New Insights into N-Doped Porous Carbons as Both Heterogeneous Catalysts and Catalyst Supports: Opportunities for the Catalytic Synthesis of Valuable Compounds
Abstract
1. Introduction
2. N-Doped Porous Carbons: Precursors and Synthesis Strategies
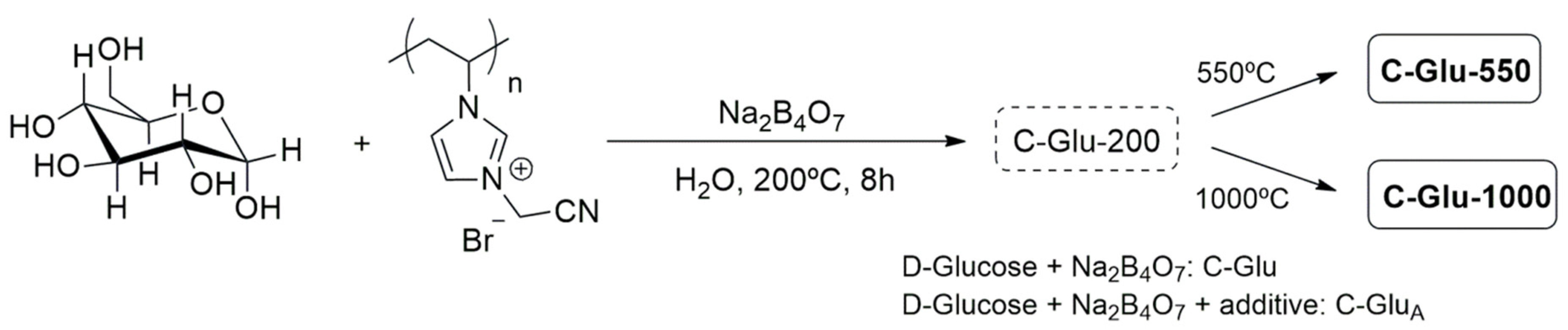
2.1. Biomass- and Waste-Derived N-Doped Carbons
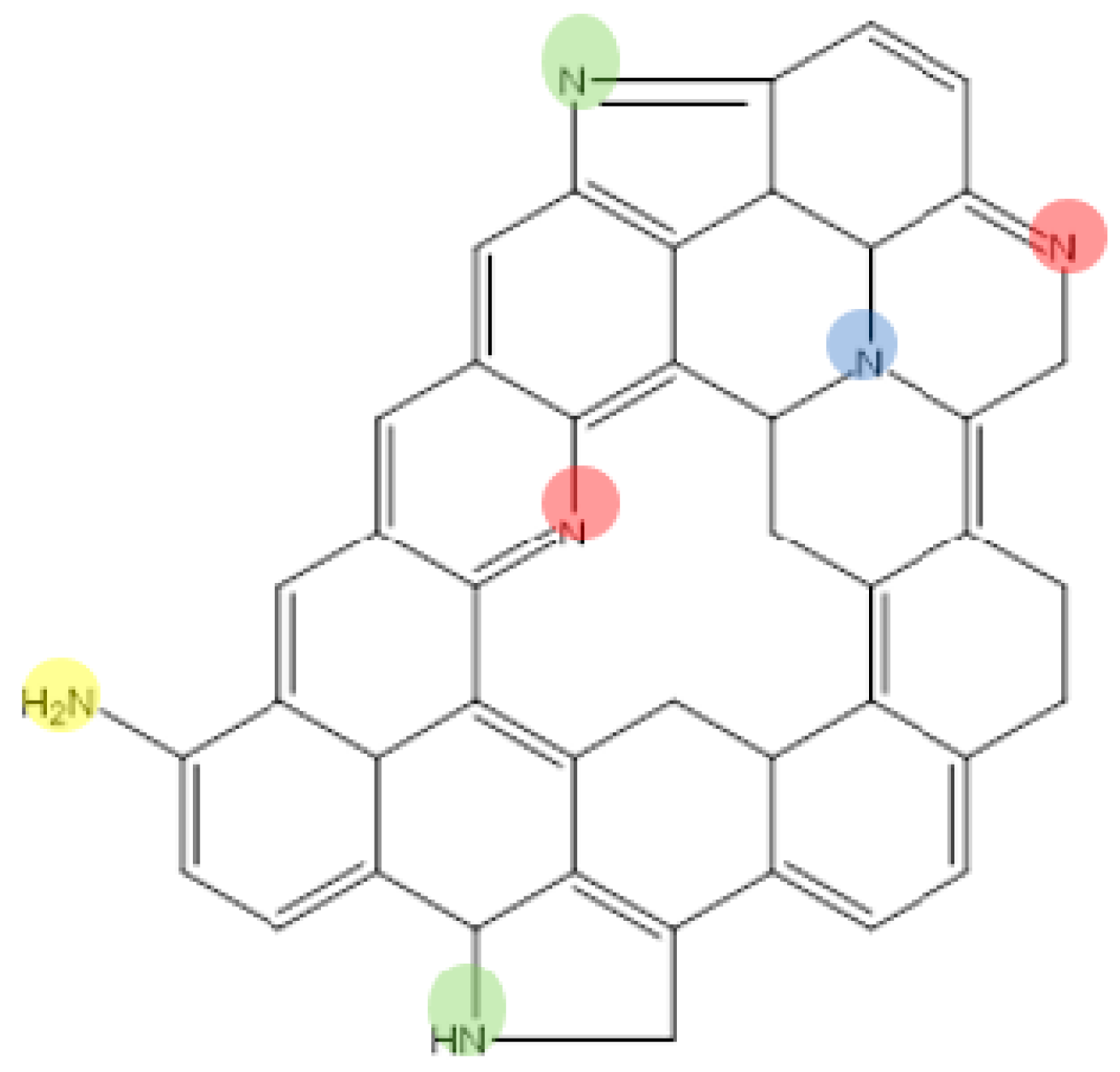
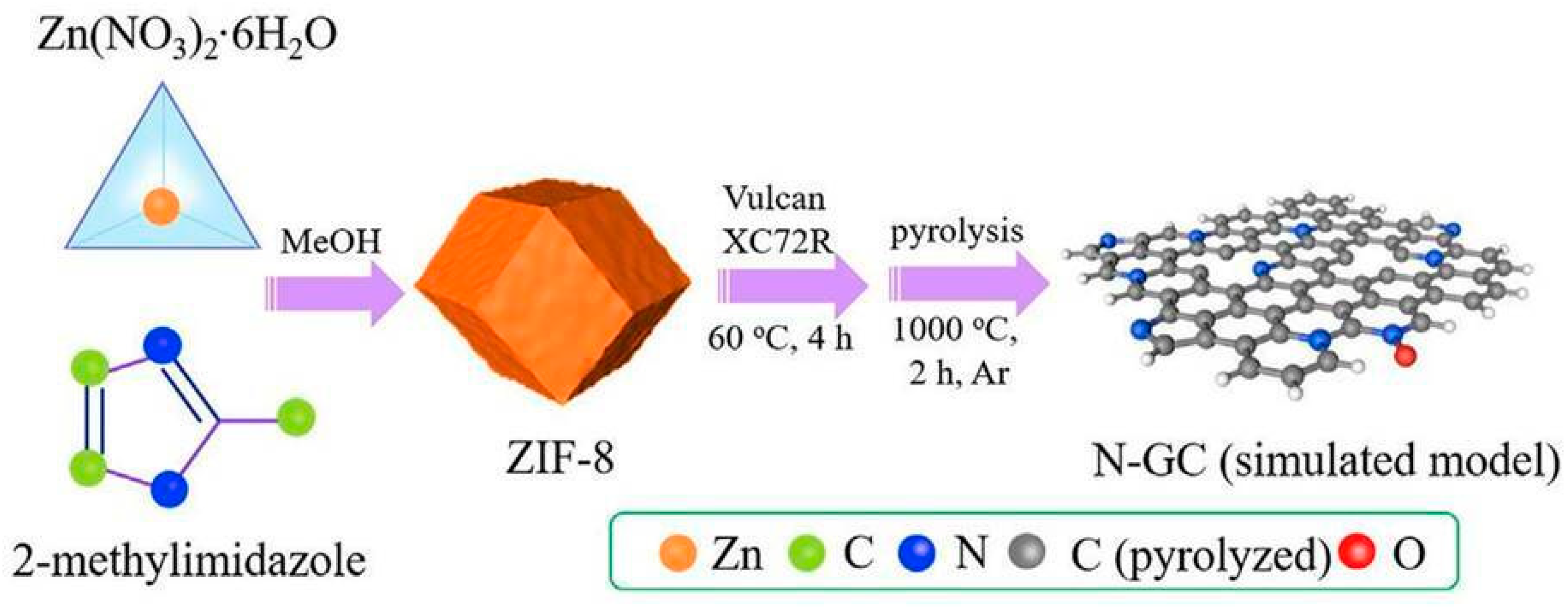
2.2. Covalent Organic Frameworks (COFs) and Metal–Organic Frameworks (MOFs) as Precursors for N-Doped Carbons
2.3. Carbon Nanotubes, Nanofibers, and Graphene N-Doped Materials
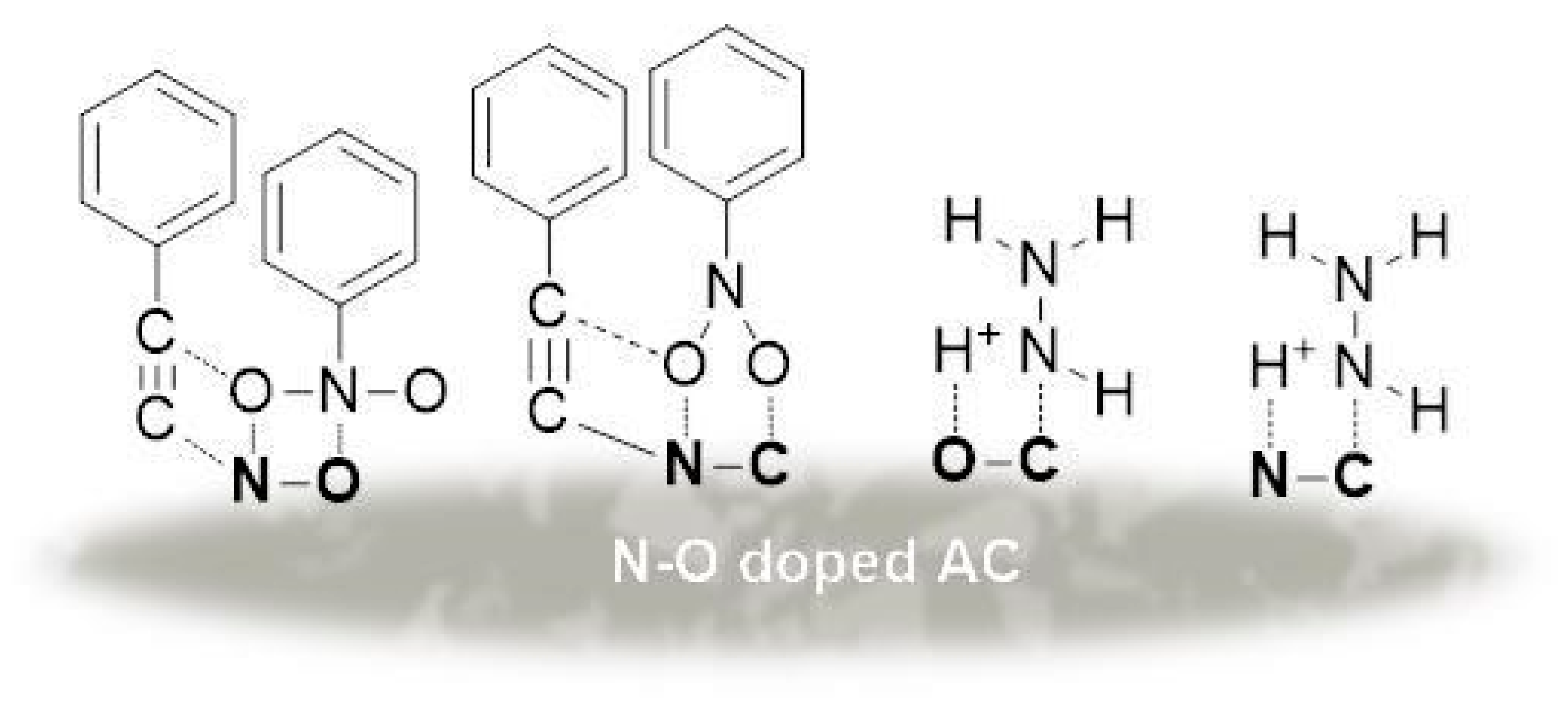
2.4. Carbonization of Nitrogen-Containing Synthetic Materials
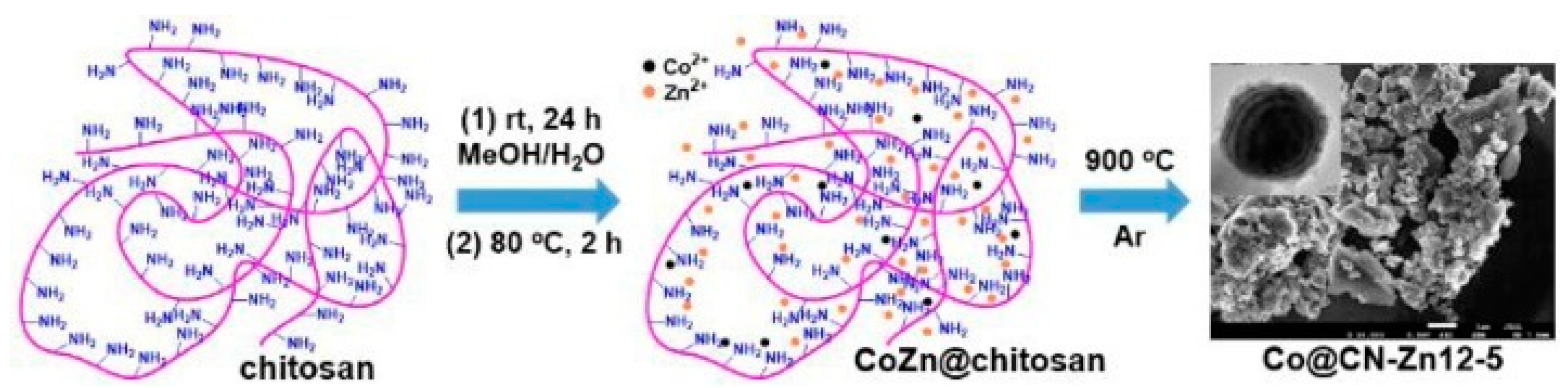
2.5. Metal-Supported N-Doped Porous Carbons
3. N-Doped Porous Carbon Materials in Fine Chemicals Synthesis
3.1. Metal-Free N-Doped Porous Carbon Catalysts
3.1.1. Base-Catalyzed Reactions
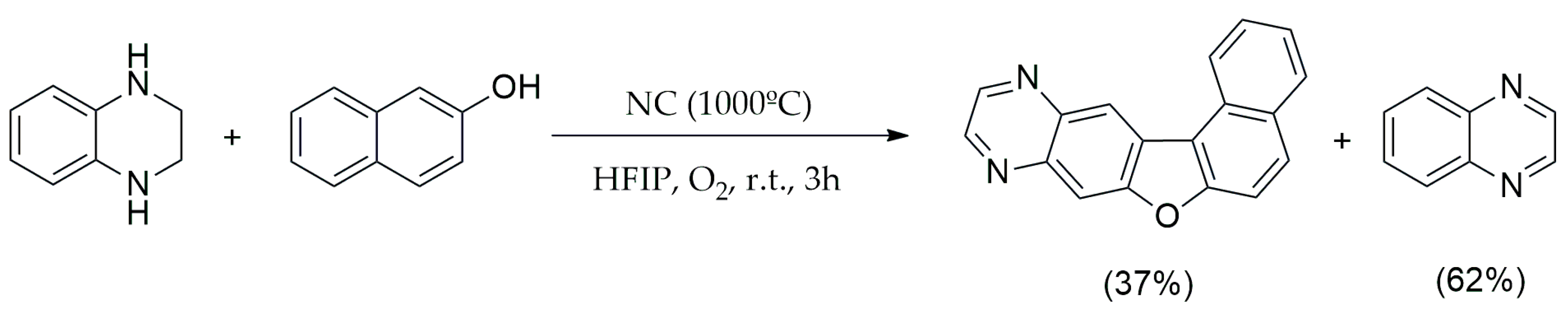
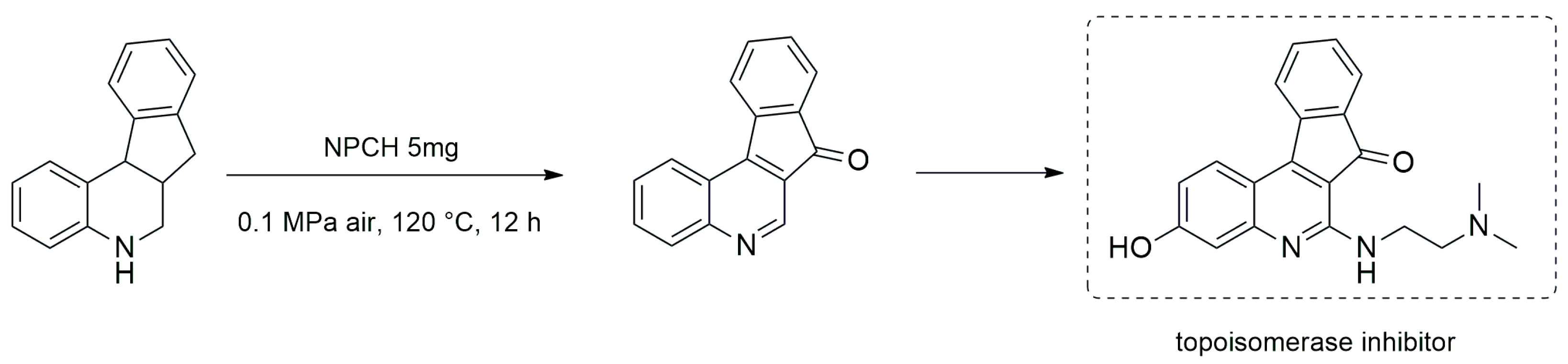
3.1.2. Oxidation–Reduction Reactions
3.2. Metal-Supported N-Doped Porous Carbon Catalysts
3.2.1. Metal-Supported N-Doped Porous from Different Carbon and Nitrogen Sources
Oxidation Reactions
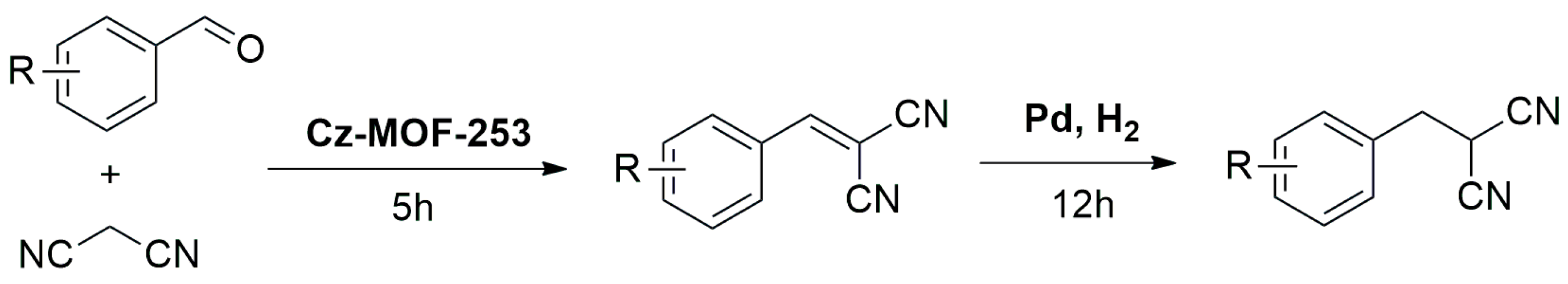
Hydrogenation Reactions
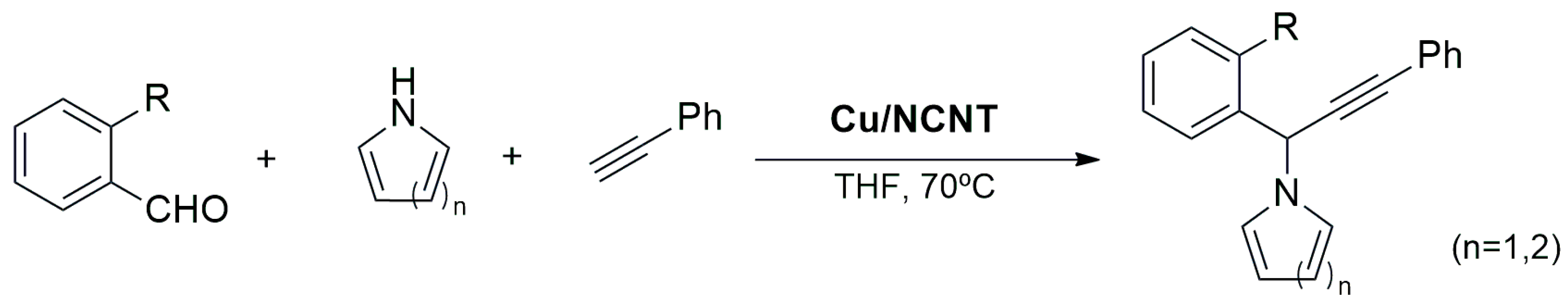
Miscellaneous
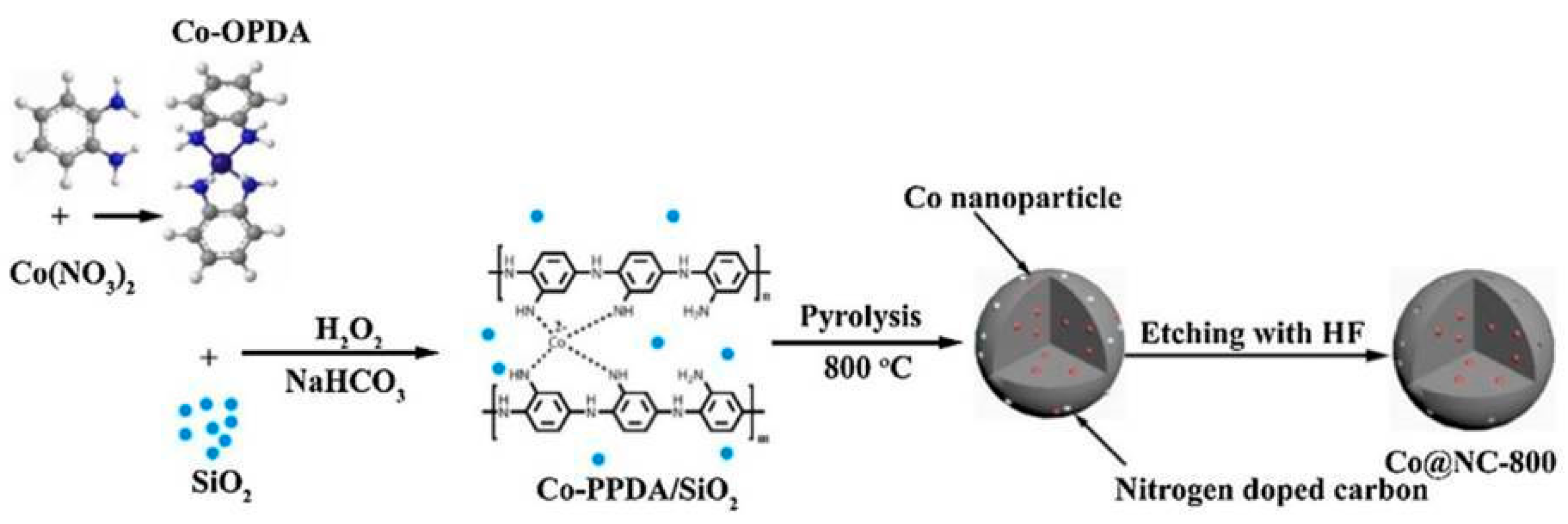
3.2.2. Metal-Supported N-Doped Porous Carbons from MOFs
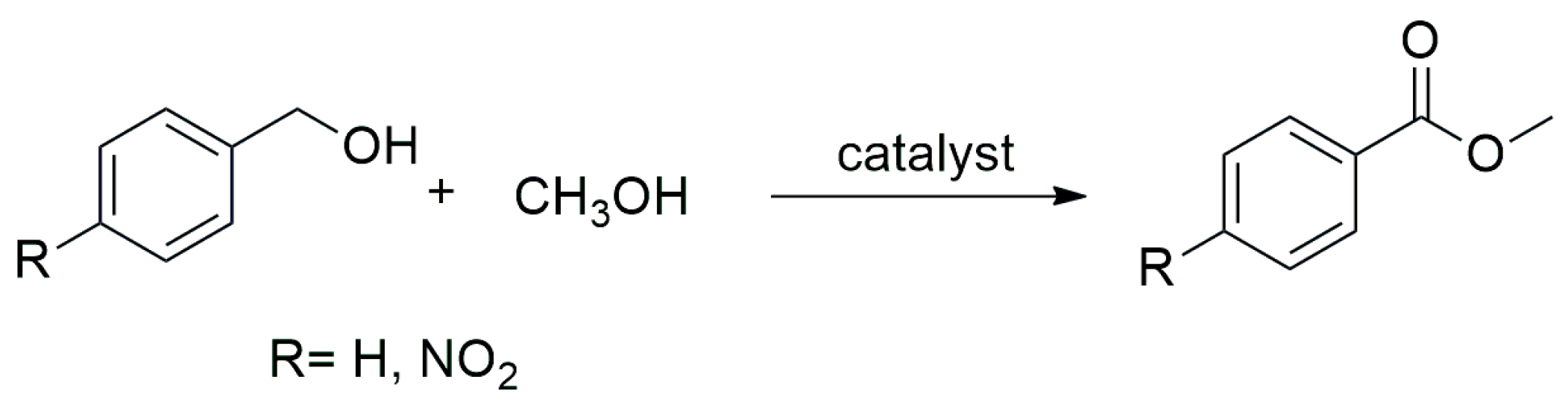
Oxidation Reaction
Reduction Reaction
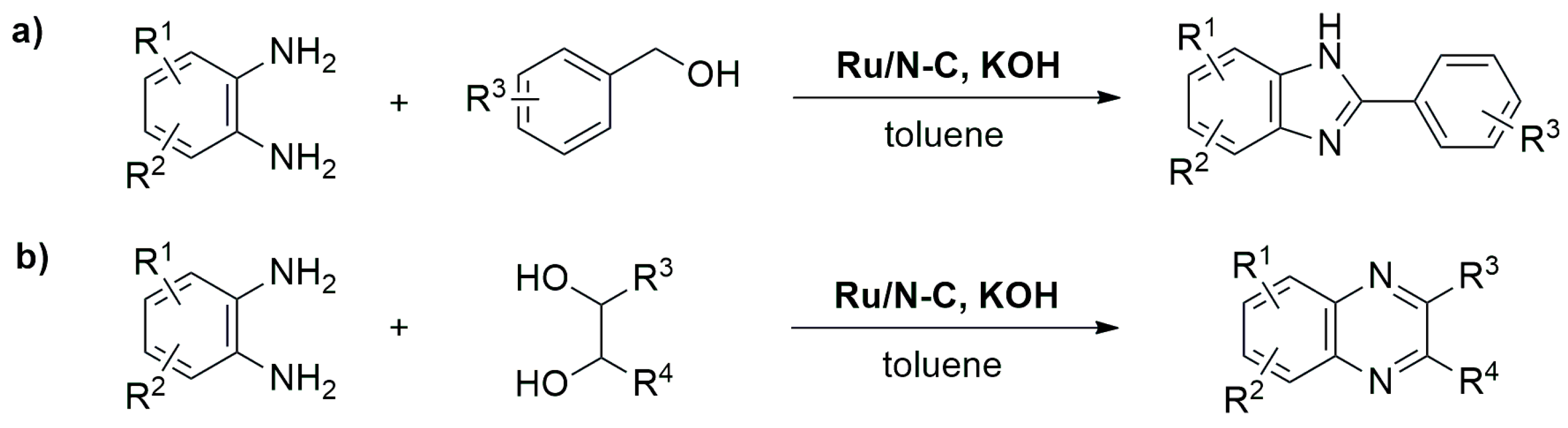
Miscellaneous
3.2.3. Single-Atom Catalysts Derived from N-Doped Porous Carbons
4. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pérez-Mayoral, E.; Matos, I.; Bernardo, M.; Fonseca, I.M. New and Advanced Porous Carbon Materials in Fine Chemical Synthesis. Emerging Precursors of Porous Carbons. Catalysts 2019, 9, 133. [Google Scholar] [CrossRef]
- Godino-Ojer, M.; Morales-Torres, S.; Pérez-Mayoral, E.; Maldonado-Hódar, F.J. Enhanced Catalytic Performance of ZnO/Carbon Materials in the Green Synthesis of Poly-Substituted Quinolines. J. Environ. Chem. Eng. 2022, 10, 106879. [Google Scholar] [CrossRef]
- Serp, P.; Figueiredo, J.L. Carbon Materials for Catalysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Al-Hajri, W.; De Luna, Y.; Bensalah, N. Review on Recent Applications of Nitrogen-Doped Carbon Materials in CO2 Capture and Energy Conversion and Storage. Energy Technol. 2022, 10, 2200498. [Google Scholar] [CrossRef]
- Li, A.; Nicolae, S.A.; Qiao, M.; Preuss, K.; Szilágyi, P.A.; Moores, A.; Titirici, M. Homogenous Meets Heterogenous and Electro-Catalysis: Iron-Nitrogen Molecular Complexes within Carbon Materials for Catalytic Applications. ChemCatChem 2019, 11, 3602–3625. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, L.; Guo, S.; Shao, S.; Li, Z.; Sun, Y.; Hao, S. N-Doped Mesoporous Carbons: From Synthesis to Applications as Metal-Free Reduction Catalysts and Energy Storage Materials. Front. Chem. 2019, 7, 761. [Google Scholar] [CrossRef]
- Lv, Q.; Si, W.; He, J.; Sun, L.; Zhang, C.; Wang, N.; Yang, Z.; Li, X.; Wang, X.; Deng, W.; et al. Selectively Nitrogen-Doped Carbon Materials as Superior Metal-Free Catalysts for Oxygen Reduction. Nat. Commun. 2018, 9, 3376. [Google Scholar] [CrossRef]
- Long, J.; Shen, K.; Li, Y. Bifunctional N-Doped Co@C Catalysts for Base-Free Transfer Hydrogenations of Nitriles: Controllable Selectivity to Primary Amines vs Imines. ACS Catal. 2017, 7, 275–284. [Google Scholar] [CrossRef]
- Gawande, M.B.; Fornasiero, P.; Zbořil, R. Carbon-Based Single-Atom Catalysts for Advanced Applications. ACS Catal. 2020, 10, 2231–2259. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Ge, R.; Zhang, J.; Cairney, J.M.; Li, Y.; Zhu, M.; Li, S.; Li, W. The Effect of Coordination Environment on the Activity and Selectivity of Single-Atom Catalysts. Coord. Chem. Rev. 2022, 461, 214493. [Google Scholar] [CrossRef]
- Jiang, J.; Jiang, P.; Wang, D.; Li, Y. The Synthetic Strategies for Single Atomic Site Catalysts Based on Metal–Organic Frameworks. Nanoscale 2020, 12, 20580–20589. [Google Scholar] [CrossRef]
- Huang, H.; Shen, K.; Chen, F.; Li, Y. Metal–Organic Frameworks as a Good Platform for the Fabrication of Single-Atom Catalysts. ACS Catal. 2020, 10, 6579–6586. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-Doped Carbon Materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Robinson, J.T.; Sanchez, H.; Diankov, G.; Dai, H. Simultaneous Nitrogen Doping and Reduction of Graphene Oxide. J. Am. Chem. Soc. 2009, 131, 15939–15944. [Google Scholar] [CrossRef] [PubMed]
- Ohtsubo, N.; Gohda, S.; Gotoh, K.; Sato, S.; Yamada, Y. Bottom-up Synthesis of Pyridinic Nitrogen-Containing Carbon Materials with C–H Groups next to Pyridinic Nitrogen from Two-Ring Aromatics. Carbon 2023, 207, 270–291. [Google Scholar] [CrossRef]
- Wee, J.-H.; Kim, C.H.; Lee, H.-S.; Choi, G.B.; Kim, D.-W.; Yang, C.-M.; Kim, Y.A. Enriched Pyridinic Nitrogen Atoms at Nanoholes of Carbon Nanohorns for Efficient Oxygen Reduction. Sci. Rep. 2019, 9, 20170. [Google Scholar] [CrossRef]
- Yamada, Y.; Tanaka, H.; Kubo, S.; Sato, S. Unveiling Bonding States and Roles of Edges in Nitrogen-Doped Graphene Nanoribbon by X-Ray Photoelectron Spectroscopy. Carbon 2021, 185, 342–367. [Google Scholar] [CrossRef]
- Prakash, A.; Yadav, S.; Yadav, U.; Saxena, P.S.; Srivastava, A. Recent Advances on Nitrogen-Doped Carbon Quantum Dots and Their Applications in Bioimaging: A Review. Bull. Mater. Sci. 2023, 46, 7. [Google Scholar] [CrossRef]
- Dhilllon, S.K.; Kundu, P.P.; Jain, R. Catalytic Advancements in Carbonaceous Materials for Bio-Energy Generation in Microbial Fuel Cells: A Review. Environ. Sci. Pollut. Res. 2022, 30, 24815–24841. [Google Scholar] [CrossRef]
