Terahertz Optical Properties and Carrier Behaviors of Graphene Oxide Quantum Dot and Reduced Graphene Oxide Quantum Dot via Terahertz Time-Domain Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of GOQDs and rGOQDs
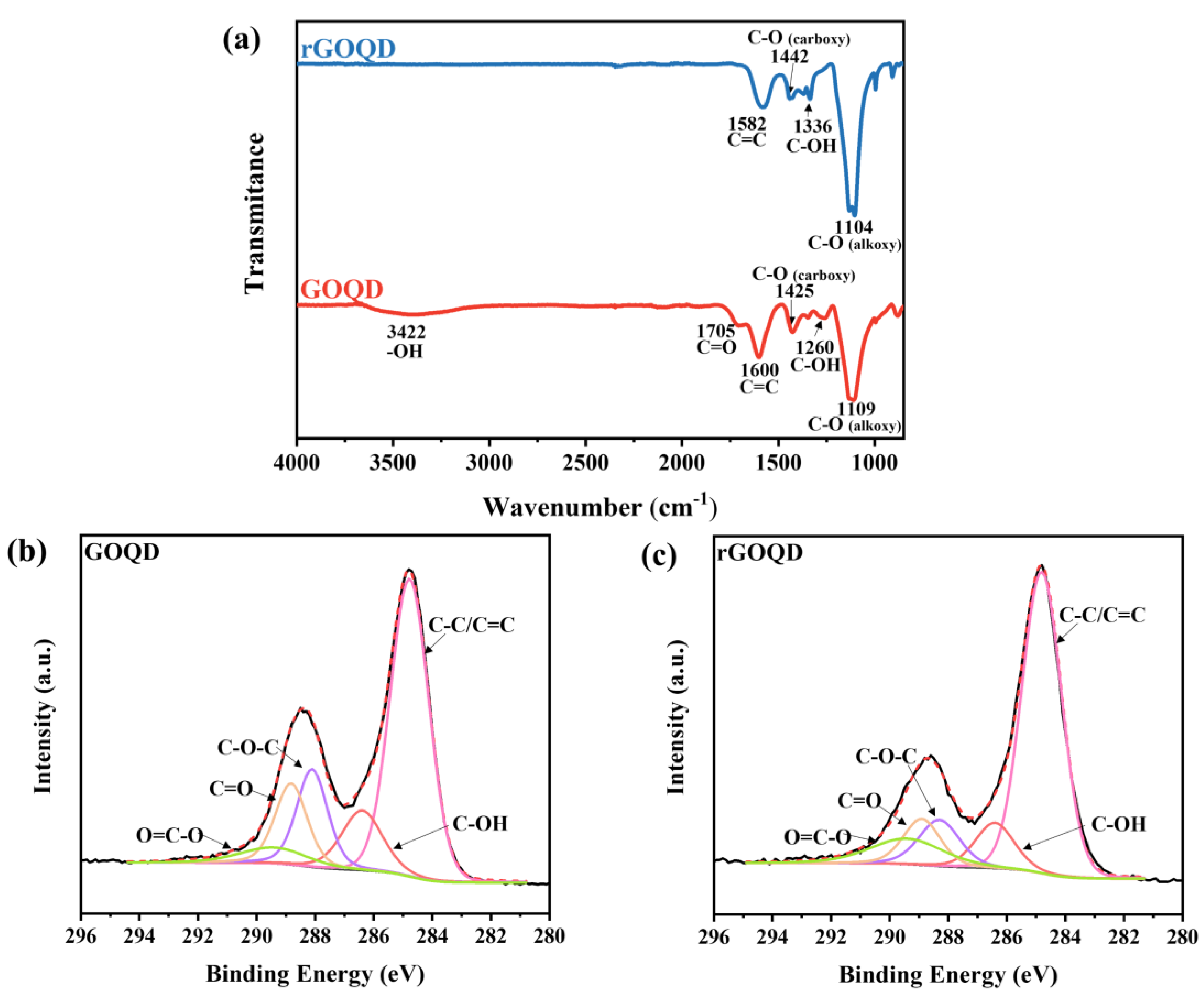
2.2. Materials Characterization
2.3. Terahertz Time-Domain Spectroscopy (THz-TDS) Measurement
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abergel, D.S.L.; Apalkov, V.; Berashevich, J.; Ziegler, K.; Chakraborty, T. Properties of graphene: A theoretical perspective. Adv. Phys. 2010, 59, 261–482. [Google Scholar] [CrossRef]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon 2010, 48, 2127–2150. [Google Scholar] [CrossRef]
- Ghaffarkhah, A.; Hosseini, E.; Kamkar, M.; Sehat, A.A.; Dordanihaghighi, S.; Allahbakhsh, A.; van der Kuur, C.; Arjmand, M. Synthesis, Applications, and Prospects of Graphene Quantum Dots: A Comprehensive Review. Small 2022, 18, 2102683. [Google Scholar] [CrossRef]
- Kumar, P.; Dhand, C.; Dwivedi, N.; Singh, S.; Khan, R.; Verma, S.; Singh, A.; Gupta, M.K.; Kumar, S.; Kumar, R.; et al. Graphene quantum dots: A contemporary perspective on scope, opportunities, and sustainability. Renew. Sustain. Energy Rev. 2022, 157, 111993. [Google Scholar] [CrossRef]
- Bokare, A.; Chinnusamy, S.; Erogbogbo, F. TiO2-Graphene Quantum Dots Nanocomposites for Photocatalysis in Energy and Biomedical Applications. Catalysts 2021, 11, 319. [Google Scholar] [CrossRef]
- Prabhu, S.A.; Kavithayeni, V.; Suganthy, R.; Geetha, K. Graphene quantum dots synthesis and energy application: A review. Carbon Lett. 2021, 31, 1–12. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Liang, Q.H.; Yan, M.; Liu, Z.F.; He, Q.Y.; Wu, T.; Luo, S.H.; Pan, Y.; Zhao, C.H.; Liu, Y. Advances in preparation, mechanism and applications of graphene quantum dots/semiconductor composite photocatalysts: A review. J. Hazard. Mater. 2022, 424, 127721. [Google Scholar] [CrossRef]
- Qian, R.F.; Zong, H.X.; Schneider, J.; Zhou, G.D.; Zhao, T.; Li, Y.L.; Yang, J.; Bahnemann, D.W.; Pan, J.H. Charge carrier trapping, recombination and transfer during TiO2 photocatalysis: An overview. Catal. Today 2019, 335, 78–90. [Google Scholar] [CrossRef]
