Characterization of Chiral Nanostructured Surfaces Made via Colloidal Lithography
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Fabrication
2.1.1. Particle Synthesis
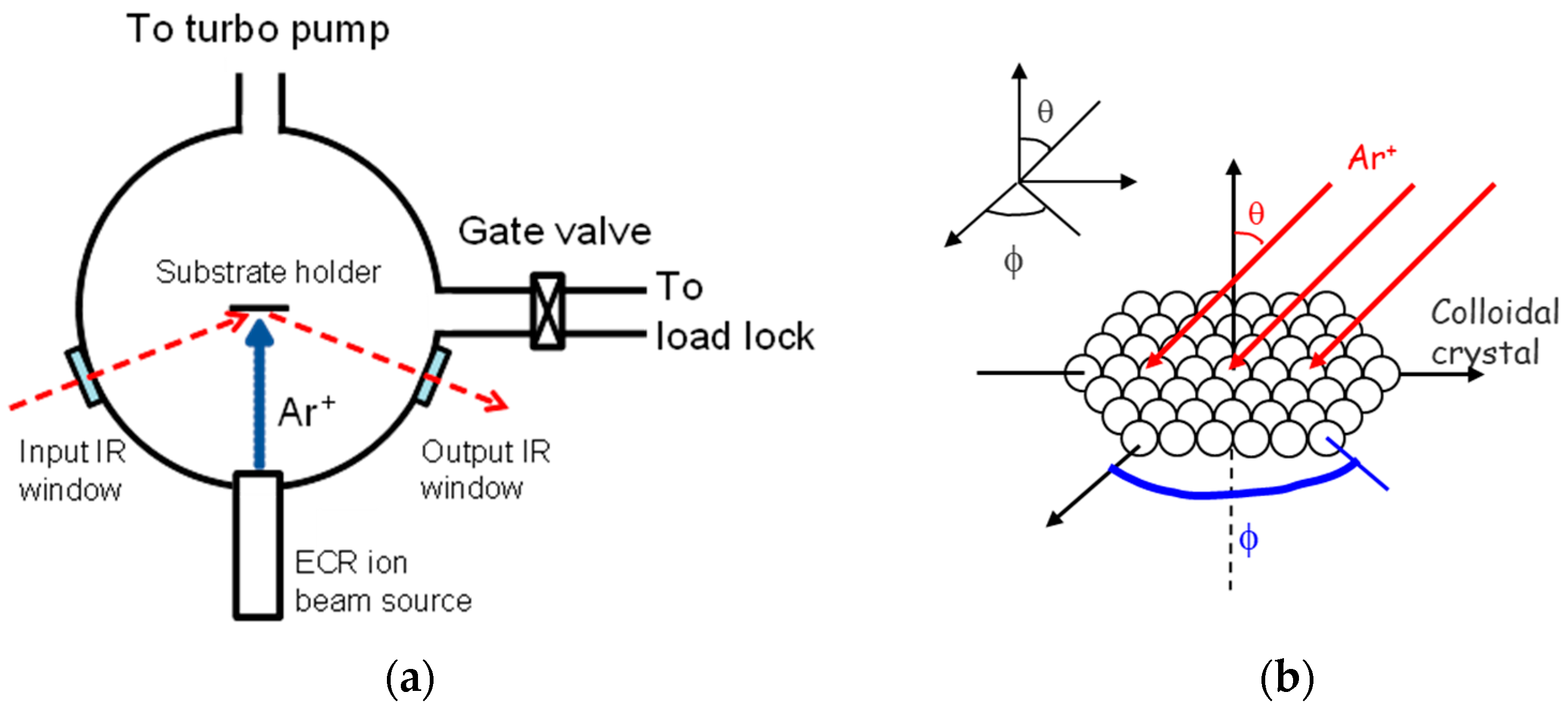
2.1.2. Colloidal Crystal Deposition
2.1.3. Directional Ion Beam Etching
2.1.4. DC Sputtering of Gold Coating
2.2. Characterization
2.2.1. SEM and Confocal Microscopy
2.2.2. Polarimetry
3. Results and Discussion
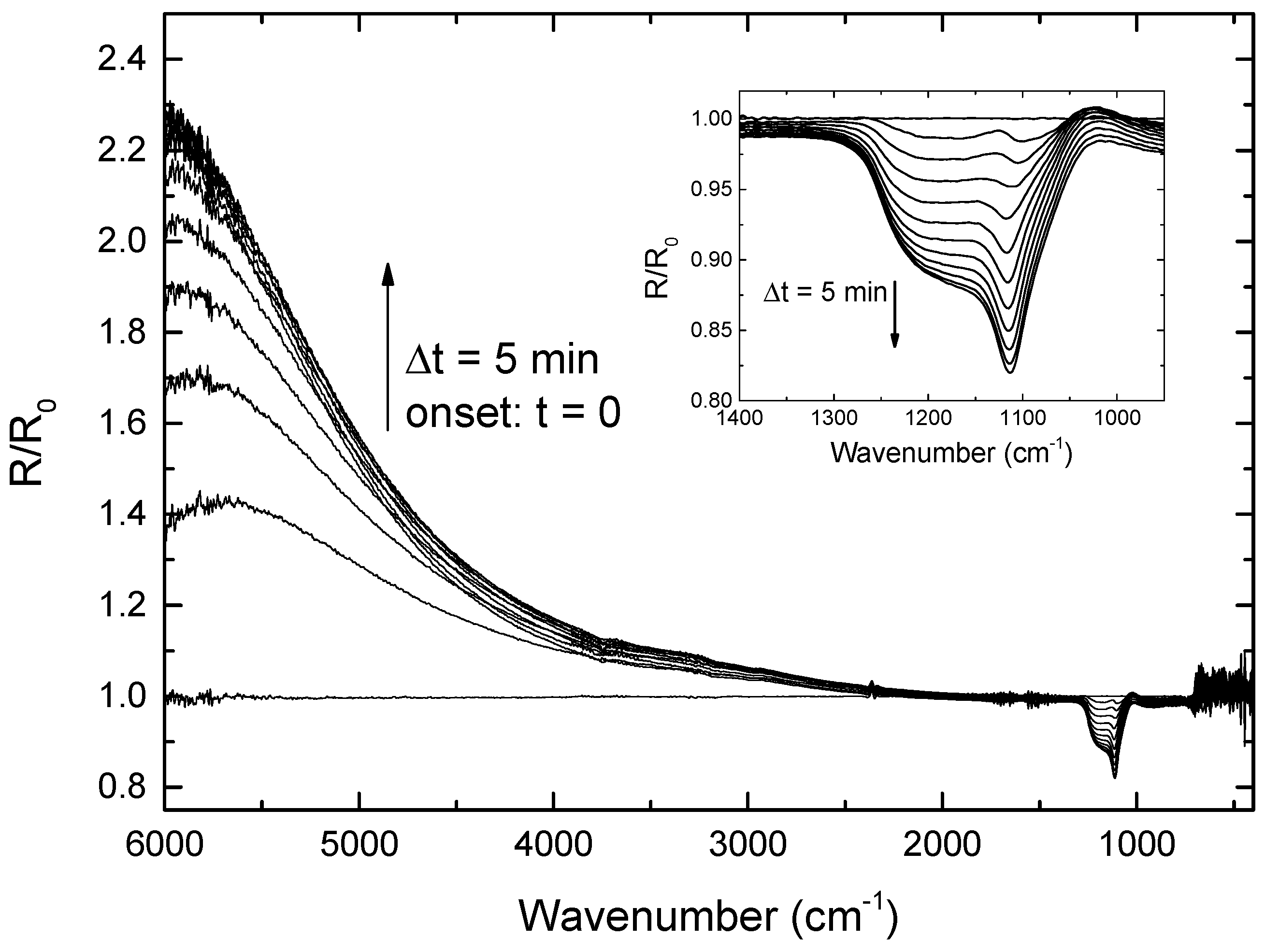
3.1. Etching Process
3.1.1. Ion Bombardment Steps
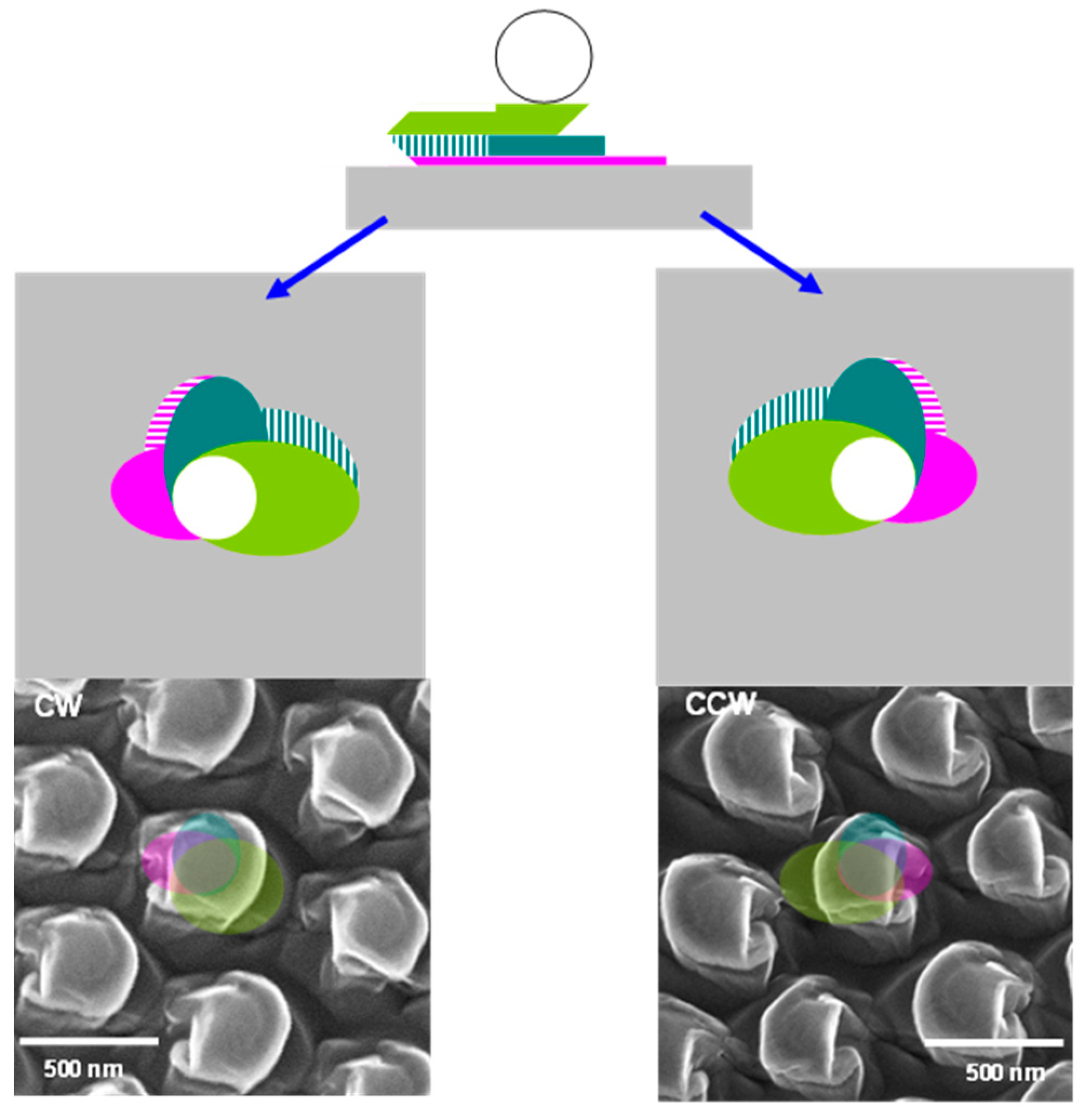
- The pillar is formed in the shadow of the etched particle (Figure 2b). Then, the crystal is rotated by ±90° around the azimuthal normal in a clockwise (CW) or counterclockwise (CCW) direction, respectively.
- Two pillars (principal and secondary), orthogonal to the first, are carved in the shadow of the etched particle and first pillar (Figure 2c1,c2). The crystal is rotated by another azimuth in its plane in the same sense as in the first rotation.
- Two other pillars (principal and secondary) are formed, whose directions are aligned with that of the first pillar in the shadows of the etched particle and the second pillar (Figure 2d1,d2).
