [CrossRef]
- Kitsou, I.; Panagopoulos, P.; Maggos, T.; Arkas, M.; Tsetsekou, A. Development of SiO2@TiO2 core-shell nanospheres for catalytic applications. Appl. Surf. Sci. 2018, 441, 223–231. [Google Scholar] [CrossRef]
- The Challenges Behind Scaling up Nanomaterials. Available online: https://www.azonano.com/article.aspx?ArticleID=6126 (accessed on 22 July 2023).
- Liu, X.; Meng, H. Consideration for the scale-up manufacture of nanotherapeutics—A critical step for technology transfer. View 2021, 2, 20200190. [Google Scholar] [CrossRef]
- Li, C. Structure controlling and process scale-up in the fabrication of nanomaterials. Front. Chem. Eng. China 2010, 4, 18–25. [Google Scholar] [CrossRef]
- Lee, J.W.; Kong, S.; Kim, W.S.; Kim, J. Preparation and characterization of SiO2/TiO2 core-shell particles with controlled shell thickness. Mater. Chem. Phys. 2007, 106, 39–44. [Google Scholar] [CrossRef]
- Tong, H.; Enomoto, N.; Inada, M.; Tanaka, Y.; Hojo, J. Hydrothermal synthesis of mesoporous TiO2–SiO2 core-shell composites for dye-sensitized solar cells. Electrochim. Acta 2014, 130, 329–334. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.S. Hydrothermal/solvothermal synthesis and treatment of TiO2 for photocatalytic degradation of air pollutants: Preparation, characterization, properties, and performance. Chemosphere 2019, 219, 804–825. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C Photochem. Rev. 2005, 6, 186–205. [Google Scholar] [CrossRef]
- El-Bery, H.M.; Saleh, M.; El-Gendy, R.A.; Saleh, M.R.; Thabet, S.M. High adsorption capacity of phenol and methylene blue using activated carbon derived from lignocellulosic agriculture wastes. Sci. Rep. 2022, 12, 5499. [Google Scholar] [CrossRef]
- Do, Y.; Cho, I.; Park, Y.; Pradhan, D.; Sohn, Y. CO Oxidation Activities of Ni and Pd-TiO@SiO Core-Shell Nanostructures. Bull. Korean Chem. Soc. 2013, 34, 3635–3640. [Google Scholar] [CrossRef]
- Huang, C.; Bai, H.; Huang, Y.; Liu, S.; Yen, S.; Tseng, Y. Synthesis of neutral SiO2/TiO2 hydrosol and its application as antireflective self-cleaning thin film. Int. J. Photoenergy 2012, 2012, 620764. [Google Scholar] [CrossRef]
- Salgado, B.C.B.; Valentini, A. Evaluation of the photocatalytic activity of Sio2@Tio2 hybrid spheres in the degradation of methylene blue and hydroxylation of benzene: Kinetic and mechanistic study. Braz. J. Chem. Eng. 2020, 36, 1501–1518. [Google Scholar] [CrossRef]
- Babyszko, A.; Wanag, A.; Sadłowski, M.; Kusiak-Nejman, E.; Morawski, A.W. Synthesis and Characterization of SiO2/TiO2 as Photocatalyst on Methylene Blue Degradation. Catalysts 2022, 12, 1372. [Google Scholar] [CrossRef]
- Ekka, B.; Sahu, M.K.; Patel, R.K.; Dash, P. Titania coated silica nanocomposite prepared via encapsulation method for the degradation of Safranin-O dye from aqueous solution: Optimization using statistical design. Water Resour. Ind. 2019, 22, 100071. [Google Scholar] [CrossRef]
- Joseph, E.; Singhvi, G. Multifunctional nanocrystals for cancer therapy: A potential nanocarrier. In Nanomaterials for Drug Delivery and Therapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 91–116. [Google Scholar] [CrossRef]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Lipid-Based Nanoparticles for Drug Delivery Systems. In Characterization and Biology of Nanomaterials for Drug Delivery: Nanoscience and Nanotechnology in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–76. [Google Scholar] [CrossRef]