- Wu, J.Y.; Cha, H.; Du, T.; Dong, Y.F.; Xu, W.D.; Lin, C.T.; Durrant, J.R. A Comparison of Charge Carrier Dynamics in Organic and Perovskite Solar Cells. Adv. Mater. 2022, 34, 2101833. [Google Scholar] [CrossRef]
- Amenabar, I.; Lopez, F.; Mendikute, A. In Introductory Review to THz Non-Destructive Testing of Composite Mater. J. Infrared Millim. Terahertz Waves 2013, 34, 152–169. [Google Scholar] [CrossRef]
- Jepsen, P.U.; Cooke, D.G.; Koch, M. Terahertz spectroscopy and imaging-Modern techniques and applications (vol 5, pg 124, 2011). Laser Photonics Rev. 2012, 6, 418. [Google Scholar] [CrossRef]
- Spies, J.A.; Neu, J.; Tayvah, U.T.; Capobianco, M.D.; Pattengale, B.; Ostresh, S.; Schmuttenmaer, C.A. Terahertz Spectroscopy of Emerging Materials. J. Phys. Chem. C 2020, 124, 22335–22346. [Google Scholar] [CrossRef]
- Kuzel, P.; Nemec, H. Terahertz Spectroscopy of Nanomaterials: A Close Look at Charge-Carrier Transport. Adv. Opt. Mater. 2020, 8, 1900623. [Google Scholar] [CrossRef]
- Baxter, J.B.; Guglietta, G.W. Terahertz Spectroscopy. Anal. Chem. 2011, 83, 4342–4368. [Google Scholar] [CrossRef]
- El Haddad, J.; Bousquet, B.; Canioni, L.; Mounaix, P. Review in terahertz spectral analysis. TrAC Trends Anal. Chem. 2013, 44, 98–105. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Tang, M.Q.; Huang, J.Q.; Liu, W.; Dong, J.Y.; Chen, X.P.; Fu, W.L.; Zhang, Y. The medical application of terahertz technology in non-invasive detection of cells and tissues: Opportunities and challenges. RSC Adv. 2019, 9, 9354–9363. [Google Scholar] [CrossRef]
- Neu, J.; Schmuttenmaer, C.A. Tutorial: An introduction to terahertz time domain spectroscopy (THz-TDS). J. Appl. Phys. 2018, 124, 231101. [Google Scholar] [CrossRef]
- Schmuttenmaer, C.A. Exploring dynamics in the far-infrared with terahertz spectroscopy. Chem. Rev. 2004, 104, 1759–1779. [Google Scholar] [CrossRef]
- Lloyd-Hughes, J.; Jeon, T.I. A Review of the Terahertz Conductivity of Bulk and Nano-Materials. J. Infrared Millim. Terahertz Waves 2012, 33, 871–925. [Google Scholar] [CrossRef]
