Antibacterial Effect of Low-Concentration ZnO Nanoparticles on Sulfate-Reducing Bacteria under Visible Light
Abstract
1. Introduction
2. Experimental
2.1. Experimental Materials and Media
2.2. Bacterial Strains and Culture Condition
2.3. Determination of Zn2+ in Water
2.4. Measurement of Reactive Oxygen Species (ROS)
2.5. Analysis of the Cell Membrane Integrity
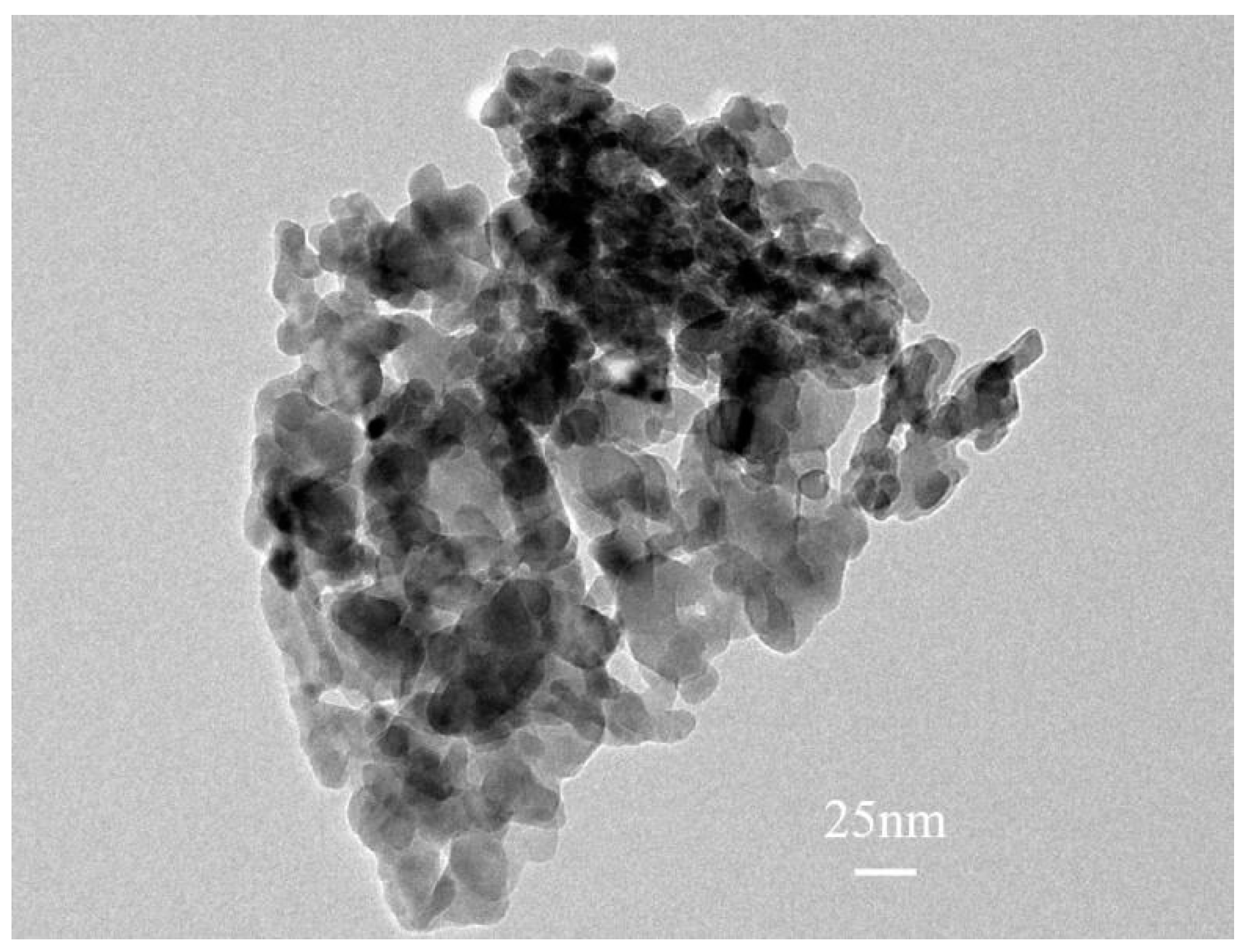
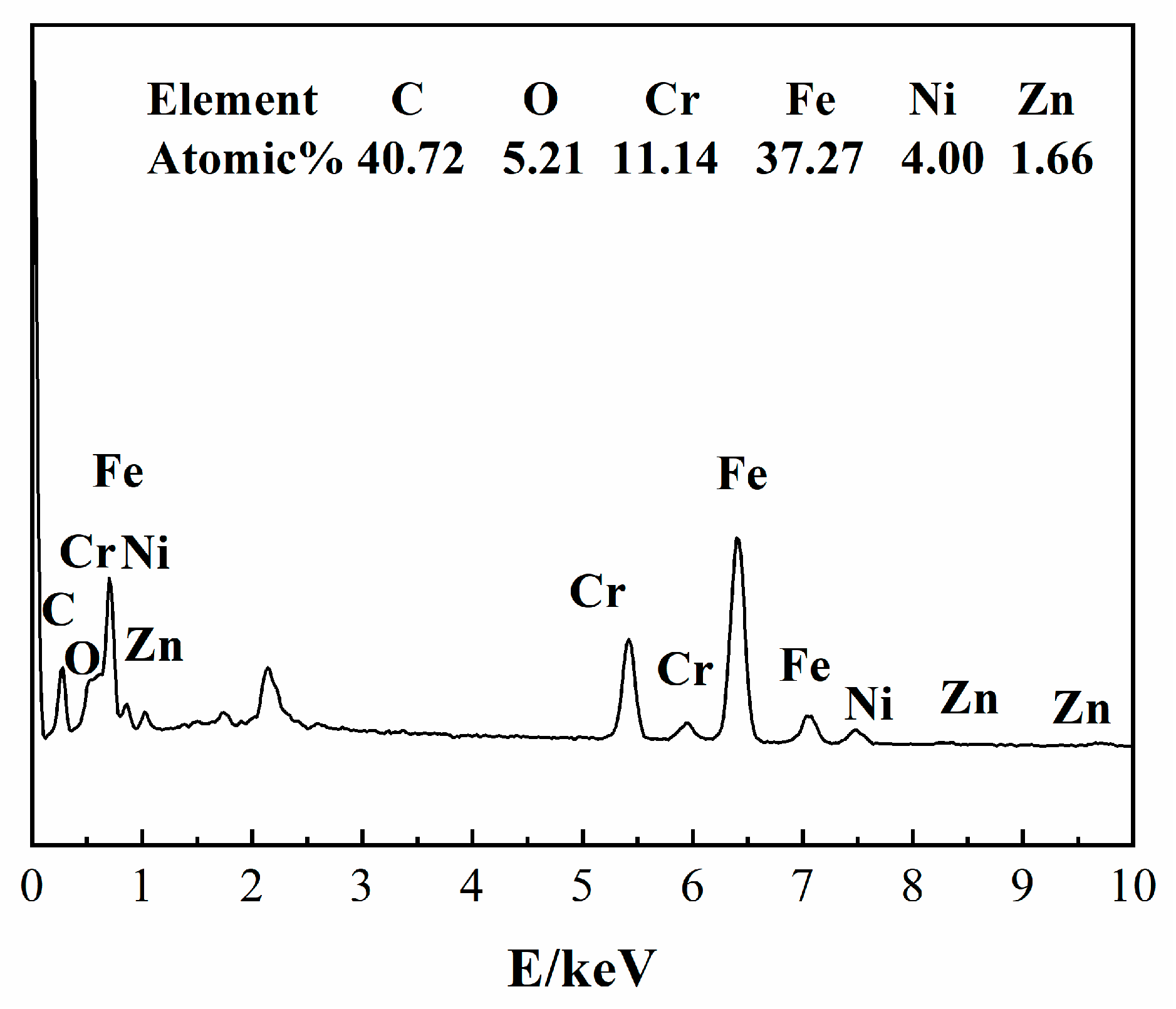
2.6. Surface Analysis
3. Results and Discussion
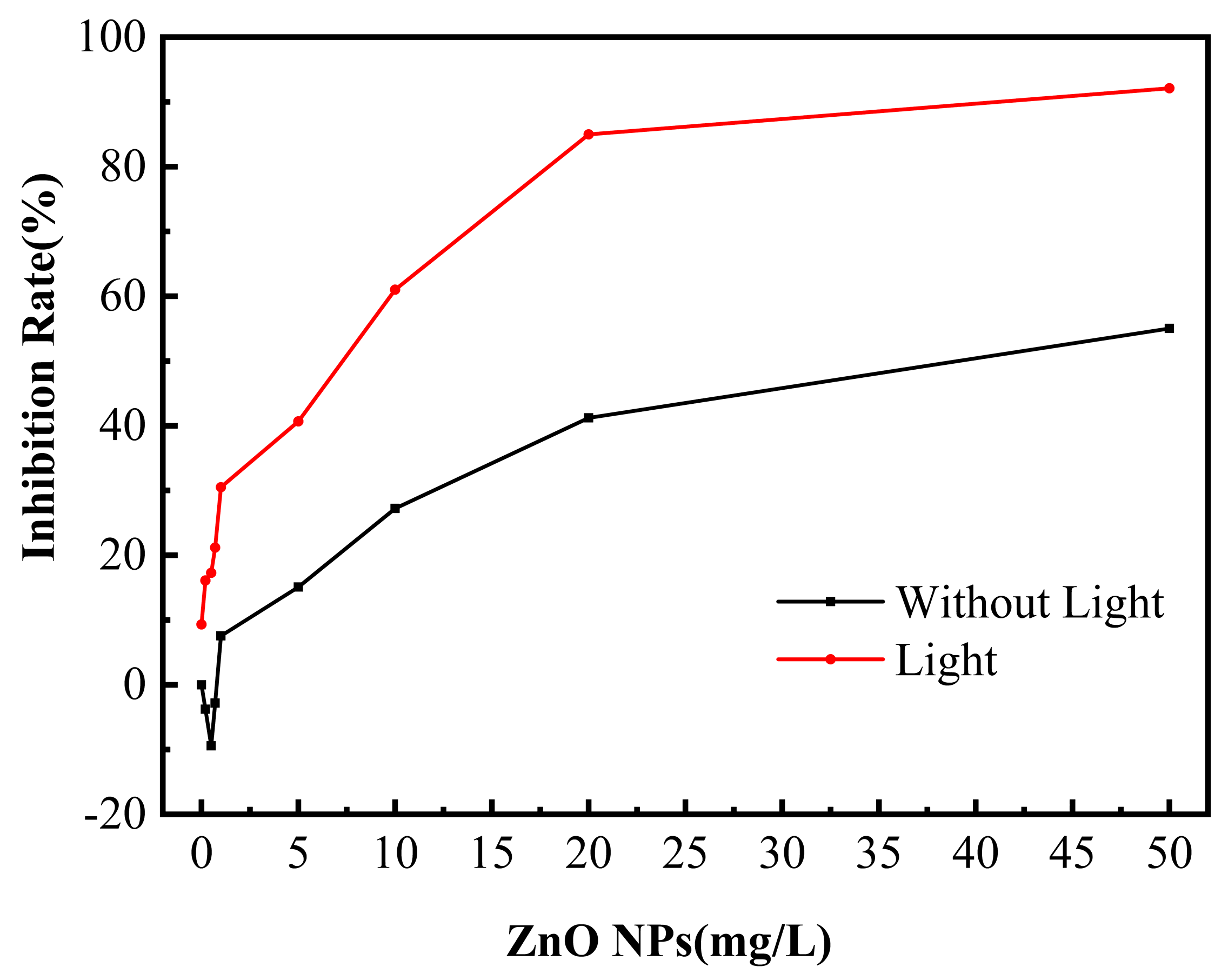
3.1. Antibacterial Effect of ZnO NPs on SRB
3.2. Zn2+ Content Released by ZnO NPs in Solution
3.3. ROS Analysis in Water
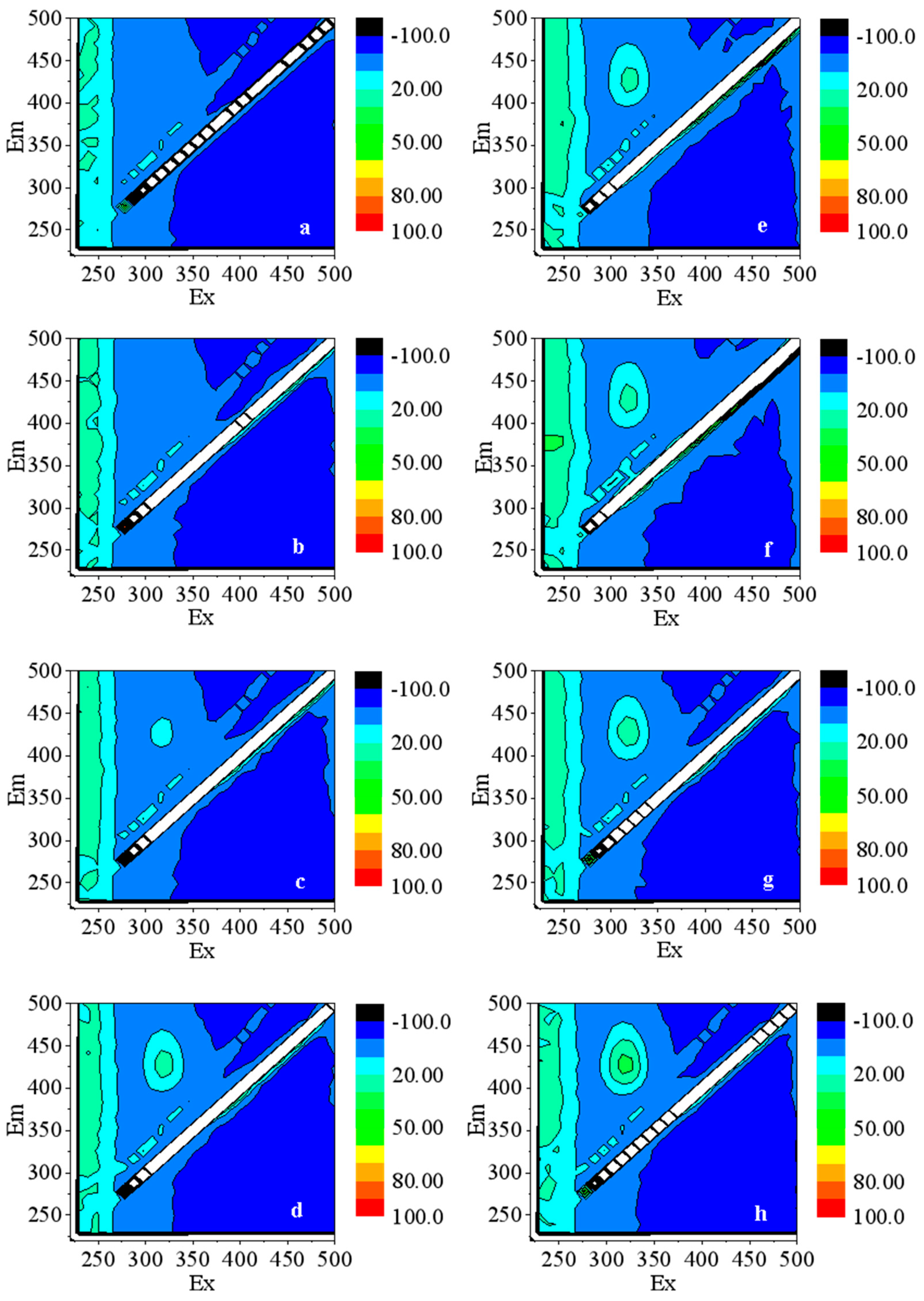
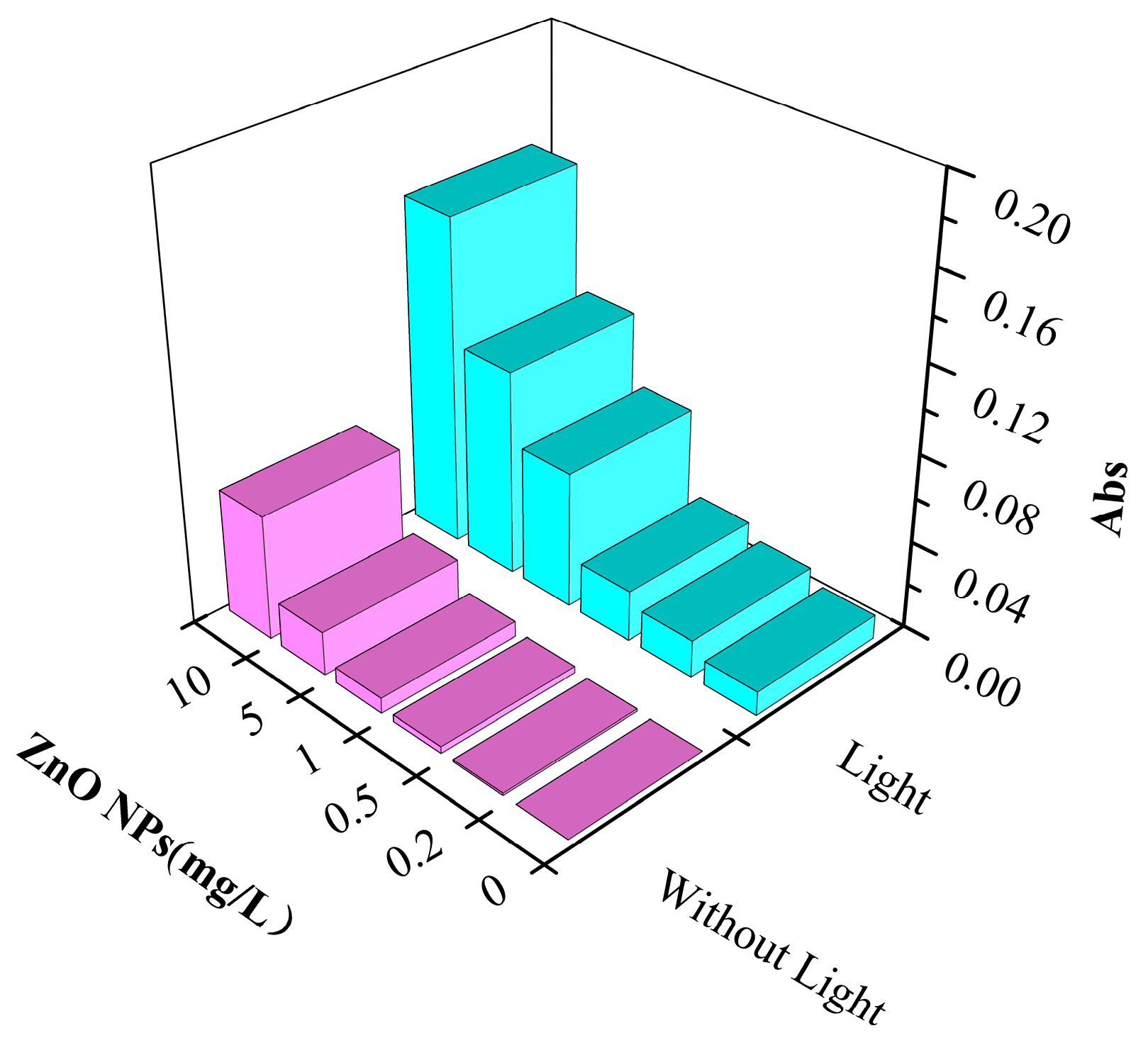
3.3.1. The Production of ·OH in Water
3.3.2. The Production of O2·− in Water
3.4. Damage Analysis of Cell Membrane
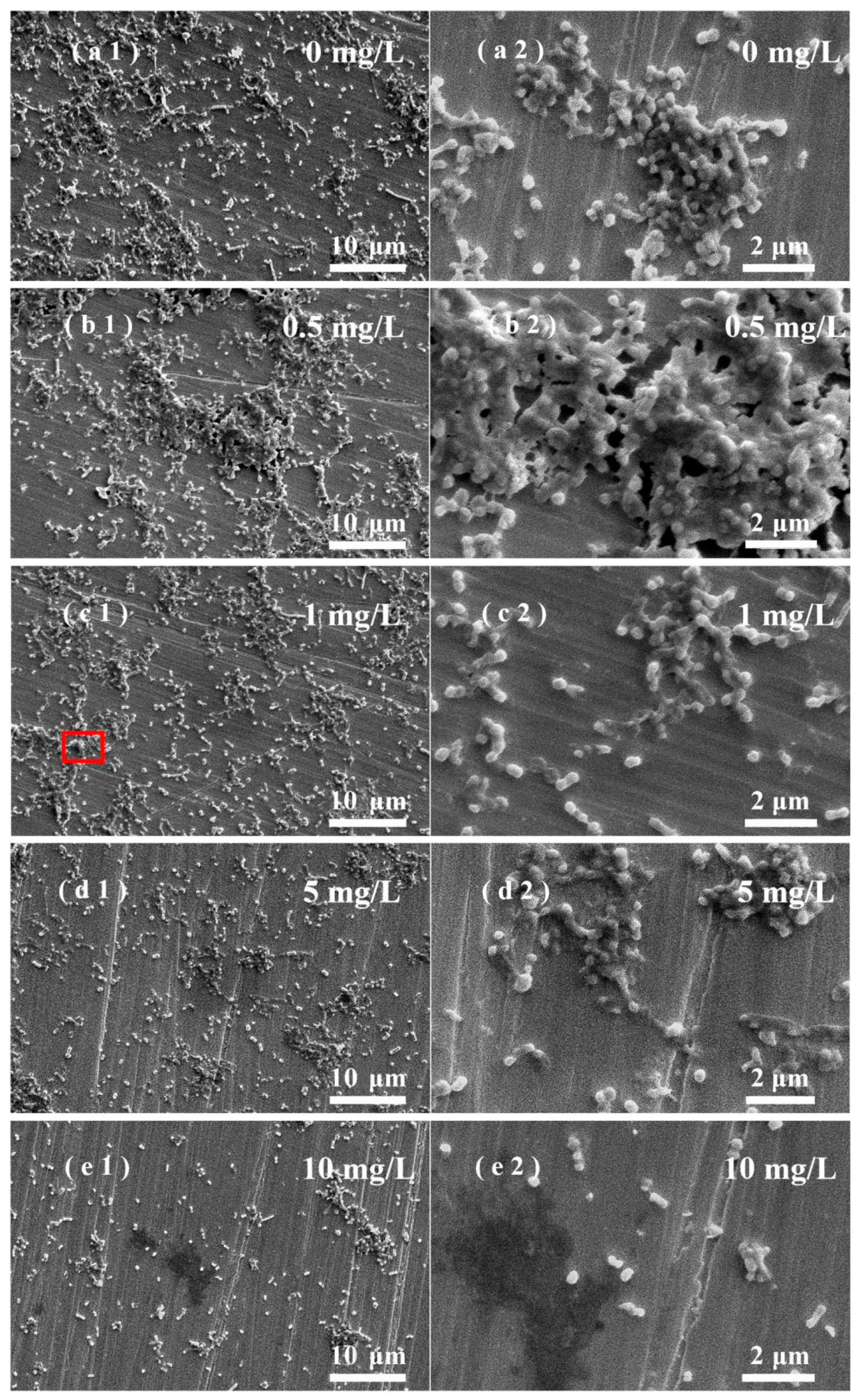
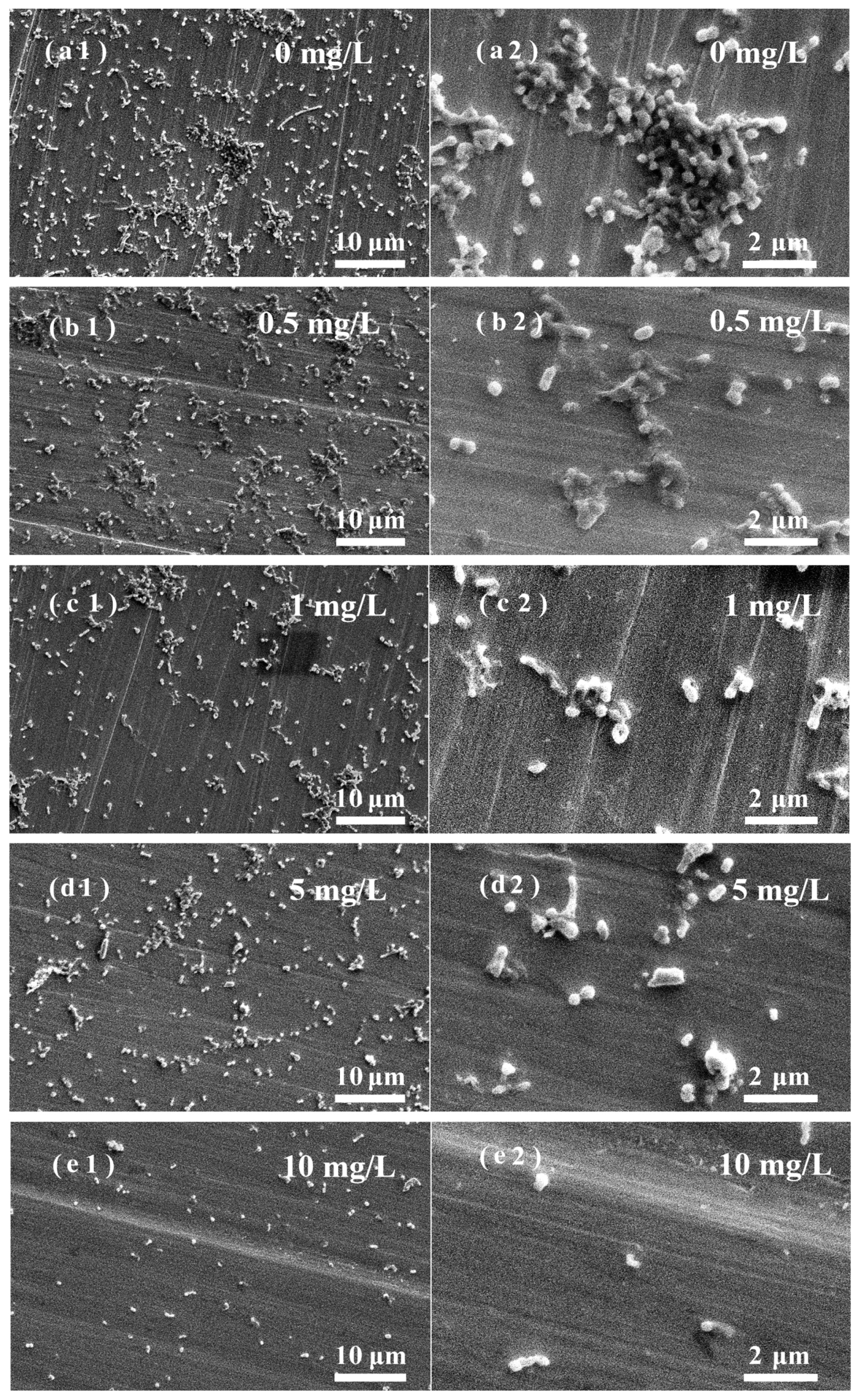
3.5. Effects of ZnO NPs on SRB Biofilm Adhesion on Stainless Steel Surfaces
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ezeuko, C.C.; Sen, A.; Gates, I.D. Modelling biofilm-induced formation damage and biocide treatment in subsurface geosystems. Microb. Biotechnol. 2012, 6, 53–66. [Google Scholar] [CrossRef]
- Ren, E.; Zhang, C.; Li, D.; Pang, X.; Liu, G. Leveraging metal oxide nanoparticles for bacteria tracing and eradicating. View 2020, 1, 108886. [Google Scholar] [CrossRef]