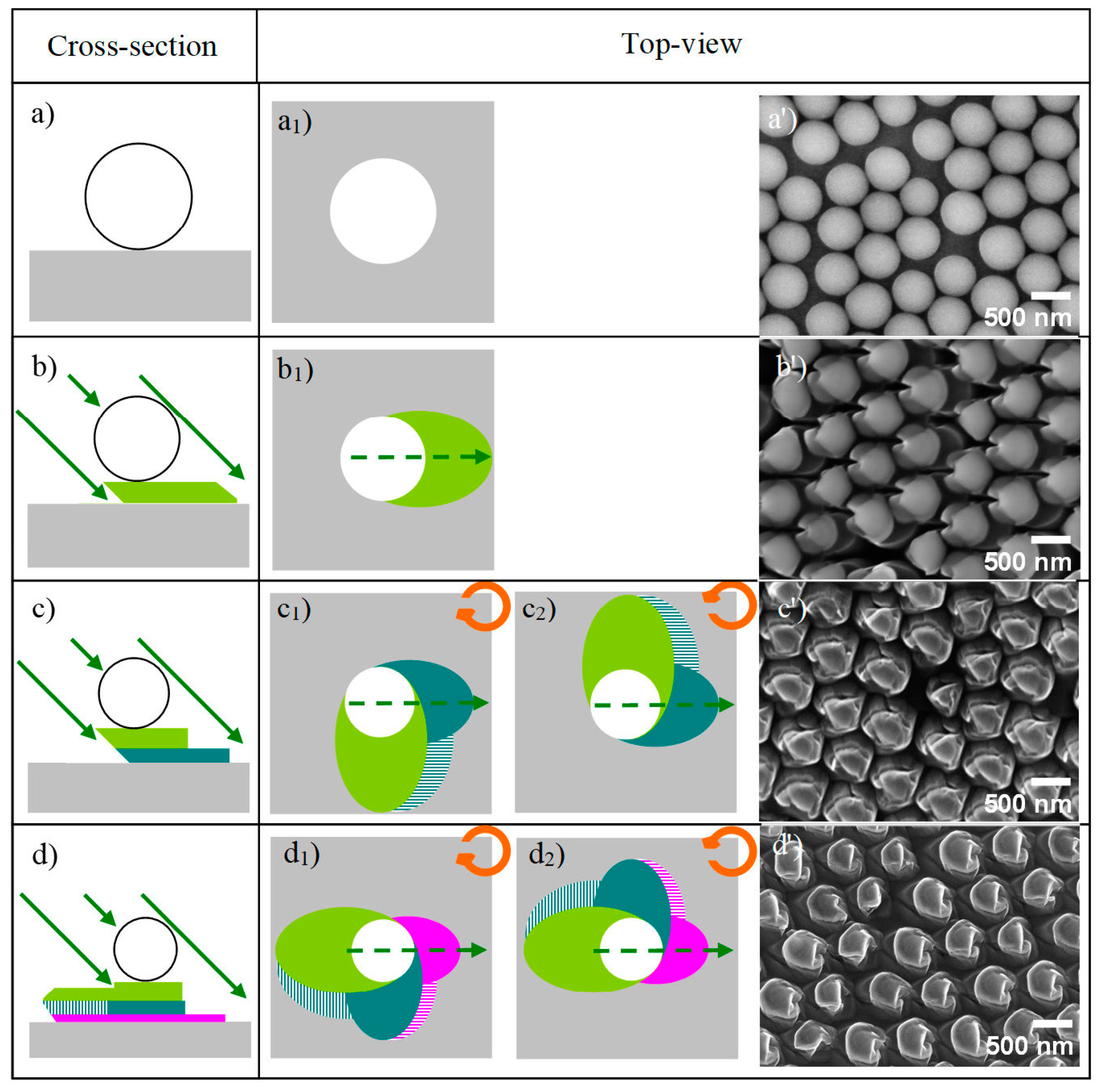
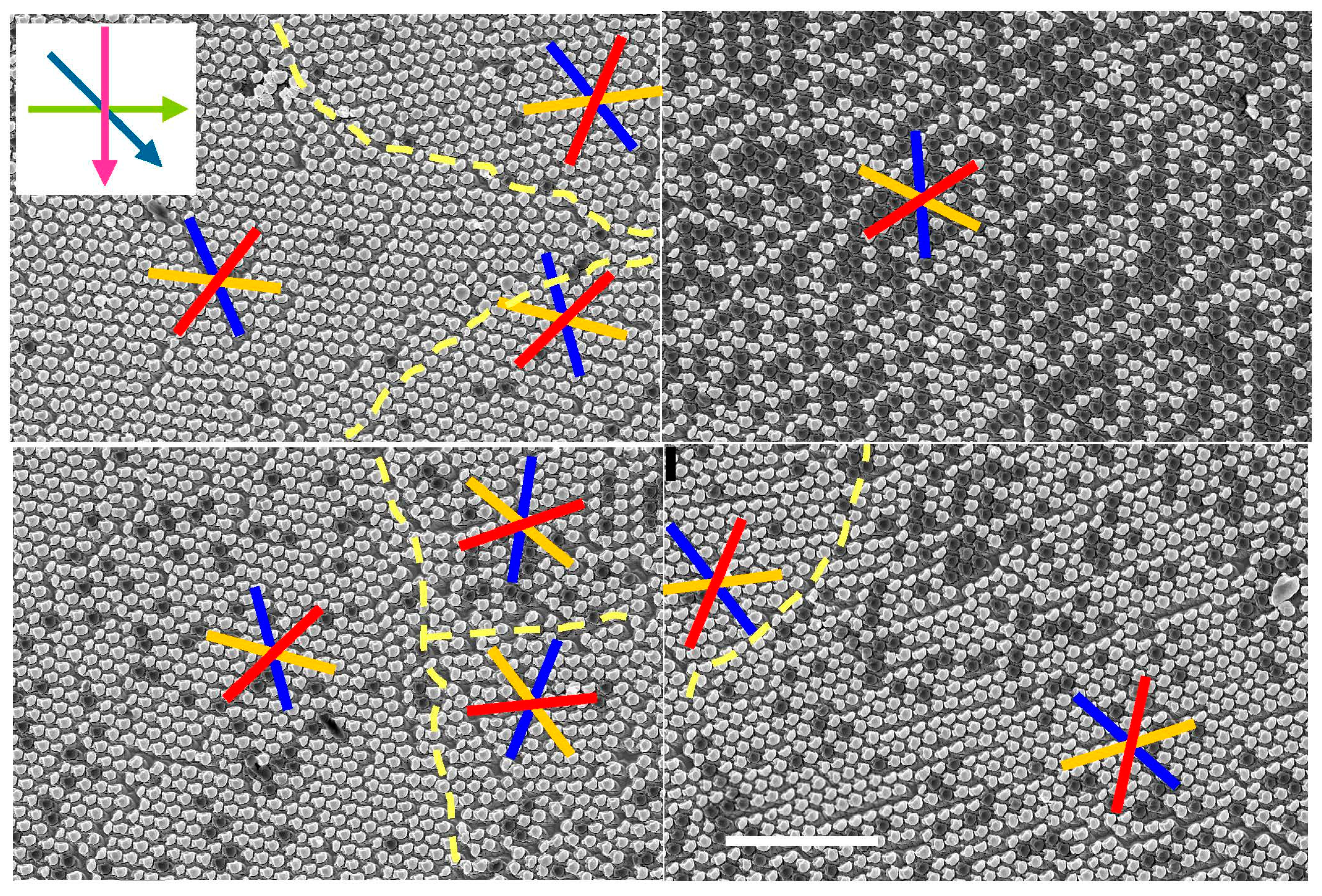
3.1.2. Enantiomorphous Structures
3.1.3. Surface Chemistry
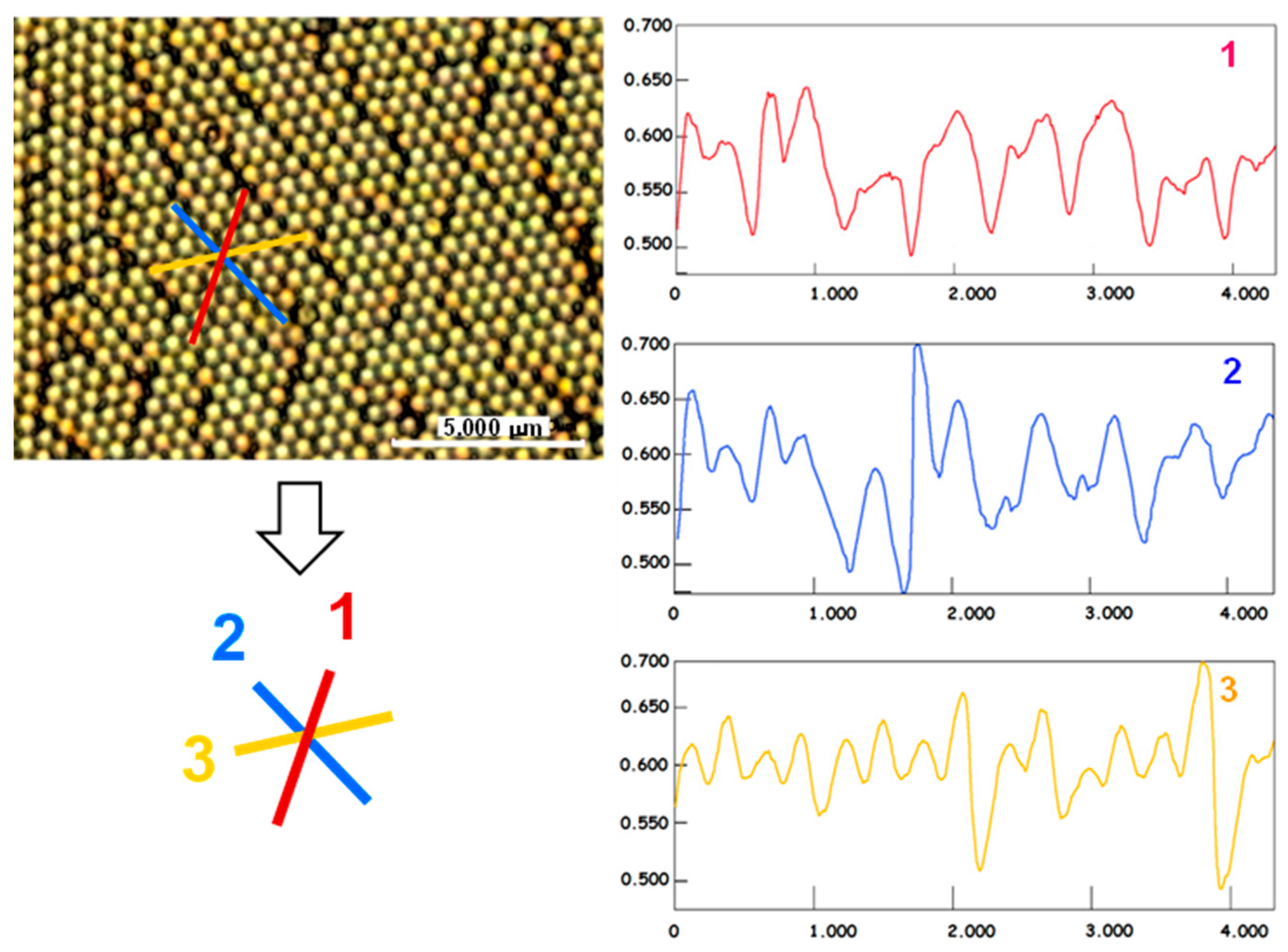
3.2. Structure and Topography
3.3. Optical Properties
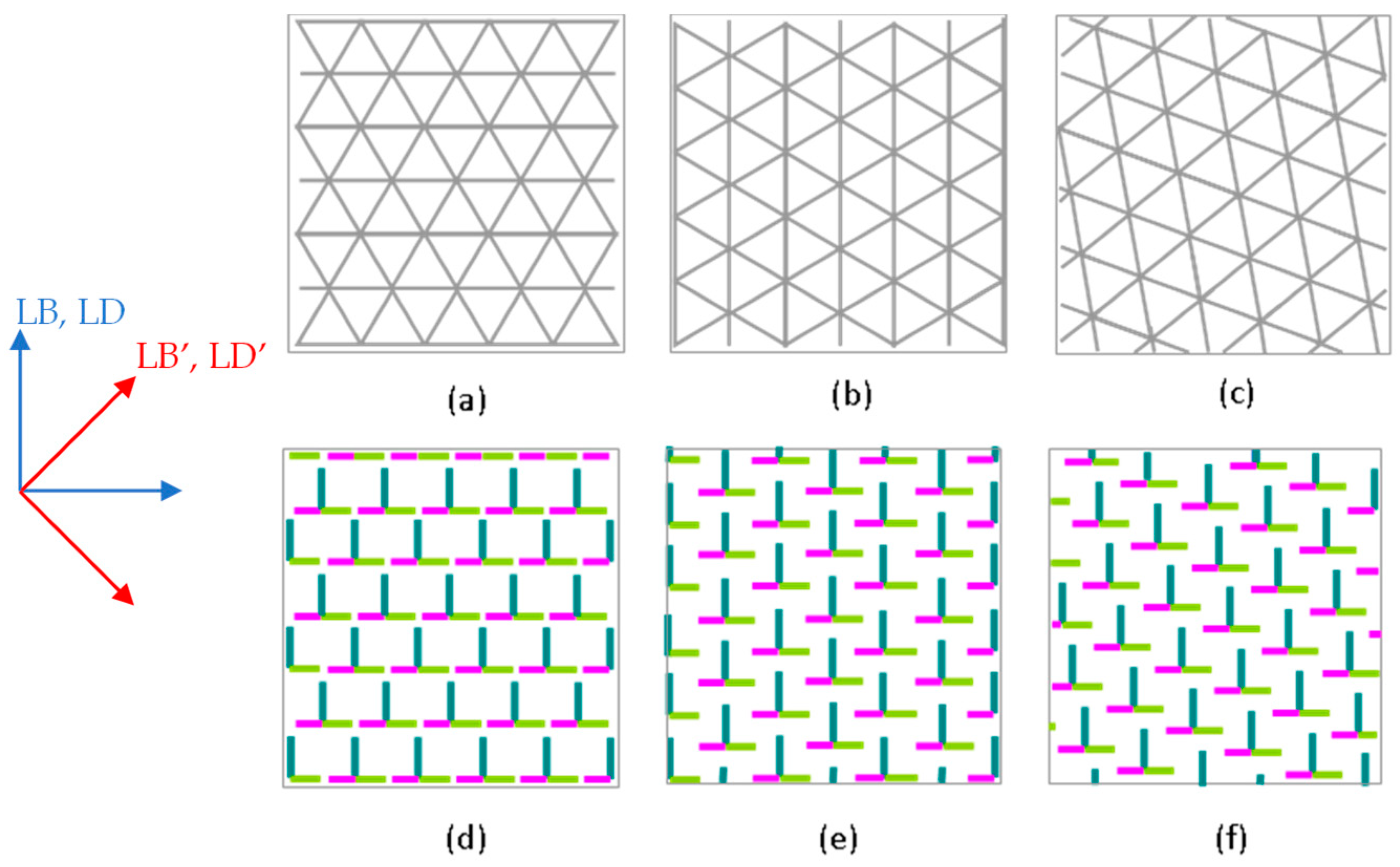
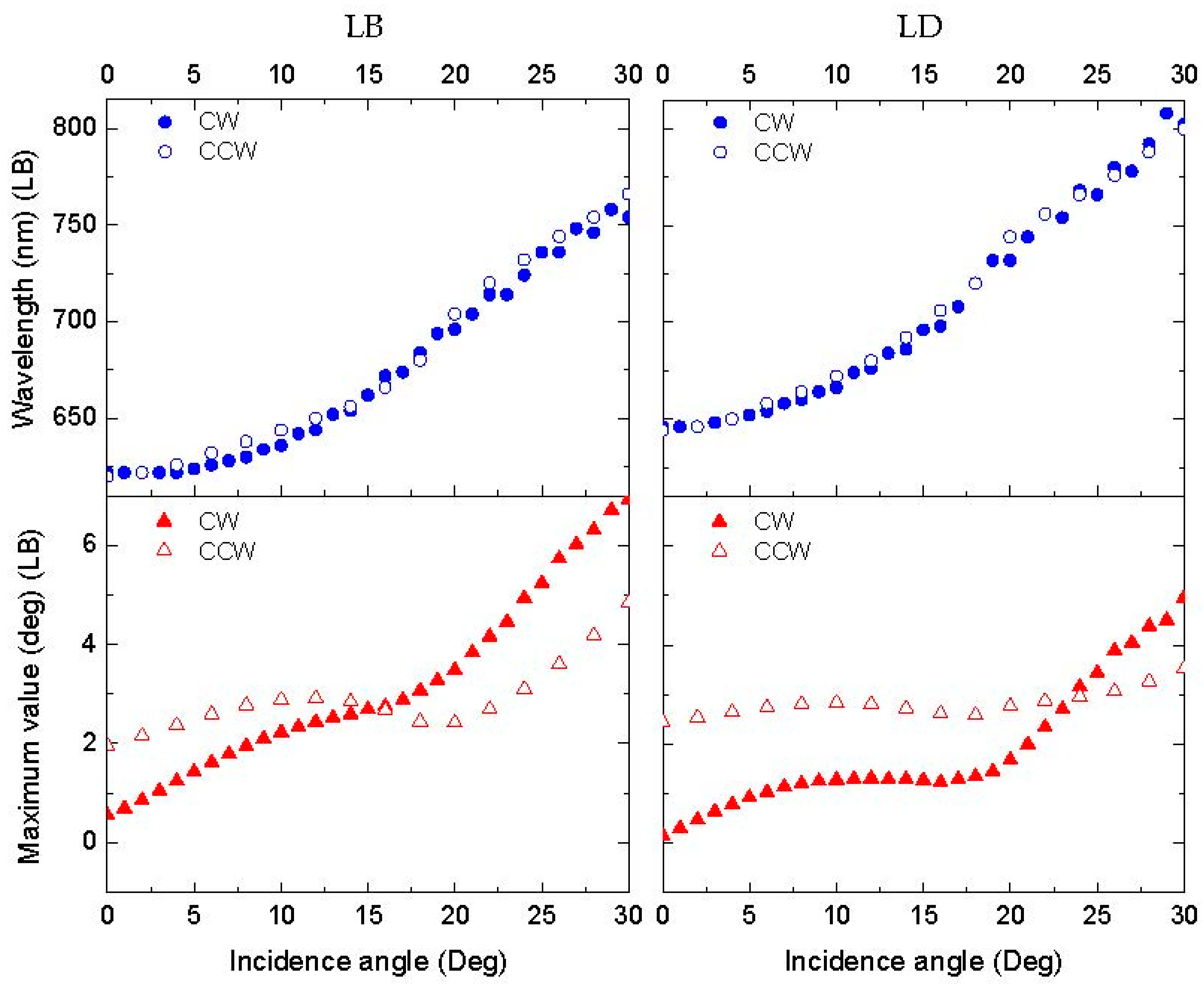
3.3.1. Linear Anisotropy Parameters
3.3.2. Circular Anisotropy Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chipman, R.A.; Lam, W.-S.T.; Young, G. Polarized Light and Optical Systems; Taylor & Francis Group: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Sacanna, S.; Pine, D.J. Shape-anisotropic colloids: Building blocks for complex assemblies. Curr. Opin. Coll. Interface Sci. 2011, 16, 96–105. [Google Scholar] [CrossRef]
- Zhang, W.; Potts, A.; Bagnall, D.M.; Davidson, B.R. Large area all-dielectric planar chiral metamaterials by electron beam lithography. J. Vac. Sci. Technol. B 2006, 24, 1455–1459. [Google Scholar] [CrossRef]
- Potts, A.; Papakostas, A.; Zheludev, N.I.; Coles, H.J.; Greef, R.; Bagnall, D.M. Planar chiral metamaterials for photonic devices. J. Mater. Sci. Mater. Electron. 2003, 14, 393–395. [Google Scholar] [CrossRef]
- Potts, A.; Papakostas, A.; Bagnall, D.M.; Zheludev, N.I. Planar chiral meta-materials for optical applications. Microelectron. Eng. 2004, 73–74, 367–371. [Google Scholar] [CrossRef]
