Advanced Photocatalytic Uranium Extraction Strategies: Progress, Challenges, and Prospects
Abstract
:1. Introduction
2. Mechanism of Photocatalytic Uranium Reduction and Identification of Reduction Products
3. Photocatalysts for Uranium Recovery from Solution
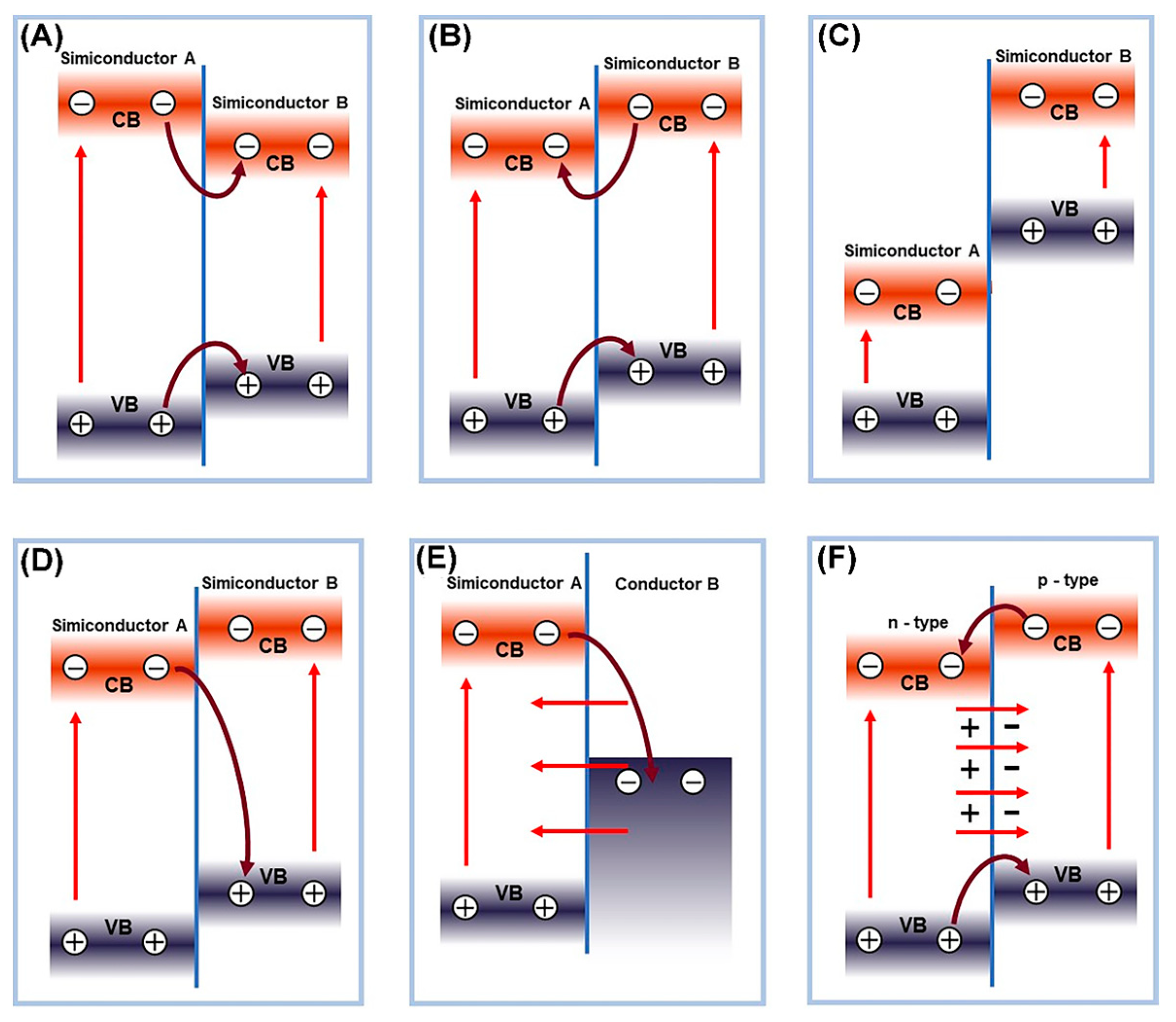
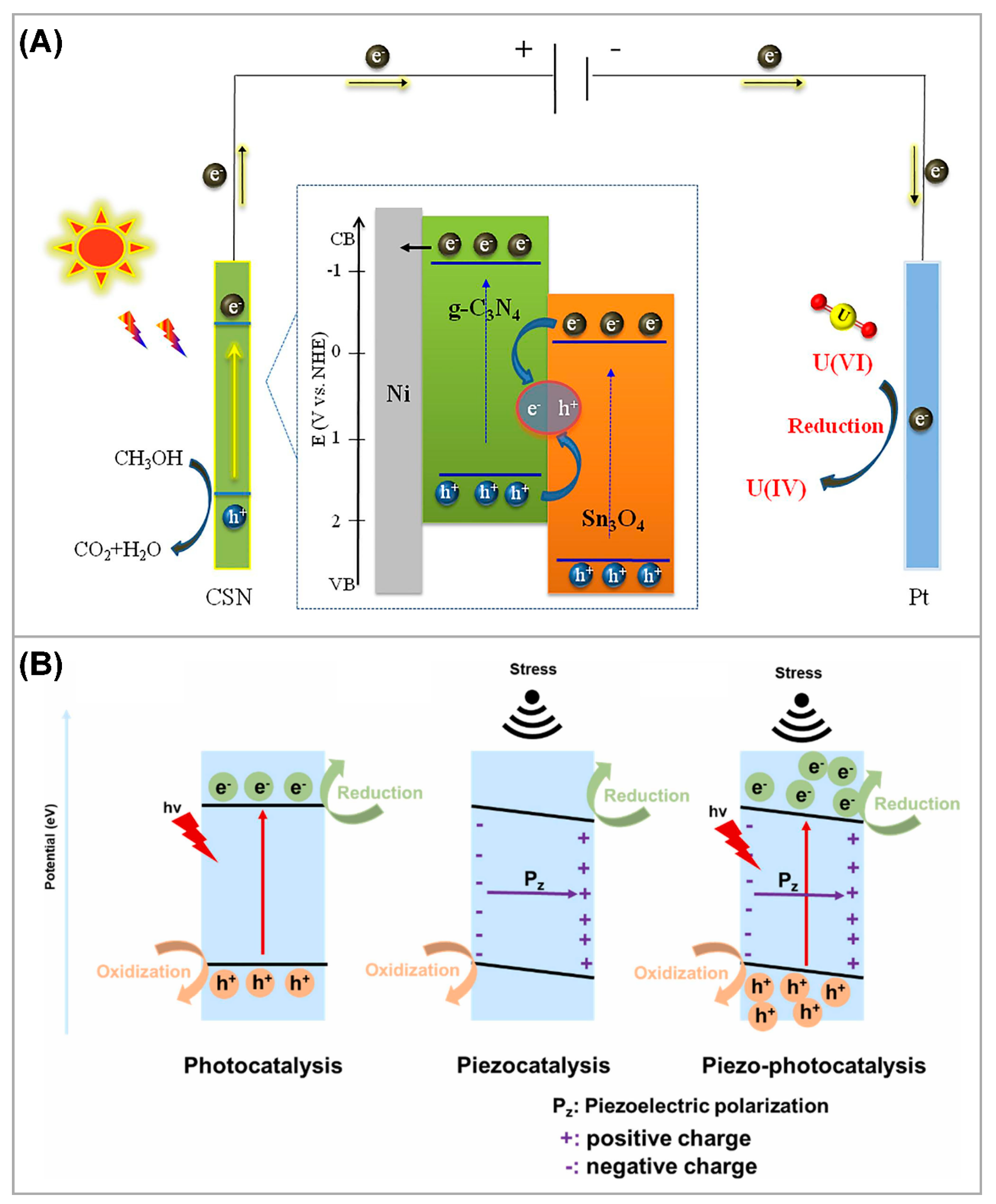
3.1. Semiconductor Heterojunction Structure
3.2. Defective Semiconductors
3.3. Elemental Doping of Semiconductors
3.4. Other Strategies
4. Photocatalytic U(VI) Extraction from Uranium-Containing Wastewater and Seawater
5. Current Challenges and Perspective
5.1. Recovery and Regeneration of Catalysts
5.2. Photocatalytic U(VI) Extraction under Sunlight
5.3. Coupling of Photocatalytic Technology with Other Techniques
5.4. Engineering Aspects of Scaling up Experiments in Real Water Environments
5.5. Cost Evaluation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chilvers, J.; Bellamy, R.; Pallett, H.; Hargreaves, T. A systemic approach to mapping participation with low-carbon energy transitions. Nat. Energy 2021, 6, 250–259. [Google Scholar] [CrossRef]
- Kaltsoyannis, N.; Liddle, S.T. Catalyst: Nuclear power in the 21st century. Chem 2016, 1, 659–662. [Google Scholar] [CrossRef] [Green Version]
- Devell, L.; Tovedal, H.; Bergström, U.; Appelgren, A.; Chyssler, J.; Andersson, L. Initial observations of fallout from the reactor accident at Chernobyl. Nature 1986, 321, 192–193. [Google Scholar] [CrossRef]
- Neidell, M.; Uchida, S.; Veronesi, M. The unintended effects from halting nuclear power production: Evidence from Fukushima Daiichi accident. J. Health Econ. 2021, 79, 102507. [Google Scholar] [CrossRef]
- Yuan, Y.; Yu, Q.; Cao, M.; Feng, L.; Feng, S.; Liu, T.; Feng, T.; Yan, B.; Guo, Z.; Wang, N. Selective extraction of uranium from seawater with biofouling-resistant polymeric peptide. Nat. Sustain. 2021, 4, 708–714. [Google Scholar] [CrossRef]
- Yu, F.; Zhu, Z.; Wang, S.; Peng, Y.; Xu, Z.; Tao, Y.; Xiong, J.; Fan, Q.; Luo, F. Tunable perylene-based donor-acceptor conjugated microporous polymer to significantly enhance photocatalytic uranium extraction from seawater. Chem. Eng. J. 2021, 412, 127558. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Shang, H.; Dong, X.; Huang, L.; Qing, Q.; Xu, C.; Chen, J.; Liu, H.; Wang, X.; et al. Photo-induced removal of uranium under air without external photocatalysts. Green Chem. 2022, 24, 7092–7099. [Google Scholar] [CrossRef]
- Shang, D.; Geissler, B.; Mew, M.; Satalkina, L.; Zenk, L.; Tulsidas, H.; Barker, L.; El-Yahyaoui, A.; Hussein, A.; Taha, M.; et al. Unconventional uranium in China’s phosphate rock: Review and outlook. Renew. Sustain. Energy Rev. 2021, 140, 110740. [Google Scholar] [CrossRef]
- Pu, Y.; Qiang, T.; Ren, L. Anti-biofouling bio-adsorbent with ultrahigh uranium extraction capacity: One uranium resource recycling solution. Desalination 2022, 531, 115721. [Google Scholar] [CrossRef]
- Liu, X.; Xie, Y.; Hao, M.; Chen, Z.; Yang, H.; Waterhouse, G.I.; Ma, S.; Wang, X. Highly efficient electrocatalytic uranium extraction from seawater over an amidoxime-functionalized In–N–C catalyst. Adv. Sci. 2022, 9, 2201735. [Google Scholar] [CrossRef]
- Gureli, L.; Apak, R. Recovery of uranium from ammonium uranyl carbonate (AUC) effluents by combined ion exchange and membrane separation. Sep. Sci. Technol. 2005, 39, 1857–1869. [Google Scholar] [CrossRef]
- Hernández, J.; Ruiz, D. Removal of chloride ions from a copper leaching solution, using electrodialysis, to improve the uranium extraction through ion-exchange. J. Hazard. Mater. 2021, 420, 126582. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, Y.; Lv, Z.; Zhang, S.; Gao, F.; Fang, M.; Kong, M.; Liu, P.; Tan, X.; Hu, B.; et al. Highly efficient uranium extraction by a piezo catalytic reduction-oxidation process. Appl. Catal. B 2022, 310, 121343. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, C.; Hui, W.; Huang, X.; Yang, C.; Liang, Y. Intrinsically morphological effect of perovskite BaTiO3 boosting piezocatalytic uranium extraction efficiency and mechanism investigation. J. Hazard. Mater. 2023, 455, 131578. [Google Scholar] [CrossRef]
- Liu, C.; Hsu, P.-C.; Xie, J.; Zhao, J.; Wu, T.; Wang, H.; Liu, W.; Zhang, J.; Chu, S.; Cui, Y. A half-wave rectified alternating current electrochemical method for uranium extraction from seawater. Nat. Energy 2017, 2, 17007. [Google Scholar] [CrossRef]
- Abney, C.W.; Mayes, R.T.; Saito, T.; Dai, S. Materials for the recovery of uranium from seawater. Chem. Rev. 2017, 117, 13935–14013. [Google Scholar] [CrossRef]
- Feng, S.; Feng, L.; Wang, M.; Yuan, Y.; Yu, Q.; Feng, T.; Cao, M.; Wang, N.; Peng, Q. Highly efficient extraction of uranium from seawater by natural marine crab carapace. Chem. Eng. J. 2022, 430, 133038. [Google Scholar] [CrossRef]
- Haji, M.N.; Drysdale, J.A.; Buesseler, K.O.; Slocum, A.H. Results of an ocean trial of the symbiotic machine for ocean uranium extraction. Environ. Sci. Technol. 2019, 53, 2229–2237. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, Q.; Ma, R.; Wang, Z.; Yang, Y.; Sha, H.; Ma, X.; Ruan, X.; Zou, X.; Yuan, Y.; et al. Constructing an ion pathway for uranium extraction from seawater. Chem 2020, 6, 1683–1691. [Google Scholar] [CrossRef]