- Xiaorui, L.; Haiping, Y. A State-of-the-Art Review of N Self-Doped Biochar Development in Supercapacitor Applications. Front. Energy Res. 2023, 11, 1135093. [Google Scholar] [CrossRef]
- Fiorio, J.L.; Garcia, M.A.S.; Gothe, M.L.; Galvan, D.; Troise, P.C.; Conte-Junior, C.A.; Vidinha, P.; Camargo, P.H.C.; Rossi, L.M. Recent Advances in the Use of Nitrogen-Doped Carbon Materials for the Design of Noble Metal Catalysts. Coord. Chem. Rev. 2023, 481, 215053. [Google Scholar] [CrossRef]
- Liu, L.-L.; Ma, M.-X.; Xu, H.; Yang, X.-Y.; Lu, X.-Y.; Yang, P.; Wang, H. S-Doped M-N-C Catalysts for the Oxygen Reduction Reaction: Synthetic Strategies, Characterization, and Mechanism. J. Electroanal. Chem. 2022, 920, 116637. [Google Scholar] [CrossRef]
- Ullah, N.; Ullah, R.; Khan, S.; Xu, Y. Boron Nitride-Based Electrocatalysts for HER, OER, and ORR: A Mini-Review. Front. Mater. Sci. 2021, 15, 543–552. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Yang, R.X.; Dutta, S.; Ok, Y.S.; Wu, K.C.W. Recent Progress in the Development of Biomass-Derived Nitrogen-Doped Porous Carbon. J. Mater. Chem. A Mater. 2021, 9, 3703–3728. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, X.; Wang, H.; Zhao, X.; Kong, F.; Liu, Y. Novel Nitrogen-Doped Porous Carbon with High Surface Areas Prepared from Industrial Alkali Lignin for Supercapacitors. ChemElectroChem 2022, 9, e202200869. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, X.; Song, S.; Sun, M.; Xue, Y.; Yang, G. Preparation of Nitrogen-Doped Cellulose-Based Porous Carbon and Its Carbon Dioxide Adsorption Properties. ACS Omega 2021, 6, 24814–24825. [Google Scholar] [CrossRef]
- Chu, M.; Zhai, Y.; Shang, N.; Guo, P.; Wang, C.; Gao, Y. N-Doped Carbon Derived from the Monomer of Chitin for High-Performance Supercapacitor. Appl. Surf. Sci. 2020, 517, 146140. [Google Scholar] [CrossRef]
- Godino-Ojer, M.; Blazquez-García, R.; Matos, I.; Bernardo, M.; Fonseca, I.M.; Pérez Mayoral, E. Porous Carbons-Derived from Vegetal Biomass in the Synthesis of Quinoxalines. Mechanistic Insights. Catal. Today 2020, 354, 90–99. [Google Scholar] [CrossRef]
- Hammi, N.; Chen, S.; Dumeignil, F.; Royer, S.; El Kadib, A. Chitosan as a Sustainable Precursor for Nitrogen-Containing Carbon Nanomaterials: Synthesis and Uses. Mater. Today Sustain. 2020, 10, 100053. [Google Scholar] [CrossRef]
- Sattayarut, V.; Wanchaem, T.; Ukkakimapan, P.; Yordsri, V.; Dulyaseree, P.; Phonyiem, M.; Obata, M.; Fujishige, M.; Takeuchi, K.; Wongwiriyapan, W.; et al. Nitrogen Self-Doped Activated Carbons: Via the Direct Activation of Samanea Saman Leaves for High Energy Density Supercapacitors. RSC Adv. 2019, 9, 21724–21732. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, Y.; Feng, Z.; Li, X.; Yi, R.; Sun, W.; Zhao, C.; Yang, L. Nitrogen-Doped Hierarchical Porous Activated Carbon Derived from Paddy for High-Performance Supercapacitors. Materials 2021, 14, 318. [Google Scholar] [CrossRef]
- Zhao, G.; Li, Y.; Zhu, G.; Shi, J.; Lu, T.; Pan, L. Biomass-Based N, P, and S Self-Doped Porous Carbon for High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 12052–12060. [Google Scholar] [CrossRef]
- Rao, L.; Ma, R.; Liu, S.; Wang, L.; Wu, Z.; Yang, J.; Hu, X. Nitrogen Enriched Porous Carbons from D-Glucose with Excellent CO2 Capture Performance. Chem. Eng. J. 2019, 362, 794–801. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Yang, H.; Shao, J.; Wang, X.; Chen, Y.; Zhang, S.; Chen, H. Preparation of Nitrogen-Doped Microporous Modified Biochar by High Temperature CO2 –NH3 Treatment for CO2 Adsorption: Effects of Temperature. RSC Adv. 2016, 6, 98157–98166. [Google Scholar] [CrossRef]
- Guo, L.; Yang, J.; Hu, G.; Hu, X.; Wang, L.; Dong, Y.; Dacosta, H.; Fan, M. Role of Hydrogen Peroxide Preoxidizing on CO2 Adsorption of Nitrogen-Doped Carbons Produced from Coconut Shell. ACS Sustain. Chem. Eng. 2016, 4, 2806–2813. [Google Scholar] [CrossRef]
- Wang, S.; Zou, K.; Qian, Y.; Deng, Y.; Zhang, L.; Chen, G. Insight to the Synergistic Effect of N-Doping Level and Pore Structure on Improving the Electrochemical Performance of Sulfur/N-Doped Porous Carbon Cathode for Li-S Batteries. Carbon 2019, 144, 745–755. [Google Scholar] [CrossRef]
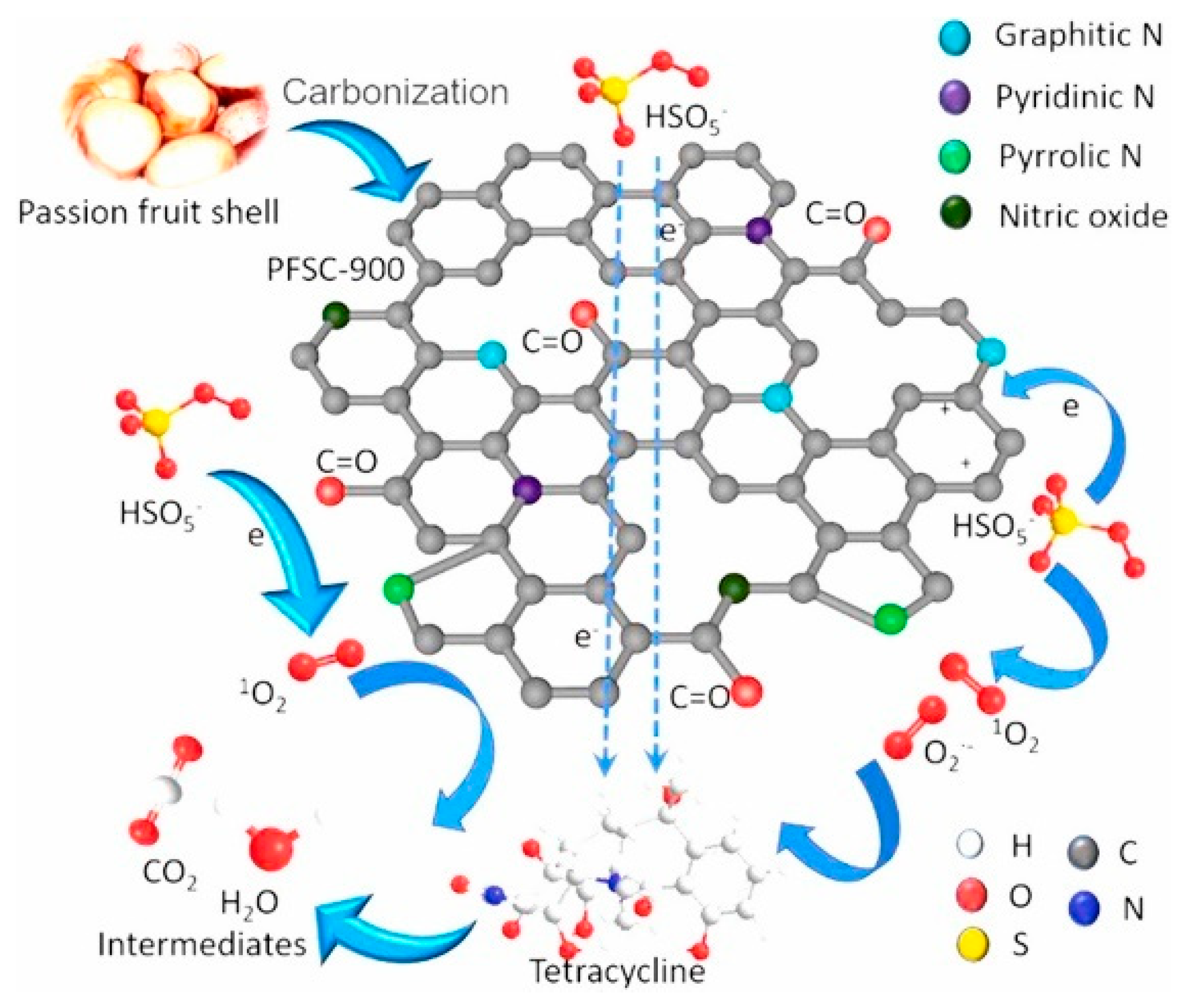
- Hu, Y.; Chen, D.; Zhang, R.; Ding, Y.; Ren, Z.; Fu, M.; Cao, X.; Zeng, G. Singlet Oxygen-Dominated Activation of Peroxymonosulfate by Passion Fruit Shell Derived Biochar for Catalytic Degradation of Tetracycline through a Non-Radical Oxidation Pathway. J. Hazard. Mater. 2021, 419, 126495. [Google Scholar] [CrossRef]
- Llatance-Guevara, L.; Flores, N.E.; Barrionuevo, G.O.; Mullo Casillas, J.L. Waste Biomass Selective and Sustainable Photooxidation to High-Added-Value Products: A Review. Catalysts 2022, 12, 1091. [Google Scholar] [CrossRef]
- Li, Y.; Xu, R.; Wang, B.; Wei, J.; Wang, L.; Shen, M.; Yang, J. Enhanced N-Doped Porous Carbon Derived from KOH-Activated Waste Wool: A Promising Material for Selective Adsorption of CO2/CH4 and CH4/N2. Nanomaterials 2019, 9, 266. [Google Scholar] [CrossRef]
- Mainali, K.; Garcia-Perez, M. Effect of H3PO4 and NaOH Additives on the Co-Carbonization of Cellulose and N-Containing Compounds to Produce N-Doped Chars. J. Anal. Appl. Pyrolysis 2023, 169, 105837. [Google Scholar] [CrossRef]
- Tsubouchi, N.; Nishio, M.; Mochizuki, Y. Role of Nitrogen in Pore Development in Activated Carbon Prepared by Potassium Carbonate Activation of Lignin. Appl. Surf. Sci. 2016, 371, 301–306. [Google Scholar] [CrossRef]