- Lu, B.-R.; Wan, J.; Shu, Z.; Xie, S.-Q.; Chen, Y.; Huq, E.; Qu, X.-P.; Liu, R. Metallic and dielectric photonic crystals with chiral elements by combined nanoimprint and reversal lithography in SU-8. Microelectron. Eng. 2009, 86, 619–621. [Google Scholar] [CrossRef]
- Raub, A.K.; Brueck, S.R.J. Large area 3D helical photonic crystals. J. Vac. Sci. Technol. B 2011, 29, 06FF02. [Google Scholar] [CrossRef]
- Retsch, M.; Tamm, M.; Bocchio, N.; Horn, N.; Förch, R.; Jonas, U.; Kreiter, M. Parallel Preparation of Densely Packed Arrays of 150-nm Gold-Nanocrescent Resonators in Three Dimensions. Small 2009, 5, 2105–2110. [Google Scholar] [CrossRef]
- Oliveira, R.D.; Mouquinho, A.; Centeno, P.; Alexandre, M.; Haque, S.; Martins, R.; Fortunato, E.; Aguas, H.; Mendes, M.J. Colloidal Lithography for Photovoltaics: An Attractive Route for Light Management. Nanomaterials 2021, 11, 1665. [Google Scholar] [CrossRef]
- Frank, B.; Yin, X.; Schäferling, M.; Zhao, J.; Hein, S.M.; Braun, P.V.; Giessen, H. Large-Area 3D Chiral Plasmonic Structures. ACS Nano 2013, 7, 6321–6329. [Google Scholar] [CrossRef]
- Ogier, R.; Fang, Y.; Svedendahl, M.; Johansson, P.; Käll, M. Macroscopic Layers of Chiral Plasmonic Nanoparticle Oligomers from Colloidal Lithography. ACS Photonics 2014, 1, 1074–1081. [Google Scholar] [CrossRef]
- Shao, L.; Zheng, J. Fabrication of plasmonic nanostructures by hole-mask colloidal lithography: Recent development. Appl. Mater. Today 2019, 15, 6–17. [Google Scholar] [CrossRef]