- Liao, D.L.; Wu, G.S.; Liao, B.Q. Zeta potential of shape-controlled TiO2 nanoparticles with surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 270–275. [Google Scholar] [CrossRef]
- Tan, Z.; Yuan, S.; Hong, M.; Zhang, L.; Huang, Q. Mechanism of negative surface charge formation on biochar and its effect on the fixation of soil Cd. J. Hazard. Mater. 2020, 384, 121370. [Google Scholar] [CrossRef]
- Ramirez, L.; Gentile, S.R.; Zimmermann, S.; Stoll, S. Behavior of TiO2 and CeO2 Nanoparticles and Polystyrene Nanoplastics in Bottled Mineral, Drinking and Lake Geneva Waters. Impact of Water Hardness and Natural Organic Matter on Nanoparticle Surface Properties and Aggregation. Water 2019, 11, 721. [Google Scholar] [CrossRef]
- Wang, K.; Zhuo, Y.; Chen, J.; Gao, D.; Ren, Y.; Wang, C.; Qi, Z. Crystalline phase regulation of anatase–rutile TiO2 for the enhancement of photocatalytic activity. RSC Adv. 2020, 10, 43592–43598. [Google Scholar] [CrossRef]
- Budiarti, H.A.; Puspitasari, R.N.; Hatta, A.M.; Sekartedjo; Risanti, D.D. Synthesis and Characterization of TiO2@SiO2 and SiO2@TiO2 Core-Shell Structure Using Lapindo Mud Extract via Sol-Gel Method. Procedia Eng. 2017, 170, 65–71. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Z.; Zhang, Q.; Jia, X.; Xi, K. Synthesis of stable Au–SiO2 composite nanospheres with good catalytic activity and SERS effect. J. Colloid Interface Sci. 2014, 418, 1–7. [Google Scholar] [CrossRef]
- González-Burciaga, L.A.; Núñez-Núñez, C.M.; Morones-Esquivel, M.M.; Avila-Santos, M.; Lemus-Santana, A.; Proal-Nájera, J.B. Characterization and Comparative Performance of TiO2 Photocatalysts on 6-Mercaptopurine Degradation by Solar Heterogeneous Photocatalysis. Catalysts 2020, 10, 118. [Google Scholar] [CrossRef]
- El-Desoky, M.M.; Morad, I.; Wasfy, M.H.; Mansour, A.F. Synthesis, structural and electrical properties of PVA/TiO2 nanocomposite films with different TiO2 phases prepared by sol–gel technique. J. Mater.Sci. Mater. Electron. 2020, 31, 17574–17584. [Google Scholar] [CrossRef]
- Murashkevich, A.N.; Lavitskaya, A.S.; Barannikova, T.I.; Zharskii, I.M. Infrared absorption spectra and structure of TiO2–SiO2 composites. J. Appl. Spectrosc. 2008, 75, 730–734. [Google Scholar] [CrossRef]
- Liu, Z.; Quan, X.; Fu, H.; Li, X.; Yang, K. Effect of embedded-silica on microstructure and photocatalytic activity of titania prepared by ultrasound-assisted hydrolysis. Appl. Catal. B 2004, 52, 33–40. [Google Scholar] [CrossRef]
- Bonelli, B.; Cozzolino, M.; Tesser, R.; Diserio, M.; Piumetti, M.; Garrone, E.; Santacesaria, E. Study of the surface acidity of TiO2/SiO2 catalysts by means of FTIR measurements of CO and NH3 adsorption. J. Catal. 2007, 246, 293–300. [Google Scholar] [CrossRef]
- Pu, S.; Hou, Y.; Chen, H.; Deng, D.; Yang, Z.; Xue, S.; Zhu, R.; Diao, Z.; Chu, W. An Efficient Photocatalyst for Fast Reduction of Cr(VI) by Ultra-Trace Silver Enhanced Titania in Aqueous Solution. Catalysts 2018, 8, 251. [Google Scholar] [CrossRef]