- Yuan, W.; Wei, Y.; Zhang, Y.; Riaz, L.; Yang, Q.X.; Wang, Q.; Wang, R.F. Resistance of multidrug resistant Escherichia coli to environmental nanoscale TiO2 and ZnO. Sci. Total Environ. 2020, 761, 144303. [Google Scholar] [CrossRef] [PubMed]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Viswanathan, K.; Kasi, G.; Kim, I.; Sadeghi, K.; Thanakkasaranee, S.; Seo, J. Preparation and characterization of positively surface charged zinc oxide nanoparticles against bacterial pathogens. Microb. Pathog. 2020, 149, 104290. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003. [Google Scholar] [CrossRef]
- Leareng, S.K.U.-J.E.; Musee, N. Toxicity of zinc oxide and iron oxide engineered nanoparticles to Bacillus subtilis in river water systems. Environ. Sci.-Nano 2020, 7, 172–185. [Google Scholar] [CrossRef]
- Happy, A.; Soumya, M.; Kumar, S.V.; Rajeshkumar, S.; Sheba, R.D.; Lakshmi, T.; Deepak Nallaswamy, V. Phyto-assisted synthesis of zinc oxide nanoparticles using Cassia alata and its antibacterial activity against Escherichia coli. Biochem. Biophys. Rep. 2019, 17, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2007, 279, 71–76. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Forsan, C.F.; Sapata, V.D.M.R.; Lopes, P.R.M.; De Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Li, H.; Zhong, X.; Hu, J.; Wang, J.; Yu, J. The inhibition of sulfate reducing bacteria adhesion and corrosion on the carbon steel surface using ZnO particles. J. Adhes. Sci. Technol. 