- Li, B.; Hu, J.; Xiong, H.; Xiao, Y. Application and Properties of Microporous Carbons Activated by ZnCl2: Adsorption Behavior and Activation Mechanism. ACS Omega 2020, 5, 9398–9407. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Cheng, T.; Liang, Z.; Wei, T. Investigation of the Mechanism of Phytate-Modified Biochar-Catalyzed Persulfate Degradation of Ponceau 2R. Biochar 2022, 4, 6. [Google Scholar] [CrossRef]
- Park, J.; Kim, T. Biomass-Derived Sustainable Electrode Material for Low-Grade Heat Harvesting. Nanomaterials 2023, 13, 1488. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wei, W.; Chen, H.; Ni, B.-J. Recent Advances in Waste-Derived Functional Materials for Wastewater Remediation. Eco-Environ. Health 2022, 1, 86–104. [Google Scholar] [CrossRef]
- Pérez-Mayoral, E.; Godino-Ojer, M.; Matos, I.; Bernardo, M. Opportunities from Metal Organic Frameworks to Develop Porous Carbons Catalysts Involved in Fine Chemical Synthesis. Catalysts 2023, 13, 541. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abdelhamid, H.N.; Abuelftooh, A.M.; Mohamed, S.G.; Wen, Z.; Sun, X. Covalent Organic Frameworks (COFs)-Derived Nitrogen-Doped Carbon/Reduced Graphene Oxide Nanocomposite as Electrodes Materials for Supercapacitors. J. Energy Storage 2022, 55, 105375. [Google Scholar] [CrossRef]
- Hu, X.; Long, Y.; Fan, M.; Yuan, M.; Zhao, H.; Ma, J.; Dong, Z. Two-Dimensional Covalent Organic Frameworks as Self-Template Derived Nitrogen-Doped Carbon Nanosheets for Eco-Friendly Metal-Free Catalysis. Appl. Catal. B 2019, 244, 25–35. [Google Scholar] [CrossRef]
- Vargheese, S.; Dinesh, M.; Kavya, K.V.; Pattappan, D.; Rajendra Kumar, R.T.; Haldorai, Y. Triazine-Based 2D Covalent Organic Framework-Derived Nitrogen-Doped Porous Carbon for Supercapacitor Electrode. Carbon. Lett. 2021, 31, 879–886. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, J.; Jiang, H.; Chen, R. Well-Defined MOF-Derived Hierarchically Porous N-Doped Carbon Materials for the Selective Hydrogenation of Phenol to Cyclohexanone. Ind. Eng. Chem. Res. 2021, 60, 5806–5815. [Google Scholar] [CrossRef]
- Song, J.P.; Wu, L.; Dong, W.D.; Li, C.F.; Chen, L.H.; Dai, X.; Li, C.; Chen, H.; Zou, W.; Yu, W.B.; et al. MOF-Derived Nitrogen-Doped Core-Shell Hierarchical Porous Carbon Confining Selenium for Advanced Lithium-Selenium Batteries. Nanoscale 2019, 11, 6970–6981. [Google Scholar] [CrossRef]
- Ping, K.; Alam, M.; Kahnert, S.R.; Bhadoria, R.; Mere, A.; Mikli, V.; Käärik, M.; Aruväli, J.; Paiste, P.; Kikas, A.; et al. Multi-Purpose Heterogeneous Catalyst Material from an Amorphous Cobalt Metal–Organic Framework. Mater. Adv. 2021, 2, 4009–4015. [Google Scholar] [CrossRef]
- Goyat, R.; Saharan, Y.; Singh, J.; Umar, A.; Akbar, S. Synthesis of Graphene-Based Nanocomposites for Environmental Remediation Applications: A Review. Molecules 2022, 27, 6433. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Tong, X.; Zhang, G.; Qiao, J.; Gong, Q.; Sun, S. Nitrogen-Doped Carbon Nanotube and Graphene Materials for Oxygen Reduction Reactions. Catalysts 2015, 5, 1574–1602. [Google Scholar] [CrossRef]
- Brownlie, L.; Shapter, J. Advances in Carbon Nanotube N-Type Doping: Methods, Analysis and Applications. Carbon 2018, 126, 257–270. [Google Scholar] [CrossRef]
- Arora, N.; Sharma, N.N. Arc Discharge Synthesis of Carbon Nanotubes: Comprehensive Review. Diam. Relat. Mater. 2014, 50, 135–150. [Google Scholar] [CrossRef]
- Ben Belgacem, A.; Hinkov, I.; Yahia, S.B.; Brinza, O.; Farhat, S. Arc Discharge Boron Nitrogen Doping of Carbon Nanotubes. Mater. Today Commun. 2016, 8, 183–195. [Google Scholar] [CrossRef]
- Pant, M.; Singh, R.; Negi, P.; Tiwari, K.; Singh, Y. A Comprehensive Review on Carbon Nano-Tube Synthesis Using Chemical Vapor Deposition. In Materials Today: Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; Volume 46, pp. 11250–11253. [Google Scholar] [CrossRef]
- Huo, Y.; Xiu, S.; Meng, L.Y.; Quan, B. Solvothermal Synthesis and Applications of Micro/Nano Carbons: A Review. Chem. Eng. J. 2023, 451, 138572. [Google Scholar] [CrossRef]
- Metwally, N.H.; Saad, G.R.; El-Wahab, E.A.A. Grafting of Multiwalled Carbon Nanotubes with Pyrazole Derivatives: Characterization, Antimicrobial Activity and Molecular Docking Study. Int. J. Nanomed. 2019, 14, 6645–6659. [Google Scholar] [CrossRef]
- Cadena, A.; Botka, B.; Kamaraś, K. Organic Molecules Encapsulated in Single-Walled Carbon Nanotubes. Oxf. Open Mater. Sci. 2021, 1, itab009. [Google Scholar] [CrossRef]
- Maria Díez-Pascual, A. Chemical Functionalization of Carbon Nanotubes with Polymers: A Brief Overview. Macromol 2021, 1, 64–83. [Google Scholar] [CrossRef]
- Wang, H.; Keum, J.K.; Hiltner, A.; Baer, E.; Freeman, B.; Rozanski, A.; Galeski, A. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 757–760. [Google Scholar] [CrossRef]
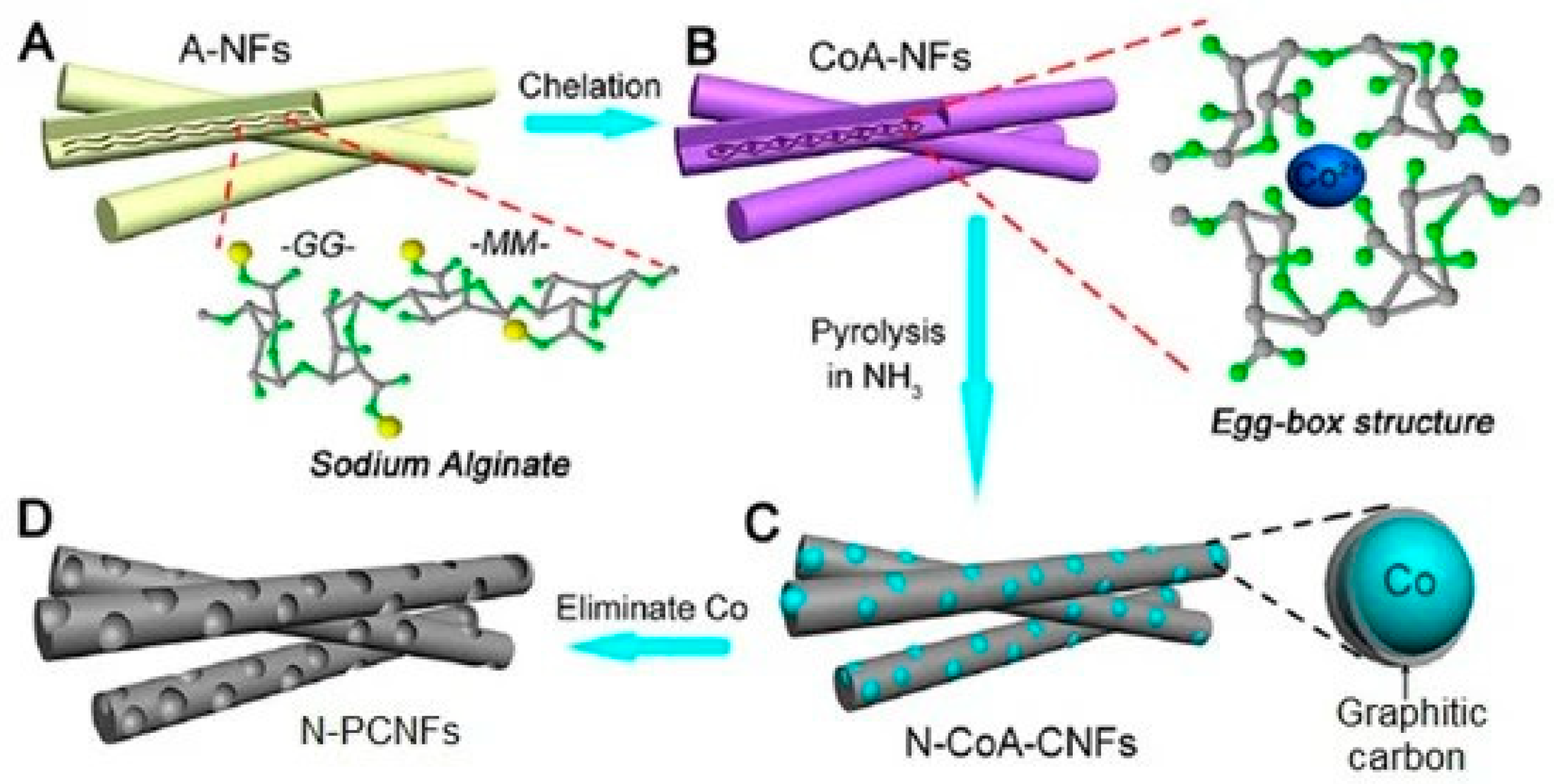
- Li, D.; Lv, C.; Liu, L.; Xia, Y.; She, X.; Guo, S.; Yang, D. Egg-Box Structure in Cobalt Alginate: A New Approach to Multifunctional Hierarchical Mesoporous N-Doped Carbon Nanofibers for Efficient Catalysis and Energy Storage. ACS Cent. Sci. 2015, 1, 261–269. [Google Scholar] [CrossRef]
- Choudhury, A.; Kim, J.H.; Sinha Mahapatra, S.; Yang, K.S.; Yang, D.J. Nitrogen-Enriched Porous Carbon Nanofiber Mat as Efficient Flexible Electrode Material for Supercapacitors. ACS Sustain. Chem. Eng. 2017, 5, 2109–2118. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.F.; Zhong, J.J.; Qian, X.; Song, S.L.; Zhang, Y.G.; Li, D.H. Nitrogen-Enriched Porous Polyacrylonitrile-Based Carbon Fibers for CO2 Capture. Ind. Eng. Chem. Res. 2018, 57, 11608–11616. [Google Scholar] [CrossRef]
- Sun, M.; Pan, D.; Ye, T.; Gu, J.; Zhou, Y.; Wang, J. Ionic Porous Polyamide Derived N-Doped Carbon towards Highly Selective Electroreduction of CO2. Chin. J. Chem. Eng. 2023, 55, 212–221. [Google Scholar] [CrossRef]