- Al-Madanat, O.; Nunes, B.N.; AlSalka, Y.; Hakki, A.; Curti, M.; Patrocinio, A.O.T.; Bahnemann, D.W. Application of EPR Spectroscopy in TiO2 and Nb2O5 Photocatalysis. Catalysts 2021, 11, 1514. [Google Scholar] [CrossRef]
- Utami, F.D.; Rahman, D.Y.; Margareta, D.O.; Rahmayanti, H.D.; Munir, R.; Sustini, E.; Abdullah, M. TiO2 Photocatalytic Degradation of Methylene Blue Using Simple Spray Method. IOP Conf. Ser. Mater. Sci. Eng. 2019, 599, 012026. [Google Scholar] [CrossRef]
- Xu, C.; Rangaiah, G.P.; Zhao, X.S. Photocatalytic Degradation of Methylene Blue by Titanium Dioxide: Experimental and Modeling Study. Ind. Eng. Chem. Res. 2014, 53, 14641–14649. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Jellali, S.; Ben Harharah Hamed, N.; El Jery, A.; Khezami, L.; Assadi, A.A.; Amrane, A. Photocatalytic treatment of wastewater containing simultaneous organic and inorganic pollution: Competition and operating parameters effects. Catalysts 2021, 11, 855. [Google Scholar] [CrossRef]
- Wei, S.; Kamali, A.R. Waste plastic derived Co3Fe7/CoFe2O4@carbon magnetic nanostructures for efficient dye adsorption. J. Alloys Compd. 2021, 886, 161201. [Google Scholar] [CrossRef]
- Kamali, A.R.; Zhu, W.; Shi, Z.; Wang, D. Combustion synthesis-aqueous hybridization of nanostructured graphene-coated silicon and its dye removal performance. Mater. Chem. Phys. 2022, 277, 125565. [Google Scholar] [CrossRef]
- Li, S.; Kamali, A.R. Fast and clean preparation of highly crystalline SnO2 nanoparticles incorporated in amorphous carbon, and its dye removal performance. Inorg. Chem. Commun. 2022, 142, 109597. [Google Scholar] [CrossRef]
- Zhu, W.; Kamali, A.R. Mechanochemical synthesis of molybdenum disulfide quantum dots with enhanced dye adsorption and photocatalytic performance. J. Water Process Eng. 2023, 53, 103903. [Google Scholar] [CrossRef]
- Kim, J.H.; Cha, B.J.; Kim, Y.D.; Seo, H.O. Kinetics and thermodynamics of methylene blue adsorption on the Fe-oxide nanoparticles embedded in the mesoporous SiO2. Adv. Powder Technol. 2020, 31, 816–826. [Google Scholar] [CrossRef]
- Nabih, S.; Shalan, A.E.; Serea, E.S.A.; Goda, M.A.; Sanad, M.F. Photocatalytic performance of TiO2@SiO2 nanocomposites for the treatment of different organic dyes. J. Mater.Sci. Mater. Electron. 2019, 30, 9623–9633. [Google Scholar] [CrossRef]
- Cabezuelo, O.; Diego-Lopez, A.; Atienzar, P.; Marin, M.L.; Bosca, F. Optimizing the use of light in supported TiO2 photocatalysts: Relevance of the shell thickness. J. Photochem. Photobiol. A Chem. 2023, 444, 114917. [Google Scholar] [CrossRef]
- Alvaro, M.; Aprile, C.; Benitez, M.; Carbonell, E.; García, H. Photocatalytic Activity of Structured Mesoporous TiO2 Materials. J. Phys. Chem. B 2006, 110, 6661–6665. [Google Scholar] [CrossRef]
- De Souza Pereira, M.; Mendes, R.P.; Bellettini, G.C.; Benetti, R.M.; Elyseu, F.; Bernardin, A.M. Photocatalytic discoloration of methylene blue by TiO2 P25 under UV light using ISO 10678 standard as a guide. J. Photochem. Photobiol. A Chem. 2023, 435, 114304. [Google Scholar] [CrossRef]
- Sun, R.; Chen, Z.; Yang, Y.; Peng, J.; Zheng, T. Effects and mechanism of SiO2 on photocatalysis and super hydrophilicity of TiO2 films prepared by sol-gel method. Mater. Res. Express 2019, 6, 046409. [Google Scholar] [CrossRef]