- Zhao, Z.; An, H.; Lin, J.; Feng, M.; Murugadoss, V.; Ding, T.; Liu, H.; Shao, Q.; Mai, X.; Wang, N.; et al. Progress on the photocatalytic reduction removal of chromium contamination. Chem. Rec. 2019, 19, 873–882. [Google Scholar] [CrossRef]
- Wang, C.-C.; Du, X.-D.; Li, J.; Guo, X.-X.; Wang, P.; Zhang, J. Photocatalytic Cr (VI) reduction in metal-organic frameworks: A mini-review. Appl. Catal. B Environ. 2016, 193, 198–216. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, K.L.; Shen, H.; Wang, C.; Zhao, Q.; Wang, D.; Fu, F.; Liang, Y. Magnetically recyclable Fe3O4@SiO2/Bi2WO6−xF2x photocatalyst with well-designed core-shell nanostructure for the reduction of Cr(VI). Chem. Eng. J. 2019, 370, 1522–1533. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, Q.; Wang, C.; Shen, H.; Han, X.; Zhang, K.; Wang, D.; Fu, F. Magnetically recyclable Fe3O4@SiO2/Bi2WO6/Bi2S3 with visible-light-driven photocatalytic oxidative desulfurization. Mater. Res. Bull. 2019, 118, 110520. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, H.; Liu, Q.; Zhu, J.; Chen, R.; Yu, J.; Li, Y.; Li, R.; Wang, J. Magnetron sputtering ultra-thin TiO2 films for photocatalytic reduction of uranium. Desalination 2022, 543, 116121. [Google Scholar] [CrossRef]
- Qin, Z.; Ye, Y.; Li, C.; Liang, Y.; Jin, J.; Tang, X.; Chen, Y.; Chen, F.; Shi, T.; Wang, Y. Removal and recovery of aqueous uranium using photocatalytic reduction method: Performance and implication. Sep. Purif. Technol. 2023, 306, 122670. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.; Wang, Y.; Dong, L.; Wang, W.; Geng, R.; Ding, Z.; Luo, D.; Pan, D.; Liang, J.; et al. Ultrafast recovery of aqueous uranium: Photocatalytic U(VI) reduction over CdS/g-C3N4. Chem. Eng. J. 2021, 425, 131552. [Google Scholar] [CrossRef]
- Li, H.; Zhai, F.; Gui, D.; Wang, X.; Wu, C.; Zhang, D.; Dai, X.; Deng, H.; Su, X.; Diwu, J.; et al. Powerful uranium extraction strategy with combined ligand complexation and photocatalytic reduction by postsynthetically modified photoactive metal-organic frameworks. Appl. Catal. B 2019, 254, 47–54. [Google Scholar] [CrossRef]
- Liu, X.; Bi, R.-X.; Zhang, C.-R.; Luo, Q.-X.; Liang, R.-P.; Qiu, J.-D. SnS2-covalent organic framework Z-scheme van der Waals heterojunction for enhanced photocatalytic reduction of uranium (VI) in rare earth tailings wastewater. Chem. Eng. J. 2023, 460, 141756. [Google Scholar] [CrossRef]
- Chen, T.; Yu, K.; Dong, C.; Yuan, X.; Gong, X.; Lian, J.; Cao, X.; Li, M.; Zhou, L.; Hu, B.; et al. Advanced photocatalysts for uranium extraction: Elaborate design and future perspectives. Coord. Chem. Rev. 2022, 467, 214615. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.; Wang, Y.; Liang, J.; Pan, D.; Qiang, S.; Fan, Q. An overview and recent progress in the heterogeneous photocatalytic reduction of U(VI). J. Photochem. Photobiol. C 2019, 41, 100320. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Wang, W.; Ding, Z.; Geng, R.; Li, P.; Pan, D.; Liang, J.; Qin, H.; Fan, Q. Tunable mesoporous g-C3N4 nanosheets as a metal-free catalyst for enhanced visible-light-driven photocatalytic reduction of U(VI). Chem. Eng. J. 2020, 383, 123193. [Google Scholar] [CrossRef]
- Chen, T.; Li, M.; Zhou, L.; Feng, X.; Lin, D.; Ding, X.; Li, C.; Yan, R.; Duan, T.; He, R.; et al. Harmonizing the energy band between adsorbent and semiconductor enables efficient uranium extraction. Chem. Eng. J. 2021, 420, 127645. [Google Scholar] [CrossRef]
- Chen, T.; Liu, B.; Li, M.; Zhou, L.; Lin, D.; Ding, X.; Lian, J.; Li, J.; He, R.; Duan, T.; et al. Efficient uranium reduction of bacterial cellulose-MoS2 heterojunction via the synergistically effect of Schottky junction and S-vacancies engineering. Chem. Eng. J. 2021, 406, 126791. [Google Scholar] [CrossRef]
- Song, S.; Huang, S.; Zhang, R.; Chen, Z.; Wen, T.; Wang, S.; Hayat, T.; Alsaedi, A.; Wang, X. Simultaneous removal of U(VI) and humic acid on defective TiO2−x investigated by batch and spectroscopy techniques. Chem. Eng. J. 2017, 325, 576–587. [Google Scholar] [CrossRef]
- Li, Z.-J.; Huang, Z.-W.; Guo, W.-L.; Wang, L.; Zheng, L.-R.; Chai, Z.-F.; Shi, W.-Q. Enhanced photocatalytic removal of uranium (VI) from aqueous solution by magnetic TiO2/Fe3O4 and its graphene composite. Environ. Sci. Technol. 2017, 51, 5666–5674. [Google Scholar] [CrossRef]
- Zhu, M.; Cai, Y.; Liu, S.; Fang, M.; Tan, X.; Liu, X.; Kong, M.; Xu, W.; Mei, H.; Hayat, T. K2Ti6O13 hybridized graphene oxide: Effective enhancement in photodegradation of RhB and photoreduction of U(VI). Environ. Pollut. 2019, 248, 448–455. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.; Wang, Y.; Liang, J.; He, B.; Pan, D.; Fan, Q.; Wang, X. Photoconversion of U(VI) by TiO2: An efficient strategy for seawater uranium extraction. Chem. Eng. J. 2019, 365, 231–241. [Google Scholar] [CrossRef]