- Yang, Y.C.; Sheu, J.-K.; Lee, M.-L.; Yen, C.H.; Lai, W.-C.; Hon, S.J.; Ko, T.K. Vertical InGaN light-emitting diode with a retained patterned sapphire layer. Opt. Express 2012, 20, A1019–A1025. [Google Scholar] [CrossRef]
- Bochenkov, V.E.; Sutherland, D.S. Chiral plasmonic nanocrescents: Large-area fabrication and optical properties. Opt. Express 2018, 26, 27101–27108. [Google Scholar] [CrossRef] [PubMed]
- Klös, G.; Miola, M.; Sutherland, D.S. Increased Refractive Index Sensitivity by Circular Dichroism Sensing through Reduced Substrate Effect. J. Phys. Chem. C 2019, 123, 7347–7355. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, Z.; Ai, B.; Chen, C.; Zhang, W.; Wang, Y.; Zhang, G. Chiral Plasmonic Metamaterials with Tunable Chirality. ACS Appl. Mater. Interfaces 2020, 12, 50192–50202. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, M.; Schäferling, M.; Duan, X.; Giessen, H.; Liu, N. Chiral plasmonics. Sci. Adv. 2017, 3, e1602735. [Google Scholar] [CrossRef] [PubMed]
- Goerlitzer, E.S.A.; Puri, A.S.; Moses, J.J.; Poulikakos, L.V.; Vogel, N. The Beginner’s Guide to Chiral Plasmonics: Mostly Harmless Theory and the Design of Large-Area Substrates. Adv. Opt. Mater. 2021, 9, 2100378. [Google Scholar] [CrossRef]
- Wu, W.; Pauly, M. Chiral plasmonic nanostructures: Recent advances in their synthesis and applications. Mater. Adv. 2022, 3, 186–215. [Google Scholar] [CrossRef]