2022, 37, 270–283. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Ferrari-Iliou, R.B.N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Zudyte, B.; Luksiene, Z. Visible light-activated ZnO nanoparticles for microbial control of wheat crop. J. Photochem. Photobiol. B Biol. 2021, 219, 112206. [Google Scholar] [CrossRef] [PubMed]
- Khalighie, J.; Ure, A.M.; West, T.S. Atom-trapping atomic absorption spectrometry of arsenic, cadmium, lead, selenium and zinc in air-acetylene and air-propane flames. Anal. Chim. Acta 1981, 131, 27–36. [Google Scholar] [CrossRef]
- Sami, R.; Elhakem, A.; Alharbi, M.; Benajiba, N.; Fikry, M.; Helal, M. The combined effect of coating treatments to nisin, nano-silica, and chitosan on oxidation processes of stored button mushrooms at 4 °C. Sci. Rep. 2021, 11, 6031. [Google Scholar] [CrossRef]
- Ziaee, E.; Razmjooei, M.; Shad, E.; Eskandari, M.H. Antibacterial mechanisms of Zataria multiflora Boiss. essential oil against Lactobacillus curvatus. LWT Food Sci. Technol. 2017, 87, 406–412. [Google Scholar] [CrossRef]
- Denyer, S.P. Mechanisms of action of antibacterial biocides. Int. Biodeter. Biodegr. 1995, 36, 227–245. [Google Scholar] [CrossRef]