- Wu, L.; Yang, X.; Chen, T.; Li, Y.; Meng, Q.; Zhu, L.; Zhu, W.; He, R.; Duan, T. Three-dimensional C3N5/RGO aerogels with enhanced visible-light response and electron-hole separation efficiency for photocatalytic uranium reduction. Chem. Eng. J. 2022, 427, 131773. [Google Scholar] [CrossRef]
- Guo, Y.; Li, L.; Li, Y.; Li, Z.; Wang, X.; Wang, G. Adsorption and photocatalytic reduction activity of uranium (VI) on zinc oxide/rectorite composite enhanced with methanol as sacrificial organics. J. Radioanal. Nucl. Chem. 2016, 310, 883–890. [Google Scholar] [CrossRef]
- Hu, L.; Yan, X.-W.; Zhang, X.-J.; Shan, D. Integration of adsorption and reduction for uranium uptake based on SrTiO3/TiO2 electrospun nanofibers. Appl. Surf. Sci. 2018, 428, 819–824. [Google Scholar] [CrossRef]
- Dutta, D.P.; Nath, S. Low cost synthesis of SiO2/C nanocomposite from corn cobs and its adsorption of uranium (VI), chromium (VI) and cationic dyes from wastewater. J. Mol. Liq. 2018, 269, 140–151. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, P.; Jiang, S.; Wu, X.; Song, S.; Zhu, M.; Lou, Z.; Li, Z.; Liu, F.; Liu, Y.; et al. Photocatalytic reduction elimination of UO22+ pollutant under visible light with metal-free sulfur doped g-C3N4 photocatalyst. Appl. Catal. B 2017, 200, 378–385. [Google Scholar] [CrossRef]
- Wang, H.; Guo, H.; Zhang, N.; Chen, Z.; Hu, B.; Wang, X. Enhanced photoreduction of U(VI) on C3N4 by Cr(VI) and bisphenol A: ESR, XPS, and EXAFS investigation. Environ. Sci. Technol. 2019, 53, 6454–6461. [Google Scholar] [CrossRef]
- Liu, X.; Du, P.; Pan, W.; Dang, C.; Qian, T.; Liu, H.; Liu, W.; Zhao, D. Immobilization of uranium (VI) by niobate/titanate nanoflakes heterojunction through combined adsorption and solar-light-driven photocatalytic reduction. Appl. Catal. B 2018, 231, 11–22. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, Z.; Dong, Z.; Xiong, Y.; Wang, Y.; Liu, Y.; Cao, X.; Dong, W.; Liu, M.; Liu, Y. Fabrication of heterostructured CdS/TiO2 nanotube arrays composites for photoreduction of U(VI) under visible light. J. Solid State Chem. 2021, 298, 122053. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, J.; Ge, H.; Li, M.; Li, Y.; Duan, T.; He, R.; Zhu, W. Efficient extraction of uranium in organics-containing wastewater over g-C3N4/GO hybrid nanosheets with type-II band structure. J. Hazard. Mater. 2020, 384, 121383. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, Z.; Sui, Y.; Xue, J.; Ding, D. Adsorption of U(VI) from aqueous solution by magnetic core–dual shell Fe3O4@PDA@TiO2. J. Radioanal. Nucl. Chem. 2018, 317, 613–624. [Google Scholar] [CrossRef]
- Dai, Z.; Zhen, Y.; Sun, Y.; Li, L.; Ding, D. ZnFe2O4/g-C3N4 S-scheme photocatalyst with enhanced adsorption and photocatalytic activity for uranium (VI) removal. Chem. Eng. J. 2021, 415, 129002. [Google Scholar] [CrossRef]
- Huang, X.; Xiao, J.; Mei, P.; Wang, H.; Ishag, A.; Sun, Y. The Synthesis of Z-Scheme MoS2/g-C3N4 Heterojunction for Enhanced Visible-Light-Driven Photoreduction of Uranium. Catal. Lett. 2022, 152, 1981–1989. [Google Scholar] [CrossRef]
- Ke, L.; Li, P.; Wu, X.; Jiang, S.; Luo, M.; Liu, Y.; Le, Z.; Sun, C.; Song, S. Graphene-like sulfur-doped g-C3N4 for photocatalytic reduction elimination of UO22+ under visible Light. Appl. Catal. B 2017, 205, 319–326. [Google Scholar] [CrossRef]
- Deng, H.; Li, Z.-J.; Wang, L.; Yuan, L.-Y.; Lan, J.-H.; Chang, Z.-Y.; Chai, Z.-F.; Shi, W.-Q. Nanolayered Ti3C2 and SrTiO3 composites for photocatalytic reduction and removal of uranium (VI). ACS Appl. Nano Mater. 2019, 2, 2283–2294. [Google Scholar] [CrossRef]
- Rao, G.; Liu, X.; Liu, P. Fabrication of MoS2@TiO2 hollow-sphere heterostructures with enhanced visible light photocatalytic reduction of U(VI). J. Radioanal. Nucl. Chem. 2022, 331, 263–273. [Google Scholar] [CrossRef]
- Niu, Y.; Lu, Z.; Li, J.; Jiang, J.; Lu, J.; Wu, K. Low-cost synthesis of cellular materials with preformed foam for uranium (VI) adsorption. Mater. Lett. 2019, 247, 36–39. [Google Scholar] [CrossRef]
- Jiang, X.-H.; Xing, Q.-J.; Luo, X.-B.; Li, F.; Zou, J.-P.; Liu, S.-S.; Li, X.; Wang, X.-K. Simultaneous photoreduction of Uranium (VI) and photooxidation of Arsenic (III) in aqueous solution over g-C3N4/TiO2 heterostructured catalysts under simulated sunlight irradiation. Appl. Catal. B 2018, 228, 29–38. [Google Scholar] [CrossRef]
- Lee, S.; Kang, U.; Piao, G.; Kim, S.; Han, D.S.; Park, H. Homogeneous photoconversion of seawater uranium using copper and iron mixed-oxide semiconductor electrodes. Appl. Catal. B 2017, 207, 35–41. [Google Scholar] [CrossRef]
- Liang, P.; Yuan, L.; Du, K.; Wang, L.; Li, Z.; Deng, H.; Wang, X.; Luo, S.-Z.; Shi, W. Photocatalytic reduction of uranium (VI) under visible light with 2D/1D Ti3C2/CdS. Chem. Eng. J. 2021, 420, 129831. [Google Scholar] [CrossRef]
- Feng, J.; Yang, Z.; He, S.; Niu, X.; Zhang, T.; Ding, A.; Liang, H.; Feng, X. Photocatalytic reduction of Uranium (VI) under visible light with Sn-doped In2S3 microspheres. Chemosphere 2018, 212, 114–123. [Google Scholar] [CrossRef]