- Burdanova, M.G.; Tsapenko, A.P.; Kharlamova, M.V.; Kauppinen, E.I.; Gorshunov, B.P.; Kono, J.; Lloyd-Hughes, J. A Review of the Terahertz Conductivity and Photoconductivity of Carbon Nanotubes and Heteronanotubes. Adv. Opt. Mater. 2021, 9, 2101042. [Google Scholar] [CrossRef]
- Senthil, R.A.; Selvi, A.; Arunachalam, P.; Amudha, L.S.; Madhavan, J.; Al-Mayouf, A.M. A sensitive electrochemical detection of hydroquinone using newly synthesized alpha-Fe2O3-graphene oxide nanocomposite as an electrode material. J. Mater. Sci.-Mater. Electron. 2017, 28, 10081–10091. [Google Scholar] [CrossRef]
- Rommozzi, E.; Zannotti, M.; Giovannetti, R.; D’Amato, C.A.; Ferraro, S.; Minicucci, M.; Gunnella, R.; Di Cicco, A. Reduced Graphene Oxide/TiO2 Nanocomposite: From Synthesis to Characterization for Efficient Visible Light Photocatalytic Applications. Catalysts 2018, 8, 598. [Google Scholar] [CrossRef]
- Sujatmiko, F.; Sahroni, I.; Fadillah, G.; Fatimah, I. Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract. Open Chem. 2021, 19, 174–183. [Google Scholar] [CrossRef]
- Tucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Cheng, M.M.; Huang, L.J.; Wang, Y.X.; Zhao, Y.C.; Tang, J.G.; Wang, Y.; Zhang, Y.; Hedayati, M.; Kipper, M.J.; Wickramasinghe, S.R. Synthesis of graphene oxide/polyacrylamide composite membranes for organic dyes/water separation in water purification. J. Mater. Sci. 2019, 54, 252–264. [Google Scholar] [CrossRef]
- Emiru, T.F.; Ayele, D.W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef]
- Alam, K.; Sim, Y.; Yu, J.H.; Gnanaprakasam, J.; Choi, H.; Chae, Y.; Sim, U.; Cho, H. In-situ Deposition of Graphene Oxide Catalyst for Efficient Photoelectrochemical Hydrogen Evolution Reaction Using Atmospheric Plasma. Materials 2020, 13, 12. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Z.; Li, J.; Zhou, B.; Shan, M.; Li, Y.; Liu, L.; Li, B.; Niu, J. Modifying graphite oxide nanostructures in various media by high-energy irradiation. RSC Adv. 2014, 4, 1025–1031. [Google Scholar] [CrossRef]
- Gusev, A.; Zakharova, O.; Muratov, D.S.; Vorobeva, N.S.; Sarker, M.; Rybkin, I.; Bratashov, D.; Kolesnikov, E.; Lapanje, A.; Kuznetsov, D.V.; et al. Medium-Dependent Antibacterial Properties and Bacterial Filtration Ability of Reduced Graphene Oxide. Nanomaterials 2019, 9, 1454. [Google Scholar] [CrossRef]
