An Aqueous Process for Preparing Flexible Transparent Electrodes Using Non-Oxidized Graphene/Single-Walled Carbon Nanotube Hybrid Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Aqueous Non-Oxidized Graphene Solution
2.3. Synthesis of Aqueous SWNT and Non-Oxidized Graphene/SWNT Hybrid Solution
2.4. Fabrication of Flexible Transparent Electrodes
2.5. Characterization
2.6. Device Fabrication and Characteristics
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, W.; Lee, S.; Zhao, K.; Lee, K.; Han, H.; Oh, J.; Lee, H.; Kim, H.; Koo, C.M.; Park, C. Flexible and transparent electrode of hybrid Ti3C2TX MXene–silver nanowires for high-performance quantum dot light-emitting diodes. ACS Nano 2022, 16, 9203–9213. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lu, K.; Xu, Z.; Lin, Z.; Ning, H.; Qiu, T.; Yang, Z.; Zheng, H.; Yao, R.; Peng, J. Recent developments in flexible transparent electrode. Crystals 2021, 11, 511. [Google Scholar] [CrossRef]
- Meng, L.; Wang, W.; Xu, B.; Qin, J.; Zhang, K.; Liu, H. Solution-processed flexible transparent electrodes for printable electronics. ACS Nano 2023, 17, 4180–4192. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Xu, R.; Han, X.; Xu, J.; Hong, W.; Xu, Y.; Fu, Z.; Zhu, H.; Sun, X.; Wang, J.; et al. Ultra-high temperature tolerant flexible transparent electrode with embedded silver nanowires bundle micromesh for electrical heater. npj Flex. Electron. 2022, 6, 48. [Google Scholar] [CrossRef]
- Scardaci, V. Copper nanowires for transparent electrodes: Properties, challenges and applications. Appl. Sci. 2021, 11, 8035. [Google Scholar] [CrossRef]
- Engel, L.F.; González-García, L.; Kraus, T. Flexible and transparent electrodes imprinted from metal nanostructures: Morphology and optoelectronic performance. Nanoscale Adv. 2022, 4, 3370. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.-Y.; Jang, J.; Park, S.; Ji, G.; Lee, S.-H.; Kim, D.-B.; Yoon, K.-H.; Chung, C.-M.; Cho, S. Flexible and transparent electrode based on Ag-nanowire embedded colorless poly(amide-imide). Nanomaterials 2022, 12, 1457. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, W. Flexible and stretchable carbon-based sensors and actuators for soft robots. Nanomaterials 2023, 13, 316. [Google Scholar] [CrossRef]
