Nanomaterial Production from Metallic Vapor Bubble Collapse in Liquid Nitrogen
Abstract
:1. Introduction
2. Experimental Setup
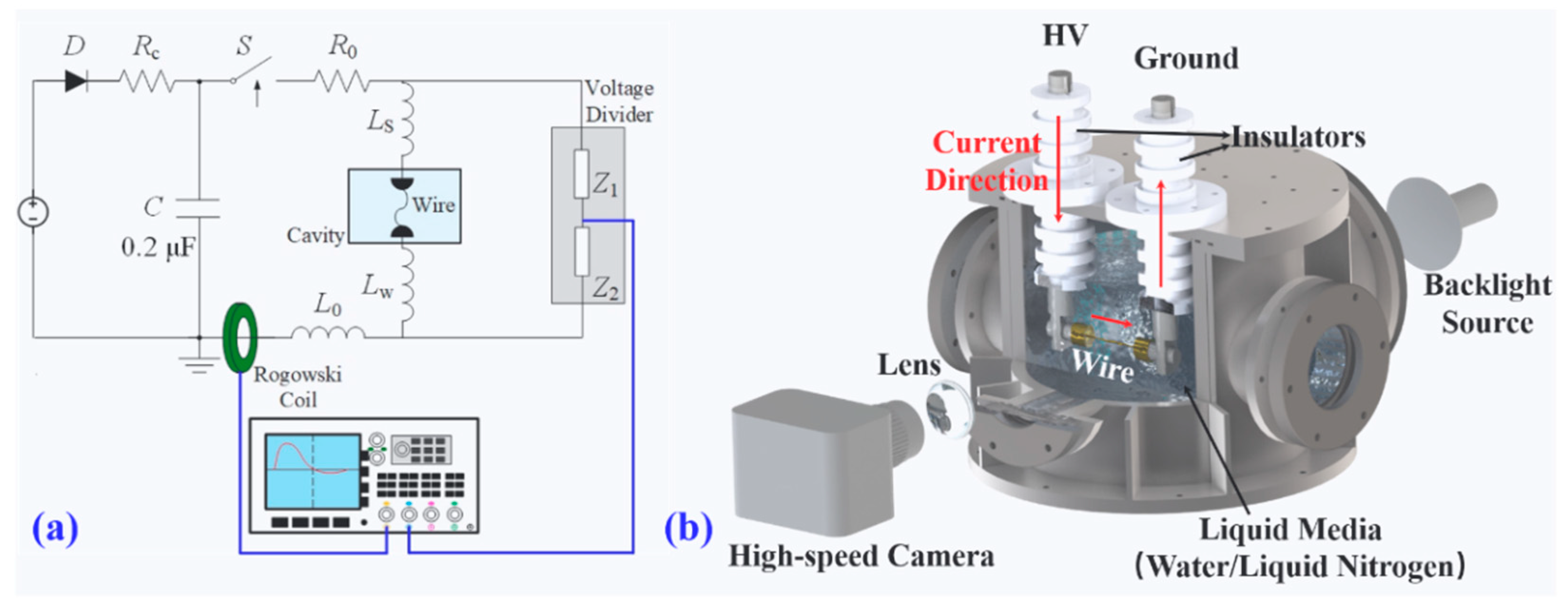
2.1. Electrical Explosion Device and Diagnosis Methods
2.2. Products Collection and Characterization Methods
2.3. Experimental Methods
3. Experimental Results
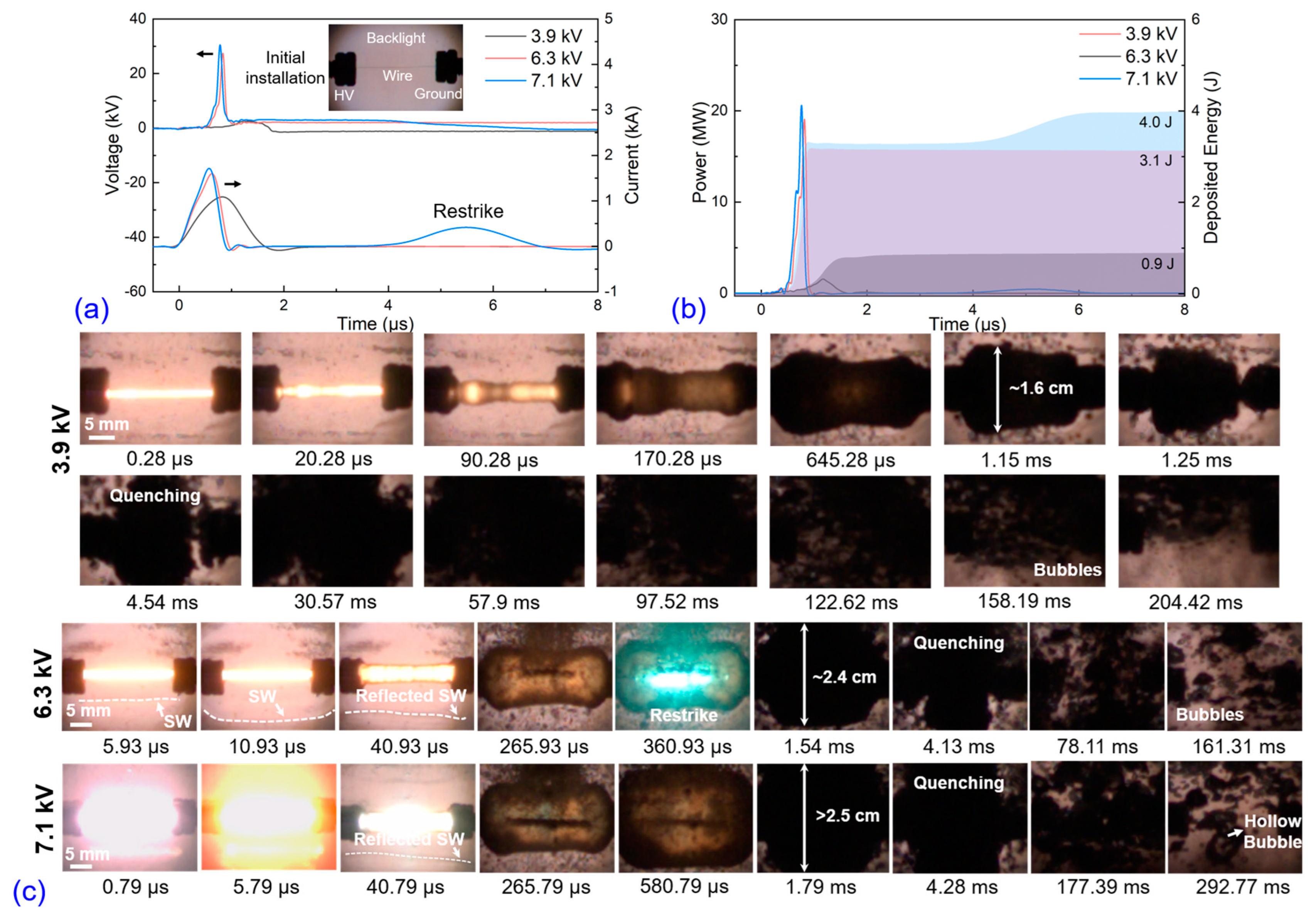
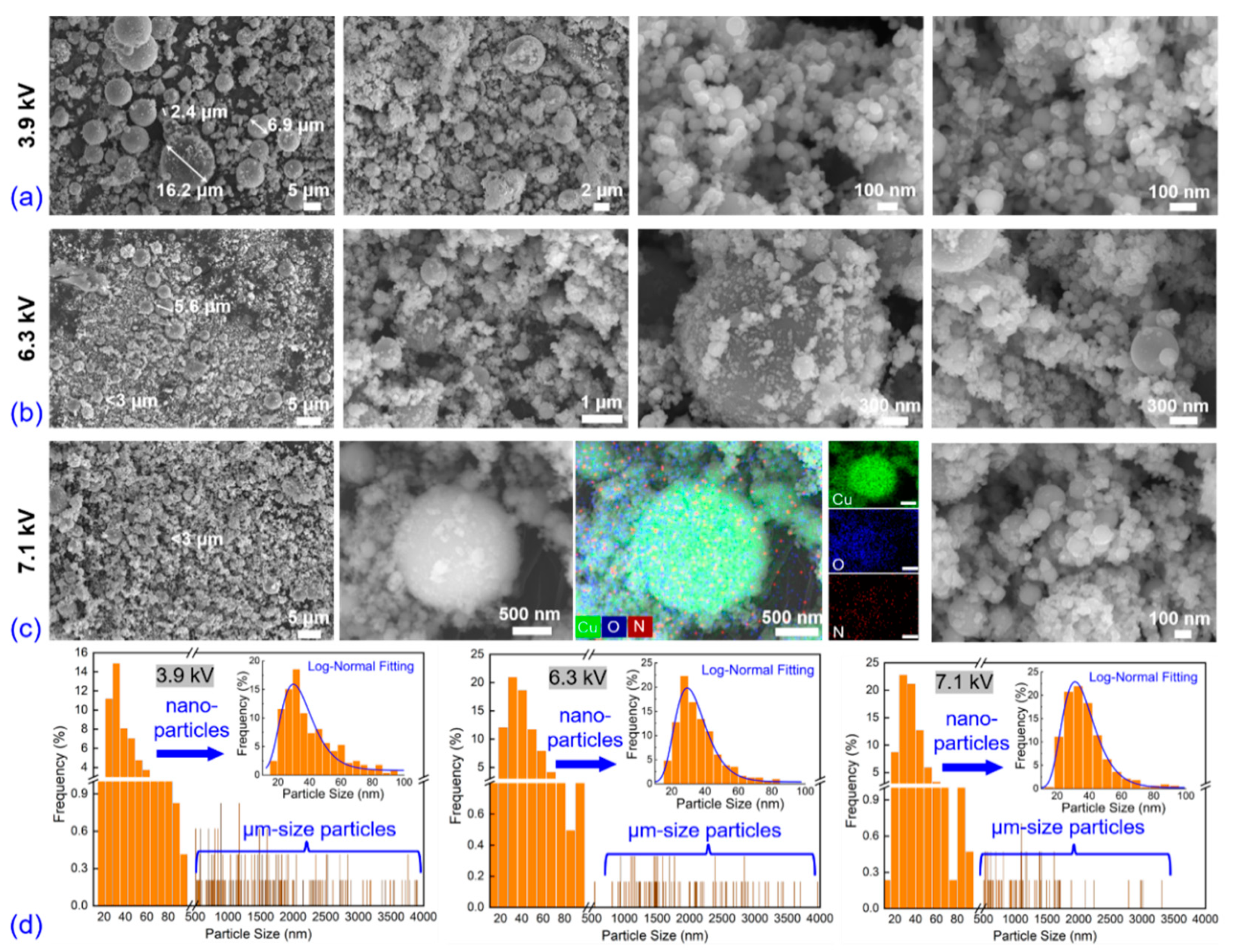
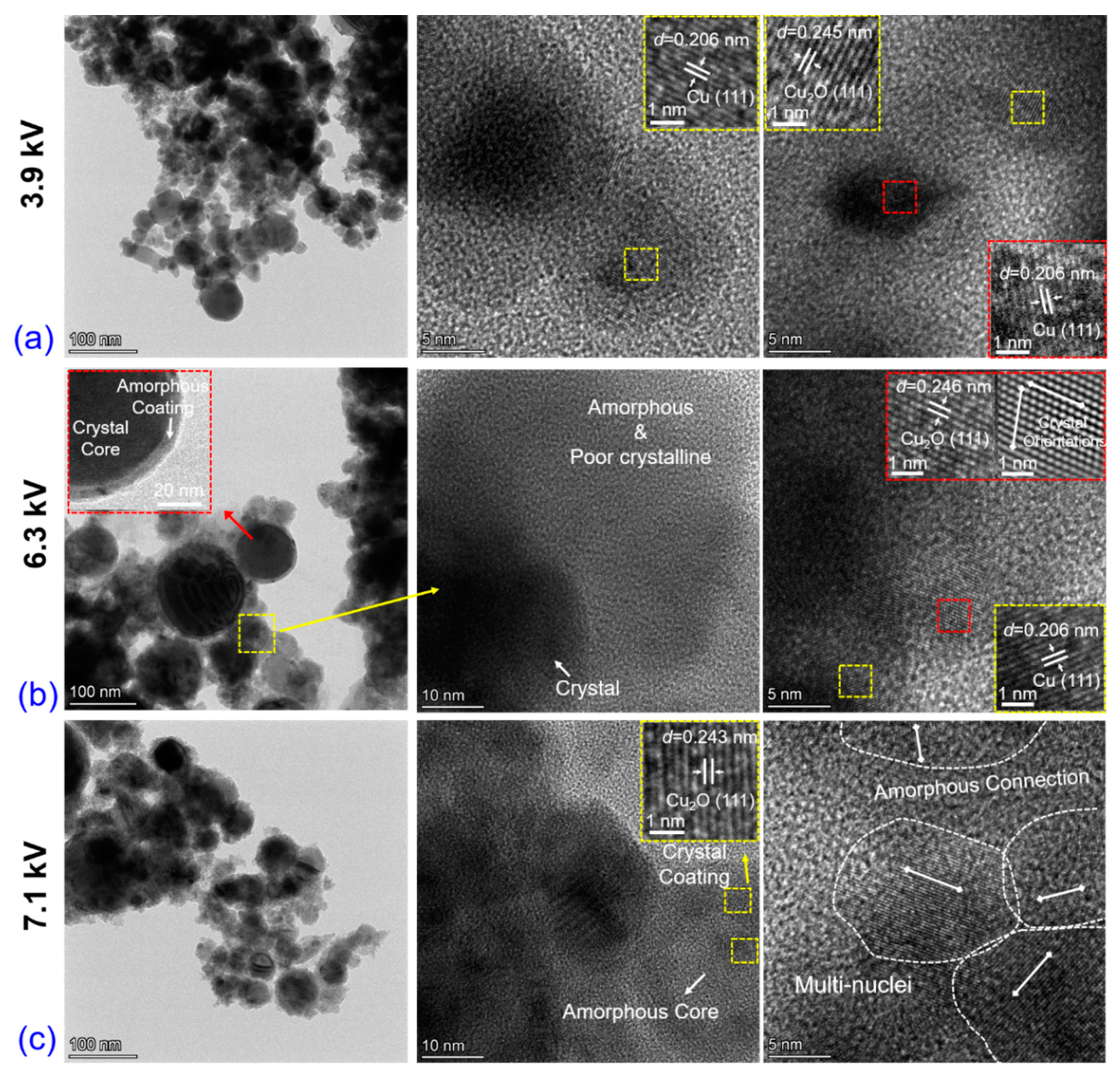
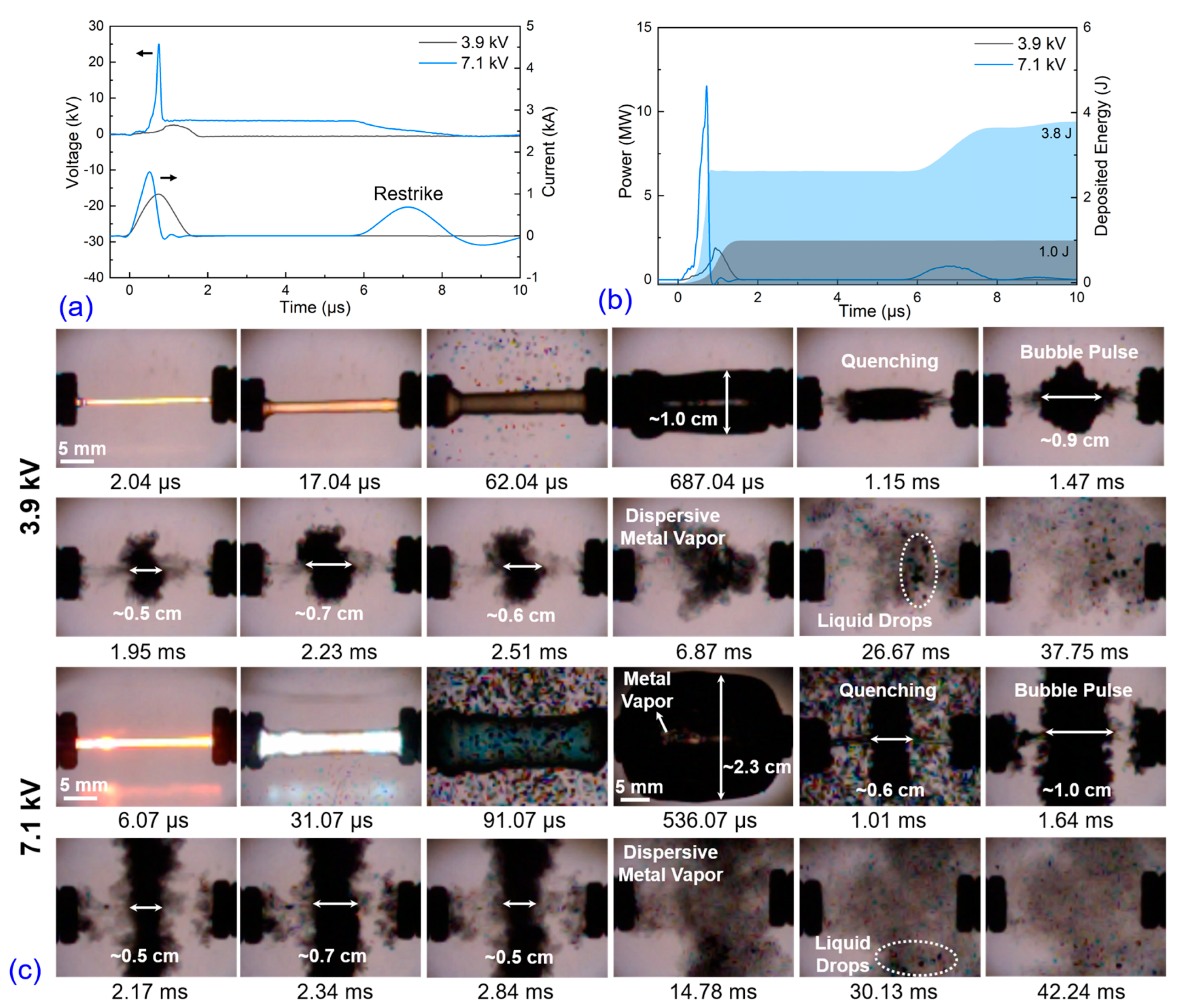
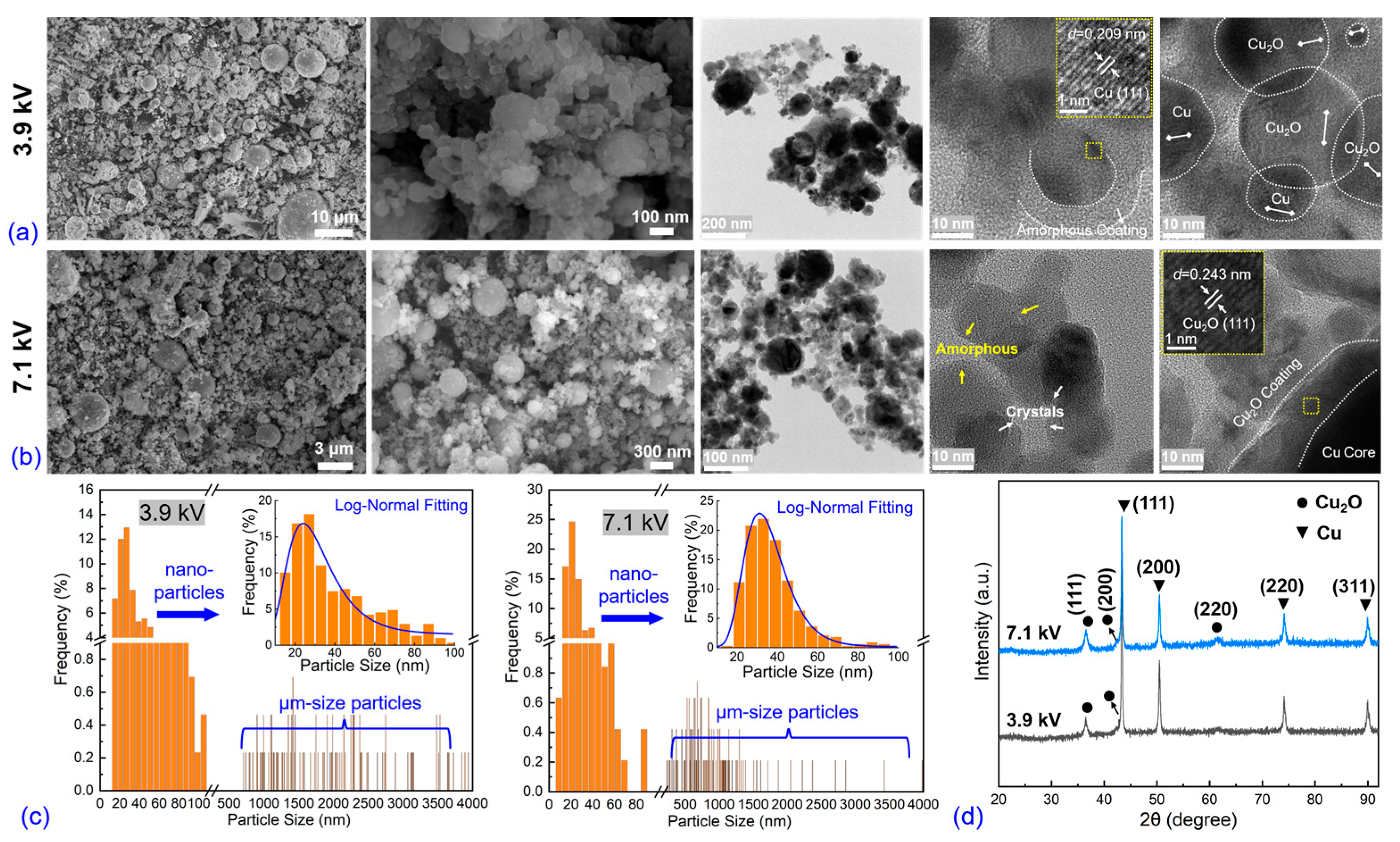
3.1. Electro-Physical Characteristics, Bubble Dynamics, and Explosion Products of EEW in Liq N2
3.2. Electro-Physical Characteristics, Bubble Dynamics, and Explosion Products of EEW in Water
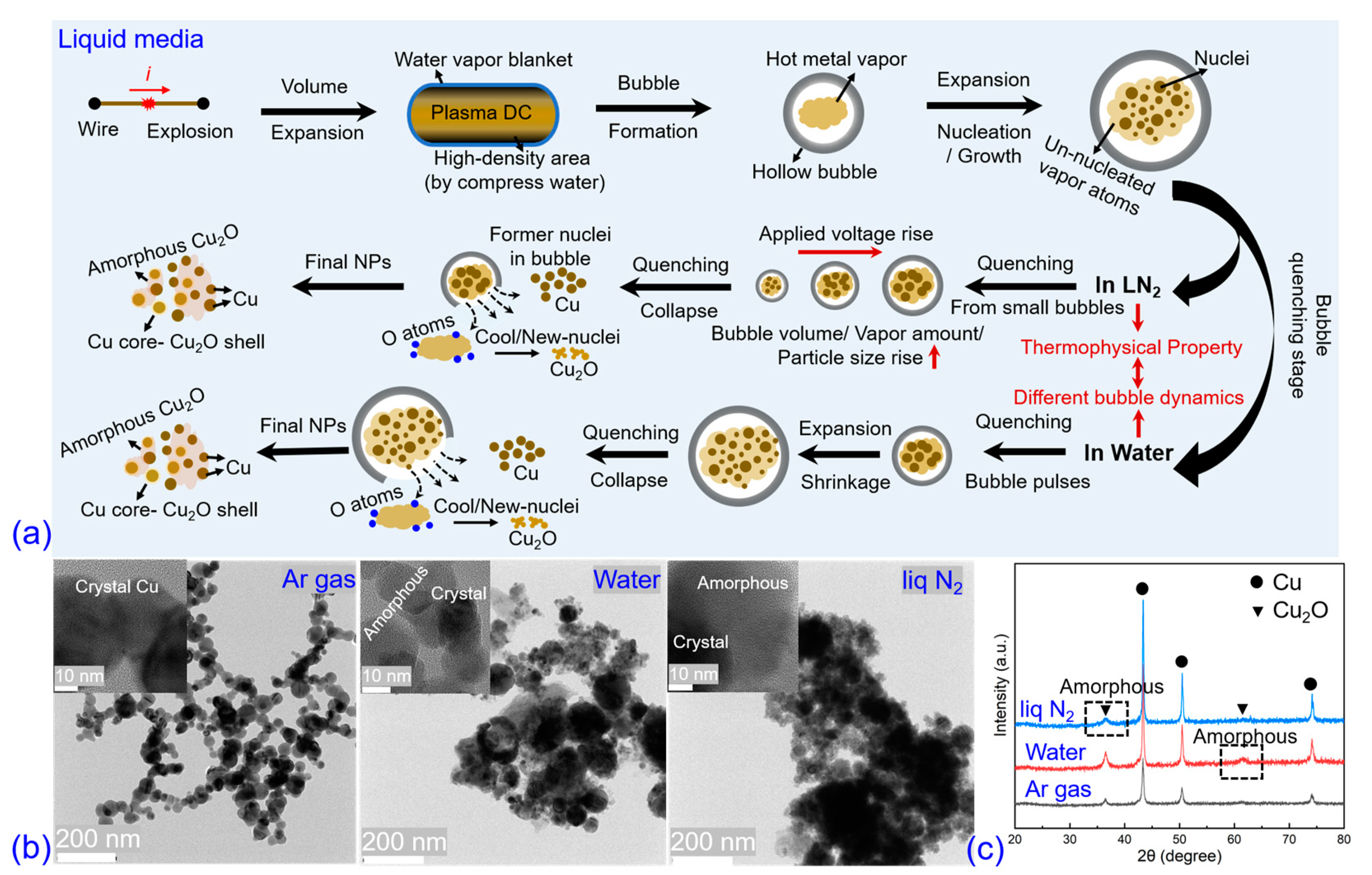
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kotov, Y.A. Electric explosion of wires as a method for preparation of nanopowders. J. Nanoparticle Res. 2003, 5, 539–550. [Google Scholar] [CrossRef]
- Han, R.; Li, C.; Gao, M.; Cao, Y.; Yuan, W.; Huang, Y. Graphite and Bismuth Selenide under Electrical Explosion in Confined Environment: Exfoliation, Phase Transition, and Surface Decoration. Adv. Mater. Interfaces 2023, 10, 2201568. [Google Scholar] [CrossRef]
