Co-Promoted CoNi Bimetallic Nanocatalyst for the Highly Efficient Catalytic Hydrogenation of Olefins
Abstract
:1. Introduction
2. Experimental and Computational Details
2.1. Catalysts Preparation
2.2. Catalyst Characterization
2.3. Catalytic Evaluation
2.4. Computational Methods
3. Results and Discussion
3.1. H2-TPR, H2-TPD and XRD Analysis
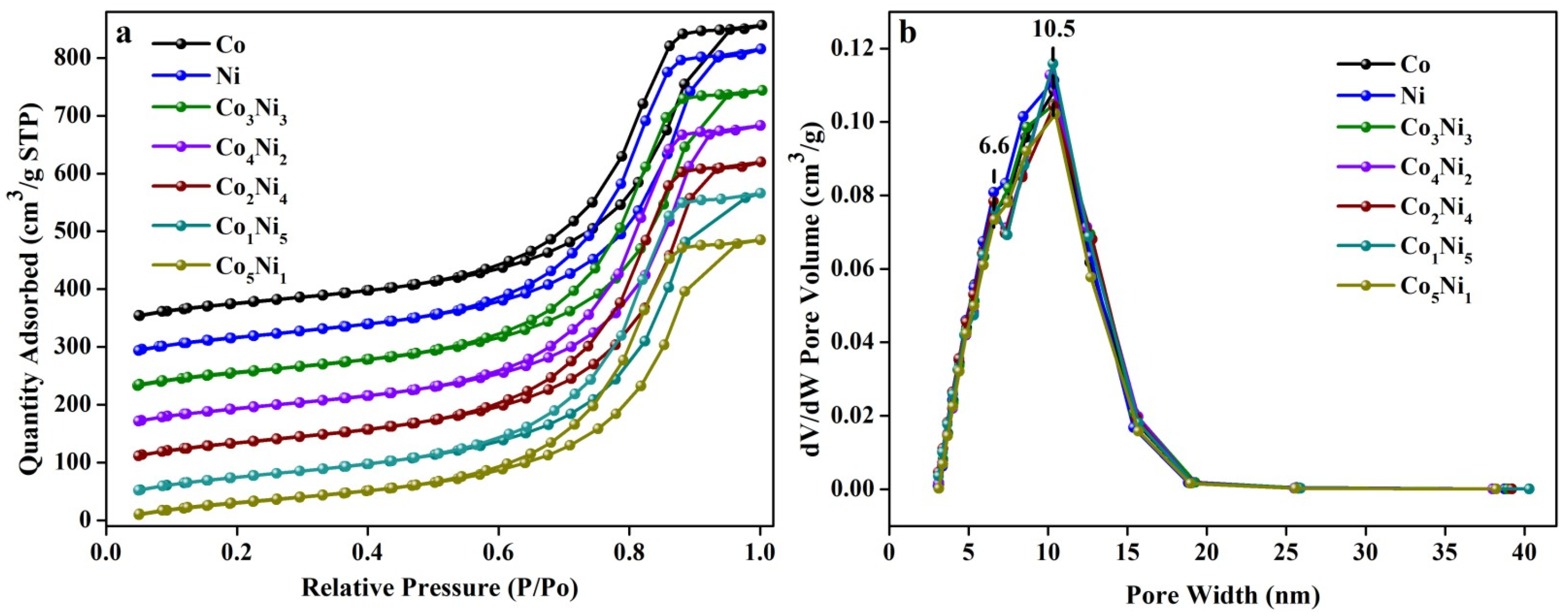
3.2. BET and BJH Analysis
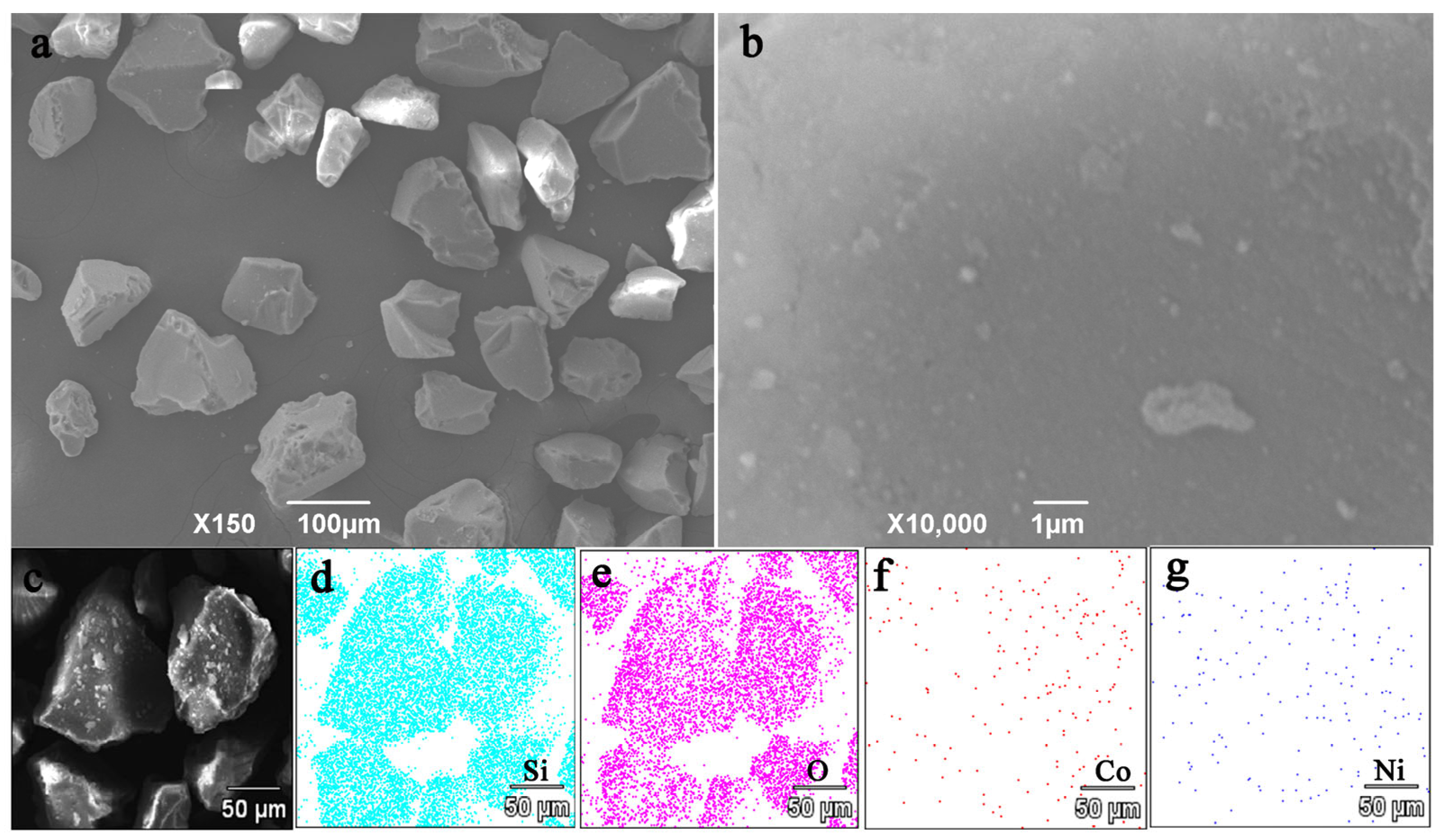
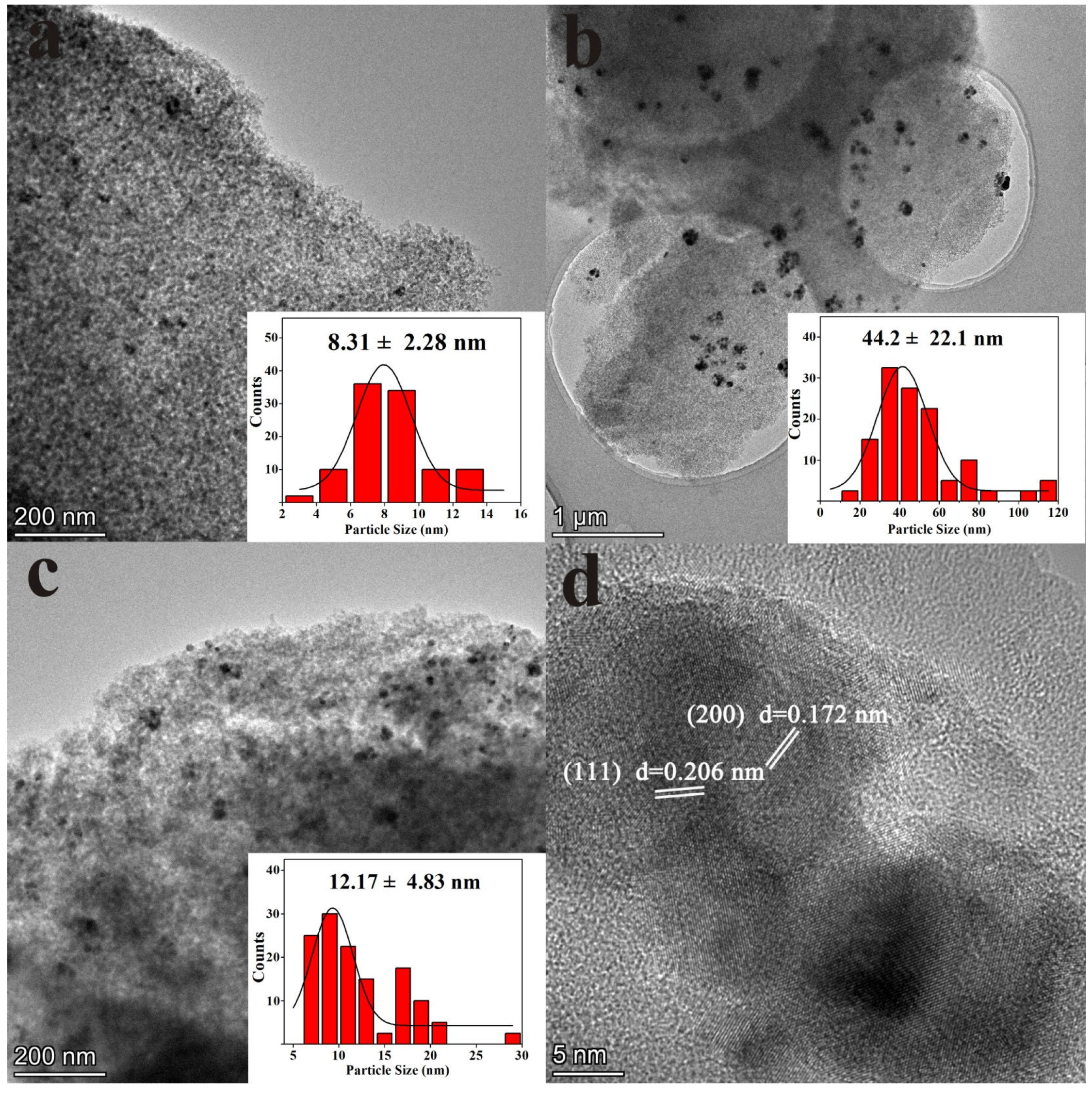
3.3. SEM and TEM Analysis
3.4. XPS Analysis
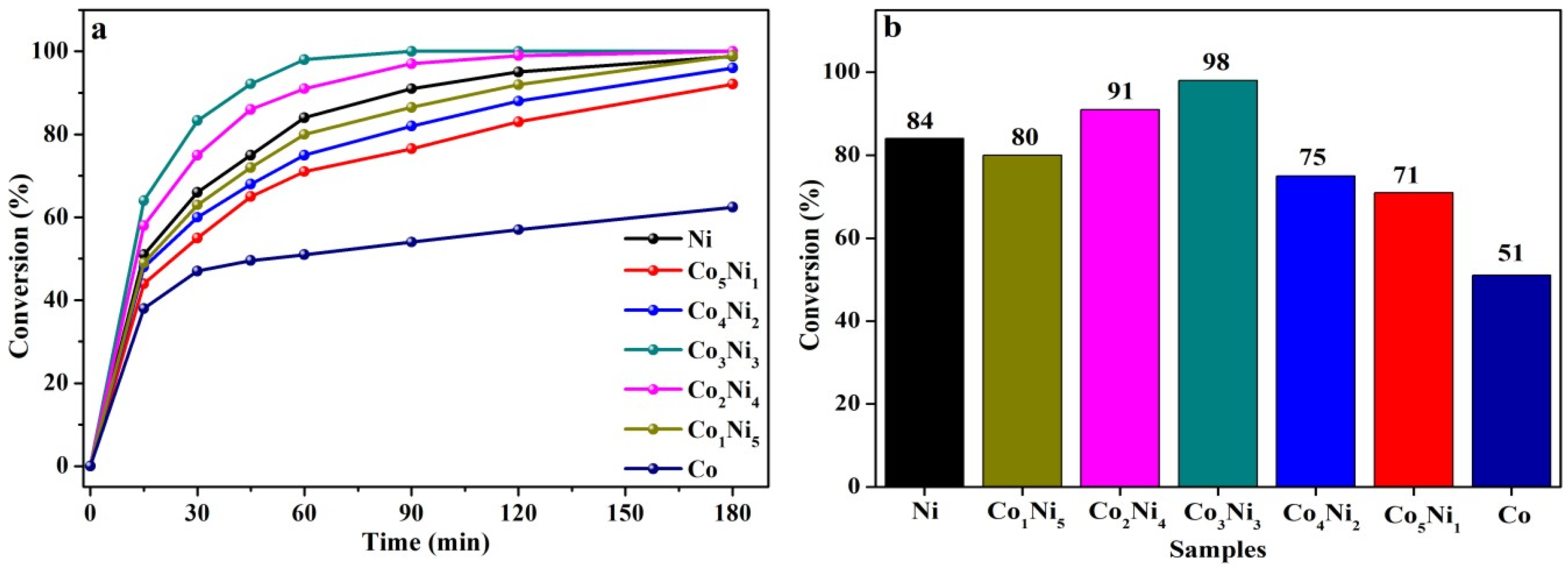
3.5. Catalytic Hydrogenation Performance
3.6. DFT Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peters, B.B.C.; Zheng, J.; Krajangsri, S.; Andersson, P.G. Stereoselective Iridium-N,P-Catalyzed Double Hydrogenation of Conjugated Enones to Saturated Alcohols. J. Am. Chem. Soc. 2022, 144, 8734–8740. [Google Scholar] [CrossRef] [PubMed]
- Knowles, W.S. Asymmetric hydrogenations (Nobel lecture). Angew. Chem. Int. Ed. 2002, 41, 1998–2007. [Google Scholar] [CrossRef]
- Woodmansee, D.H.; Pfaltz, A. Asymmetric hydrogenation of alkenes lacking coordinating groups. Chem. Commun. 2011, 47, 7912–7916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, X.J.; Shi, F.; Deng, Y.Q. Nano-Gold Catalysis in Fine Chemical Synthesis. Chem. Rev. 2012, 112, 2467–2505. [Google Scholar] [CrossRef]
- Margarita, C.; Andersson, P.G. Evolution and Prospects of the Asymmetric Hydrogenation of Unfunctionalized Olefins. J. Am. Chem. Soc. 2017, 139, 1346–1356. [Google Scholar] [CrossRef] [Green Version]
- Kraft, S.; Ryan, K.; Kargbo, R.B. Recent Advances in Asymmetric Hydrogenation of Tetrasubstituted Olefins. J. Am. Chem. Soc. 2017, 139, 11630–11641. [Google Scholar] [CrossRef]
- Fish, R.H.; Thormodsen, A.D.; Cremer, G.A. Homogeneous catalytic hydrogenation. 1. Regiospecific Reductions of Polynuclear Aromatic and Polynuclear Heteroaromatic Nitrogen Compounds Catalyzed by Transition Metal Carbonyl Hydrides. J. Am. Chem. Soc. 1982, 104, 5234–5237. [Google Scholar] [CrossRef]