- Das, H.T.; Dutta, S.; Balaji, T.E.; Das, N.; Das, P.; Dheer, N.; Kanojia, R.; Ahuja, P.; Ujjain, S.K. Recent trends in carbon nanotube electrodes for flexible supercapacitors: A review of smart energy storage device assembly and performance. Chemosensors 2022, 10, 223. [Google Scholar] [CrossRef]
- Ding, E.-X.; Liu, P.; Yoon, H.H.; Ahmed, F.; Du, M.; Shafi, A.M.; Mehmood, N.; Kauppinen, E.I.; Sun, Z.; Lipsanen, H. Highly sensitive MoS2 photodetectors enabled with a dry-transferred transparent carbon nanotube electrode. ACS Appl. Mater. Interfaces 2023, 15, 4216–4225. [Google Scholar] [CrossRef]
- Cui, T.-R.; Li, D.; Huang, X.-R.; Yan, A.-Z.; Dong, Y.; Xu, J.-D.; Guo, Y.-Z.; Wang, Y.; Chen, Z.-K.; Shao, W.-C.; et al. Graphene-based flexible electrode for electrocardiogram signal monitoring. Appl. Sci. 2022, 12, 4526. [Google Scholar] [CrossRef]
- Miao, J.; Fan, T. Flexible and stretchable transparent conductive graphene-based electrodes for emerging wearable electronics. Carbon 2023, 202, 495–527. [Google Scholar] [CrossRef]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhi, L.; Müllen, K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef]
- King, P.J.; Khan, U.; Lotya, M.; De, S.; Coleman, J.N. Improvement of transparent conducting nanotube films by addition of small quantities of graphene. ACS Nano 2010, 4, 4238–4246. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Min, D.-H. Durable large-area thin films of graphene/carbon nanotube double layers as a transparent electrode. Langmuir 2009, 25, 11302–11306. [Google Scholar] [CrossRef]
- Tung, V.C.; Chen, L.-M.; Allen, M.J.; Wassei, J.K.; Nelson, K.; Kaner, R.B.; Yang, Y. Low-temperature solution processing of graphene-carbon nanotube hybrid materials for high-performance transparent conductors. Nano Lett. 2009, 9, 1949–1955. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, B.; Lin, X.; Shen, X.; Yousefi, N.; Huang, Z.-D.; Li, Z.; Kim, J.-K. Highly transparent and conducting ultralarge graphene oxide/single-walled carbon nanotube hybrid films produced by Langmuir–Blodgett assembly. J. Mater. Chem. 2012, 22, 25072–25082. [Google Scholar] [CrossRef]