- Shi, H.; Wu, J.; Li, X.; Murphy, A.B.; Li, X.; Li, C.; Li, P. Understanding the nanoparticle formation during electrical wire explosion using a modified moment model. Plasma Sources Sci. Technol. 2019, 28, 085010. [Google Scholar] [CrossRef]
- Bora, B.; Wong, C.S.; Bhuyan, H.; Lee, Y.S.; Yap, S.L.; Favre, M. Understanding the mechanism of nanoparticle formation in wire explosion process. J. Quant. Spectrosc. Radiat. Transf. 2013, 117, 1–6. [Google Scholar] [CrossRef]
- Strucka, J.; Lukic, B.; Koerner, M.; Halliday, J.W.D.; Yao, Y.; Mughal, K.; Maler, D.; Efimov, S.; Skidmore, J.; Rack, A.; et al. Synchrotron radiography of Richtmyer-Meshkov instability driven by exploding wire arrays. Phys. Fluids 2023, 35, 044108. [Google Scholar] [CrossRef]
- Han, R.; Li, C.; Yao, W.; Yuan, W.; Cao, Y.; Zhang, Y. Electrical explosion in confined space: From warm dense matter to fragmentation. Phys. Fluids 2022, 34, 087108. [Google Scholar] [CrossRef]
- Cho, C.H.; Park, S.H.; Choi, Y.W.; Kim, B.G. Production of nanopowders by wire explosion in liquid media. Surf. Coat. Technol. 2007, 201, 4847–4849. [Google Scholar] [CrossRef]
- Aravinth, S.; Sankar, B.; Chakravarthi, S.R.; Sarathi, R. Generation and characterization of nano tungsten oxide particles by wire explosion process. Mater. Charact. 2011, 62, 248–255. [Google Scholar] [CrossRef]
