Abstract
We designed and synthesized a new indolocarbazole-based polymer, poly(N,N-diphenyl(5,11-dihexylindolo[3,2,1-jk]carbazol-2-yl)amine) (PICA), for solution-processed organic light-emitting diodes (OLEDs). The highest occupied and lowest unoccupied molecular orbital energy levels of this polymer, −5.25 and −2.46 eV, respectively, are suitable for hole transport from the anode to the emissive layer. PICA was photo-crosslinked by UV irradiation with ethane-1,2-diyl bis(4-azido-2,3,5,6-tetrafluorobenzoate) (FPA) as the photoinitiator. Successful crosslinking was confirmed by a decreased intensity in the azide-stretching FT-IR peak and solvent test with toluene (a suitable solvent for PICA). The PICA film photo-crosslinked with 3 wt% FPA showed enhanced solvent resistance (90%) compared to the non-crosslinked neat PICA film (<20%). Moreover, OLED devices using PICA-based hole-transporting layers exhibited better device performance (EQE/LE/PE: 8.88%/12.97/8.12 in red devices, 10.84%/38.47 cd/A/25.06 lm/W in green devices) than those using poly-TPD:FPA. We demonstrated that the photo-crosslinked PICA can be applied as a hole-transporting layer in solution-processed OLEDs.
1. Introduction
Recently, organic light-emitting diodes (OLEDs) have become representative displays because of their high brightness and contrast, fast response, flexibility, high color purity, and low power consumption [1,2,3]. OLEDs show high device efficiency by adopting multiple organic thin layers consisting of hole and electron injection layers, hole and electron transport layers, and emissive layers [4,5,6,7]. In the OLED, holes and electrons generated at the anode and cathode, respectively, move to the emissive layer through the injection and the transport layers. Subsequently, light is generated through the recombination of the excitons. Vacuum sublimation is one of the major OLED device fabrication methods that has high device efficiency and stable device characteristics [6]. In contrast, the solution-processed OLED manufacturing strategy is attracting attention because it is favorable to large-area technologies such as inkjet, roll-to-roll printing, or spin casting [8,9,10,11]. A major problem in device fabrication through solution processing is that the lower organic layer can be damaged by solvent used in forming the upper layer. The dissolution or interfacial mixing makes it difficult to manufacture a multilayered thin film by adopting solution processing. This is one of the reasons that the solution-processed OLED device shows relatively lower device performances such as efficiency and lifetime than those of the vacuum-deposited OLED device. Many efforts are being made to create a thin film with high solvent resistance in order to prevent thin films from being damaged during the solution process [12,13,14,15,16,17,18,19,20,21,22].
An approach to create an insoluble network film through chemical crosslinking has been extensively studied as a method of increasing solvent resistance. Additionally, using an azide-based photo-crosslinking group in the side chain of a polymer is known as a good crosslinking method because of its excellent chemical stabilities and it does not significantly degrade the performance of the devices [23]. In particular, ethane-1,2-diylbis(4-azido-2,3,5,6-tetrafluorobenzoate) (FPA) has been used as a photo initiator and cross-linker, which has high efficiency of insertion to the alkyl position [24,25].
The terminal azide group is transformed into the active species, nitrene, by the loss of N2 upon relatively low-dose UV irradiation. The nitrene can be inserted into the alkyl position (C–H) to form a new covalent bond creating an insoluble network film. Our group also reported a blend of poly(bis-4-butylphenyl-N,N-bisphenyl)benzidine (poly-TPD) and FPA as a photo-crosslinkable hole-transporting layer (HTL) [26].
We have studied a new indolocarbazole-based photo-crosslinkable hole-transporting polymer with the proper HOMO level and alkyl side chains. Indolocarbazole derivatives have been studied as efficient and stable hole transport materials. The planar fused ring system with less ring strain and the strong electron donating ability of indolo[3,2,1-jk] carbazole is advantageous for hole transporting in organic thin-film transistors, perovskite solar cells, or dye-sensitized solar cells [27,28,29,30,31,32,33]. Taking the above into account, we develop an indolo[3,2,1-jk]carbazole-based novel polymer as a photo-crosslinkable HTL material with FPA photo initiator to achieve efficient solution-processed OLEDs.