- Gómez, I.J.; Sulleiro, M.V.; Mantione, D.; Alegret, N. Carbon nanomaterials embedded in conductive polymers: A state of the art. Polymers 2021, 13, 745. [Google Scholar] [CrossRef]
- Jin, S.W.; Lee, Y.H.; Yeom, K.M.; Yun, J.; Park, H.; Jeong, Y.R.; Hong, S.Y.; Lee, G.; Oh, S.Y.; Lee, J.H.; et al. Highly durable and flexible transparent electrode for flexible optoelectronic applications. ACS Appl. Mater. Interfaces 2018, 10, 30706–30715. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [PubMed]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fulvio, P.F.; Baker, G.A.; Veith, G.M.; Unocic, R.R.; Mahurin, S.M.; Chi, M.; Dai, S. Direct exfoliation of natural graphite into micrometre size few layers graphene sheets using ionic liquids. Chem. Commun. 2010, 46, 4487–4489. [Google Scholar] [CrossRef]
- Du, W.; Jiang, X.; Zhu, L. From graphite to graphene: Direct liquid-phase exfoliation of graphite to produce single- and few-layered pristine graphene. J. Mater. Chem. A 2013, 1, 10592–10606. [Google Scholar] [CrossRef]
- Bagri, A.; Mattevi, C.; Acik, M.; Chabal, Y.J.; Chhowalla, M.; Shenoy, V.B. Structural evolution during the reduction of chemically derived graphene oxide. Nat. Chem. 2010, 2, 581–587. [Google Scholar] [CrossRef]
- Shih, C.-J.; Vijayaraghavan, A.; Krishnan, R.; Sharma, R.; Han, J.-H.; Ham, M.-H.; Jin, Z.; Lin, S.; Paulus, G.L.C.; Reuel, N.F.; et al. Bi- and trilayer graphene solutions. Nat. Nanotechnol. 2011, 6, 439–445. [Google Scholar] [CrossRef]
- Lin, S.; Blankschtein, D. Role of the bile salt surfactant sodium cholate in enhancing the aqueous dispersion stability of single-walled carbon nanotubes: A molecular dynamics simulation study. J. Phys. Chem. B 2010, 114, 15616–15625. [Google Scholar] [CrossRef]