| Samples | Zeta Potential (mV) | pH | T (°C) |
|---|---|---|---|
| SiO2 | −20.5 ± 0.4 | 5.6 | 25 |
| SiO2@TiO2 | −21.4 ± 0.9 | 4.9 | |
| SiO2@TiO2 (HT1) | −33.9 ± 0.4 | 6.9 | |
| SiO2@TiO2 (HT2) | −37.3 ± 0.5 | 6.6 | |
| SiO2@TiO2 (HT1) upscaled | −28.1 ± 0.7 | 5.9 |
| Samples | Si %wt | Ti %wt | O %wt | C+ Impurities |
|---|---|---|---|---|
| SiO2 | 9.9 ± 0.1 | - | 68.0 ± 0.2 | 21.2 ± 0.2 |
| SiO2@TiO2 | 0.7 ± 0.0 | 0.5 ± 0.1 | 72.1 ± 0.5 | 26.7 ± 0.3 |
| SiO2@TiO2 (HT1) | 3.9 ± 0.1 | 1.9 ± 0.1 | 26.1 ± 0.4 | 67.5 ± 0.4 |
| SiO2@TiO2 (HT2) | 5.0 ± 0.1 | 0.9 ± 0.0 | 34.9 ± 0.4 | 59.1 ± 0.4 |
| SiO2@TiO2 (HT1) upscaled | 8.1 ± 0.2 | 11.2 ± 2.4 | 29.5 ± 0.8 | 51.0 ± 1.4 |
| Samples | BET SSA (m2/g) | V Micropore (cm3/g) | V Meso/Macropore (cm3/g) | V Total (cm3/g) |
|---|---|---|---|---|
| SiO2 | 18 | - | 0.04 | 0.04 |
| TiO2 P25 | 52 | 0.01 | 0.10 | 0.11 |
| SiO2@TiO2 | 52 | 0.01 | 0.07 | 0.08 |
| SiO2@TiO2 (HT1) | 304 | 0.05 | 0.20 | 0.25 |
| SiO2@TiO2 (HT2) | 394 | 0.07 | 0.23 | 0.30 |
| SiO2@TiO2 (HT1) upscaled | 280 | 0.04 | 0.14 | 0.18 |
| SiO2@TiO2 | |
|---|---|
| SiO2 TiO2 Calcined HT1 HT2 HT1 US 1 MB | |
| 0 min |  |
| 480 min |  |
| Samples | Adsorption (µg/g) |
|---|---|
| SiO2 | 328 |
| TiO2 | 79 |
| SiO2@TiO2 | 502 |
| SiO2@TiO2 (HT1) | 714 |
| SiO2@TiO2 (HT2) | 718 |
| SiO2@TiO2 (HT1) upscaled | 718 |
| # | Material | Exposure Time (min) | Conditions | Efficiency (%) | Ref. |
|---|---|---|---|---|---|
| 1 | SiO2-TiO2 nanoparticles | 30 | Dark | 88.6 | [7] |
| Sunlight | 90 | ||||
| UV light | 85 | ||||
| 120 | Sunlight | 98 | |||
| 2 | SiO2-TiO2 composite | 360 | UV light | 90 | [65] |
| 3 | TiO2–SiO2 hollow nanospheres | 120 | UV light | >90 | |
| 4 | TiO2 prior calcination | 300 | UV light | 7 | [36] |
| TiO2 after calcination | 30 | ||||
| SiO2-TiO2 prior calcination | 43 | ||||
| SiO2-TiO2 after calcination | 76 | ||||
| 5 | SiO2 | 30 | UV light | 45.8 | This work |
| TiO2 | 30.0 | ||||
| SiO2@TiO2 calcined | 77.8 | ||||
| SiO2@TiO2 HT1 upscaled | 97.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, B.R.; Lopes, J.L.; Coelho, L.; Ligonzo, M.; Rigoletto, M.; Magnacca, G.; Deganello, F. Development and Upscaling of SiO2@TiO2 Core-Shell Nanoparticles for Methylene Blue Removal. Nanomaterials 2023, 13, 2276. https://doi.org/10.3390/nano13162276
Gomes BR, Lopes JL, Coelho L, Ligonzo M, Rigoletto M, Magnacca G, Deganello F. Development and Upscaling of SiO2@TiO2 Core-Shell Nanoparticles for Methylene Blue Removal. Nanomaterials. 2023; 13(16):2276. https://doi.org/10.3390/nano13162276
Chicago/Turabian StyleGomes, Bárbara R., Joana L. Lopes, Lorena Coelho, Mattia Ligonzo, Monica Rigoletto, Giuliana Magnacca, and Francesca Deganello. 2023. "Development and Upscaling of SiO2@TiO2 Core-Shell Nanoparticles for Methylene Blue Removal" Nanomaterials 13, no. 16: 2276. https://doi.org/10.3390/nano13162276
APA StyleGomes, B. R., Lopes, J. L., Coelho, L., Ligonzo, M., Rigoletto, M., Magnacca, G., & Deganello, F. (2023). Development and Upscaling of SiO2@TiO2 Core-Shell Nanoparticles for Methylene Blue Removal. Nanomaterials, 13(16), 2276. https://doi.org/10.3390/nano13162276