- Hessling, M.; Spellerberg, B.; Hoenes, K. Photoinactivation of bacteria by endogenous photosensitizers and exposure to visible light of different wavelengths—A review on existing data. FEMS Microbiol. Lett. 2016, 364, fnw270. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 35004. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, K.L.; Sun, H.H.; Ren, H.Q.; Zhang, X.X.; Ye, L. Comparison of the impacts of zinc ions and zinc nanoparticles on nitrifying microbial community. J. Hazard. Mater. 2017, 343, 166–175. [Google Scholar] [CrossRef]
- Applerot, G.; Lipovsky, A.; Dror, R.; Nina, P.; Yeshayahu, N.; Rachel, L.; Aharon, G. Enhanced Antibacterial Activity of Nanocrystalline ZnO Due to Increased ROS-Mediated Cell Injury. Adv. Funct. Mater. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Mangavati, S.; Rao, A.; Devadiga, D.; Selvakumar, M. Defects and band gap shrinkage in ZnO/rGO composite nano-pebbles prepared by solid–state reaction. Diam. Relat. Mater. 2022, 123, 108886. [Google Scholar] [CrossRef]
- Wang, J.P.; Wang, Z.Y.; Huang, B.; Ma, Y.D.; Liu, Y.Y.; Qin, X.Y.; Zhang, X.Y.; Dai, Y. Oxygen Vacancy Induced Band-Gap Narrowing and Enhanced Visible Light Photocatalytic Activity of ZnO. ACS Appl. Mater. Interfaces 2012, 4, 4024–4030. [Google Scholar] [CrossRef] [PubMed]
- Lipovsky, A.; Tzitrinovich, Z.; Friedmann, H.; Applerot, G.; Gedanken, A.; Lubart, R. EPR Study of Visible Light-Induced ROS Generation by Nanoparticles of ZnO. J. Phys. Chem. C 2009, 113, 15997–16001. [Google Scholar] [CrossRef]