- Hsieh, A.G.; Korkut, S.; Punckt, C.; Aksay, I.A. Dispersion stability of functionalized graphene in aqueous sodium dodecyl sulfate solutions. Langmuir 2013, 29, 14831–14838. [Google Scholar] [CrossRef]
- Cunha, R.; Paupitz, R.; Yoon, K.; Duin, A.C.T.V.; Elías, A.L.; Carozo, V.; Dasgupta, A.; Fujisawa, K.; Lopez, N.P.; Araujo, P.T.; et al. Raman spectroscopy revealing noble gas adsorption on single-walled carbon nanotube bundles. Carbon 2018, 127, 312–319. [Google Scholar] [CrossRef]
- Son, M.; Chee, S.-S.; Kim, S.-Y.; Lee, W.; Kim, Y.H.; Oh, B.-Y.; Hwang, J.Y.; Lee, B.H.; Ham, M.-H. High-quality nitrogen-doped graphene films synthesized from pyridine via two-step chemical vapor deposition. Carbon 2020, 159, 579–585. [Google Scholar] [CrossRef]
- Son, M.; Jang, J.; Kim, G.-H.; Lee, J.-H.; Chun, D.W.; Bae, J.-H.; Kim, I.S.; Ham, M.-H.; Chee, S.-S. Large-area Bernal-stacked bilayer graphene film on a uniformly rough Cu surface via chemical vapor deposition. ACS Appl. Electron. Mater. 2021, 3, 2497–2503. [Google Scholar] [CrossRef]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Ki, H.; Kang, Y.; Son, M.; Auxilia, F.M.; Seo, H.; Kim, I.-H.; Kim, K.-H.; Park, K.-H.; Kim, Y.; et al. Chemically prelithiated graphene for anodes of Li-ion batteries. Energy Fuels 2020, 34, 13048–13055. [Google Scholar] [CrossRef]
- Yang, C.-R.; Tseng, S.-F.; Chen, Y.-T. Characteristics of graphene oxide films reduced by using an atmospheric plasma system. Nanomaterials 2018, 8, 802. [Google Scholar] [CrossRef]
- Geng, H.-Z.; Kim, K.K.; So, K.P.; Lee, Y.S.; Chang, Y.; Lee, Y.H. Effect of acid treatment on carbon nanotube-based flexible transparent conducting films. J. Am. Chem. Soc. 2007, 129, 7758–7759. [Google Scholar] [CrossRef]
- Tenent, R.C.; Barnes, T.M.; Bergeson, J.D.; Ferguson, A.J.; To, B.; Gedvilas, L.M.; Heben, M.J.; Blackburn, J.L. Ultrasmooth, large-area, high-uniformity, conductive transparent single-walled-carbon-nanotube films for photovoltaics produced by ultrasonic spraying. Adv. Mater. 2009, 21, 3210–3216. [Google Scholar] [CrossRef]