- Ma, C.; Bai, J.; Demir, M.; Yu, Q.; Hu, X.; Jiang, W.; Wang, L. Polyacrylonitrile-Derived Nitrogen Enriched Porous Carbon Fiber with High CO2 Capture Performance. Sep. Purif. Technol. 2022, 303, 122299. [Google Scholar] [CrossRef]
- Zhao, X.; Nie, G.; Luan, Y.; Wang, X.; Yan, S.; Long, Y.Z. Nitrogen-Doped Carbon Networks Derived from the Electrospun Polyacrylonitrile@branched Polyethylenimine Nanofibers as Flexible Supercapacitor Electrodes. J. Alloys Compd. 2019, 808, 151737. [Google Scholar] [CrossRef]
- Mooste, M.; Kibena-Põldsepp, E.; Vassiljeva, V.; Merisalu, M.; Kook, M.; Treshchalov, A.; Kisand, V.; Uibu, M.; Krumme, A.; Sammelselg, V.; et al. Electrocatalysts for Oxygen Reduction Reaction Based on Electrospun Polyacrylonitrile, Styrene–Acrylonitrile Copolymer and Carbon Nanotube Composite Fibres. J. Mater. Sci. 2019, 54, 11618–11634. [Google Scholar] [CrossRef]
- Song, B.; Zhu, X.; Wang, W.; Wang, L.; Pei, X.; Qian, X.; Liu, L.; Xu, Z. Toughening of Melamine–Formaldehyde Foams and Advanced Applications Based on Functional Design. J. Ind. Eng. Chem. 2023, 119, 130–152. [Google Scholar] [CrossRef]
- Civioc, R.; Lattuada, M.; Koebel, M.M.; Galmarini, S. Monolithic Resorcinol–Formaldehyde Alcogels and Their Corresponding Nitrogen-Doped Activated Carbons. J. Sol-Gel Sci. Technol. 2020, 95, 719–732. [Google Scholar] [CrossRef]
- Qian, M.; Wang, Z.; Li, Z.; Xu, J.; Sun, P.; Lin, J.; Lin, T.; Huang, F. Sol-Gel Assisted Chemical Activation for Nitrogen Doped Porous Carbon. Microporous Mesoporous Mater. 2019, 286, 18–24. [Google Scholar] [CrossRef]
- Brzeczek-Szafran, A.; Erfurt, K.; Blacha-Grzechnik, A.; Krzywiecki, M.; Boncel, S.; Chrobok, A. Carbohydrate Ionic Liquids and Salts as All-in-One Precursors for N-Doped Carbon. ACS Sustain. Chem. Eng. 2019, 7, 19880–19888. [Google Scholar] [CrossRef]
- Xu, X.; Li, H.; Wang, Y. Selective Hydrogenation of Phenol to Cyclohexanone in Water over Pd@N-Doped Carbon Derived from Ionic-Liquid Precursors. ChemCatChem 2014, 6, 3328–3332. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, Z.; Qin, F.; Gao, J.; Wang, H.; Xu, Z.; Tan, X.; Liu, X.; Li, X.; Yin, Z. Electrocatalytic Activity Enhancement of N,P-Doped Carbon Nanosheets Derived from Polymerizable Ionic Liquids. J. Appl. Electrochem. 2021, 51, 669–679. [Google Scholar] [CrossRef]
- Stanton Ribeiro, M.; Zanatta, M.; Corvo, M.C. Ionic Liquids and Biomass as Carbon Precursors: Synergistically Answering a Call for CO2 Capture and Conversion. Fuel 2022, 327, 125164. [Google Scholar] [CrossRef]
- She, Y.; Chen, J.; Zhang, C.; Lu, Z.; Ni, M.; Sit, P.H.-L.; Leung, M.K.H. Nitrogen-Doped Graphene Derived from Ionic Liquid as Metal-Free Catalyst for Oxygen Reduction Reaction and Its Mechanisms. Appl. Energy 2018, 225, 513–521. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Li, Q.; Wang, Y.; Ma, J. Guanidine-Embedded Poly(Ionic Liquid) as a Versatile Precursor for Self-Templated Synthesis of Nitrogen-Doped Carbons: Tailoring the Microstructure for Enhanced CO2 Capture. Fuel 2022, 329, 125357. [Google Scholar] [CrossRef]
- Kong, Y.; Luo, Y.; Ma, J.; Tang, J.; Huang, Y.; Zhou, M.; Han, S. Pomegranate Clusters of N-Doped Carbon-Coated CoOxSupported on Graphene as a Flexible Lithium-Ion Battery Anode. ACS Appl. Energy Mater. 2022, 5, 5010–5017. [Google Scholar] [CrossRef]
- Feng, J.; Luo, S.-H.; Lin, Y.-C.; Zhan, Y.; Yan, S.-X.; Hou, P.-Q.; Wang, Q.; Zhang, Y.-H. Metal-Organic Framework Derived CoSe2/N-Doped Carbon Core-Shell Nanoparticles Encapsulated in Porous N-Doped Carbon Nanotubes as High-Performance Anodes for Sodium-Ion Batteries. J. Power Sources 2022, 535, 231444. [Google Scholar] [CrossRef]
- Niu, Y.; Teng, X.; Gong, S.; Chen, Z. A Bimetallic Alloy Anchored on Biomass-Derived Porous N-Doped Carbon Fibers as a Self-Supporting Bifunctional Oxygen Electrocatalyst for Flexible Zn-Air Batteries. J. Mater. Chem. A Mater. 2020, 8, 13725–13734. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, H.; Wu, K.; Wang, X.; Xiong, D.; He, M. Fe3C Encapsulated in N-Doped Carbon as Potassium Ion Battery Anode with High Capacity and Long-Term Cycling Performance. J. Alloys Compd. 2022, 910, 164845. [Google Scholar] [CrossRef]
- Li, J.; Song, J.; Luo, L.; Zhang, H.; Feng, J.; Zhao, X.; Guo, X.; Dong, H.; Chen, S.; Liu, H.; et al. Synergy of MXene with Se Infiltrated Porous N-Doped Carbon Nanofibers as Janus Electrodes for High-Performance Sodium/Lithium–Selenium Batteries. Adv. Energy Mater. 2022, 12, 2200894. [Google Scholar] [CrossRef]
- Huang, B.; Jiang, J. N-Doped Carbon Nanosheets Derived from Lignin as a Novel Bifunctional Electrocatalyst for Rechargeable Zinc-Air Battery. Diam. Relat. Mater. 2022, 128, 109291. [Google Scholar] [CrossRef]
- Qiang, F.; Feng, J.; Wang, H.; Yu, J.; Shi, J.; Huang, M.; Shi, Z.; Liu, S.; Li, P.; Dong, L. Oxygen Engineering Enables N-Doped Porous Carbon Nanofibers as Oxygen Reduction/Evolution Reaction Electrocatalysts for Flexible Zinc-Air Batteries. ACS Catal. 2022, 12, 4002–4015. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, W.; Yang, Q.; Dong, J.; Ma, L.; Jiang, Z.; Huang, Y. 3D Hierarchical Oxygen-Deficient AlCoNi-(Oxy)Hydroxides/N-Doped Carbon Hybrids Enable Efficient Battery-Type Asymmetric Supercapacitor. J. Energy Chem. 2022, 72, 416–423. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, S.; Wang, D.; Qing, Y.; Yan, G.; Li, L.; Wu, Y. Low-Tortuosity Carbon Electrode Derived from Wood@ZIF-67 for Supercapacitor Applications. Chem. Eng. J. 2023, 454, 140410. [Google Scholar] [CrossRef]
- Li, S.; Feng, C.; Xie, Y.; Guo, C.; Zhang, L.; Wang, J. Synthesis of Nitrogen-Rich Porous Carbon Nanotubes Coated Co Nanomaterials as Efficient ORR Electrocatalysts via MOFs as Precursor. J. Alloys Compd. 2022, 911, 165060. [Google Scholar] [CrossRef]