- Ruffato, G.; Romanato, F. Grating-coupled surface plasmon resonance in conical mounting with polarization modulation. Opt. Lett. 2012, 37, 2718–2720. [Google Scholar] [CrossRef]
- Hendry, E.; Carpy, T.; Johnston, J.; Popland, M.; Mikhaylovskiy, R.V.; Lapthorn, A.J.; Kelly, S.M.; Barron, L.D.; Gadegaard, N.; Kadodwala, M. Ultrasensitive detection and characterization of biomolecules using superchiral fields. Nat. Nanotechnol. 2010, 5, 783–787. [Google Scholar] [CrossRef]
- Portal, S.; Rubio-Roy, M.; Corbella, C.; Vallvé, M.A.; Ignes-Mullol, J.; Bertran, E. Influence of incident ion beam angle on dry etching of silica sub-micron particles deposited on Si substrates. Thin Solid Films 2009, 518, 1543–1548. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Portal, S.; Vallvé, M.A.; Arteaga, O.; Ignés-Mullol, J.; Canillas, A.; Bertran, E. Optical characterization of colloidal crystals based on dissymmetric metal-coated oxide submicrospheres. Thin Solid Films 2008, 517, 1053–1057. [Google Scholar] [CrossRef]
- Takeuchi, T.; Corbella, C.; Grosse-Kreul, S.; von Keudell, A.; Ishikawa, K.; Kondo, H.; Takeda, K.; Sekine, M.; Hori, M. Development of the sputtering yields of ArF photoresist after the onset of argon ion bombardment. J. Appl. Phys. 2013, 113, 014306. [Google Scholar] [CrossRef]
- Corbella, C.; Grosse-Kreul, S.; Kreiter, O.; de los Arcos, T.; Benedikt, J.; von Keudell, A. Particle beam experiments for the analysis of reactive sputtering processes in metals and polymer surfaces. Rev. Sci. Instrum. 2013, 84, 103303. [Google Scholar] [CrossRef] [PubMed]