- Fang, M.F.; Sanchez-Delgado, R.A. Ruthenium Nanoparticles Supported on Magnesium Oxide: A Versatile and Recyclable Dual Site Catalyst for Hydrogenation of Mono- and Poly-cyclic Arenes, N-Heteroaromatics, and S-Heteroaromatics. J. Catal. 2014, 311, 357–368. [Google Scholar] [CrossRef]
- Campanati, M.; Casagrande, M.; Fagiolino, I.; Lenarda, M.; Storaro, L.; Battagliarin, M.; Vaccari, A. Mild Hydrogenation of Quinoline-2. A Novel Rh-Containing Pillared Layered Clay Catalyst. J. Mol. Catal. A Chem. 2002, 184, 267–272. [Google Scholar] [CrossRef]
- Sanchez, A.; Fang, M.F.; Ahmed, A.; Sanchez-Delgado, R.A. Hydrogenation of Arenes, N-Heteroaromatic Compounds, and Alkenes Catalyzed by Rhodium Nanoparticles Supported on Magnesium Oxide. Appl. Catal. A 2014, 477, 117–124. [Google Scholar] [CrossRef]
- Ge, D.H.; Hu, L.; Wang, J.Q.; Li, X.M.; Qi, F.Q.; Lu, J.M.; Cao, X.Q.; Gu, H.W. Reversible Hydrogenation-Oxidative Dehydrogenation of Quinolines over A Highly Active Pt Nanowire Catalyst under Mild Conditions. ChemCatChem 2013, 5, 2183–2186. [Google Scholar] [CrossRef]
- Bai, L.C.; Wang, X.; Chen, Q.; Ye, Y.F.; Zheng, H.Q.; Guo, J.H.; Yin, Y.D.; Gao, C.B. Explaining the Size Dependence in Platinum-Nanoparticle-Catalyzed Hydrogenation Reactions. Angew. Chem. Int. Ed. 2016, 55, 15656–15661. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, Z.M.; Ni, T.; Zhang, Z.Y.; Ma, Y.Y.; Qu, Y.Q. Strong Electronic Metal-support Interaction of Pt/CeO2 Enables Efficient and Selective Hydrogenation of Quinolines at Room Temperature. J. Catal. 2018, 359, 101–111. [Google Scholar] [CrossRef]
- Ren, D.; He, L.; Yu, L.; Ding, R.S.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. An Unusual Chemoselective Hydrogenation of Quinoline Compounds Using Supported Gold Catalysts. J. Am. Chem. Soc. 2012, 134, 17592–17598. [Google Scholar] [CrossRef]