- Liu, M.; Wang, L.; Zhao, K.; Shi, S.; Shao, Q.; Zhang, L.; Sun, X.; Zhao, Y.; Zhang, J. Atomically Dispersed Metal Catalysts for the Oxygen Reduction Reaction: Synthesis, Characterization, Reaction Mechanisms and Electrochemical Energy Applications. Energy Environ. Sci. 2019, 12, 2890–2923. [Google Scholar] [CrossRef]
- Ma, J.; Sun, A.; Liu, Y.; Hoang, T.K.A.; Yang, L. Encapsulation of Fe2P-Co2P Nanoparticles in N-Doped Biomass-Based Carbon and Carbon Nanotube Composite as an Efficient Oxygen Reduction Electrocatalyst. Electrochim. Acta 2022, 427, 140893. [Google Scholar] [CrossRef]
- Sarapuu, A.; Kibena-Põldsepp, E.; Borghei, M.; Tammeveski, K. Electrocatalysis of Oxygen Reduction on Heteroatom-Doped Nanocarbons and Transition Metal–Nitrogen–Carbon Catalysts for Alkaline Membrane Fuel Cells. J. Mater. Chem. A 2018, 6, 776–804. [Google Scholar] [CrossRef]
- Alarawi, A.; Ramalingam, V.; He, J.H. Recent Advances in Emerging Single Atom Confined Two-Dimensional Materials for Water Splitting Applications. Mater. Today Energy 2019, 11, 1–23. [Google Scholar] [CrossRef]
- Li, B.; Zhang, L.; Zhang, J.; Su, Y. Recent Insight in Transition Metal Anchored on Nitrogen-Doped Carbon Catalysts: Preparation and Catalysis Application. Electrochem 2022, 3, 520–537. [Google Scholar] [CrossRef]
- Li, M.; Chen, S.; Jiang, Q.; Chen, Q.; Wang, X.; Yan, Y.; Liu, J.; Lv, C.; Ding, W.; Guo, X. Origin of the Activity of Co–N–C Catalysts for Chemoselective Hydrogenation of Nitroarenes. ACS Catal. 2021, 11, 3026–3039. [Google Scholar] [CrossRef]
- Cao, Y.; Mao, S.; Li, M.; Chen, Y.; Wang, Y. Metal/Porous Carbon Composites for Heterogeneous Catalysis: Old Catalysts with Improved Performance Promoted by N-Doping. ACS Catal. 2017, 7, 8090–8112. [Google Scholar] [CrossRef]
- Wei, S.; Li, A.; Liu, J.-C.; Li, Z.; Chen, W.; Gong, Y.; Zhang, Q.; Cheong, W.-C.; Wang, Y.; Zheng, L.; et al. Direct Observation of Noble Metal Nanoparticles Transforming to Thermally Stable Single Atoms. Nat. Nanotechnol. 2018, 13, 856–861. [Google Scholar] [CrossRef]
- Yang, J.; Qiu, Z.; Zhao, C.; Wei, W.; Chen, W.; Li, Z.; Qu, Y.; Dong, J.; Luo, J.; Li, Z.; et al. In Situ Thermal Atomization To Convert Supported Nickel Nanoparticles into Surface-Bound Nickel Single-Atom Catalysts. Angew. Chem. Int. Ed. 2018, 57, 14095–14100. [Google Scholar] [CrossRef]
- Munnik, P.; De Jongh, P.E.; De Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef]
- Campelo, J.M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A. Sustainable Preparation of Supported Metal Nanoparticles and Their Applications in Catalysis. ChemSusChem 2009, 2, 18–45. [Google Scholar] [CrossRef]
- Wang, J.; Kim, J.; Choi, S.; Wang, H.; Lim, J. A Review of Carbon-Supported Nonprecious Metals as Energy-Related Electrocatalysts. Small Methods 2020, 4, 2000621. [Google Scholar] [CrossRef]
- He, L.; Weniger, F.; Neumann, H.; Beller, M. Synthesis, Characterization, and Application of Metal Nanoparticles Supported on Nitrogen-Doped Carbon: Catalysis beyond Electrochemistry. Angew. Chem. Int. Ed. 2016, 55, 12582–12594. [Google Scholar] [CrossRef]
- Chen, P.; Chew, L.M.; Xia, W. The Influence of the Residual Growth Catalyst in Functionalized Carbon Nanotubes on Supported Pt Nanoparticles Applied in Selective Olefin Hydrogenation. J. Catal. 2013, 307, 84–93. [Google Scholar] [CrossRef]
- Ombaka, L.M.; Ndungu, P.G.; Nyamori, V.O. Pyrrolic Nitrogen-Doped Carbon Nanotubes: Physicochemical Properties, Interactions with Pd and Their Role in the Selective Hydrogenation of Nitrobenzophenone. RSC Adv. 2015, 5, 109–122. [Google Scholar] [CrossRef]
- Castillejos, E.; Chico, R.; Bacsa, R.; Coco, S.; Espinet, P.; Pérez-Cadenas, M.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I.; Serp, P. Selective Deposition of Gold Nanoparticles on or inside Carbon Nanotubes and Their Catalytic Activity for Preferential Oxidation of CO. Eur. J. Inorg. Chem. 2010, 2010, 5096–5102. [Google Scholar] [CrossRef]
- Campisi, S.; Chan-Thaw, C.E.; Villa, A. Understanding Heteroatom-Mediated Metal-Support Interactions in Functionalized Carbons: A Perspective Review. Appl. Sci. 2018, 8, 1159. [Google Scholar] [CrossRef]
- Westerhaus, F.A.; Jagadeesh, R.V.; Wienhöfer, G.; Pohl, M.M.; Radnik, J.; Surkus, A.E.; Rabeah, J.; Junge, K.; Junge, H.; Nielsen, M.; et al. Heterogenized Cobalt Oxide Catalysts for Nitroarene Reduction by Pyrolysis of Molecularly Defined Complexes. Nat. Chem. 2013, 5, 537–543. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Yu, A.; Zhang, L.; Zhang, J. A Review on Non-Precious Metal Electrocatalysts for PEM Fuel Cells. Energy Environ. Sci. 2011, 4, 3167–3192. [Google Scholar] [CrossRef]
- Wood, K.N.; O’Hayre, R.; Pylypenko, S. Recent Progress on Nitrogen/Carbon Structures Designed for Use in Energy and Sustainability Applications. Energy Environ. Sci. 2014, 7, 1212–1249. [Google Scholar] [CrossRef]
- Cui, X.; Li, Y.; Bachmann, S.; Scalone, M.; Surkus, A.E.; Junge, K.; Topf, C.; Beller, M. Synthesis and Characterization of Iron-Nitrogen-Doped Graphene/Core-Shell Catalysts: Efficient Oxidative Dehydrogenation of N-Heterocycles. J. Am. Chem. Soc. 2015, 137, 10652–10658. [Google Scholar] [CrossRef]
- Chung, D.Y.; Jun, S.W.; Yoon, G.; Kwon, S.G.; Shin, D.Y.; Seo, P.; Yoo, J.M.; Shin, H.; Chung, Y.H.; Kim, H.; et al. Highly Durable and Active PtFe Nanocatalyst for Electrochemical Oxygen Reduction Reaction. J. Am. Chem. Soc. 2015, 137, 15478–15485. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, K.; Zhu, Q.; Kong, X. Design of N-Doped Carbon Materials Assisted Co–CoOx Anchoring on Mesoporous Silica Spheres Catalyst for Levulinic Acid Valorization. ChemCatChem 2022, 14, e202201001. [Google Scholar] [CrossRef]