- Yu, F.; Yu, Z.; Xu, Z.; Xiong, J.; Fan, Q.; Feng, X.; Tao, Y.; Hua, J.; Luo, F. Heteroatom engineering of polymeric carbon nitride heterojunctions for boosting photocatalytic reduction of hexavalent uranium. Mol. Syst. Des. Eng. 2020, 5, 882–889. [Google Scholar] [CrossRef]
- Wang, G.; Zhen, J.; Zhou, L.; Wu, F.; Deng, N. Adsorption and photocatalytic reduction of U (VI) in aqueous TiO2 suspensions enhanced with sodium formate. J. Radioanal. Nucl. Chem. 2015, 304, 579–585. [Google Scholar] [CrossRef]
- Lu, C.; Chen, R.; Wu, X.; Fan, M.; Liu, Y.; Le, Z.; Jiang, S.; Song, S. Boron doped g-C3N4 with enhanced photocatalytic UO22+ reduction performance. Appl. Surf. Sci. 2016, 360, 1016–1022. [Google Scholar] [CrossRef]
- Le, Z.; Xiong, C.; Gong, J.; Wu, X.; Pan, T.; Chen, Z.; Xie, Z. Self-cleaning isotype g-C3N4 heterojunction for efficient photocatalytic reduction of hexavalent uranium under visible light. Environ. Pollut. 2020, 260, 114070. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, Y.; Wang, S.; Zhu, Y.; Hu, B. In-situ growth of COF on BiOBr 2D material with excellent visible-light-responsive activity for U(VI) photocatalytic reduction. Sep. Purif. Technol. 2021, 279, 119627. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Cui, Z.; Xu, Y.; Niu, Z.; Li, P.; Pan, D.; Wu, W. Efficient photoreduction strategy for uranium immobilization based on graphite carbon nitride/perovskite oxide heterojunction nanocomposites. Appl. Catal. B 2021, 298, 120625. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, Z.; Wang, S.; Hu, B. The photocatalytic reduction of U (VI) into U (IV) by ZIF-8/g-C3N4 composites at visible light. Environ. Res. 2021, 196, 110349. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, M.; Zhang, S.; Cai, Y.; Lv, Z.; Fang, M.; Tan, X.; Wang, X. Highly efficient removal of U(VI) by the photoreduction of SnO2/CdCO3/CdS nanocomposite under visible light irradiation. Appl. Catal. B 2020, 279, 119390. [Google Scholar]
- Lei, J.; Liu, H.; Wen, F.; Jiang, X.; Yuan, C.; Chen, Q.; Liu, J.-A.; Cui, X.; Yang, F.; Zhu, W.; et al. Tellurium nanowires wrapped by surface oxidized tin disulfide nanosheets achieves efficient photocatalytic reduction of U(VI). Chem. Eng. J. 2021, 426, 130756. [Google Scholar]
- He, S.; Yang, Z.; Cui, X.; Zhang, X.; Niu, X. Fabrication of the novel Ag-doped SnS2@ InVO4 composite with high adsorption-photocatalysis for the removal of uranium (VI). Chemosphere 2020, 260, 127548. [Google Scholar] [CrossRef]
- Yan, Z.-Y.; Xi, H.-L.; Yuan, L.-Y. Metal organic framework MIL-53 (Fe) as a photocatalyst for visible-light catalytic reduction of U(Ⅵ) in aqueous solution. Huan Jing Ke Xue-Huanjing Kexue 2019, 40, 1819–1825. [Google Scholar]
- Chen, J.; Ollis, D.F.; Rulkens, W.H.; Bruning, H. Photocatalyzed deposition and concentration of soluble uranium (VI) from TiO2 suspensions. Colloid Surf. A 1999, 151, 339–349. [Google Scholar] [CrossRef]
- Dong, C.; Qiao, T.; Huang, Y.; Yuan, X.; Lian, J.; Duan, T.; Zhu, W.; He, R. Efficient photocatalytic extraction of uranium over ethylenediamine capped cadmium sulfide telluride nanobelts. ACS Appl. Mater. Inter. 2021, 13, 11968–11976. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Lei, Z.; Huang, L.; Wu, T.; Liu, S.; Ye, G.; Lu, Y.; Wang, X. Graphene aerogel for photocatalysis-assist uranium elimination under visible light and air atmosphere. Chem. Eng. J. 2020, 402, 126256. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lee, S.; Ryu, J.; Park, H. Solar conversion of seawater uranium (VI) using TiO2 electrodes. Appl. Catal. B 2015, 163, 584–590. [Google Scholar] [CrossRef]
- Hu, Y.; Tang, D.; Shen, Z.; Yao, L.; Zhao, G.; Wang, X. Photochemically triggered self-extraction of uranium from aqueous solution under ambient conditions. Appl. Catal. B 2023, 322, 122092. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Shyichuk, A.; Mironyuk, I.; Naushad, M. A review on removal of uranium (VI) ions using titanium dioxide based sorbents. J. Mol. Liq. 2019, 293, 111563. [Google Scholar] [CrossRef]
- Bonato, M.; Allen, G.; Scott, T. Reduction of U(VI) to U(IV) on the surface of TiO2 anatase nanotubes. Micro Nano Lett. 2008, 3, 57–61. [Google Scholar] [CrossRef]
- Litter, M.I. Last advances on TiO2-photocatalytic removal of chromium, uranium and arsenic. Curr. Opin. Green Sustain. 2017, 6, 150–158. [Google Scholar] [CrossRef]
- Miyake, C.; Nakase, T.; Sano, Y. EPR study of uranium (V) species in photo-and electrolytic reduction processes of UO2(NO3)2-2TBP. J. Nucl. Sci. Technol. 1993, 30, 1256–1260. [Google Scholar] [CrossRef]
- Salomone, V.N.; Meichtry, J.M.; Zampieri, G.; Litter, M.I. New insights in the heterogeneous photocatalytic removal of U(VI) in aqueous solution in the presence of 2-propanol. Chem. Eng. J. 2015, 261, 27–35. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, Y.; Wang, X.; Li, P.; Kong, L.; Wang, G.; Li, X.; Liu, Y. Enhanced photocatalytic reduction activity of uranium (vi) from aqueous solution using the Fe2O3–graphene oxide nanocomposite. Dalton Trans. 2017, 46, 14762–14770. [Google Scholar] [CrossRef]