- Wang, Z.C.; Wei, R.B.; Liu, X.B. Dielectric properties of copper phthalocyanine nanocomposites incorporated with graphene oxide. J. Mater. Sci.-Mater. Electron. 2017, 28, 7437–7448. [Google Scholar] [CrossRef]
- Jung, G.B.; Myung, Y.; Cho, Y.J.; Sohn, Y.J.; Jang, D.M.; Kim, H.S.; Lee, C.W.; Park, J.; Maeng, I.; Son, J.H.; et al. Terahertz Spectroscopy of Nanocrystal-Carbon Nanotube and -Graphene Oxide Hybrid Nanostructures. J. Phys. Chem. C 2010, 114, 11258–11265. [Google Scholar] [CrossRef]
- Kang, C.; Maeng, I.H.; Oh, S.J.; Lim, S.C.; An, K.H.; Lee, Y.H.; Son, J.H. Terahertz optical and electrical properties of hydrogen-functionalized carbon nanotubes. Phys. Rev. B 2007, 75, 085410. [Google Scholar] [CrossRef]
- Dutta, P.; Afalla, J.; Halder, A.; Datta, S.; Tominaga, K. Temperature-Dependent Conductivity of Graphene Oxide and Graphene Oxide-Polyaniline Nanocomposites Studied by Terahertz Time-Domain Spectroscopy. J. Phys. Chem. C 2017, 121, 1442–1448. [Google Scholar] [CrossRef]
- Koroliov, A.; Chen, G.Y.; Goodfellow, K.M.; Vamivakas, A.N.; Staniszewski, Z.; Sobolewski, P.; El Fray, M.; Laszcz, A.; Czerwinski, A.; Richter, C.P.; et al. Terahertz Time-Domain Spectroscopy of Graphene Nanoflakes Embedded in Polymer Matrix. Appl. Sci. 2019, 9, 391. [Google Scholar] [CrossRef]
- Chen, S.Z.; Kuhne, P.; Stanishev, V.; Knight, S.; Brooke, R.; Petsagkourakis, I.; Crispin, X.; Schubert, M.; Darakchieva, V.; Jonsson, M.P. On the anomalous optical conductivity dispersion of electrically conducting polymers: Ultra-wide spectral range ellipsometry combined with a Drude-Lorentz model. J. Mater. Chem. C 2019, 7, 4350–4362. [Google Scholar] [CrossRef]
- Chung, D.C.; Park, Y.S.; Lee, K.S.; Kang, S.B. Terahertz characterization of multi-walled carbon nanotubes/high-density polyethylene nanocomposites. J. Korean Phys. Soc. 2022, 80, 265–272. [Google Scholar] [CrossRef]
- Ulbricht, R.; Hendry, E.; Shan, J.; Heinz, T.F.; Bonn, M. Carrier dynamics in semiconductors studied with time-resolved terahertz spectroscopy. Rev. Mod. Phys. 2011, 83, 543–586. [Google Scholar] [CrossRef]