- Niu, H.J.; Zhang, L.; Feng, J.J.; Zhang, Q.L.; Huang, H.; Wang, A.J. Graphene-Encapsulated Cobalt Nanoparticles Embedded in Porous Nitrogen-Doped Graphitic Carbon Nanosheets as Efficient Electrocatalysts for Oxygen Reduction Reaction. J. Colloid. Interface Sci. 2019, 552, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Zai, S.F.; Zhou, Y.T.; Du, L.; Jiang, Q. Fe3C-Co Nanoparticles Encapsulated in a Hierarchical Structure of N-Doped Carbon as a Multifunctional Electrocatalyst for ORR, OER, and HER. Adv. Funct. Mater. 2019, 29, 1901949. [Google Scholar] [CrossRef]
- Kumar, Y.; Mooste, M.; Tammeveski, K. Recent Progress of Transition Metal-Based Bifunctional Electrocatalysts for Rechargeable Zinc–Air Battery Application. Curr. Opin. Electrochem. 2023, 38, 101229. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, T.; Ren, K.; Yu, S.; Liu, M.; Wang, Z.; Li, X.; Wang, L.; Wang, H. Metal-Organic Frameworks-Derived Ru-Doped Co2P/N-Doped Carbon Composite Nanosheet Arrays as Bifunctional Electrocatalysts for Hydrogen Evolution and Urea Oxidation. Chem. Eng. J. 2021, 408, 127308. [Google Scholar] [CrossRef]
- Chen, X.; Ma, D.D.; Chen, B.; Zhang, K.; Zou, R.; Wu, X.T.; Zhu, Q.L. Metal–Organic Framework-Derived Mesoporous Carbon Nanoframes Embedded with Atomically Dispersed Fe–Nx Active Sites for Efficient Bifunctional Oxygen and Carbon Dioxide Electroreduction. Appl. Catal. B 2020, 267, 118720. [Google Scholar] [CrossRef]
- Hu, X.M.; Hval, H.H.; Bjerglund, E.T.; Dalgaard, K.J.; Madsen, M.R.; Pohl, M.M.; Welter, E.; Lamagni, P.; Buhl, K.B.; Bremholm, M.; et al. Selective CO2 Reduction to CO in Water Using Earth-Abundant Metal and Nitrogen-Doped Carbon Electrocatalysts. ACS Catal. 2018, 8, 6255–6264. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, D.; Li, J.; Dong, L.; Ong, W.J.; He, Y. A Highly Efficient Fenton-like Catalyst Based on Isolated Diatomic Fe-Co Anchored on N-Doped Porous Carbon. Chem. Eng. J. 2021, 404, 126376. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Zeng, Z.; Zeng, G.; Xu, P.; Xiao, R.; Huang, D.; Chen, X.; He, L.; Zhou, C.; et al. Metal-Organic Frameworks Derived Bi2O2CO3/Porous Carbon Nitride: A Nanosized Z-Scheme Systems with Enhanced Photocatalytic Activity. Appl. Catal. B 2020, 267, 118700. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Z.; Wang, H.; Wang, Y. Toward Efficient Single-Atom Catalysts for Renewable Fuels and Chemicals Production from Biomass and CO2. Appl. Catal. B 2021, 292, 120162. [Google Scholar] [CrossRef]
- Li, S.; Yao, N.; Zhao, F.; Li, X. Nitrogen-Doped Carbon Species: A Promising Nonmetallic Promoter for the Co/SiO2 Fischer-Tropsch Synthesis Catalyst. Catal. Sci. Technol. 2016, 6, 2188–2194. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Xia, C.; Li, F. Nitrogen-Functionalized Ordered Mesoporous Carbons as Multifunctional Supports of Ultrasmall Pd Nanoparticles for Hydrogenation of Phenol. ACS Catal. 2013, 3, 2440–2448. [Google Scholar] [CrossRef]
- Nongwe, I.; Ravat, V.; Meijboom, R.; Coville, N.J. Efficient and Reusable Co/Nitrogen Doped Hollow Carbon Sphere Catalysts for the Aerobic Oxidation of Styrene. Appl. Catal. A Gen. 2013, 466, 1–8. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, S.; Liu, Z. Influence of the Synergistic Effect between Co-N-C and Ceria on the Catalytic Performance for Selective Oxidation of Ethylbenzene. Phys. Chem. Chem. Phys. 2015, 17, 14012–14020. [Google Scholar] [CrossRef]
- Cui, T.; Ma, L.; Wang, S.; Ye, C.; Liang, X.; Zhang, Z.; Meng, G.; Zheng, L.; Hu, H.S.; Zhang, J.; et al. Atomically Dispersed Pt-N3C1Sites Enabling Efficient and Selective Electrocatalytic C-C Bond Cleavage in Lignin Models under Ambient Conditions. J. Am. Chem. Soc. 2021, 143, 9429–9439. [Google Scholar] [CrossRef]
- Ramu, V.G.; Bordoloi, A.; Nagaiah, T.C.; Schuhmann, W.; Muhler, M.; Cabrele, C. Copper Nanoparticles Stabilized on Nitrogen-Doped Carbon Nanotubes as Efficient and Recyclable Catalysts for Alkyne/Aldehyde/Cyclic Amine A 3-Type Coupling Reactions. Appl. Catal. A Gen. 2012, 431–432, 88–94. [Google Scholar] [CrossRef]
- Liu, X.; Dai, L. Carbon-Based Metal-Free Catalysts. Nat. Rev. Mater. 2016, 1, 16064. [Google Scholar] [CrossRef]
- Van Dommele, S.; de Jong, K.P.; Bitter, J.H. Nitrogen-Containing Carbon Nanotubes as Solid Base Catalysts. Chem. Commun. 2006, 46, 4859–4861. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Jin, H.; Bing, N. Nitrogen-Doped Carbon Nanotubes with Variable Basicity: Preparation and Catalytic Properties. Catal. Commun. 2011, 15, 78–81. [Google Scholar] [CrossRef]
- Kan-nari, N.; Okamura, S.; Fujita, S.; Ozaki, J.; Arai, M. Nitrogen-Doped Carbon Materials Prepared by Ammoxidation as Solid Base Catalysts for Knoevenagel Condensation and Transesterification Reactions. Adv. Synth. Catal. 2010, 352, 1476–1484. [Google Scholar] [CrossRef]
- Fujita, S.; Katagiri, A.; Watanabe, H.; Asano, S.; Yoshida, H.; Arai, M. Preparation of Nitrogen-Doped Carbon from Polyacrylonitrile and Its Application as a Solid-Base Catalyst. ChemCatChem 2015, 7, 2965–2970. [Google Scholar] [CrossRef]
- Li, X.; Zhang, B.; Fang, Y.; Sun, W.; Qi, Z.; Pei, Y.; Qi, S.; Yuan, P.; Luan, X.; Goh, T.W.; et al. Metal–Organic-Framework-Derived Carbons: Applications as Solid-Base Catalyst and Support for Pd Nanoparticles in Tandem Catalysis. Chem.–A Eur. J. 2017, 23, 4266–4270. [Google Scholar] [CrossRef] [PubMed]
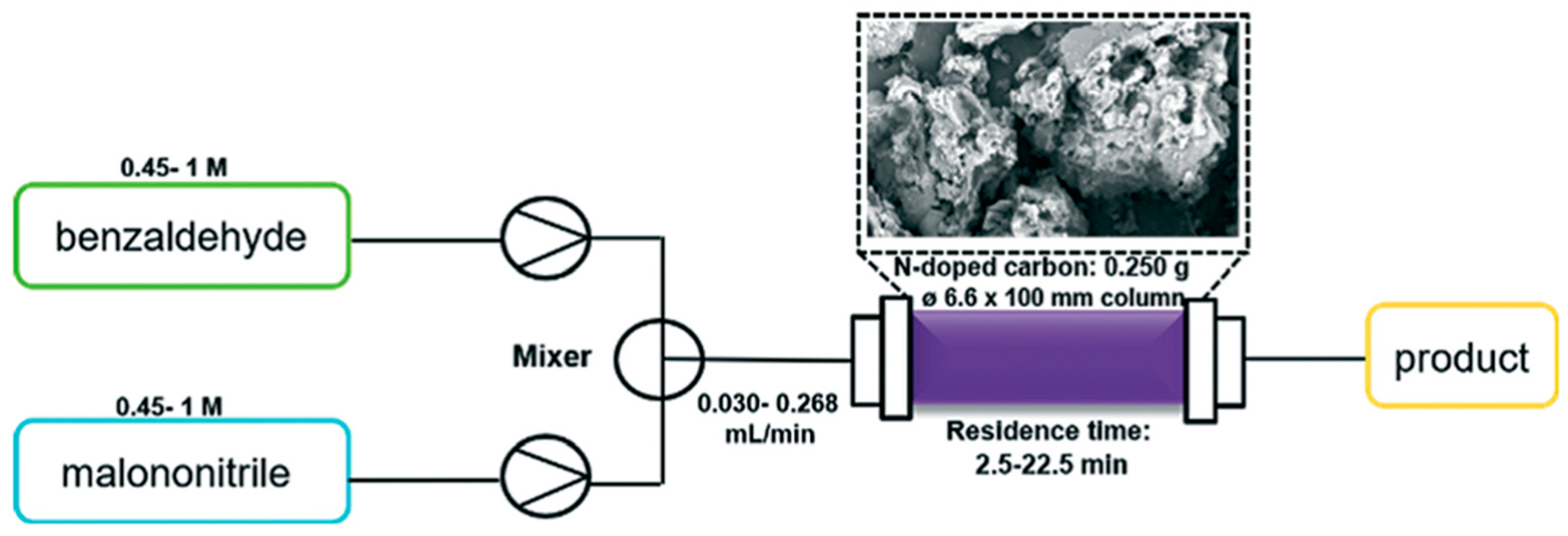
- Brzęczek-Szafran, A.; Gwóźdź, M.; Kolanowska, A.; Krzywiecki, M.; Latos, P.; Chrobok, A. N-Doped Carbon as a Solid Base Catalyst for Continuous Flow Knoevenagel Condensation. React. Chem. Eng. 2021, 6, 1246–1253. [Google Scholar] [CrossRef]