- Gao, X.; Chen, P.W.; Wang, X.G.; Xu, C.X.; Song, Q.Z.; Yin, H. Production of AlN nanopowders by electrical wire explosion in liquid nitrogen. Mater. Sci. Forum 2018, 910, 46–51. [Google Scholar] [CrossRef]
- Lee, Y.S.; Bora, B.; Yap, S.L.; Wong, C.S. Effect of ambient air pressure on synthesis of copper and copper oxide nanoparticles by wire explosion process. Curr. Appl. Phys. 2012, 12, 199–203. [Google Scholar] [CrossRef]
- Antony, J.K.; Vasa, N.J.; Chakravarthy, S.R.; Sarathi, R. Understanding the mechanism of nano-aluminum particle formation by wire explosion process using optical emission technique. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 2509–2516. [Google Scholar] [CrossRef]
- Sindhu, T.K.; Sarathi, R.; Chakravarthy, S.R. Understanding nanoparticle formation by a wire explosion process through experimental and modelling studies. Nanotechnology 2007, 19, 025703. [Google Scholar] [CrossRef]
- Bai, J.; Shi, Z.; Huang, C.; Wu, Z.; Jia, S. Electrical explosion of aluminum wire for preparation of nanoparticles: An experimental study. J. Phys. D Appl. Phys. 2019, 52, 425201. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Jiang, L.; Li, Z.; Wang, X.; Zhai, J.; Zhang, S. Understanding the effect of ambient gas pressure on the nanoparticle formation in electrically exploding wires. Phys. Plasmas 2023, 30, 033511. [Google Scholar] [CrossRef]