- Dychalska, A.; Popielarski, P.; Frankow, W.; Fabisiak, K.; Paprocki, K.; Szybowicz, M. Study of CVD diamond layers with amorphous carbon admixture by Raman scattering spectroscopy. Mater. Sci.-Pol. 2015, 33, 799–805. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cancado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef]
- Maeng, I.; Kang, C.; Oh, S.J.; Son, J.H.; An, K.H.; Lee, Y.H. Terahertz electrical and optical characteristics of double-walled carbon nanotubes and their comparison with single-walled carbon nanotubes. Appl. Phys. Lett. 2007, 90, 051914. [Google Scholar] [CrossRef]



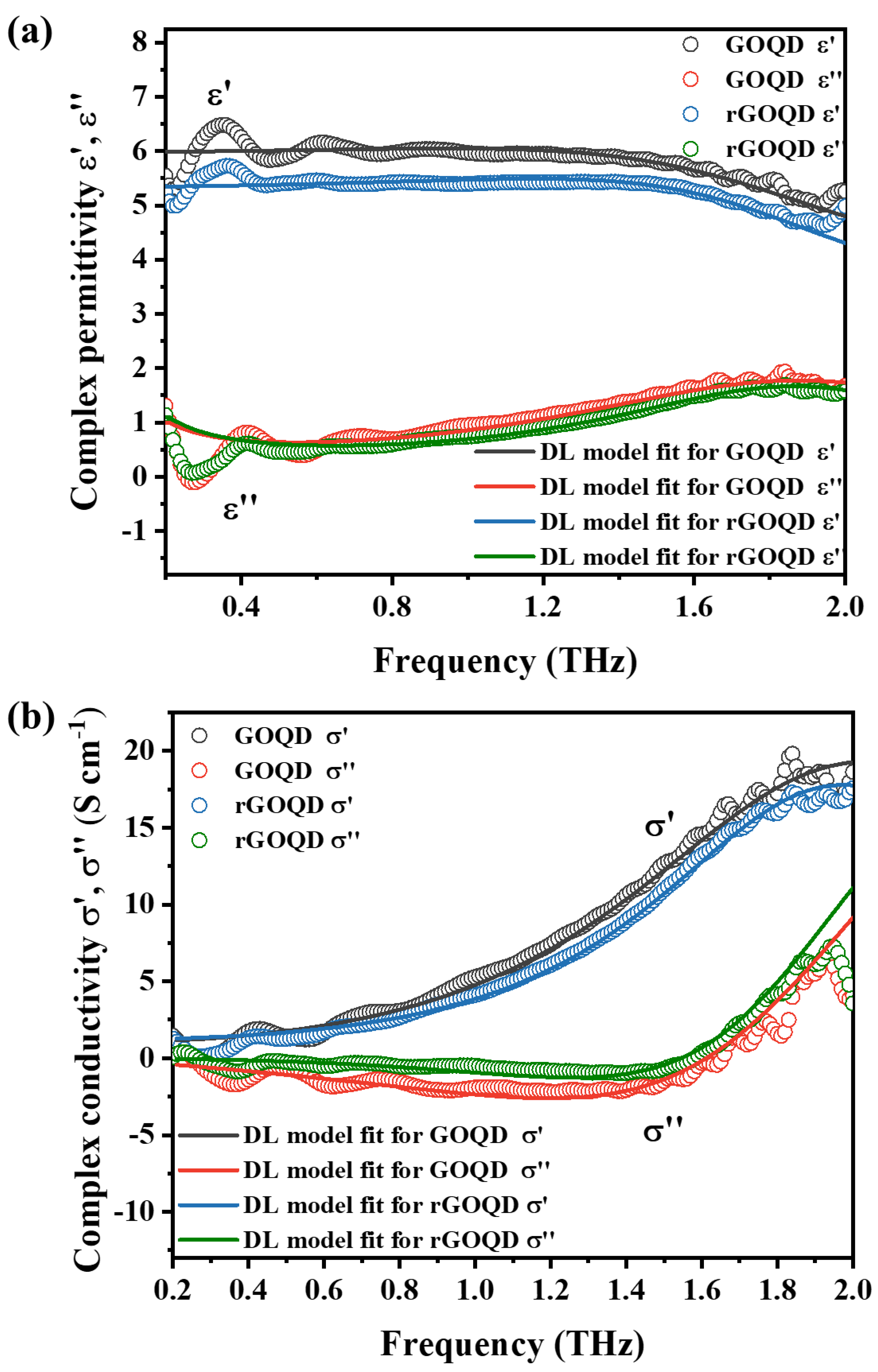

| Sample | ωp [THz] | ΓD [THz] | AL | ωL [THz] | ΓL [THz] | N [1015 cm−3] | μ [cm2 V−1 s−1] |
|---|---|---|---|---|---|---|---|
| GOQD | 2.55 ± 0.18 | 18.53 ± 0.76 | 1.42 ± 0.21 | 13.47 ± 0.79 | 11.64 ± 2.49 | 2.05 ± 0.29 | 256.80 ± 10.74 |
| rGOQD | 3.13 ± 0.13 | 20.71 ± 0.52 | 1.04 ± 0.26 | 12.57 ± 0.26 | 8.33 ± 0.75 | 3.07 ± 0.25 | 229.61 ± 5.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Kim, H.; Kang, C.; Bae, J. Terahertz Optical Properties and Carrier Behaviors of Graphene Oxide Quantum Dot and Reduced Graphene Oxide Quantum Dot via Terahertz Time-Domain Spectroscopy. Nanomaterials 2023, 13, 1948. https://doi.org/10.3390/nano13131948
Song S, Kim H, Kang C, Bae J. Terahertz Optical Properties and Carrier Behaviors of Graphene Oxide Quantum Dot and Reduced Graphene Oxide Quantum Dot via Terahertz Time-Domain Spectroscopy. Nanomaterials. 2023; 13(13):1948. https://doi.org/10.3390/nano13131948
Chicago/Turabian StyleSong, Seunghyun, Hyeongmun Kim, Chul Kang, and Joonho Bae. 2023. "Terahertz Optical Properties and Carrier Behaviors of Graphene Oxide Quantum Dot and Reduced Graphene Oxide Quantum Dot via Terahertz Time-Domain Spectroscopy" Nanomaterials 13, no. 13: 1948. https://doi.org/10.3390/nano13131948
APA StyleSong, S., Kim, H., Kang, C., & Bae, J. (2023). Terahertz Optical Properties and Carrier Behaviors of Graphene Oxide Quantum Dot and Reduced Graphene Oxide Quantum Dot via Terahertz Time-Domain Spectroscopy. Nanomaterials, 13(13), 1948. https://doi.org/10.3390/nano13131948