- Watanabe, H.; Asano, S.; Fujita, S.; Yoshida, H.; Arai, M. Nitrogen-Doped, Metal-Free Activated Carbon Catalysts for Aerobic Oxidation of Alcohols. ACS Catal. 2015, 5, 2886–2894. [Google Scholar] [CrossRef]
- Fujita, S.-I.; Yamada, K.; Katagiri, A.; Watanabe, H.; Yoshida, H.; Arai, M. Nitrogen-Doped Metal-Free Carbon Catalysts for Aerobic Oxidation of Xanthene. Appl. Catal. A Gen. 2014, 488, 171–175. [Google Scholar] [CrossRef]
- Yang, S.; Peng, L.; Huang, P.; Wang, X.; Sun, Y.; Cao, C.; Song, W. Nitrogen, Phosphorus, and Sulfur Co-Doped Hollow Carbon Shell as Superior Metal-Free Catalyst for Selective Oxidation of Aromatic Alkanes. Angew. Chem. Int. Ed. 2016, 55, 4016–4020. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y. Nanoporous Carbons Derived from MOFs as Metal-Free Catalysts for Selective Aerobic Oxidations. J. Mater. Chem. 2016, 4, 5247–5257. [Google Scholar] [CrossRef]
- Ren, Y.; Yuan, Z.; Lv, K.; Sun, J.; Zhang, Z.; Chi, Q. Selective and Metal-Free Oxidation of Biomass-Derived 5-Hydroxymethylfurfural to 2,5-Diformylfuran over Nitrogen-Doped Carbon Materials. Green Chem. 2018, 20, 4946–4956. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, J.; Guan, R.; Song, Q.; Jiang, H.; Zhang, M. Selective Construction of Fused Heterocycles by Mild Oxidative C-H Functionalization Using Non-Metallic Catalysis. Cell Rep. Phys. Sci. 2021, 2, 100383. [Google Scholar] [CrossRef]
- Sun, K.; Shan, H.; Ma, R.; Wang, P.; Neumann, H.; Lu, G.-P.; Beller, M. Catalytic Oxidative Dehydrogenation of N-Heterocycles with Nitrogen/Phosphorus Co-Doped Porous Carbon Materials. Chem. Sci. 2022, 13, 6865–6872. [Google Scholar] [CrossRef]
- Fujita, S.; Watanabe, H.; Katagiri, A.; Yoshida, H.; Arai, M. Nitrogen and Oxygen-Doped Metal-Free Carbon Catalysts for Chemoselective Transfer Hydrogenation of Nitrobenzene, Styrene, and 3-Nitrostyrene with Hydrazine. J. Mol. Catal. A Chem. 2014, 393, 257–262. [Google Scholar] [CrossRef]
- Fujita, S.; Asano, S.; Arai, M. Nitrobenzene-Assisted Reduction of Phenylacetylene with Hydrazine over Nitrogen-Doped Metal-Free Activated Carbon Catalyst: Significance of Interactions among Substrates and Catalyst. J. Mol. Catal. A Chem. 2016, 423, 181–184. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Ye, R.; Yan, X.; Wang, L.; Jian, P. Versatile Bifunctional Nitrogen-Doped Porous Carbon Derived from Biomass in Catalytic Reduction of 4-Nitrophenol and Oxidation of Styrene. Chin. J. Catal. 2020, 41, 1217–1229. [Google Scholar] [CrossRef]
- Liu, W.; Li, S.-Q.; Liu, W.-X.; Zhang, Q.; Shao, J.; Tian, J.-L. MOF-Derived B, N Co-Doped Porous Carbons as Metal-Free Catalysts for Highly Efficient Nitro Aromatics Reduction. J. Environ. Chem. Eng. 2021, 9, 105689. [Google Scholar] [CrossRef]
- Wang, L.; Ye, R.; Jian, P.; Liu, J. Pumpkin-Derived N-Doped Porous Carbon for Enhanced Liquid-Phase Reduction of 2-Methyl-4-Nitrophenol. J. Colloid Interface Sci. 2022, 606, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Kaiser, S.K.; Hauert, R.; Pérez-Ramírez, J. Descriptors for High-Performance Nitrogen-Doped Carbon Catalysts in Acetylene Hydrochlorination. ACS Catal. 2018, 8, 1114–1121. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, Y.; Li, H.; Chen, Z.; Wang, Y. Solvent-Free Aerobic Oxidation of Hydrocarbons and Alcohols with Pd@N-Doped Carbon from Glucose. Nat. Commun. 2013, 4, 1593. [Google Scholar] [CrossRef]
- Gil, S.; Lucas, P.J.; Nieto-Márquez, A.; Sánchez-Silva, L.; Giroir-Fendler, A.; Romero, A.; Valverde, J.L. Synthesis and Characterization of Nitrogen-Doped Carbon Nanospheres Decorated with Au Nanoparticles for the Liquid-Phase Oxidation of Glycerol. Ind. Eng. Chem. Res. 2014, 53, 16696–16706. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, G.-P.; Zhao, X.; Cao, X.; Yang, L.; Zhou, B.; Zhong, Q.; Chen, Z. Porous Cobalt@N-Doped Carbon Derived from Chitosan for Oxidative Esterification of 5-Hydroxymethylfurfural: The Roles of Zinc in the Synthetic and Catalytic Process. Mol. Catal. 2020, 482, 110695. [Google Scholar] [CrossRef]
- Sikora, E.; Kiss, A.; Göndör, Z.H.; Pekker, P.; Kristály, F.; Szőri, M.; Rágyanszki, A.; Viskolcz, B.; Fiser, B.; Vanyorek, L. Fine-Tuning the Catalytic Activity by Applying Nitrogen-Doped Carbon Nanotubes as Catalyst Supports for the Hydrogenation of Olefins. React. Kinet. Mech. Catal. 2020, 129, 95–106. [Google Scholar] [CrossRef]
- Liu, J.; Sun, J.; Singh, T.; Lin, S.; Ma, L. Facile Synthesis of N-Doped Graphene Encapsulated Ni@N/C Catalyst and Its Catalysis for Highly Selective Semi-Hydrogenation of Alkynes. Green Chem. Eng. 2022, 3, 395–404. [Google Scholar] [CrossRef]
- Gong, W.; Chen, C.; Zhang, H.; Zhang, Y.; Zhang, Y.; Wang, G.; Zhao, H. Highly Selective Liquid-Phase Hydrogenation of Furfural over N-Doped Carbon Supported Metallic Nickel Catalyst under Mild Conditions. Mol. Catal. 2017, 429, 51–59. [Google Scholar] [CrossRef]
- Lv, J.; Liu, Z.; Dong, Z. Iron Oxide Modified N-Doped Porous Carbon Derived from Porous Organic Polymers as a Highly-Efficient Catalyst for Reduction of Nitroarenes. Mol. Catal. 2020, 498, 111249. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, B.; Zhou, P.; Zhang, Z.; Chi, Q. Preparation of Nitrogen-Doped Carbon Supported Cobalt Catalysts and Its Application in the Reductive Amination. J. Catal. 2019, 370, 347–356. [Google Scholar] [CrossRef]
- Guo, B.; Li, H.-X.; Zhang, S.-Q.; Young, D.J.; Lang, J.-P. C-N Bond Formation Catalyzed by Ruthenium Nanoparticles Supported on N-Doped Carbon via Acceptorless Dehydrogenation to Secondary Amines, Imines, Benzimidazoles and Quinoxalines. ChemCatChem 2018, 10, 5627–5636. [Google Scholar] [CrossRef]