- Han, R.; Li, C.; Wang, K.; Yuan, W.; Wang, Y.; Ding, W.; Ouyang, J. “Breakdown” of stratified electrical explosion products: Plasma development and its mechanical effect. Phys. Fluids 2021, 33, 107119. [Google Scholar] [CrossRef]
- Cho, C.; Choi, Y.W.; Kang, C.; Lee, G.W. Effects of the medium on synthesis of nanopowders by wire explosion process. Appl. Phys. Lett. 2007, 91, 141501. [Google Scholar] [CrossRef]
- Yun, G.S.; Bac, L.H.; Kim, J.S.; Kwon, Y.S.; Choi, H.S.; Kim, J.C. Preparation and dispersive properties of Ag colloid by electrical explosion of wire. J. Alloys Compd. 2011, 509, S348–S352. [Google Scholar] [CrossRef]
- Green, D.W.; Southard, M.Z. Thermal Conductivity: Perry’s Handbook for Chemical Engineers, 5th ed.; McGraw-Hill Education: New York, NY, USA, 1995. [Google Scholar]
- Krishnan, S.; Haseeb, A.S.M.A.; Johan, M.R. Synthesis and growth kinetics of spindly CuO nanocrystals via pulsed wire explosion in liquid medium. J. Nanoparticle Res. 2013, 15, 1410. [Google Scholar] [CrossRef]
- Peng, C.; Wang, J.; Zhou, N.; Sun, G. Fabrication of nanopowders by electrical explosion of a copper wire in water. Curr. Appl. Phys. 2016, 16, 284–287. [Google Scholar] [CrossRef]