- Wang, T.L.; Zhuo, L.G.; Li, Z.W.; Chen, F.; Ding, Z.Y.; He, Y.M.; Fan, Q.H.; Xiang, J.F.; Yu, Z.X.; Chan, A.S. Highly Enantioselective Hydrogenation of Quinolines Using Phosphine-Free Chiral Cationic Ruthenium Catalysts: Scope, Mechanism, and Origin of Enantioselectivity. J. Am. Chem. Soc. 2011, 133, 9878–9891. [Google Scholar] [CrossRef]
- Hashimoto, N.; Takahashi, Y.; Hara, T.; Shimazu, S.; Mitsudome, T.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Fine Tuning of Pd-0 Nanoparticle Formation on Hydroxyapatite and Its Application for Regioselective Quinoline Hydrogenation. Chem. Lett. 2010, 39, 832–834. [Google Scholar] [CrossRef]
- Gong, Y.T.; Zhang, P.F.; Xu, X.; Li, Y.; Li, H.R.; Wang, Y. A Novel Catalyst Pd@ompg-C3N4 for Highly Chemoselective Hydrogenation of Quinoline under Mild Conditions. J. Catal. 2013, 297, 272–280. [Google Scholar] [CrossRef]
- Liu, L.J.; Lou, H.; Chen, M. Selective hydrogenation of furfural to tetrahydrofurfuryl alcohol over Ni/CNTs and bimetallic Cu-Ni/CNTs catalysts. Int. J. Hydrogen Energy 2016, 41, 14721–14731. [Google Scholar] [CrossRef]
- Qiu, M.; Li, Y.; Zhang, Z.Y. The synthesis mechanism of nitrogen to ammonia on the Fe, Co, Ni-doped Cu(100) Surface: A DFT study. Appl. Surf. Sci. 2022, 573, 151477. [Google Scholar] [CrossRef]
- Liu, L.C.; Gao, F.; Concepcion, P.; Corma, A. A new strategy to transform mono and bimetallic non-noble metal nanoparticles into highly active and chemoselective hydrogenation catalysts. J. Catal. 2017, 350, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Dou, N.N.; Qu, J.Y. Fast Synthesis of Copper-Nickel Bimetal and Reduced Graphene Hybrid Nanomaterial for Sensitive Sensing of 4-Aminophenol and Acetaminophen Simultaneously. J. Electrochem. Soc. 2020, 167, 136513. [Google Scholar] [CrossRef]
- Chen, C.; Zhong, Y.; Cheng, S.Y.; Huang, Y.Y.; Li, T.X.; Shi, T.L.; Liao, G.L.; Tang, Z.R. In Situ Fabrication of Porous Nanostructures Derived from Bimetal- Organic Frameworks for Highly Sensitive Non-Enzymatic Glucose Sensors. J. Electrochem. Soc. 2020, 167, 027531. [Google Scholar] [CrossRef]