- Song, T.; Ren, P.; Ma, Z.; Xiao, J.; Yang, Y. Highly Dispersed Single-Phase Ni2P Nanoparticles on N,P-Codoped Porous Carbon for Efficient Synthesis of N-Heterocycles. ACS Sustain. Chem. Eng. 2020, 8, 267–277. [Google Scholar] [CrossRef]
- Pérez-Mayoral, E.; Matos, I.; Bernardo, M.; Ventura, M.; Fonseca, I.M. Carbon-Based Materials for the Development of Highly Dispersed Metal Catalysts: Towards Highly Performant Catalysts for Fine Chemical Synthesis. Catalysts 2020, 10, 1407. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, H.; Bai, C.; Liao, S.; Li, Y. Base-Free Oxidation of Alcohols to Esters at Room Temperature and Atmospheric Conditions Using Nanoscale Co-Based Catalysts. ACS Catal. 2015, 5, 1850–1856. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Chen, Y.-Z.; Cao, L.; Lu, J.; Jiang, H.-L. Conversion of a Metal–Organic Framework to N-Doped Porous Carbon Incorporating Co and CoO Nanoparticles: Direct Oxidation of Alcohols to Esters. Chem. Commun. 2015, 51, 8292–8295. [Google Scholar] [CrossRef]
- Long, J.; Zhou, Y.; Li, Y. Transfer Hydrogenation of Unsaturated Bonds in the Absence of Base Additives Catalyzed by a Cobalt-Based Heterogeneous Catalyst. Chem. Commun. 2015, 51, 2331–2334. [Google Scholar] [CrossRef]
- Long, J.; Shen, K.; Chen, L.; Li, Y. Multimetal-MOF-Derived Transition Metal Alloy NPs Embedded in an N-Doped Carbon Matrix: Highly Active Catalysts for Hydrogenation Reactions. J. Mater. Chem. 2016, 4, 10254–10262. [Google Scholar] [CrossRef]
- Bennedsen, N.R.; Kramer, S.; Mielby, J.J.; Kegnæs, S. Cobalt–Nickel Alloy Catalysts for Hydrosilylation of Ketones Synthesized by Utilizing Metal–Organic Framework as Template. Catal. Sci. Technol. 2018, 8, 2434–2440. [Google Scholar] [CrossRef]
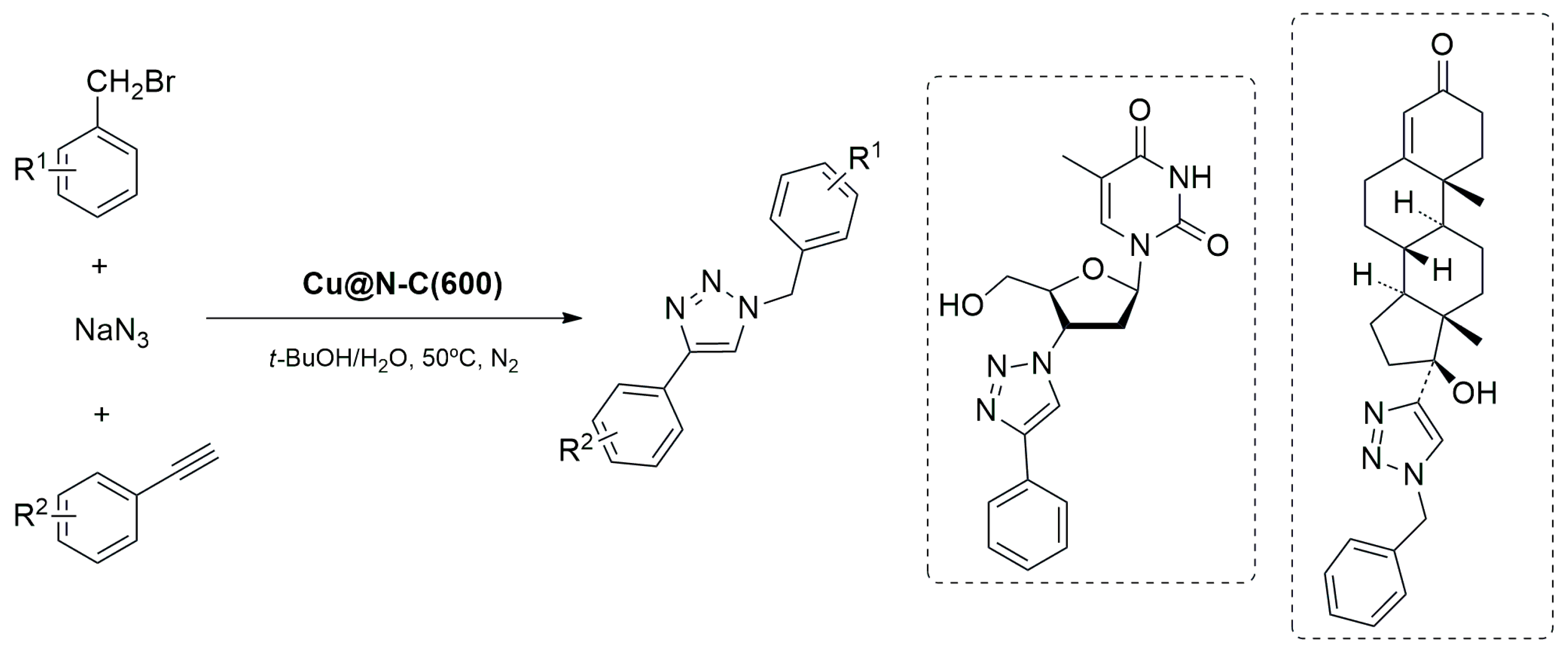
- Wang, Z.; Zhou, X.; Gong, S.; Xie, J. MOF-Derived Cu@N-C Catalyst for 1,3-Dipolar Cycloaddition Reaction. Nanomaterials 2022, 12, 1070. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Li, D.; Lu, G.; Cai, C. Hydrogen Auto-transfer Synthesis of Quinoxalines from o-Nitroanilines and Biomass-based Diols Catalyzed by MOF-derived N,P Co-doped Cobalt Catalysts. ChemCatChem 2021, 13, 373–381. [Google Scholar] [CrossRef]
- Panja, D.; Paul, B.; Balasubramaniam, B.; Gupta, R.K.; Kundu, S. Application of a Reusable Co-Based Nanocatalyst in Alcohol Dehydrogenative Coupling Strategy: Synthesis of Quinoxaline and Imine Scaffolds. Catal. Commun. 2020, 137, 105927. [Google Scholar] [CrossRef]
- Deng, D.; Chen, X.; Yu, L.; Wu, X.; Liu, Q.; Liu, Y.; Yang, H.; Tian, H.; Hu, Y.; Du, P.; et al. A Single Iron Site Confined in a Graphene Matrix for the Catalytic Oxidation of Benzene at Room Temperature. Sci. Adv. 2015, 1, e1500462. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Li, H.; Wang, Y.; Hu, Y.; Hua, L.; Li, H.; Han, X.; Liu, Q.; Yang, F.; He, L.; et al. Room-Temperature Methane Conversion by Graphene-Confined Single Iron Atoms. Chem 2018, 4, 1902–1910. [Google Scholar] [CrossRef]
- Bi, W.; Li, X.; You, R.; Chen, M.; Yuan, R.; Huang, W.; Wu, X.; Chu, W.; Wu, C.; Xie, Y. Surface Immobilization of Transition Metal Ions on Nitrogen-Doped Graphene Realizing High-Efficient and Selective CO2 Reduction. Adv. Mater. 2018, 30, 1706617. [Google Scholar] [CrossRef]
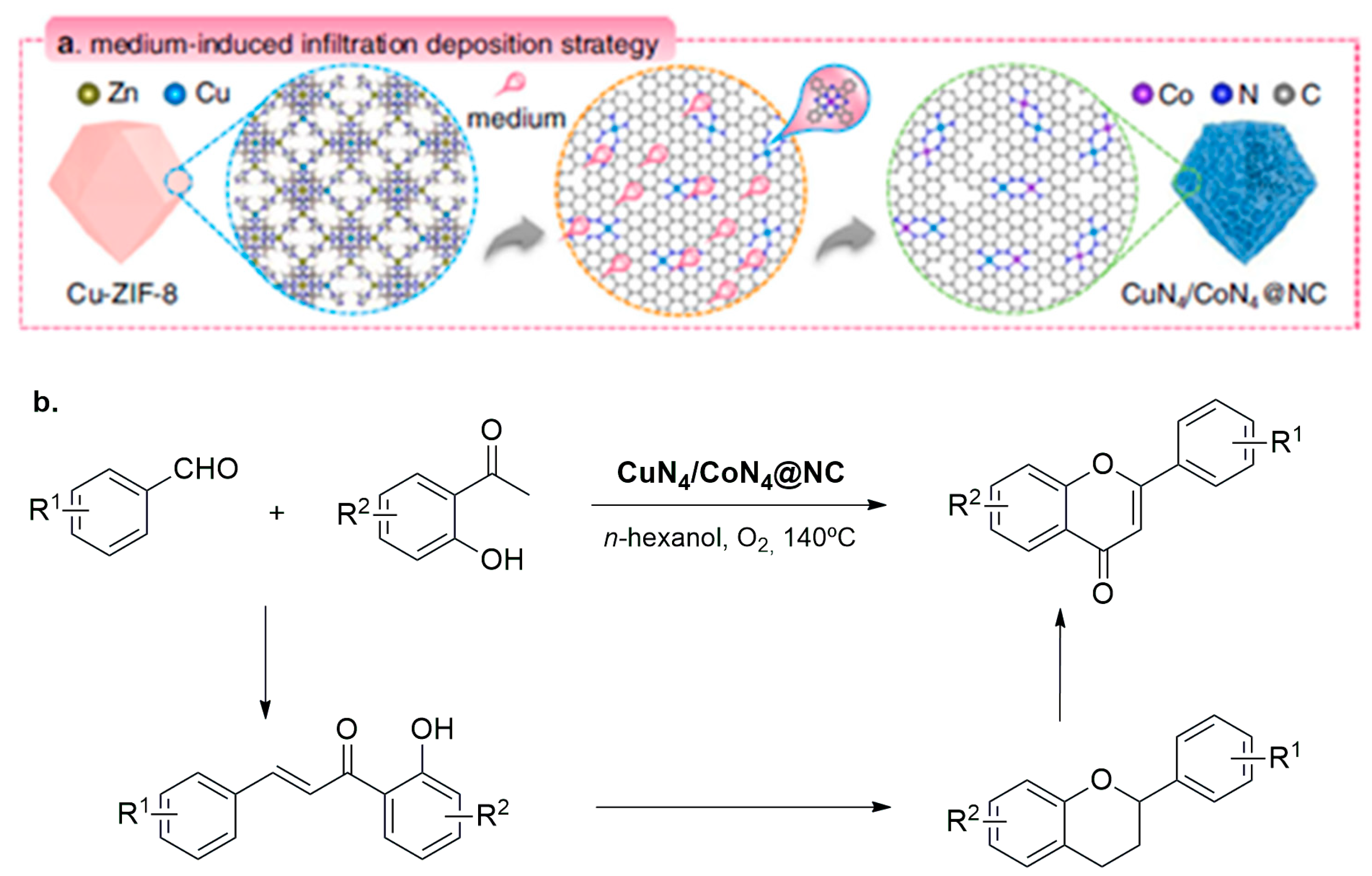
- Zhao, X.; Fang, R.; Wang, F.; Kong, X.; Li, Y. Atomic Design of Dual-Metal Hetero-Single-Atoms for High-Efficiency Synthesis of Natural Flavones. Nat. Commun. 2022, 13, 7873. [Google Scholar] [CrossRef]
- Wang, X.; Chen, W.; Zhang, L.; Yao, T.; Liu, W.; Lin, Y.; Ju, H.; Dong, J.; Zheng, L.; Yan, W.; et al. Uncoordinated Amine Groups of Metal–Organic Frameworks to Anchor Single Ru Sites as Chemoselective Catalysts toward the Hydrogenation of Quinoline. J. Am. Chem. Soc. 2017, 139, 9419–9422. [Google Scholar] [CrossRef]
- Guan, Y.; Chaffart, D.; Liu, G.; Tan, Z.; Zhang, D.; Wang, Y.; Li, J.; Ricardez-Sandoval, L. Machine Learning in Solid Heterogeneous Catalysis: Recent Developments, Challenges and Perspectives. Chem. Eng. Sci. 2022, 248, 117224. [Google Scholar] [CrossRef]
- Wang, L.; Sofer, Z.; Pumera, M. Will Any Crap We Put into Graphene Increase Its Electrocatalytic Effect? ACS Nano 2020, 14, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Kiciński, W.; Dyjak, S. Transition Metal Impurities in Carbon-Based Materials: Pitfalls, Artifacts and Deleterious Effects. Carbon 2020, 168, 748–845. [Google Scholar] [CrossRef]

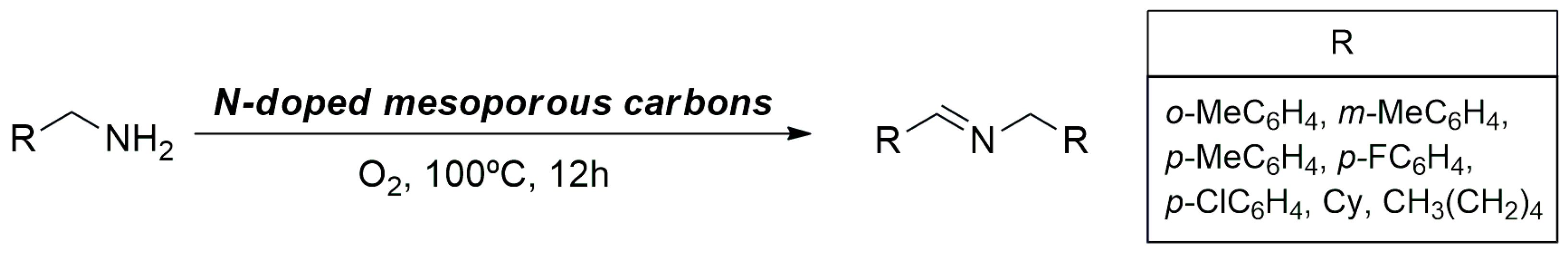


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez Mayoral, E.; Godino Ojer, M.; Ventura, M.; Matos, I. New Insights into N-Doped Porous Carbons as Both Heterogeneous Catalysts and Catalyst Supports: Opportunities for the Catalytic Synthesis of Valuable Compounds. Nanomaterials 2023, 13, 2013. https://doi.org/10.3390/nano13132013
Pérez Mayoral E, Godino Ojer M, Ventura M, Matos I. New Insights into N-Doped Porous Carbons as Both Heterogeneous Catalysts and Catalyst Supports: Opportunities for the Catalytic Synthesis of Valuable Compounds. Nanomaterials. 2023; 13(13):2013. https://doi.org/10.3390/nano13132013
Chicago/Turabian StylePérez Mayoral, Elena, Marina Godino Ojer, Márcia Ventura, and Ines Matos. 2023. "New Insights into N-Doped Porous Carbons as Both Heterogeneous Catalysts and Catalyst Supports: Opportunities for the Catalytic Synthesis of Valuable Compounds" Nanomaterials 13, no. 13: 2013. https://doi.org/10.3390/nano13132013
APA StylePérez Mayoral, E., Godino Ojer, M., Ventura, M., & Matos, I. (2023). New Insights into N-Doped Porous Carbons as Both Heterogeneous Catalysts and Catalyst Supports: Opportunities for the Catalytic Synthesis of Valuable Compounds. Nanomaterials, 13(13), 2013. https://doi.org/10.3390/nano13132013