- Li, C.; Han, R.; Li, J.; Deng, C.; Yang, B.; Ouyang, J. Exploring the Influence of the Evolution of Discharge Plasma Channel on the Characteristics of Electric Explosion Products. IEEE Trans. Ind. Appl. 2023, 59, 456–464. [Google Scholar] [CrossRef]
- Romanova, V.M.; Ivanenkov, G.V.; Mingaleev, A.R.; Ter-Oganesyan, A.E.; Tilikin, I.N.; Shelkovenko, T.A.; Pikuz, S.A. On the phase state of thin silver wire cores during a fast electric explosion. Phys. Plasmas 2018, 25, 112704. [Google Scholar] [CrossRef]
- Buogo, S.; Cannelli, G.B. Implosion of an underwater spark-generated bubble and acoustic energy evaluation using the Rayleigh model. J. Acoust. Soc. Am. 2002, 111, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Hokamotoa, K.; Wada, N.; Tomoshigec, R.; Kai, S.; Ujimoto, Y. Synthesis of TiN powders through electrical wire explosion in liquid nitrogen. J. Alloys Compd. 2009, 485, 573–576. [Google Scholar] [CrossRef]
- Shi, H.; Li, T.; Hu, Y.; Li, X.; Wu, J.; Chen, L.; Qiu, A. Two-dimensional simulation of microsecond-timescale underwater electrical explosion of a copper wire. J. Phys. D Appl. Phys. 2022, 55, 405501. [Google Scholar] [CrossRef]