- Arteaga, O.; Freudenthal, J.; Wang, B.; Kahr, B. Mueller matrix polarimetry with four photoelastic modulators: Theory and calibration. Appl. Opt. 2012, 51, 6805–6817. [Google Scholar] [CrossRef]
- Arteaga, O.; Canillas, A. Analytic inversion of the Mueller-Jones polarization matrices for homogeneous media: Erratum. Opt. Lett. 2010, 35, 3525. [Google Scholar] [CrossRef]
- Corbella, C.; Portal-Marco, S.; Rubio-Roy, M.; Bertran, E.; Oncins, G.; Vallvé, M.A.; Ignés-Mullol, J.; Andújar, J.L. Modifying surface properties of diamond-like carbon films via nanotexturing. J. Phys. D Appl. Phys. 2011, 44, 395301. [Google Scholar] [CrossRef]
- Kajiyama, K.; Yoneda, T.; Fujioka, Y.; Kido, Y. Si-O bond formation by oxygen implantation into silicon. Nucl. Instrum. Meth. Phys. Res. B 1997, 121, 315–318. [Google Scholar] [CrossRef]
- Portal-Marco, S.; Vallvé, M.A.; Arteaga, O.; Ignés-Mullol, J.; Corbella, C.; Bertran, E. Structure and physical properties of colloidal crystals made of silica particles. Colloids Surf. A Physicochem. Eng. Asp. 2012, 401, 38–47. [Google Scholar] [CrossRef]
- Elliott, J.; Smolyaninov, I.I.; Zheludev, N.I.; Zayats, A.V. Wavelength dependent birefringence of surface plasmon polaritonic crystals. Phys. Rev. B 2004, 70, 233403. [Google Scholar] [CrossRef]