- Meng, R.J.; Du, Q.J.; Zhong, N.; Zhou, X.; Liu, S.H.; Yin, S.F.; Liang, X. A Tandem Electrocatalysis of Sulfur Reduction by Bimetal 2D MOFs. Adv. Energy Mater. 2021, 11, 210281. [Google Scholar] [CrossRef]
- Qiu, B.C.; Cai, L.J.; Wang, Y.; Lin, Z.Y.; Zuo, Y.P.; Wang, M.Y.; Chai, Y. Fabrication of Nickel–Cobalt Bimetal Phosphide Nanocages for Enhanced Oxygen Evolution Catalysis. Adv. Funct. Mater. 2018, 28, 170600. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.B.; Zhang, H.; Zhu, Y.M.; Mi, G. Porously hierarchical Cu@Ni cubic-cage microstructure: Very active and durable catalyst for hydrolytically liberating H2 gas from ammonia borane. Renew Energy 2016, 99, 1038–1045. [Google Scholar] [CrossRef]
- Wang, H.Q.; Wei, L.L.; Liu, J.T.; Shen, J.Q. Hollow N-doped bimetal carbon spheres with superior ORR catalytic performance for microbial fuel cells. J. Colloid Interf. Sci. 2020, 575, 177–182. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, K.G.; Chen, J.H.; Zhu, L.J.; Wang, S.R. Mild hydrogenation of bio-oil and its derived phenolic monomers over Pt–Ni bimetal-based catalysts. Appl. Energy 2020, 275, 115154. [Google Scholar] [CrossRef]
- De, S.; Mandal, S. Surfactant-assisted shape control of copper nanostructures. Colloid Surf. A 2013, 421, 72–83. [Google Scholar] [CrossRef]
- Liu, H.X.; Li, S.Q.; Wang, W.W.; Yu, W.Z.; Zhang, W.J.; Ma, C.; Jia, C.J. Partially sintered copper—ceria as excellent catalyst for the high-temperature reverse water gas shift reaction. Nat. Commun. 2022, 13, 867. [Google Scholar] [CrossRef]
- Fei, W.; Lia, H.G.; Cheng, C.; Zhang, H.; Fei, S.X. An Efficient FeCo Bimetallic Catalyst for Olefin Hydrogenation. Russ. J. Phys. Chem. A 2022, 96, 2634–2641. [Google Scholar]