- Lei, J.; Liu, H.; Yuan, C.; Chen, Q.; Liu, J.-A.; Wen, F.; Jiang, X.; Deng, W.; Cui, X.; Duan, T.; et al. Enhanced photoreduction of U(VI) on WO3 nanosheets by oxygen defect engineering. Chem. Eng. J. 2021, 416, 129164. [Google Scholar] [CrossRef]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Qin, Z.; Yang, C.; Shuai, W.; Jin, J.; Tang, X.; Chen, F.; Shi, T.; Ye, Y.; Liang, Y.; Wang, Y. NiS@CdS interfacial Schottky junction boosting spatial charge separation for highly efficient photocatalytic reduction of U(VI). Sep. Purif. Technol. 2023, 307, 122816. [Google Scholar] [CrossRef]
- Chen, L.; Hang, J.; Chen, B.; Kang, J.; Yan, Z.; Wang, Z.; Zhang, Y.; Chen, S.; Wang, Y.; Jin, Y.; et al. Photocatalytic uranium removal from basic effluent by porphyrin-Ni COF as the photocatalyst. Chem. Eng. J. 2023, 454, 140378. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Hao, M.; Xie, Y.; Liu, X.; Yang, H.; Waterhouse, G.I.; Wang, X.; Ma, S. Tuning excited state electronic structure and charge transport in covalent organic frameworks for enhanced photocatalytic performance. Nat. Commun. 2023, 14, 1106. [Google Scholar] [CrossRef]
- Amadelli, R.; Maldotti, A.; Sostero, S.; Carassiti, V. Photodeposition of uranium oxides onto TiO2 from aqueous uranyl solutions. J. Chem. Soc. 1991, 87, 3267–3273. [Google Scholar] [CrossRef]
- Lyulyukin, M.; Filippov, T.; Cherepanova, S.; Solovyeva, M.; Prosvirin, I.; Bukhtiyarov, A.; Kozlov, D.; Selishchev, D. Synthesis, characterization and visible-light photocatalytic activity of solid and TiO2-supported uranium oxycompounds. Nanomaterials 2021, 11, 1036. [Google Scholar] [CrossRef]
- Gong, X.; Tang, L.; Zou, J.; Guo, Z.; Li, Y.; Lei, J.; Liu, H.; Liu, M.; Zhou, L.; Huang, P.; et al. Introduction of cation vacancies and iron doping into TiO2 enabling efficient uranium photoreduction. J. Hazard. Mater. 2022, 423, 126935. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, G. Unraveling the photocatalytic mechanisms for U(VI) reduction by TiO2. Mol. Catal. 2022, 533, 112770. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, H.; Liu, Q.; Liu, J.; Chen, R.; Yu, J.; Zhu, J.; Li, R.; Wang, J. Surface hybridization of π-conjugate structure cyclized polyacrylonitrile and radial microsphere shaped TiO2 for reducing U(VI) to U(IV). J. Hazard. Mater. 2021, 416, 125812. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Brillas, E. Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters. J. Photochem. Photobiol. C 2017, 31, 1–35. [Google Scholar] [CrossRef]
- Ye, Y. Micropollutant Degradation in Water by Photochemical Processes. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2018. [Google Scholar]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Z.; Shen, S.; Zhong, W.; Cao, S. Advances in designing heterojunction photocatalytic materials. Chin. J. Catal. 2021, 42, 710–730. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, C.; Dong, Z.; Dai, Y.; Xiong, G.; Liu, Y.; Wang, Y.; Wang, Y.; Liu, Y. Synthesis of flower-like MoS2/g-C3N4 nanosheet heterojunctions with enhanced photocatalytic reduction activity of uranium (VI). Appl. Surf. Sci. 2020, 520, 146352. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.; Gao, F.; Zhang, S.; Han, Q.; Li, J.; Fang, M.; Cai, Y.; Hu, B.; Tan, X.; et al. Insights into photothermally enhanced photocatalytic U(VI) extraction by a step-scheme heterojunction. Research 2022, 2022, 9790320. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; He, J.; Lo, I.M. Recent developments and challenges in practical application of visible–light–driven TiO2–based heterojunctions for PPCP degradation: A critical review. Water Res. 2020, 170, 115356. [Google Scholar] [CrossRef]
- Yao, X.; Chen, L.; Liu, M.; Feng, D.; Wang, C.; Lu, F.; Wang, W.; Wang, X.; Cheng, Y.; Liu, H.; et al. Rational design of Si/TiO2 heterojunction photocatalysts: Transfer matrix method. Appl. Catal. B 2018, 221, 70–76. [Google Scholar] [CrossRef]
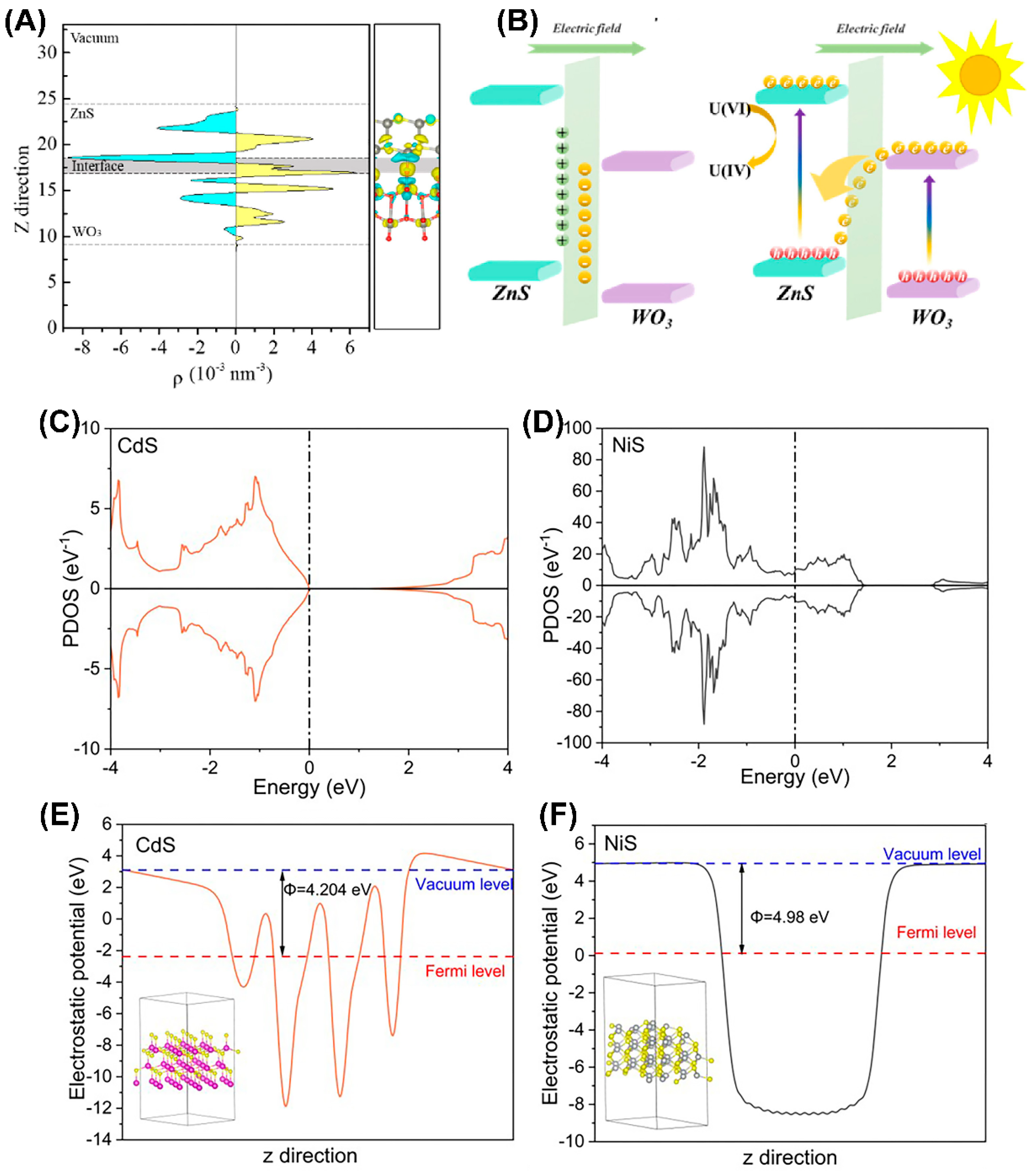
- Wu, F.; Zhang, Z.; Cheng, Z.; Zhou, R.; Lin, Y.; Liu, Y.; Wang, Y.; Cao, X.; Liu, M.; Liu, Y. The enhanced photocatalytic reduction of uranium (VI) by ZnS@g-C3N4 heterojunctions under sunlight. J. Radioanal. Nucl. Chem. 2021, 329, 1125–1133. [Google Scholar] [CrossRef]
- Liu, N.; Li, R.; Zhu, J.; Liu, Q.; Chen, R.; Yu, J.; Li, Y.; Zhang, H.; Wang, J. Z-scheme heterojunction ZnS/WO3 composite: Photocatalytic reduction of uranium and band gap regulation mechanism. J. Colloid Interface Sci. 2023, 630, 727–737. [Google Scholar] [CrossRef]
- Dai, Z.; Sun, Y.; Zhang, H.; Ding, D.; Li, L. Photocatalytic reduction of U(VI) in wastewater by mGO/g-C3N4 nanocomposite under visible LED light irradiation. Chemosphere 2020, 254, 126671. [Google Scholar] [CrossRef]