- Ault, A.P.; Zhao, D.; Ebben, C.J.; Tauber, M.J.; Geiger, F.M.; Prather, K.A.; Grassian, V.H. Raman microspectroscopy and vibrational sum frequency generation spectroscopy as probes of the bulk and surface compositions of size-resolved sea spray aerosol particles. Phys. Chem. Chem. Phys. 2013, 15, 6206. [Google Scholar] [CrossRef]
- Bauhofer, W.; Kovacs, J.Z. A review and analysis of electrical percolation in carbon nanotube polymer composites. Compos. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Ning, H.; Zeng, X.; Zhang, H.; Zhang, X.; Yao, R.; Liu, X.; Luo, D.; Xu, Z.; Ye, Q.; Peng, J. Transparent flexible IGZO thin film transistors fabricated at room temperature. Membranes 2022, 12, 29. [Google Scholar] [CrossRef]
- Li, Y.; Yao, R.; Wang, H.; Wu, X.; Wu, J.; Wu, X.; Qin, W. Enhanced performance in Al-doped ZnO based transparent flexible transparent thin-film transistors due to oxygen vacancy in ZnO film with Zn−Al−O interfaces fabricated by atomic layer deposition. ACS Appl. Electron. Mater. 2017, 9, 11711–11720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-R.; Huang, C.-Y.; Li, G.-M.; Zhou, L.; Wu, W.-J.; Xu, M.; Wang, L.; Ning, H.-L.; Yao, R.-H.; Peng, J.-B. A Low-Power High-Stability Flexible Scan Driver Integrated by IZO TFTs. IEEE Trans. Electron. Devices 2016, 63, 1779–1782. [Google Scholar] [CrossRef]
- Tiwari, N.; Rajput, M.; John, R.A.; Kulkarni, M.; Nguyen, A.C.; Mathews, N. Indium tungsten oxide thin films for flexible high performance transistors and neuromorphic electronics. ACS Appl. Electron. Mater. 2018, 10, 30506–30513. [Google Scholar] [CrossRef] [PubMed]
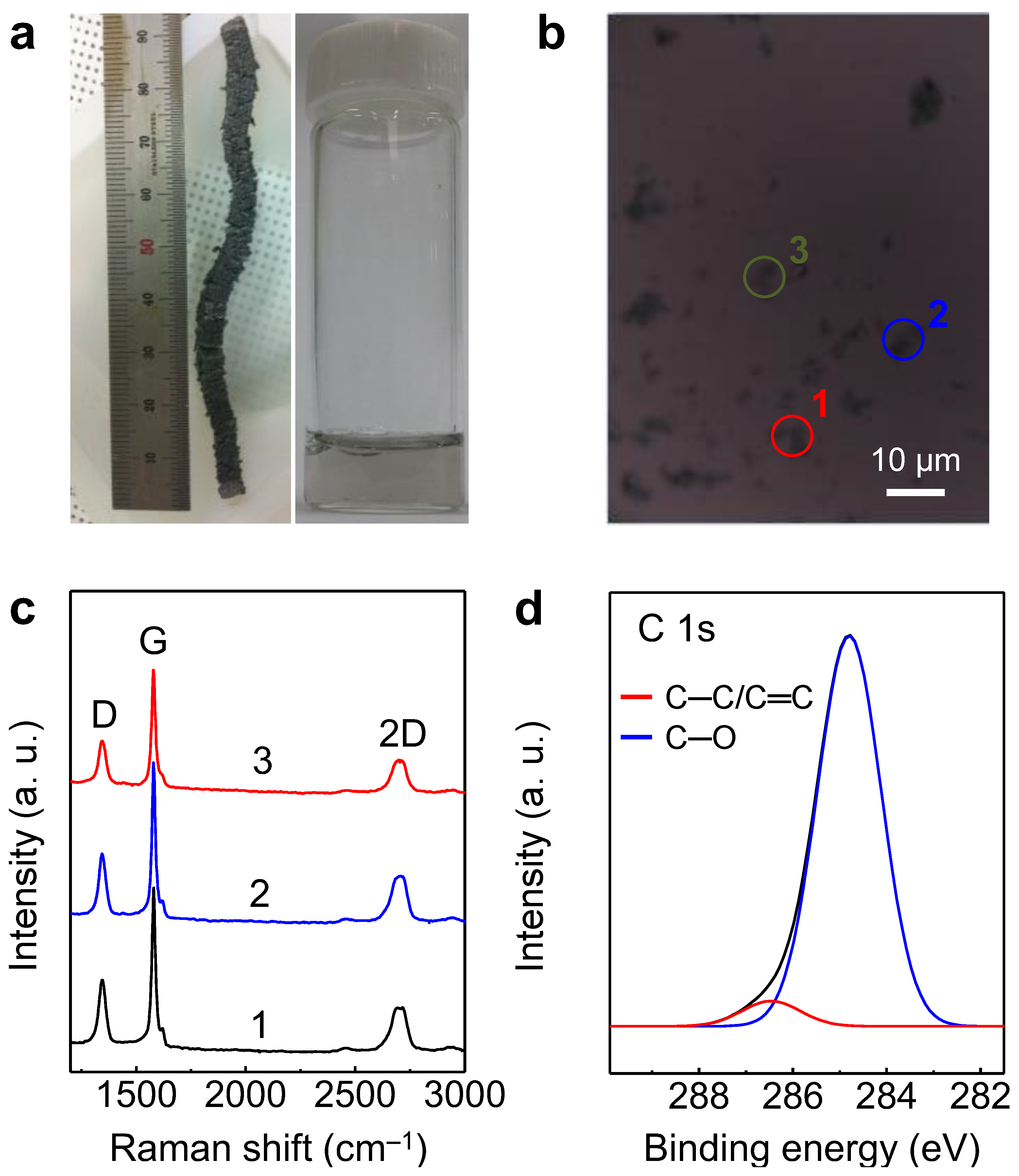
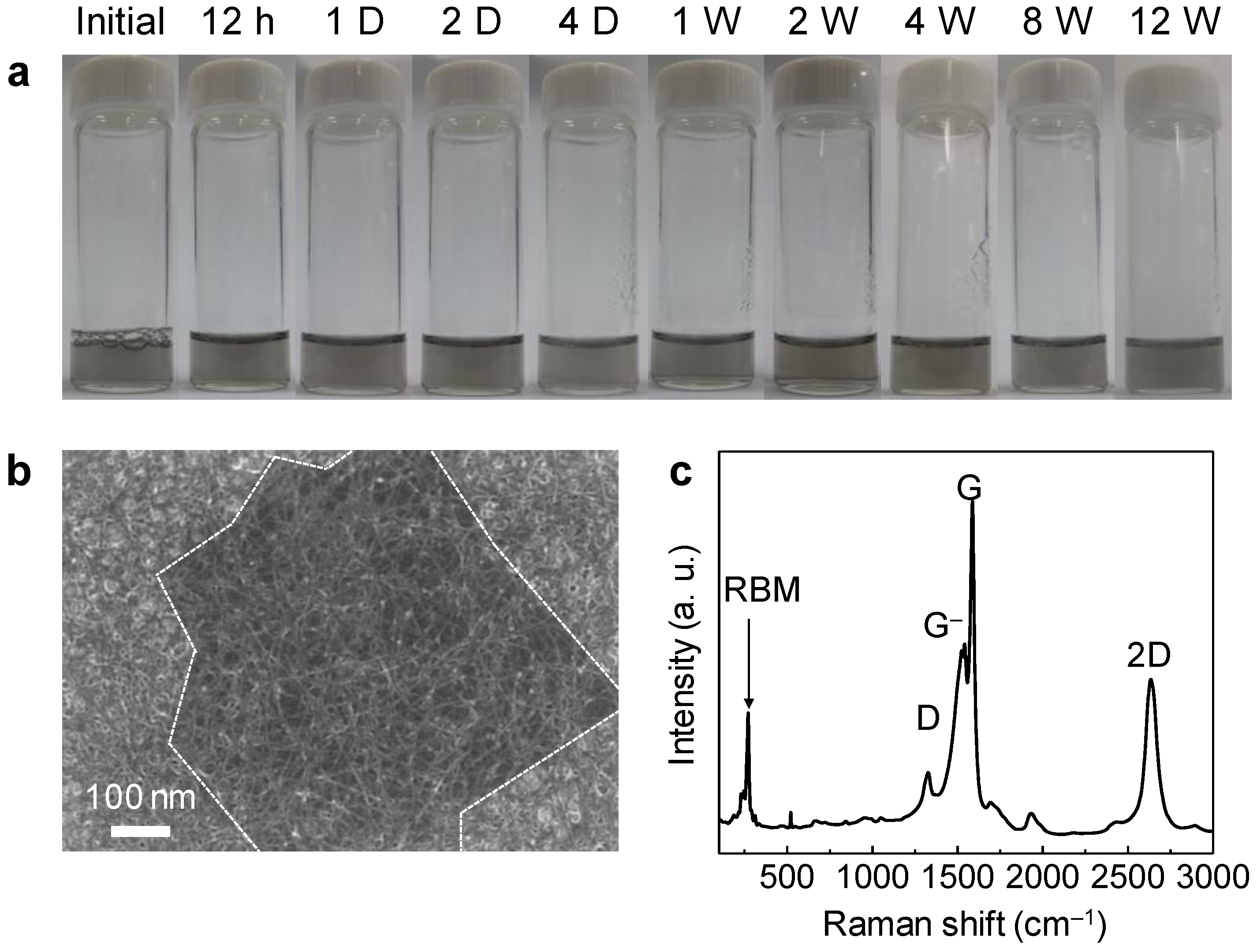

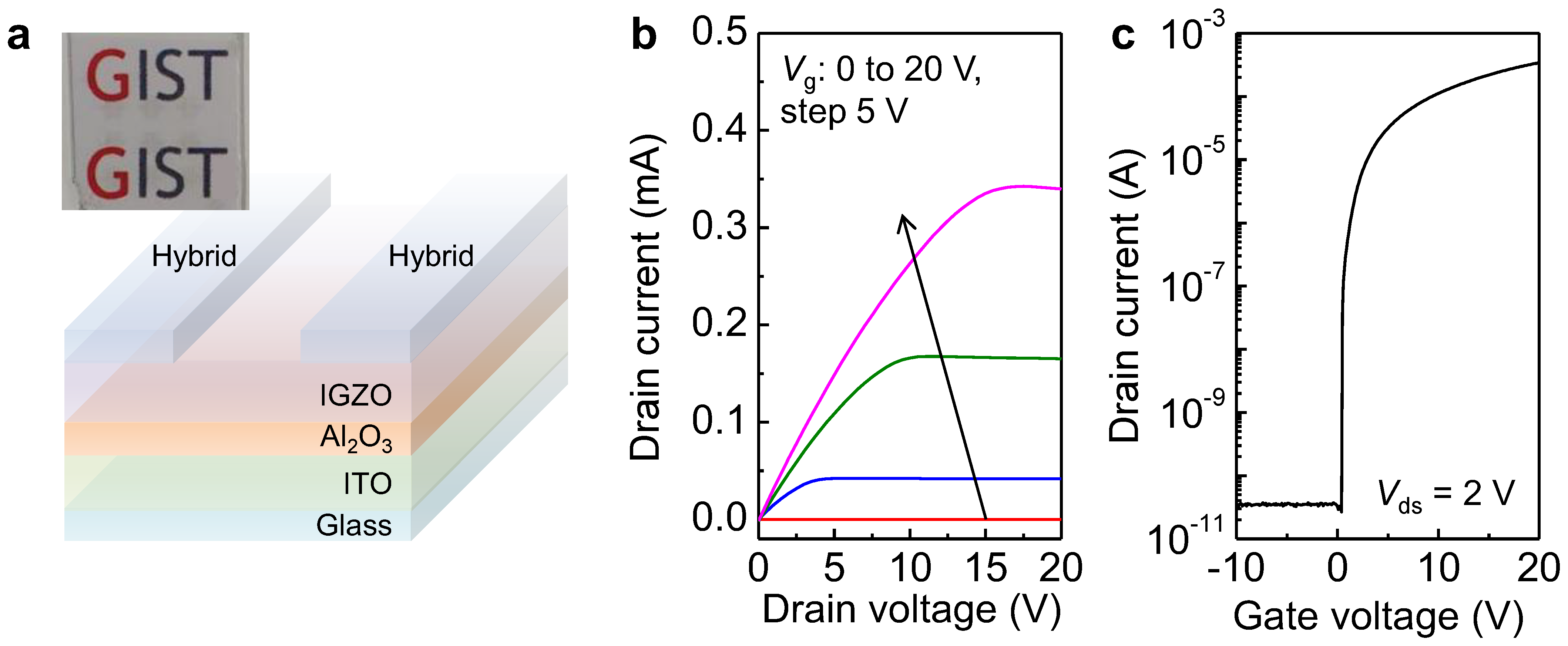
| Materials | Graphene Synthesis | Deposition | Transparency (%) | Sheet Resistance (Ω/sq.) | Ref. |
|---|---|---|---|---|---|
| RGO | Hummers’ | Spin-coating | 80 | 100–1000 | [13] |
| RGO | Hummers’ | Filtration | 95 | 43,000 | [14] |
| RGO | Hummers’ | Dip-coating | 70 | 1800 | [15] |
| Graphene/SWNT | Hummers’ | Filtration | 80 | 100 | [16] |