- Delley, B. Fast Calculation of Electrostatics in Crystals and Large Molecules. J. Phys. Chem. 1996, 100, 6107–6110. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Sheng, J.; Yi, X.; Li, F.; Fang, W.P. Effects of tungsten on the catalytic activity of Ni–W catalysts for the hydrogenation of aromatic hydrocarbons. Ract. Kinet. Mech. Cat. 2010, 2, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Yang, H.; Xiong, Z. Hollow core-shell CoNi@C and CoNi@NC composites as high-performance microwave absorbers. J. Alloys Compd. 2021, 871, 159574–159583. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, H.Y.; Cai, Y.S.; Ferrando, R.; Cheng, D.J. Origin of enhanced stability and oxygen adsorption capacity of medium-sized Pt–Ni nanoclusters. J. Phys. Condens. Matter. 2018, 30, 285503. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Lan, X.; Wang, T.F. Supported Ultrafine NiCo Bimetallic Alloy Nanoparticles Derived from Bimetal–Organic Frameworks: A Highly Active Catalyst for Furfuryl Alcohol Hydrogenation. ACS Catal. 2018, 8, 2121–2128. [Google Scholar] [CrossRef]
- An, H.Q.; Hu, P.; Hu, X.J.; Zhao, W.L.; Zhu, B.L.; Wang, S.R.; Zhang, S.M.; Huang, W.P. Characterization of Pt catalysts supported by three forms of TiO2 and their catalytic activities for hydrogenation. React. Kinet. Mech. Catal. 2013, 108, 117–126. [Google Scholar] [CrossRef]
- Fei, S.X.; Xia, K.S.; Tian, X.L.; Mei, P.; Yan, H.; Cheng, H.S. Fabrication of ordered mesoporous MoO3 for olefin catalytic hydrogenation. Int. J. Hydrogen Energy 2016, 41, 5652–5660. [Google Scholar] [CrossRef]
- Mei, P.; Yang, M.; Bai, Y.; Fei, S.X.; Dong, Y.; Cheng, H.S. Facile hydrothermal synthesis of nanorod-structured Mo0.6W0.4O3 catalyst for olefin hydrogenation with high activity. J. Catal. 2018, 360, 213–220. [Google Scholar] [CrossRef]
- Jung, C.W.; Lee, C.S.; Bang, K.H.; Lim, J.H.; Lee, H.; Ryu, H.J.; Cho, E.A.; Lee, H.M. Synthesis of Chemically Ordered Pt3Fe/C Intermetallic Electrocatalysts for Oxygen Reduction Reaction with Enhanced Activity and Durability via a Removable Carbon Coating. ACS Appl. Mater. Interfaces 2017, 9, 31806–31815. [Google Scholar] [CrossRef]