- Wan, H.; Li, Y.; Wang, M.; Zhao, Q.; Fu, Y.; Chen, Y.; He, P.; Wu, L.; Meng, Q.; Ma, T.; et al. Boosting efficient U(VI) immobilization via synergistic Schottky heterojunction and hierarchical atomic-level injected engineering. Chem. Eng. J. 2022, 430, 133139. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y. Defect engineering in photocatalytic materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Xue, J.; Fujitsuka, M.; Majima, T. Defect-mediated electron transfer in photocatalysts. Chem. Commun. 2021, 57, 3532–3542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Fu, H. Defect-mediated electron–hole separation in semiconductor photocatalysis. Inorg. Chem. Front. 2018, 5, 1240–1254. [Google Scholar] [CrossRef]
- Yang, X.; Li, F.; Liu, W.; Chen, L.; Qi, J.; Sun, W.; Pan, F.; Duan, T.; Sun, F. Oxygen vacancy-induced spin polarization of tungsten oxide nanowires for efficient photocatalytic reduction and immobilization of uranium (VI) under simulated solar light. Appl. Catal. B 2023, 324, 122202. [Google Scholar] [CrossRef]
- Li, S.; Pan, D.; Cui, Z.; Xu, Y.; Shang, H.; Hua, W.; Wu, F.; Wu, W. Synergistic effects of oxygen vacancies and heterostructures for visible-light-driven photoreduction of uranium. Sep. Purif. Technol. 2022, 301, 121966. [Google Scholar] [CrossRef]
- Devi, L.G.; Kavitha, R. A review on non metal ion doped titania for the photocatalytic degradation of organic pollutants under UV/solar light: Role of photogenerated charge carrier dynamics in enhancing the activity. Appl. Catal. B 2013, 140, 559–587. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.; Weng, H.; Wang, L.; Chen, J.; Cheng, S.; Zhang, P.; Wang, M.; Ge, X.; Chen, H.; et al. Carbon-doped boron nitride nanosheets with adjustable band structure for efficient photocatalytic U(VI) reduction under visible light. Chem. Eng. J. 2021, 410, 128280. [Google Scholar] [CrossRef]
- Li, G.; Dong, C.; Sun, G.; Li, M.; Cao, X.; Zhou, L.; Chen, T.; Yang, F. Ag-doped CdSe nanosheets as photocatalysts for uranium reduction. ACS Appl. Nano Mater. 2022, 5, 16178–16187. [Google Scholar] [CrossRef]
- Gong, X.; Tang, L.; Wang, R.; Guo, Z.; Huang, P.; Zhou, L.; Zou, J.; Lei, J.; Liu, H.; Li, N.; et al. Achieving efficient photocatalytic uranium extraction within a record short period of 3 min by Up-conversion erbium doped ZnO nanosheets. Chem. Eng. J. 2022, 450, 138044. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Lu, Y.; Li, H.; Chen, X.; Wei, G.; Wu, T.; Maguire, D.-J.; Ye, G.; Chen, J. Sunlight-induced uranium extraction with triazine-based carbon nitride as both photocatalyst and adsorbent. Appl. Catal. B 2021, 282, 119523. [Google Scholar] [CrossRef]
- Ye, Y.; Jin, J.; Chen, F.; Dionysiou, D.D.; Feng, Y.; Liang, B.; Cheng, H.-Y.; Qin, Z.; Tang, X.; Li, H.; et al. Removal and recovery of aqueous U(VI) by heterogeneous photocatalysis: Progress and challenges. Chem. Eng. J. 2022, 450, 138317. [Google Scholar] [CrossRef]
- Wu, Y.; Xie, Y.; Liu, X.; Li, Y.; Wang, J.; Chen, Z.; Yang, H.; Hu, B.; Shen, C.; Tang, Z.; et al. Functional nanomaterials for selective uranium recovery from seawater: Material design, extraction properties and mechanisms. Coord. Chem. Rev. 2023, 483, 215097. [Google Scholar] [CrossRef]
- Ye, Y.; Jin, J.; Liang, Y.; Qin, Z.; Tang, X.; Feng, Y.; Lv, M.; Miao, S.; Li, C.; Chen, Y.; et al. Efficient and durable uranium extraction from uranium mine tailings seepage water via a photoelectrochemical method. iScience 2021, 24, 103230. [Google Scholar] [CrossRef]
- Ye, Y.; Fan, B.; Qin, Z.; Tang, X.; Feng, Y.; Lv, M.; Miao, S.; Li, H.; Chen, Y.; Chen, F.; et al. Electrochemical removal and recovery of uranium: Effects of operation conditions, mechanisms, and implications. J. Hazard. Mater. 2022, 432, 128723. [Google Scholar] [CrossRef]
- Endrizzi, F.; Leggett, C.J.; Rao, L. Scientific basis for efficient extraction of uranium from seawater. I: Understanding the chemical speciation of uranium under seawater conditions. Ind. Eng. Chem. Res. 2016, 55, 4249–4256. [Google Scholar] [CrossRef]
- Kushwaha, S.; Patel, K. Catalyst: Uranium extraction from seawater, a paradigm shift in resource recovery. Chem 2021, 7, 271–274. [Google Scholar] [CrossRef]
- Dai, S. Catalyst: Challenges in development of adsorbents for recovery of uranium from seawater. Chem 2021, 7, 537–539. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, Z.; Geng, Y.; Li, H.; Wang, N.; Song, Y.; Wang, X.; Chen, J.; Wang, J.; Ma, S.; et al. Uranium extraction from seawater: Material design, emerging technologies and marine engineering. Chem. Soc. Rev. 2023, 52, 97–162. [Google Scholar] [CrossRef]
- Liu, T.; Gu, A.; Wei, T.; Chen, M.; Guo, X.; Tang, S.; Yuan, Y.; Wang, N. Ligand-assistant iced photocatalytic reduction to synthesize atomically dispersed Cu implanted metal-organic frameworks for photo-enhanced uranium extraction from seawater. Small 2023, 19, e2208002. [Google Scholar] [CrossRef]
- Yu, Z.; Ye, D.; Zhao, J.; Wu, X.; Wu, Y. Photocatalytic anti-biofouling coatings with dynamic surfaces of hybrid metal-organic framework nanofibrous mats for uranium (VI) separation from seawater. Chem. Eng. J. 2021, 420, 129691. [Google Scholar] [CrossRef]