- Lakshmi Prasanna, V.; Vijayaraghavan, R. Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species Even in the Dark. Langmuir ACS J. Surf. Colloids 2015, 31, 9155–9162. [Google Scholar] [CrossRef]
- Talebian, N.; Amininezhad, S.M.; Doudi, M. Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. Photochem. Photobiol. B 2013, 120, 66–73. [Google Scholar] [CrossRef]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomedicine 2010, 7, 184–192. [Google Scholar] [CrossRef]
- Borovanský, J.; Riley, P.A. Cytotoxicity of zinc in vitro. Chem. Biol. Interact. 1989, 69, 279–291. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.Z.; Lin, D.H. Toxicity of ZnO nanoparticles to Escherichia coli: Mechanism and the influence of medium components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef]
- Verma, R.; Pathak, S.; Srivastava, A.K.; Prawer, S.; Tomljenovic-Hanic, S. ZnO nanomaterials: Green synthesis, toxicity evaluation and new insights in biomedical applications. J. Alloys Compd. 2021, 876, 160175. [Google Scholar] [CrossRef]
- Karli, G.; Buford, S.; Mark, K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar]
- Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J. Microbiol. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef]
- Hirakawa, T.; Yawata, K.; Nosaka, Y. Photocatalytic reactivity for O2− and OH radical formation in anatase and rutile TiO2 suspension as the effect of H2O2 addition. Appl. Catal. A Gen. 2007, 325, 105–111. [Google Scholar] [CrossRef]