- Liu, Z.; Kim, S.C.; Wang, J.; Shin, W.G.; Fissan, H.; Pui, D.Y.H. Measurement of Metal Nanoparticle Agglomerates Generated by Spark Discharge Using the Universal Nanoparticle Analyzer (UNPA). Aerosol. Sci. Tech. 2012, 46, 333–346. [Google Scholar] [CrossRef]
- Parks, E.K.; Zhu, L.; Ho, J.; Riley, S.J. The structure of small nickel clusters. I. Ni3-Ni15. J. Chem. Phys. 1994, 100, 7206–7222. [Google Scholar] [CrossRef]
- Reuse, F.A.; Khanna, S.N. Geometry, electronic structure, and magnetism of small Nin (n = 2–6, 8, 13) clusters. Chem. Phys. Lett. 1995, 234, 77–81. [Google Scholar] [CrossRef]
- Desmarais, N.; Jamorski, C.; Reuse, F.A.; Khanna, S.N. Atomic Arrangements in Ni7 and Ni8 Clusters. Chem. Phys. Lett. 1998, 294, 480–486. [Google Scholar] [CrossRef]
- Zhou, C.G.; Yao, S.J.; Zhang, Q.F.; Wu, J.P.; Yang, M.; Forrey, R.C.; Cheng, H.S. Hydrogen Sequential Dissociative Chemisorption on Nin (n = 2∼9,13) Clusters: Comparison with Pt and Pd. J. Mol. Model. 2011, 17, 2305–2311. [Google Scholar]
- Dong, J.; Wen, X.; Zhu, T.L.; Qin, J.R.; Wu, Z.L.; Chen, L.G.; Bai, G.Y. Hierarchically nanostructured bimetallic NiCo/MgxNiyO catalyst with enhanced activity for phenol hydrogenation. Mol. Catal. 2020, 485, 110846. [Google Scholar] [CrossRef]
- Warczinski, L.; Hättig, C. A quantum chemical study of hydrogen adsorption on carbon-supported palladium clusters. Phys. Chem. Chem. Phys. 2019, 21, 21577–21587. [Google Scholar] [CrossRef]
- Liu, X.J.; Tian, D.X.; Meng, C.G. DFT study on the adsorption and dissociation of H2 on Pdn (n = 4, 6, 13, 19, 55) clusters. J. Mol. Struct. 2015, 1080, 105–110. [Google Scholar] [CrossRef]
- Dong, H.S.; Shin, H.; Kang, K.; Kim, H.J.; Han, S.S. First-Principles Design of Hydrogen Dissociation Catalysts Based on Isoelectronic Metal Solid Solutions. J. Phys. Chem. Lett. 2014, 5, 1819–1824. [Google Scholar]