- Cui, W.R.; Li, F.F.; Xu, R.H.; Zhang, C.R.; Chen, X.R.; Yan, R.H.; Liang, R.P.; Qiu, J.D. Regenerable covalent organic frameworks for photo-enhanced uranium adsorption from seawater. Angew. Chem. Int. Ed. 2020, 132, 17837–17843. [Google Scholar] [CrossRef]
- Liang, P.-L.; Yuan, L.-Y.; Deng, H.; Wang, X.-C.; Wang, L.; Li, Z.-J.; Luo, S.-Z.; Shi, W.-Q. Photocatalytic reduction of uranium (VI) by magnetic ZnFe2O4 under visible light. Appl. Catal. B 2020, 267, 118688. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, Y.; Lin, Y.; Zheng, Z.; Liu, Y.; Jing, Q.; Luo, F. Constructing dual-functional porphyrin-based thorium metal-organic framework toward photocatalytic uranium (VI) reduction integrated with organic oxidation. Sci. China Chem. 2022, 65, 1544–1551. [Google Scholar] [CrossRef]
- Liu, H.; Lei, J.; Gong, C.; Li, Y.; Chen, H.; Chen, J.; Wen, F.; Fu, D.; Liu, Y.; Zhu, W.; et al. In-situ oxidized tungsten disulfide nanosheets achieve ultrafast photocatalytic extraction of uranium through hydroxyl-mediated binding and reduction. Nano Res. 2022, 15, 8810–8818. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, B.; Zhu, Y.; Xue, J.; Nie, Y.; Xie, Z.; Le, Z. Synthesis of crystalline carbon nitride with molten salt thermal treatment for efficient photocatalytic reduction and removal of U(VI). Res. Chem. Intermed. 2023, 49, 1801–1817. [Google Scholar] [CrossRef]
- Alhindawy, I.G.; Mira, H.I.; Youssef, A.O.; Abdelwahab, S.M.; Zaher, A.A.; El-Said, W.A.; Elshehy, E.A.; Abdelkader, A.M. Cobalt doped titania-carbon nanosheets with induced oxygen vacancies for photocatalytic degradation of uranium complexes in radioactive wastes. Nanoscale Adv. 2022, 4, 5330–5342. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.; Peng, T.; Wang, Y.; Liang, J.; Pan, D.; Fan, Q. Heterostructure of anatase-rutile aggregates boosting the photoreduction of U(VI). Appl. Surf. Sci. 2019, 483, 670–676. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Wang, W.; Peng, T.; Liang, J.; Li, P.; Pan, D.; Fan, Q.; Wu, W. Visible light driven Ti3+ self-doped TiO2 for adsorption-photocatalysis of aqueous U(VI). Environ. Pollut. 2020, 262, 114373. [Google Scholar] [CrossRef]
- Guo, H.; Wang, J.; Chen, D.; Tian, W.; Cao, S.; Chen, D.; Tan, C.; Deng, Q.; Qin, Z. Boro/carbothermal reduction synthesis of uranium tetraboride and its oxidation behavior in dry air. J. Am. Ceram. Soc. 2019, 102, 1049–1056. [Google Scholar] [CrossRef]
- Dai, Z.; Lian, J.; Sun, Y.; Li, L.; Zhang, H.; Hu, N.; Ding, D. Fabrication of g-C3N4/Sn3O4/Ni electrode for highly efficient photoelectrocatalytic reduction of U(VI). Chem. Eng. J. 2022, 433, 133766. [Google Scholar] [CrossRef]
- Jing, L.; Xu, Y.; Xie, M.; Li, Z.; Wu, C.; Zhao, H.; Wang, J.; Wang, H.; Yan, Y.; Zhong, N.; et al. Piezo-photocatalysts in the field of energy and environment: Designs, applications, and prospects. Nano Energy 2023, 112, 108508. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, Y.; Ma, J.; He, Y.; Huang, J.; Feng, Y.; Ban, C.; Gan, L.-Y.; Zhou, X. Oxygen vacancy engineering of zinc oxide for boosting piezo-electrocatalytic hydrogen evolution. Appl. Surf. Sci. 2023, 616, 156556. [Google Scholar] [CrossRef]
- Liu, J.; Qi, W.; Xu, M.; Thomas, T.; Liu, S.; Yang, M. Piezocatalytic techniques in environmental remediation. Angew. Chem. Int. Ed. 2023, 62, e202213927. [Google Scholar]
- Chen, Z.; Zhou, H.; Kong, F.; Wang, M. Piezocatalytic oxidation of 5-hydroxymethylfurfural to 5-formyl-2-furancarboxylic acid over Pt decorated hydroxyapatite. Appl. Catal. B 2022, 309, 121281. [Google Scholar] [CrossRef]
- Ma, J.; Xiong, X.; Wu, D.; Wang, Y.; Ban, C.; Feng, Y.; Meng, J.; Gao, X.; Dai, J.Y.; Han, G.; et al. Band position-independent piezo-electrocatalysis for ultrahigh CO2 conversion. Adv. Mater. 2023, 35, 2300027. [Google Scholar] [CrossRef]
- Su, R.; Wang, Z.; Zhu, L.; Pan, Y.; Zhang, D.; Wen, H.; Luo, Z.D.; Li, L.; Li, F.T.; Wu, M.; et al. Strain-engineered nano-ferroelectrics for high-efficiency piezocatalytic overall water splitting. Angew. Chem. Int. Ed. 2021, 60, 16019–16026. [Google Scholar] [CrossRef]
- Yuan, J.; Huang, X.; Zhang, L.; Gao, F.; Lei, R.; Jiang, C.; Feng, W.; Liu, P. Tuning piezoelectric field for optimizing the coupling effect of piezo-photocatalysis. Appl. Catal. B 2020, 278, 119291. [Google Scholar] [CrossRef]
- Chen, P.; Ni, J.; Liang, Y.; Yang, B.; Jia, F.; Song, S. Piezo-photocatalytic reduction of Au(I) by defect-rich MoS2 nanoflowers for efficient gold recovery from a thiosulfate solution. ACS Sustain. Chem. Eng. 2020, 9, 589–598. [Google Scholar] [CrossRef]
- Kim, H.; Eggert, R.G.; Carlsen, B.W.; Dixon, B.W. Potential uranium supply from phosphoric acid: A US analysis comparing solvent extraction and Ion exchange recovery. Resour. Policy 2016, 49, 222–231. [Google Scholar] [CrossRef] [Green Version]

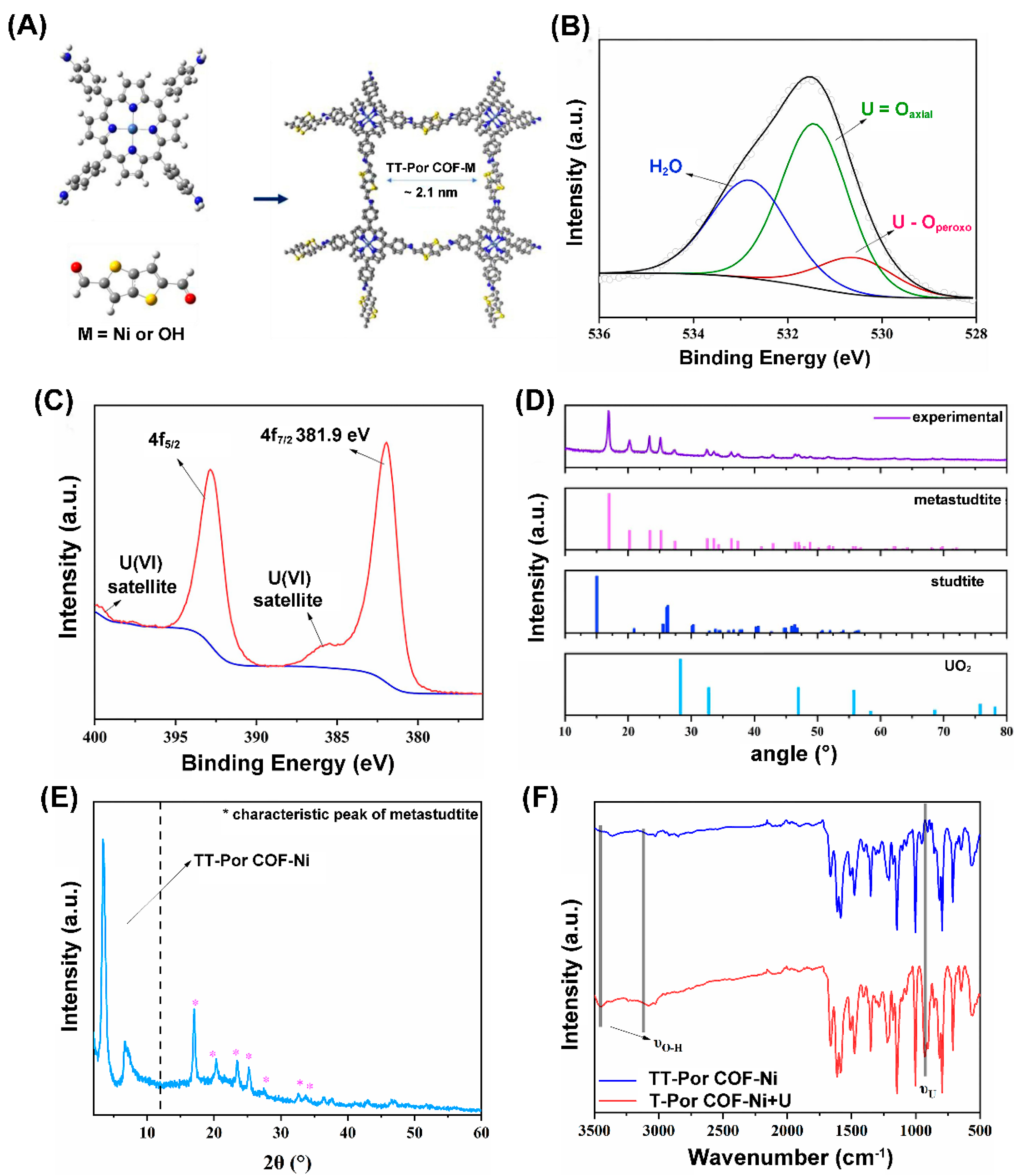
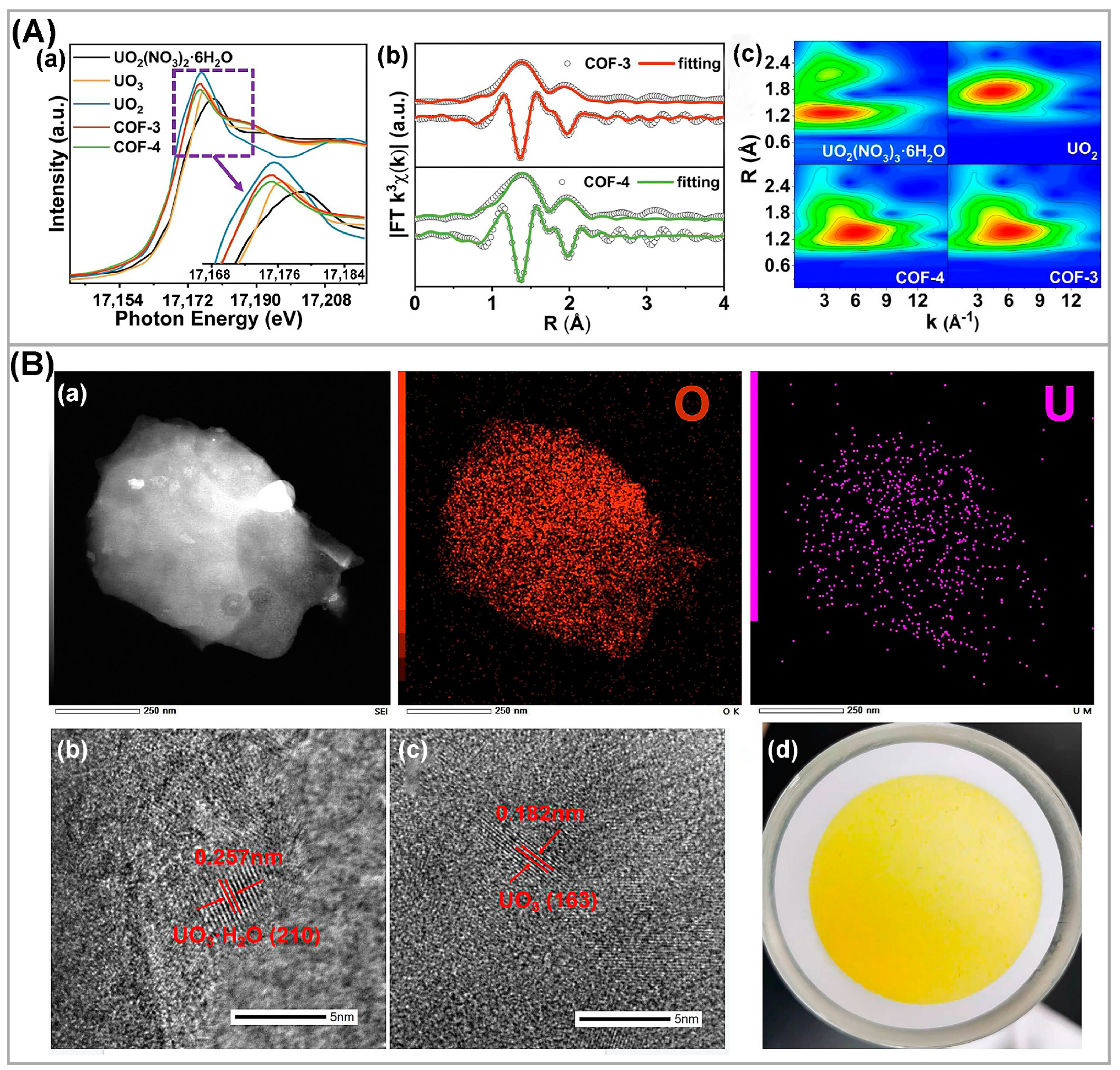

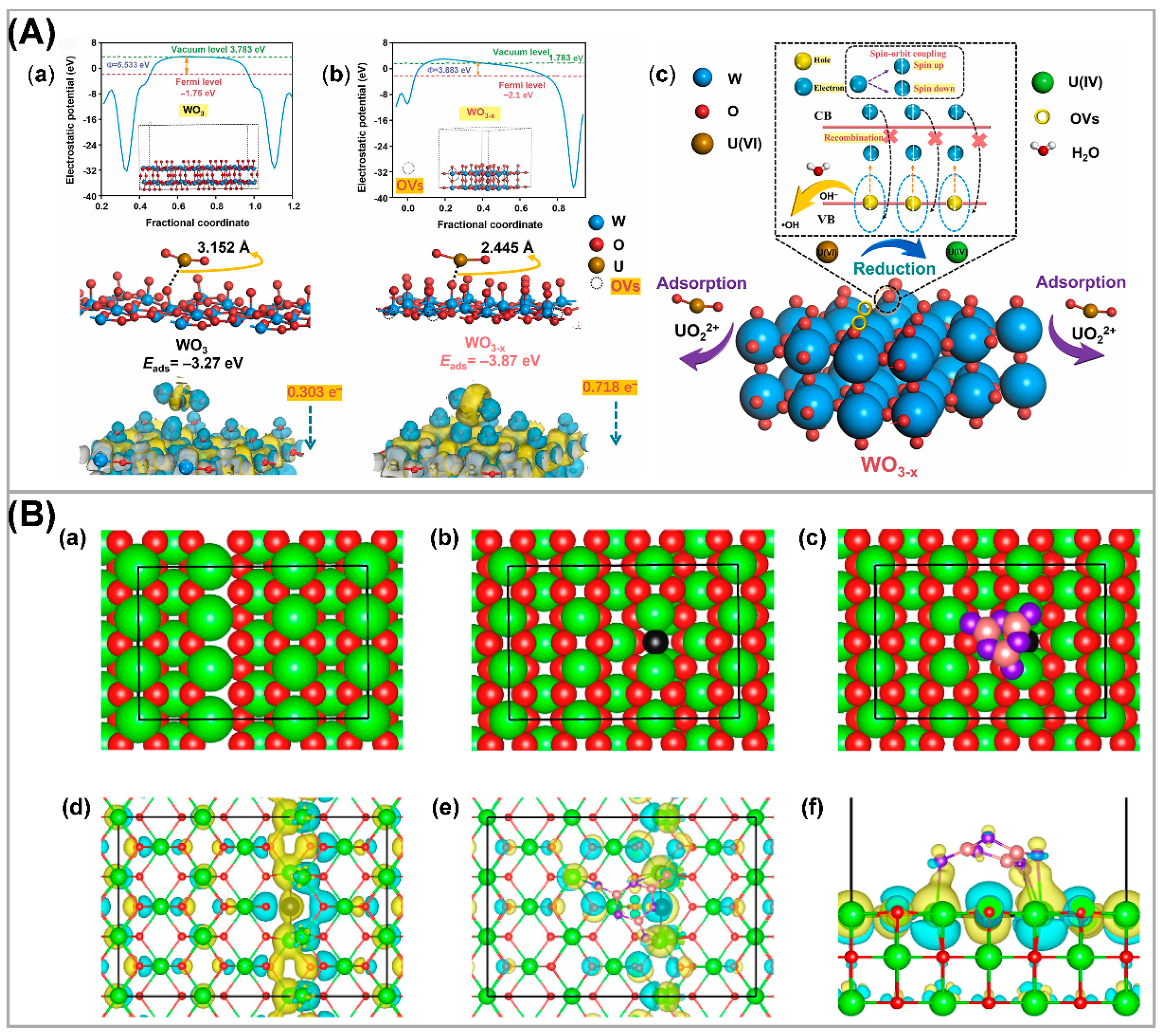
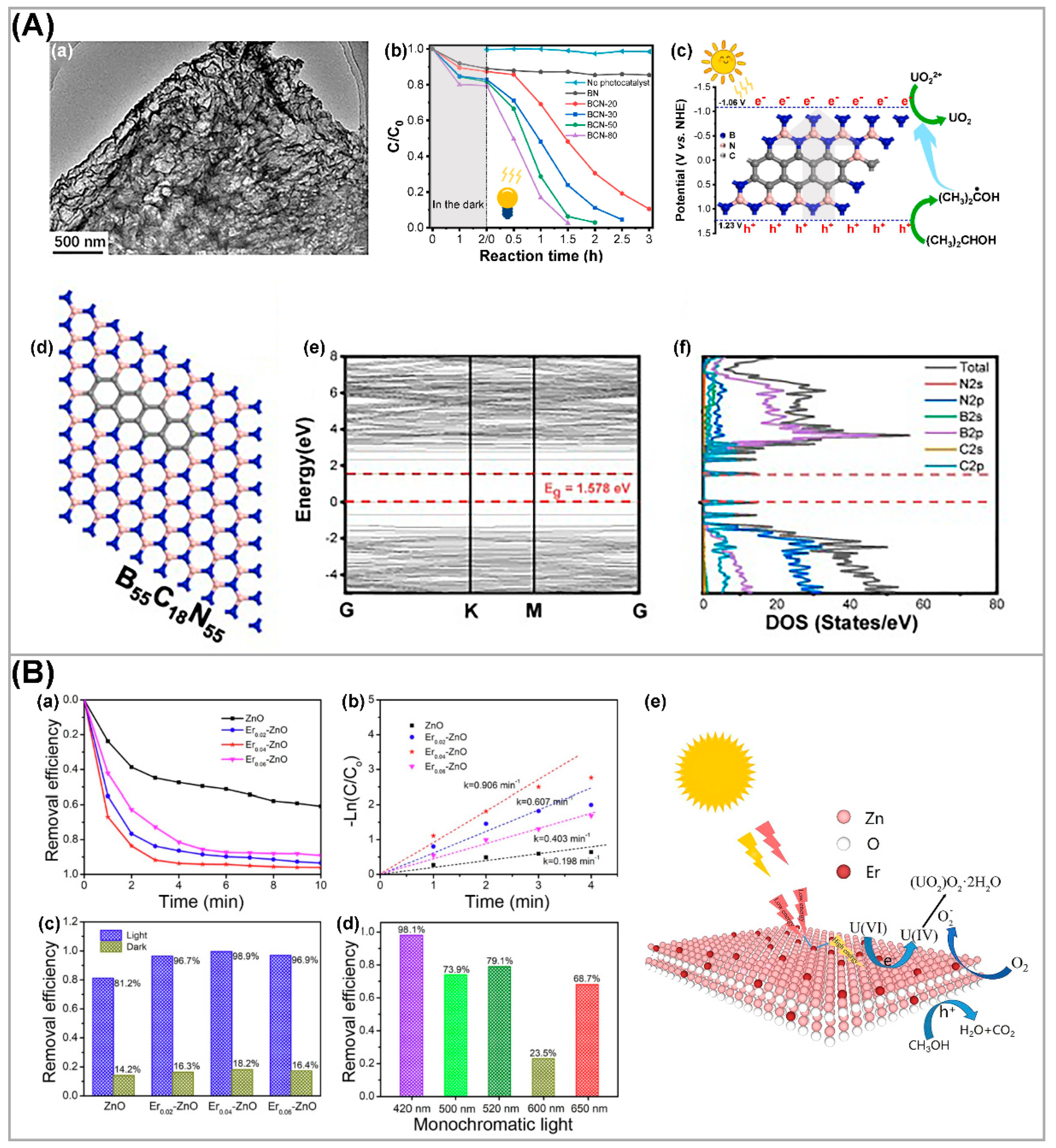
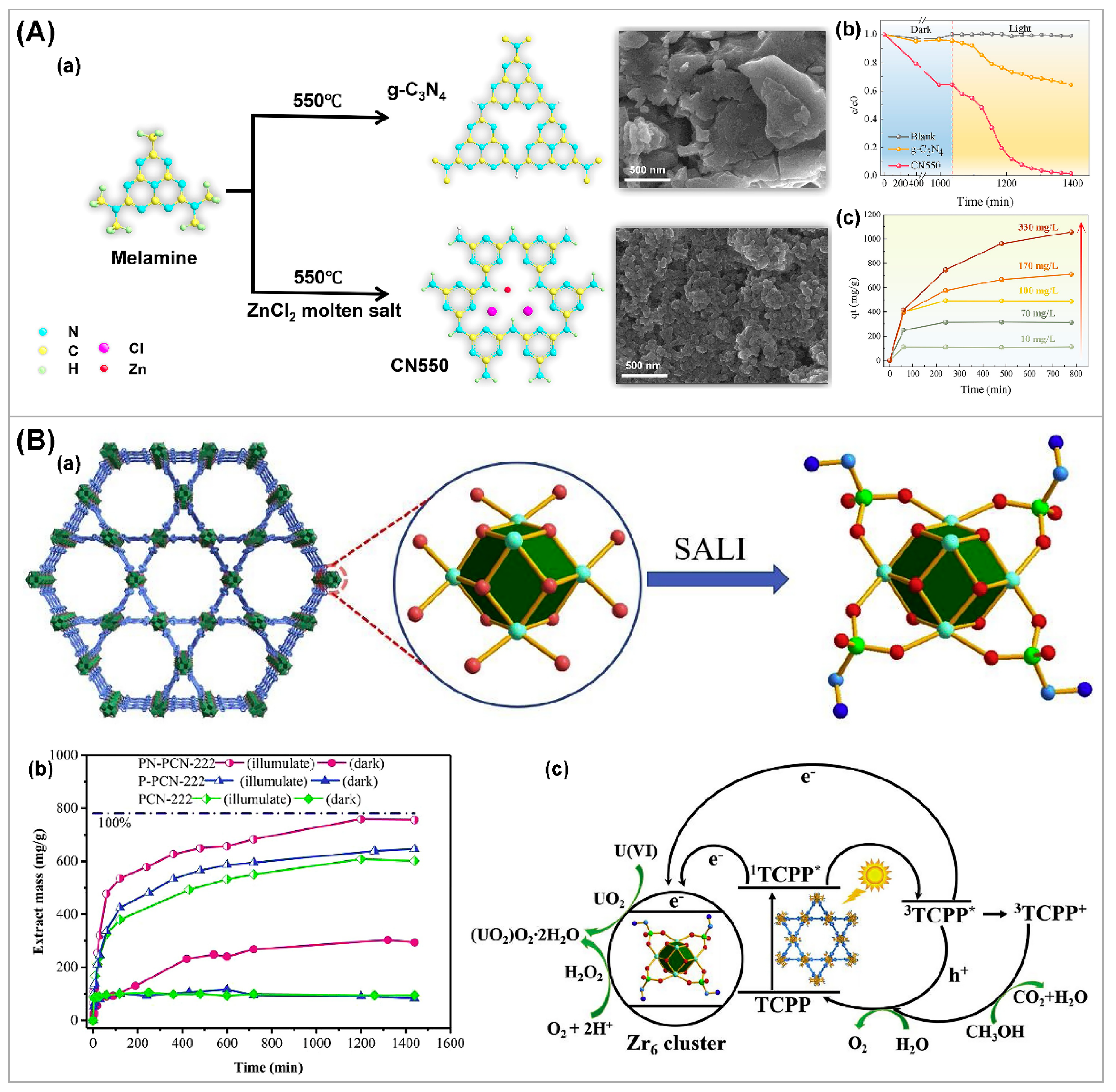
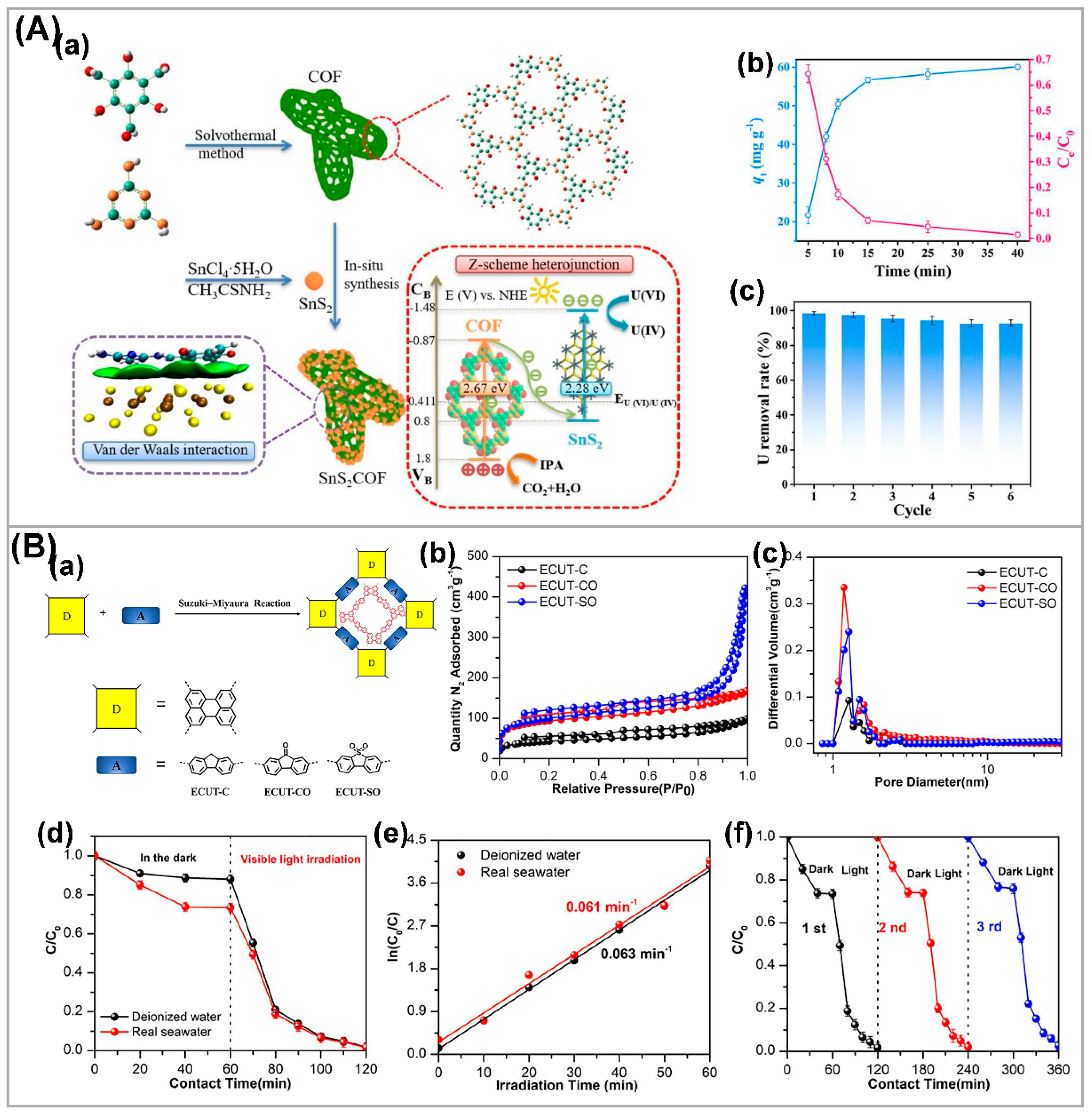