- Qian, D.; Liu, Z.; Li, L.; Zou, X.; Wang, X. Observation of Early Stage of Underwater Electrical Wire Explosion by Shadowgraph. IEEE Access 2020, 8, 85968–85972. [Google Scholar] [CrossRef]
- Shi, H.; Yin, G.; Li, X.; Wu, J.; Murphy, A.B.; Zhang, Y.; Qiu, A. Electrical wire explosion as a source of underwater shock waves. J. Phys. D Appl. Phys. 2021, 54, 403001. [Google Scholar] [CrossRef]
- Krishnan, S.; Haseeba, A.S.M.A.; Johan, M.R. One dimensional CuO nanocrystals synthesis by electrical explosion: A study on structural, optical and electronic properties. J. Alloys Compd. 2014, 586, 360–367. [Google Scholar] [CrossRef]
- Krishnan, S.; Haseeba, A.S.M.A.; Johan, M.R. Low dimensional CuO nanocomposites synthesis by pulsed wire explosion and their crystal growth mechanism. Ceram. Int. 2014, 40, 9907–9916. [Google Scholar] [CrossRef]
- Park, S.; Lee, H.; Ryu, J.; Chung, K.-J.; Hwang, Y.S.; Lee, K.; Kim, D.-K. Measurement on the electrical conductivity of copper along the binodal curve in warm dense regime. Appl. Phys. Lett. 2021, 119, 174102. [Google Scholar] [CrossRef]
- Fedotov, A.; Sheftman, D.; Gurovich, V.T.; Efimov, S.; Bazilitski, G.; Krasik, Y.E.; Oreshkin, V.I. Spectroscopic research of underwater electrical wire explosion. Phys. Plasmas 2008, 15, 082704. [Google Scholar] [CrossRef]