- Eftekhari, F.; Davis, T.J. Strong chiral optical response from planar arrays of subwavelength metallic structures supporting surface plasmon resonances. Phys. Rev. B 2012, 86, 075428. [Google Scholar] [CrossRef]

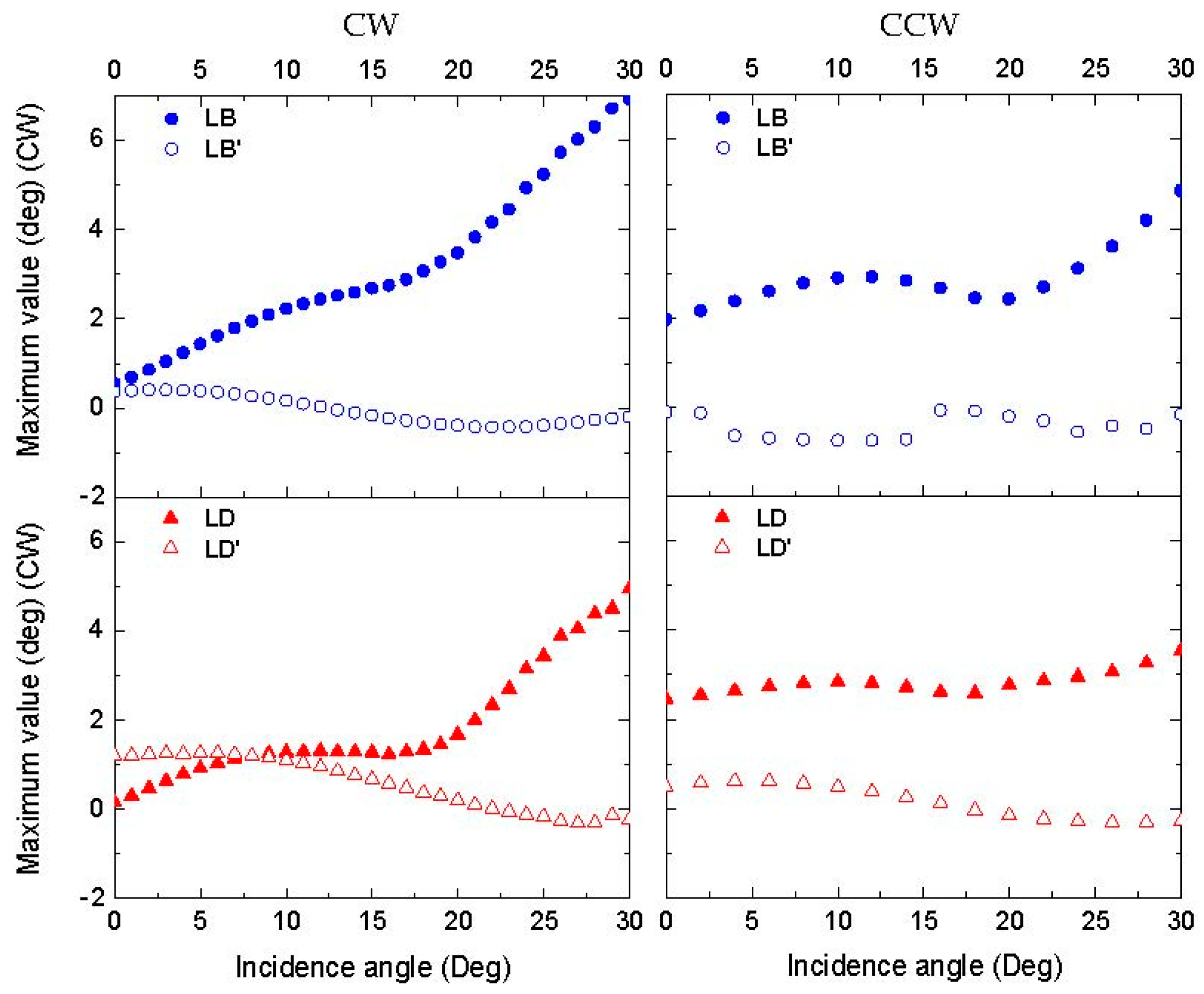
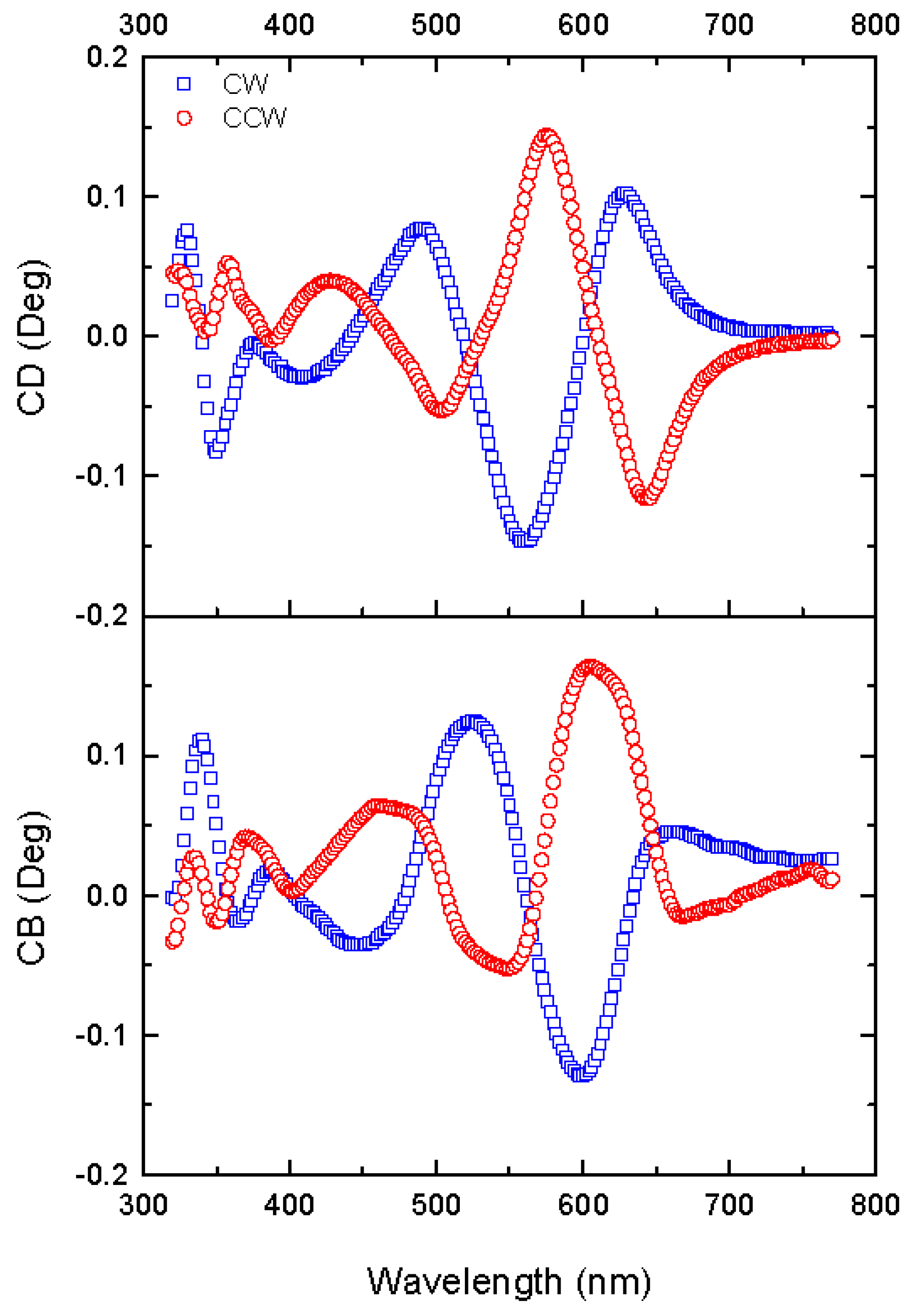
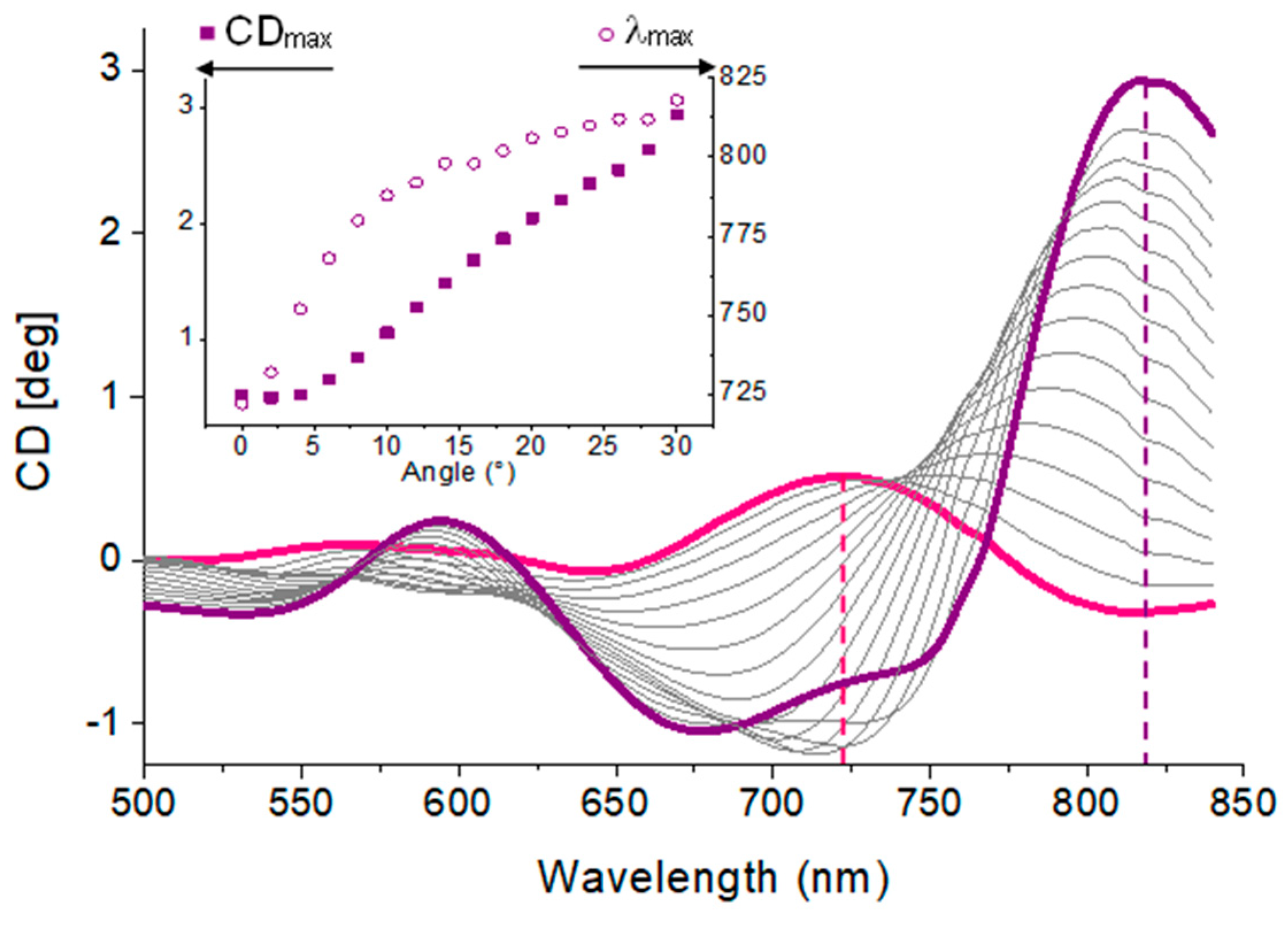
| Parameter | Definition |
|---|---|
| Linear birefringence | |
| Linear dichroism | |
| 45° LB projection | |
| 45° LD projection | |
| Circular birefringence | |
| Circular dichroism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portal, S.; Corbella, C.; Arteaga, O.; Martin, A.; Mandal, T.; Kahr, B. Characterization of Chiral Nanostructured Surfaces Made via Colloidal Lithography. Nanomaterials 2023, 13, 2235. https://doi.org/10.3390/nano13152235
Portal S, Corbella C, Arteaga O, Martin A, Mandal T, Kahr B. Characterization of Chiral Nanostructured Surfaces Made via Colloidal Lithography. Nanomaterials. 2023; 13(15):2235. https://doi.org/10.3390/nano13152235
Chicago/Turabian StylePortal, Sabine, Carles Corbella, Oriol Arteaga, Alexander Martin, Trinanjana Mandal, and Bart Kahr. 2023. "Characterization of Chiral Nanostructured Surfaces Made via Colloidal Lithography" Nanomaterials 13, no. 15: 2235. https://doi.org/10.3390/nano13152235
APA StylePortal, S., Corbella, C., Arteaga, O., Martin, A., Mandal, T., & Kahr, B. (2023). Characterization of Chiral Nanostructured Surfaces Made via Colloidal Lithography. Nanomaterials, 13(15), 2235. https://doi.org/10.3390/nano13152235