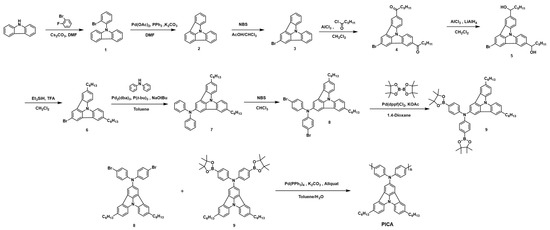
In this work, we successfully synthesized an indolo[3,2,1-jk] carbazole-based new polymer, poly(N,N-diphenyl(5,11-dihexylindolo[3,2,1-jk]carbazol-2-yl)amine) (PICA), as a hole-transporting material for solution-processed OLEDs. In order to raise the deep highest occupied molecular orbital (HOMO) energy level of indolo[3,2,1-jk]carbazole (−5.81 eV) [34] to an energy level between PEDOT:PSS (−5.0 eV) and light-emitting layers (above −5.6 eV), an electron-rich diphenylamine group was introduced on the 2-position of indolo[3,2,1-jk]carbazole, which is suitable for use as an HTL material with hole injection. Two hexyl groups were substituted on 5- and 11-positions of indolo[3,2,1-jk]carbazole unit not only to improve the molecular solubility required during the synthetic process, but also to increase the chemical crosslinking sites. The synthetic route and chemical structure of the PICA are shown in Scheme 1. The detailed synthetic procedures are described in Section 3. To the best of our knowledge, this study is the first to use an indolo[3,2,1-jk]carbazole-containing polymer as a crosslinkable HTL material for solution-processed OLEDs. As a result, the photo-crosslinked PICA:FPA (3 wt%) exhibited superior device performances in the red and green OLED devices (EQE/LE/PE: 8.88%/12.97 cd/A/8.12 lm/W in the red devices, 10.84%/38.47 cd/A, 25.06 lm/W in the green devices) compared to those of the reference material, photo-crosslinked poly-TPD: FPA (3 wt%).
Scheme 1.
Synthesis of PICA, the new polymer for HTL.
2. Materials and Methods
2.1. Materials
Carbazole, 1-bromo-2-fluorobenzene, cesium carbonate, aluminum chloride, potassium carbonate, potassium acetate triphenylphosphine, tetrabutylammonium bromide, N-bromosuccinimide, hexanoyl chloride, triethylsilane, trifluoroacetic acid, tris(dibenzylideneacetone)dipalladium, lithium aluminum hydride solution, tetrakis(triphenylphosphine)palladium, and bis(pinacolato)diboron, 4,4′,4-tris(carbazol-9-yl)triphenylamine (TCTA), 2,2′,2”-(1,3,5-Benzinetriyl)-tris(1-phenyl-1-H-benzimidazole (TPBi), 4,4′-bis(N-carbazolyl)-1,1′-biphenyl (CBP), poly(9-vinylcarbazole) (PVK) were purchased from Sigma-Aldrich, Alfa Aesar, and Tokyo Chemical Industry. They were used without further purification. Tris[2-(p-tolyl)pyridine]iridium(III) (Ir(mppy)3), iridium(III) bis[4-methyl-2-(3,5-dimethylphenyl)quinolinato-N,C2′]tetramethylheptadionate (Ir(mphmq)2(tmd)), poly-TPD, and FPA were synthesized according to reported methods [35,36,37,38,39,40].
2.2. Synthesis
Synthesis of 9-(2-bromophenyl)-9H-carbazole (1)
Carbazole (20.0 g, 119.6 mmol), cesium carbonate (97.4 g, 299.0 mmol), and 1-bromo-2-fluorobenzene (27.2 g, 155.5 mmol) were dissolved in dimethyl sulfoxide (400 mL). The mixture was refluxed for 12 h. The reaction mixture was then cooled to room temperature, extracted with dichloromethane, and the organic layer was dried over magnesium sulfate. After removing the solvent under reduced pressure, the crude product was purified using silica gel column chromatography (dichloromethane/hexane, 1:5 v/v) to afford compound 1 (30.2 g, 79%). 1H-NMR (CDCl3, 400 MHz, ppm): δ 8.18 (d, J = 7.6 Hz, 2H), 7.89 (dd, J = 8 Hz, 1.2 Hz, 1H), 7.57–7.49 (m, 2H), 7.45–7.40 (m, 3H), 7.32(td, J = 6.8 Hz, 0.8 Hz, 2H), 7.09 (d, J = 8.4 Hz, 2H). 13C-NMR (CDCl3, 100 MHz, ppm): δ 141.0, 134.3, 131.3, 130.3, 129.0, 126.2, 124.0, 123.4, 120.5, 120.2, 110.2. HRMS (ESI): m/z calcd. for C18H12BrN [M + H]+ 322.0226, found 322.0207.