- Glod, S.; Poulikakos, D.; Zhao, Z.; Yadigaroglu, G. An investigation of microscale explosive vaporization of water on an ultrathin Pt wire. Int. J. Heat Mass Transf. 2002, 45, 367–379. [Google Scholar] [CrossRef]
- Chen, J.; Chen, T.; Han, L.; Geng, H.; Tan, S. Experimental investigation on dynamic characteristics of liquid nitrogen single bubble in the free field. Chin. J. Theor. Appl. Mech. 2022, 5, 9. [Google Scholar] [CrossRef]
- Chen, T.; Chen, H.; Liang, W.; Huang, B.; Xiang, L. Experimental investigation of liquid nitrogen cavitating flows in converging-diverging nozzle with special emphasis on thermal transition. Int. J. Heat Mass Transf. 2019, 132, 618–630. [Google Scholar] [CrossRef]
- Rud, A.D.; Kuskova, N.I.; Ivaschuk, L.I.; Zelinskaya, G.M.; Biliy, N.M. Structure State of Carbon Nanomaterials Produced by High-Energy Electric Discharge Techniques. Fuller. Nanotub. Carbon Nanostructures 2011, 19, 120–126. [Google Scholar] [CrossRef]
- Kima, J.C.; Ryua, H.J.; Kima, J.S.; Kimb, B.K.; Kimb, Y.J.; Kim, H.J. Synthesis and densification of Cu added Fe-based BMG composite powders by gas atomization and electrical explosion of wire. J. Alloys Compd. 2009, 483, 28–31. [Google Scholar] [CrossRef]