- Hu, J.L.; Ma, W.; Pan, Y.Z.; Cheng, Z.H.; Yu, S.G.; Gao, J.; Zhang, Z.; Wan, C.X.; Qiu, C.X. Insights on the mechanism of Fe doped ZnO for tightly-bound extracellular polymeric substances tribo-catalytic degradation: The role of hydration layers at the interface. Chemosphere 2021, 276, 130170. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J.J. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef]
- Michal, R.; Dworniczek, E.; Caplovicova, M.; Olivier, M.; Panagiotis, L.; Lubomir, C.; Gustav, P. Photocatalytic properties and selective antimicrobial activity of TiO2(Eu)/CuO nanocomposite. Appl. Surf. Sci. 2016, 371, 538–546. [Google Scholar] [CrossRef]
- Xu, X.L.; Zhou, Z.W.; Zhu, W.J. Studies on the Active Oxygen in Zinc Oxides with Different Morphologies. Mater. Sci. Forum 2009, 610–613, 229–232. [Google Scholar] [CrossRef]
- Qiu, T.A.; Gallagher, M.J.; Hudson-Smith, N.V.; Wu, J.W.; Krause, M.O.P.; Fortner, J.D.; Haynes, C.L. Research highlights: Unveiling the mechanisms underlying nanoparticle-induced ROS generation and oxidative stress. Environ. Sci.-Nano 2016, 3, 940–945. [Google Scholar] [CrossRef]
- Yang, L.; Yang, H.; Hao, W.; Yingchang, L.; Qiuying, L.; Tong, S. Fabrication, characterization and antibacterial mechanism of in-situ modification nano-CaCO3/TiO2/CS coatings. Int. J. Food Sci. Technol. 2020, 56, 2675–2686. [Google Scholar] [CrossRef]