| RGO on MWNT | Hummers’ | Electrostatic adsorption | 93 | 151,000 | [17] |
| Chemically converted graphene/SWNT | Hummers’ | Spin-coating | 92 | 636 | [18] |
| Ultra-large GO/SWNT | Hummers’ | Langmuir–Blodgett | 77–86 | 180–560 | [19] |
| Non-oxidized graphene/SWNT | Halogen intercalation | Spray coating | 90 | 3500 | This work |
| S/D Electrodes | Deposition | Channel Layers | Dielectric | Gate Electrode | Mobility (cm2 V−1 s−1) | Ion/Ioff | SS (V/Decade) | Ref. |
|---|---|---|---|---|---|---|---|---|
| AZO | PLD | IGZO/Al2O3 | Al2O3 | AZO | 5.61 | 3.05 × 105 | 0.594 | [40] |
| ZnO/AZO | ALD | ZnO | Al2O3 | ZnO/AZO | 2 | ~107 | 1.4 | [41] |
| Mo | Sputtering | IZO | Al2O3 | Al | 6.32 | 9.7 × 107 | 0.39 | [42] |
| ITO | Sputtering | IWO | Al2O3 | Ti | 24.86 | ~105 | 0.28 | [43] |
| Non-oxidized graphene/SWNT | Spray coating | IGZO | Al2O3 | ITO | ~6.7 | ~107 | 0.122 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, M.J.; Son, G.-C.; Kim, M.; Jeon, J.; Kim, Y.H.; Son, M. An Aqueous Process for Preparing Flexible Transparent Electrodes Using Non-Oxidized Graphene/Single-Walled Carbon Nanotube Hybrid Solution. Nanomaterials 2023, 13, 2249. https://doi.org/10.3390/nano13152249
Oh MJ, Son G-C, Kim M, Jeon J, Kim YH, Son M. An Aqueous Process for Preparing Flexible Transparent Electrodes Using Non-Oxidized Graphene/Single-Walled Carbon Nanotube Hybrid Solution. Nanomaterials. 2023; 13(15):2249. https://doi.org/10.3390/nano13152249
Chicago/Turabian StyleOh, Min Jae, Gi-Cheol Son, Minkook Kim, Junyoung Jeon, Yong Hyun Kim, and Myungwoo Son. 2023. "An Aqueous Process for Preparing Flexible Transparent Electrodes Using Non-Oxidized Graphene/Single-Walled Carbon Nanotube Hybrid Solution" Nanomaterials 13, no. 15: 2249. https://doi.org/10.3390/nano13152249
APA StyleOh, M. J., Son, G.-C., Kim, M., Jeon, J., Kim, Y. H., & Son, M. (2023). An Aqueous Process for Preparing Flexible Transparent Electrodes Using Non-Oxidized Graphene/Single-Walled Carbon Nanotube Hybrid Solution. Nanomaterials, 13(15), 2249. https://doi.org/10.3390/nano13152249