- Molina, L.M.; Benito, A.; Alonso, J.A. Ab Initio Studies of Ethanol Dehydrogenation at Binary AuPd Nanocatalysts. Mol. Catal. 2018, 449, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Granja-DelRío, A.; Alonso, J.A.; López, M.J. Steric and chemical effects on the hydrogen adsorption and dissociation on free and graphene-supported palladium clusters. Comput. Theor. Chem. 2017, 1107, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Wu, J.; Nie, A.; Forrey, R.C.; Tachibana, A.; Cheng, H. On the Sequential Hydrogen Dissociative Chemisorption on Small Platinum Clusters: A Density Functional Theory Study. J. Phys. Chem. C 2007, 111, 12773–12778. [Google Scholar] [CrossRef]
- Chen, L.; Cooper, A.C.; Pez, G.P.; Cheng, H. Density Functional Study of Sequential H2 Dissociative Chemisorption on a Pt6 Cluster. J. Phys. Chem. C 2007, 111, 5514–5519. [Google Scholar] [CrossRef]
- Bertani, V.; Cavallotti, C.; Masi, M.; Carrà, S. Density Functional Study of the Interaction of Palladium Clusters with Hydrogen and CHxSpecies. J. Phys. Chem. A 2000, 104, 11390–11397. [Google Scholar] [CrossRef]
- Roques, J.; Lacaze-Dufaure, C.; Mijoule, C. Dissociative Adsorption of Hydrogen and Oxygen on Palladium Clusters: A Comparison with the (111) Infinite Surface. J. Chem. Theory Comput. 2007, 3, 878–884. [Google Scholar] [CrossRef]




| Samples | H2 Desorbed (umol/g) | Dispersion (%) |
|---|---|---|
| 5 wt% Co/SiO2 | 9.8 | 10.6 |
| 5 wt% Ni/SiO2 | 5.1 | 5.8 |
| 5 wt% Co3Ni3/SiO2 | 11.8 | 10.2 |
| Samples | SBET (m2/g) | Average Pore Size (nm) a | Vtot (cm3/g) | Grain Size (nm) b |
|---|---|---|---|---|
| SiO2 | 322.74 | 8.64 | 0.95 | - |
| 5 wt% Co/SiO2 | 299.52 | 8.73 | 0.87 | 10.42 |
| 5 wt% Ni/SiO2 | 306.10 | 8.64 | 0.89 | 23.65 |
| 5 wt% Co5Ni1/SiO2 | 280.96 | 8.69 | 0.82 | 13.19 |
| 5 wt% Co4Ni2/SiO2 | 294.60 | 8.87 | 0.88 | 12.18 |
| 5 wt% Co3Ni3/SiO2 | 299.14 | 8.81 | 0.88 | 12.06 |
| 5 wt% Co2Ni4/SiO2 | 298.44 | 8.70 | 0.87 | 11.89 |
| 5 wt% Co1Ni5/SiO2 | 298.87 | 8.79 | 0.88 | 11.98 |