- Zhong, L.; Gao, X.; Qiao, J.; Zhang, X.; Xiao, Z.; Chen, P. Formation of Si nanoparticles by pulsed discharge of Si strips in distilled water. J. Appl. Phys. 2022, 132, 113303. [Google Scholar] [CrossRef]

| Stored Energy (Applied Voltage) | Ambient Medium | Overheat Coefficient (ξ) 1 | |
|---|---|---|---|
| In Liq N2 | In Water | ||
| 1.5 J (3.9 kV) | Liq N2 and Water | 0.4 | 0.4 |
| 4.0 J (6.3 kV) | Liq N2 | 1.4 | / |
| 5.0 J (7.1 kV) | Liq N2 and Water | 1.8 | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Han, R.; Li, J.; Cao, Y.; Yuan, W.; Li, Q. Nanomaterial Production from Metallic Vapor Bubble Collapse in Liquid Nitrogen. Nanomaterials 2023, 13, 2021. https://doi.org/10.3390/nano13132021
Li C, Han R, Li J, Cao Y, Yuan W, Li Q. Nanomaterial Production from Metallic Vapor Bubble Collapse in Liquid Nitrogen. Nanomaterials. 2023; 13(13):2021. https://doi.org/10.3390/nano13132021
Chicago/Turabian StyleLi, Chen, Ruoyu Han, Jingran Li, Yuchen Cao, Wei Yuan, and Qifan Li. 2023. "Nanomaterial Production from Metallic Vapor Bubble Collapse in Liquid Nitrogen" Nanomaterials 13, no. 13: 2021. https://doi.org/10.3390/nano13132021
APA StyleLi, C., Han, R., Li, J., Cao, Y., Yuan, W., & Li, Q. (2023). Nanomaterial Production from Metallic Vapor Bubble Collapse in Liquid Nitrogen. Nanomaterials, 13(13), 2021. https://doi.org/10.3390/nano13132021