- Liu, X.; Xia, W.; Jiang, Q.; Xu, Y.S.; Yu, P.P. Effect of kojic acid-grafted-chitosan oligosaccharides as a novel antibacterial agent on cell membrane of gram-positive and gram-negative bacteria. J. Biosci. Bioeng. 2015, 120, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Campanha, N.H.; Pavarina, A.C.; Brunetti, I.L.; Carlos Eduardo, V.; Ana Lucia, M.; Denise Madalena Palomari, S. Candida albicans inactivation and cell membrane integrity damage by microwave irradiation. Mycoses 2007, 50, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, K.; Mortimer, M.; Holden, P.A.; Peng, C.; Wu, Y.C.; Gao, C.H.; Huang, Q.Y. Towards a better understanding of Pseudomonas putida biofilm formation in the presence of ZnO nanoparticles (NPs): Role of NP concentration. Environ. Int. 2020, 137, 105485. [Google Scholar] [CrossRef] [PubMed]
| NaCl | NaHCO3 | Na2SO4 | MgSO4 | CaCl2 |
|---|---|---|---|---|
| 7.50 | 2.00 | 3.50 | 0.25 | 0.50 |
| Element | C | Si | Mn | Cr | Ni | S | P |
|---|---|---|---|---|---|---|---|
| wt% | 0.07 | 0.59 | 1.16 | 17.58 | 8.25 | 0.015 | 0.026 |
| ZnO NPs (mg/L) | 0.5 | 1 | 5 | 10 | 50 | |
|---|---|---|---|---|---|---|
| Zn2+ concentration (mg/L) | Non-Light treated | 0.061 | 0.146 | 0.184 | 0.221 | 0.261 |
| Light treated | 0.070 | 0.144 | 0.180 | 0.231 | 0.263 | |
| Zn2+ (mg/L) | 0 | 0.05 | 0.25 | 0.5 |