| Photocatalysts | CU(VI) a | pH | Light | RR (%) b t (min) c | Ref. |
|---|---|---|---|---|---|
| TiO2/Fe3O4 | 0.1 mM | 4.0 | UV light (100 W high-pressure mercury lamp) | 100, 30 | [35] |
| GO/KTO | 0.21 mM | 6.0–8.0 | UV-visible light (500 W Xe lamp) | 100, 60 | [36] |
| TiO2 | 0.2 mM | 5.0 | UV light (350 W mercury discharge lamp) | 100, 100 | [37] |
| C3N5/RGO | 10 mg L−1 | 5.0 | 300 W Xe lamp (λ ≥ 425 nm, 3.08 mW/cm2) | 94.9, 100 | [38] |
| ZnO/rectorite | 5 mg L−1 | --- | 300 W Xe arc lamp | 75, 150 | [39] |
| SrTiO3/TiO2 electrospun nanofibers | 100 mg L−1 | 4.0 | 400 nm with monochromatic light | - | [40] |
| SiO2/C nanocomposite | 100 mg L−1 | 5.0 | UV–vis absorption spectra | 94.2, 120 | [41] |
| S-g-C3N4 | 0.12 mM | 7.0 | visible light (A 350 W Xe lamp with a 420 nm cutoff filter) | 95, 20 | [42] |
| g-C3N4 | 27 mg L−1 | 6.0 | 300 W Xe lamp (λ ≥ 420 nm) | 100, 20 | [31] |
| C3N4 | 20 mg L−1 | 4.0–8.0 | 300 W Xe lamp (λ ≥ 420 nm) | 92, 60 | [43] |
| Nb/Ti NFs | 20 mg L−1 | 5.0 | simulated solar light (450 W xenon lamp) | 90, 240 | [44] |
| CdS/TiO2 | 50 mg L−1 | 6.0 | solar simulator with a 420 nm cut-off filter | 97, 240 | [45] |
| g-C3N4/GO | 80 mg L−1 | 5.0 | 300-W Xe lamp | - | [46] |
| Fe3O4@PDA@TiO2 | 50 mg L−1 | 8.2 | --- | 73, 300 | [47] |
| ZnFe2O4/g-C3N4 | 20 mg L−1 | 5.0 | 8 W LED light | 94.62, 2400 | [48] |
| MoS2/g-C3N4 | 50 mg L−1 | 4.5 | photocatalytic reaction box (CEL-HXF 300, Beijing Aulight Co. Ltd.) | 81.9, 120 | [49] |
| graphene-like S-C3N4 | 0.12 mM | 7.0 | sodium hydroxide aqueous solution. A 350 W Xe lamp (λ ≥ 420 nm) | 92, 30 | [50] |
| Ti3C2/SrTiO3 | 0.21 mM | 4.0 | UV-visible light (wavelength, 320–2500 nm, 300 W Xe lamp) | 80, 180 | [51] |
| MoS2/THS | 50 mg L−1 | 6.0 | 300 W Xe lamp equipped with an ultraviolet cutoff filter (λ ≥ 420 nm) | 98, 80 | [52] |
| bentonite/Portland cement composite | 50 mg L−1 | --- | --- | 96.97, 1140 | [53] |
| g-C3N4/TiO2 | 0.25 g·L−1 | 6.9 | UV-visible light (wavelength, 320–780 nm, 300 W Xe lamp) | ∼80, 240 | [54] |
| CuO/CuFeO2 | 0.000126 mM | 8.2 | photoelectrochemical method: constant potential (−0.6 V vs. SCE) + simulated sunlight (150 W Xenon arc lamp with an AM1.5 G filter) | 100, 90 | [55] |
| TiO2/Fe3O4 | 13.5–108 mg L−1 | 3.5–9.3 | 100 W high—pressure mercury lamp (λ = 365 nm) | 100, 30 | [35] |
| PCN-222 MOF | 500 mg L−1 | 3–11 | 300 W Xe lamp (λ ≥ 420 nm) | 100, 5 | [27] |
| Ti3C2/CdS | 25–200 mg L−1 | 4–10 | 300 W Xe lamp (λ ≥ 420 nm, 500 mW/cm2) | 97, 40 | [56] |
| ECUT-SO | 50 mg L−1 | 4 | 300 W Xe lamp (λ ≥ 400 nm) | 97.8, 60 | [6] |
| Sn/In2S3 | 16,200 mg L−1 | 3–9 | 500 W Xe lamp (λ ≥ 400 nm) | 95, 40 | [57] |
| PCB/g-C3N4 | 100 mg L−1 | 4 | 300 W Xe lamp (λ ≥ 400 nm) | --- | [58] |
| TiO2 | 400 mg L−1 | 5.5 | UV light (400 W mercury discharge lamp) | 95, 135 | [59] |
| B-g-C3N4 | 500 mg L−1 | 7.0 | visible light (A 500 W Xe lamp with a 420 nm cutoff filter) | 93, 20 | [60] |
| isotype g-C3N4 | 0.168 mM | 5.32 | --- | 98, 20 | [61] |
| BiOBr@TpPa-1 | 30 mg L−1 | 2.0–7.0 | 500 W Xe lamp (λ ≥ 420 nm) | 91, 540 | [62] |
| g-C3N4/LaFeO3 | 0.1 mM | 5.0 | 300 W Xe lamp (AM 1.5 G) | 94, 120 | [63] |
| BC-MoS2-x | 8 mg L−1 | 5.0 | 300-W Xe lamp with AM 1.5 G | 92, 70 | [33] |
| ZIF-8/g-C3N4 | 10 mg L−1 | --- | 300 W Xe lamp (λ ≥ 420 nm) | 100, 30 | [64] |
| CdS/CN-33 | 0.1 mM | 6.0 | 500 W Xe lamp (λ ≥ 420 nm) | 100, 6 | [26] |
| SnO2/CdCO3/CdS | 50 mg L−1 | --- | 500 W Xe lamp (λ ≥ 420 nm) | 100, 70 | [65] |
| Te/SnS2 | 8 mg L−1 | 4.8 | 300 W Xe lamp with AM 1.5 G filter | 98.6, 90 | [66] |
| Ag-doped SnS2@InVO4 | 60 mg L−1 | 6.0 | 450 W Xe lamp (λ ≥ 400 nm) | 97.6, 100 | [67] |
| MIL-53 (Fe) | 0.21 mM | 4.5 | visible light (285 W Xe lamp, λ ≥ 420 nm) | 80, 120 | [68] |
| TiO2 | 0.21 mM | 2.7 | 15 W black-light fluorescent bulb (λ = 320∼400 nm) | 50, 780 | [69] |
| CdS0.95Te0.05-EDA nanobelt | 10–300 mg L−1 | 3–9 | 300 W Xe lamp (λ ≥ 420 nm) | 97.4, 80 | [70] |
| graphene aerogel | 95.2 mg L−1 | 5.0 | 350 W Xe lamp (λ = 420– 800 nm, 160 mW/cm2) | 96, 200 | [71] |
| TiO2 | 0.42 mM | 3.0 | 150 W Xenon arc lamp with an AM 1.5 G filter | 80, 240 | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Li, X.; Wang, D.; Fu, F.; Liang, Y. Advanced Photocatalytic Uranium Extraction Strategies: Progress, Challenges, and Prospects. Nanomaterials 2023, 13, 2005. https://doi.org/10.3390/nano13132005
Zhu W, Li X, Wang D, Fu F, Liang Y. Advanced Photocatalytic Uranium Extraction Strategies: Progress, Challenges, and Prospects. Nanomaterials. 2023; 13(13):2005. https://doi.org/10.3390/nano13132005
Chicago/Turabian StyleZhu, Wangchuan, Xiang Li, Danjun Wang, Feng Fu, and Yucang Liang. 2023. "Advanced Photocatalytic Uranium Extraction Strategies: Progress, Challenges, and Prospects" Nanomaterials 13, no. 13: 2005. https://doi.org/10.3390/nano13132005
APA StyleZhu, W., Li, X., Wang, D., Fu, F., & Liang, Y. (2023). Advanced Photocatalytic Uranium Extraction Strategies: Progress, Challenges, and Prospects. Nanomaterials, 13(13), 2005. https://doi.org/10.3390/nano13132005