| Catalysts | Temperature (°C) | H2 Pressure (MPa) | Time (min) | Conversion (%) |
|---|---|---|---|---|
| 5 wt% Co/SiO2 | 100 | 2 | 180 | 62.4 |
| 5 wt% Ni/SiO2 | 100 | 2 | 180 | 98.8 |
| 5 wt% Co5Ni1/SiO2 | 100 | 2 | 180 | 92.1 |
| 5 wt% Co4Ni2/SiO2 | 100 | 2 | 180 | 96.0 |
| 5 wt% Co3Ni3/SiO2 | 100 | 2 | 90 | 100 |
| 5 wt% Co2Ni4/SiO2 | 100 | 2 | 180 | 100 |
| 5 wt% Co1Ni5/SiO2 | 100 | 2 | 180 | 99.0 |
| Catalysts | Temperature (°C) | H2 Pressure (MPa) | Time (min) | Conversion (%) |
|---|---|---|---|---|
| 5 wt% Co3Ni3/SiO2 | 25 | 2 | 360 | 55.5 |
| 5 wt% Co3Ni3/SiO2 | 60 | 2 | 360 | 100 |
| 5 wt% Co3Ni3/SiO2 | 80 | 2 | 240 | 100 |
| 5 wt% Co3Ni3/SiO2 | 100 | 0.1 | 90 | 74.5 |
| 5 wt% Co3Ni3/SiO2 | 100 | 0.5 | 90 | 85.2 |
| 5 wt% Co3Ni3/SiO2 | 100 | 1 | 90 | 100 |
| 5 wt% Co3Ni3/SiO2 | 100 | 2 | 90 | 100 |
| 5 wt% Co3Ni3/SiO2 | 100 | 3 | 90 | 100 |
| 2 wt% Pt/TiO2-P [38] | 20 | 3 | 170 | 19.45 |
| 5 wt% Fe2Co4/SiO2 [30] | 100 | 2 | 300 | 100 |
| 0.1 wt% Pd/MoO3 [39] | 150 | 3 | 270 | 100 |
| Commercial 5 wt% Ru/Al2O3 [40] | 150 | 3 | 180 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Wang, Y.; Fei, S.; Zhu, G. Co-Promoted CoNi Bimetallic Nanocatalyst for the Highly Efficient Catalytic Hydrogenation of Olefins. Nanomaterials 2023, 13, 1939. https://doi.org/10.3390/nano13131939
Wu F, Wang Y, Fei S, Zhu G. Co-Promoted CoNi Bimetallic Nanocatalyst for the Highly Efficient Catalytic Hydrogenation of Olefins. Nanomaterials. 2023; 13(13):1939. https://doi.org/10.3390/nano13131939
Chicago/Turabian StyleWu, Fei, Yueying Wang, Shunxin Fei, and Gang Zhu. 2023. "Co-Promoted CoNi Bimetallic Nanocatalyst for the Highly Efficient Catalytic Hydrogenation of Olefins" Nanomaterials 13, no. 13: 1939. https://doi.org/10.3390/nano13131939
APA StyleWu, F., Wang, Y., Fei, S., & Zhu, G. (2023). Co-Promoted CoNi Bimetallic Nanocatalyst for the Highly Efficient Catalytic Hydrogenation of Olefins. Nanomaterials, 13(13), 1939. https://doi.org/10.3390/nano13131939