|---|---|---|---|---|
| Bacteria quantity (CFU/mL) | 7.0 × 106 | 7.3 × 106 | 7.1 × 106 | 6.8 × 106 |
| ZnO (mg/L) | 0.5 | 1 | 5 | 10 | |
|---|---|---|---|---|---|
| O2·− Concentration (mg/L) | Without Light | 0 | 0.047 | 0.103 | 0.137 |
| With light | 0.205 | 0.239 | 0.318 | 0.588 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Zhang, J.; Li, Z.; Huang, J.; Wu, J.; Zhang, Y.; Ge, H.; Zhao, Y. Antibacterial Effect of Low-Concentration ZnO Nanoparticles on Sulfate-Reducing Bacteria under Visible Light. Nanomaterials 2023, 13, 2033. https://doi.org/10.3390/nano13142033
Yang H, Zhang J, Li Z, Huang J, Wu J, Zhang Y, Ge H, Zhao Y. Antibacterial Effect of Low-Concentration ZnO Nanoparticles on Sulfate-Reducing Bacteria under Visible Light. Nanomaterials. 2023; 13(14):2033. https://doi.org/10.3390/nano13142033
Chicago/Turabian StyleYang, Hua, Jialin Zhang, Zhuoran Li, Jinrong Huang, Jun Wu, Yixuan Zhang, Honghua Ge, and Yuzeng Zhao. 2023. "Antibacterial Effect of Low-Concentration ZnO Nanoparticles on Sulfate-Reducing Bacteria under Visible Light" Nanomaterials 13, no. 14: 2033. https://doi.org/10.3390/nano13142033
APA StyleYang, H., Zhang, J., Li, Z., Huang, J., Wu, J., Zhang, Y., Ge, H., & Zhao, Y. (2023). Antibacterial Effect of Low-Concentration ZnO Nanoparticles on Sulfate-Reducing Bacteria under Visible Light. Nanomaterials, 13(14), 2033. https://doi.org/10.3390/nano13142033