Synthesis of indolo[3,2,1-jk]carbazole (2)
Compound 1 (25.0 g, 53.9 mmol), palladium acetate (1.7 g, 7.7 mmol), potassium carbonate (26.8 g, 193.8 mmol), triphenylphosphine (8.1 g, 30.8 mmol), and tetrabutylammonium bromide (0.7 g, 2.3 mmol) were dissolved in dimethylformamide (300 mL). The mixture was refluxed for 6 h. After the reaction mixture cooled to room temperature, it was extracted with chloroform and dried over magnesium sulfate. After removing the solvent under reduced pressure, the crude product was purified using silica gel column chromatography (dichloromethane/hexane, 1:7 v/v) to afford compound 2 (14.3 g, 77%). 1H-NMR (CDCl3, 400 MHz, ppm): δ 8.17 (dt, J = 8.0 Hz, 0.8 Hz, 2H), 8.07 (d, J = 7.6 Hz, 2H), 7.94 (dt, J = 8.0 Hz, 0.8 Hz, 2H), 7.62–7.55 (m, 3H), 7.39 (td, J = 7.6Hz, 0.8 Hz, 2H). 13C-NMR (CDCl3, 100 MHz, ppm): δ 143.8, 138.7, 130.1, 126.8, 123.1, 122.8, 121.7, 119.4, 118.5, 112.2. HRMS (ESI): m/z calcd. for C18H11N [M + H]+ 242.0964, found 242.0965.
Synthesis of 2-bromoindolo[3,2,1-jk]carbazole (3)
Compound 2 (13.0 g, 53.9 mmol) was dissolved in a mixture of chloroform (150 mL) and acetic acid (150 mL). N-bromosuccinimide was added to the mixture at 0 °C. After the mixture was heated at 50 °C for 6 h, it was extracted with chloroform and dried over magnesium sulfate. After removing the solvent under reduced pressure, the crude product was washed with glacial acetic acid to obtain compound 3 as a pure product (6.2 g, 36%). 1H-NMR (CDCl3, 400 MHz, ppm): δ 8.17 (s, 2H), 8.10 (dd, J = 7.6 Hz, 0.8 Hz, 2H), 7.91 (dd, J = 7.6 Hz, 0.8 Hz, 2H), 7.62 (td, J = 7.6 Hz, 0.8 Hz, 2H), 7.39 (td, J = 7.6 Hz, 0.8 Hz, 2H). 13C-NMR (CDCl3, 100 MHz, ppm): δ 142.0 139.1, 129.3, 127.6, 123.5, 122.5, 122.2, 119.8, 115.9, 112.5. HRMS (ESI): m/z calcd. for C18H11BrN [M + H]+ 320.0069, found 320.0040.
Synthesis of 1,1′-(2-bromoindolo[3,2,1-jk]carbazole-5,11-diyl) bis(hexan-1-one) (4)
AlCl3 (6.5 g, 48.2 mmol) was added to a dry two-neck flask, followed by anhydrous CH2Cl2 (100 mL). Then, hexanoyl chloride (48.2 mmol, 7.3 g) was added slowly, and the mixture was stirred for 20 min at 0 °C. After adding compound 3 (6.2 g, 19.3 mmol), the mixture was slowly warmed up to room temperature and stirred for 12 h. The mixture was then cooled to 0 °C and quenched on ice. The mixture was extracted with chloroform and dried over magnesium sulfate. After removing the solvent under reduced pressure, the crude product was recrystallized in dichloromethane/MeOH to give compound 4 as a pure product (8.1 g, 82%). 1H-NMR (CDCl3, 400 MHz, ppm): δ 8.60 (d, J = 1.6 Hz, 2H), 8.22 (dd, J = 8.4 Hz, 1.6 Hz, 2H), 8.10 (s, 2H), 7.80 (d, J = 8.4 Hz, 2H), 3.10 (t, J = 7.6 Hz, 4H), 1.89–1.82 (m, 4H), 1.47–1.43 (m, 8H), 0.98–0.95 (m, 6H). 13C-NMR (CDCl3, 400 MHz, ppm): δ 199.2, 142.6, 140.1, 131.8, 129.0, 127.9, 123.4, 122.9, 119.1, 117.0, 111.8, 38.8, 31.8, 24.2, 22.8, 14.2. HRMS (ESI): m/z calcd. for C30H30BrNO2 [M + H]+ 516.1543, found 516.1534.
Synthesis of 1,1′-(2-bromoindolo[3,2,1-jk]carbazole-5,11-diyl) bis(hexan-1-ol) (5)
AlCl3 (37.1 mmol, 4.9 g) was added to a dry one-necked flask, followed by anhydrous tetrahydrofuran (150 mL). LiAlH4 solution (47 mL, 2.0 M in THF) was added slowly and stirred for 20 min at 0 °C. Compound 4 (8.1 g, 15.7 mmol) was added, and the mixture was slowly warmed up to room temperature and stirred for 3 h. The mixture was then cooled to 0 °C and quenched on ice. The mixture was extracted with chloroform and dried over magnesium sulfate. After removing the solvent under reduced pressure, the crude product was washed with MeOH to obtain compound 5 as a pure product (7.8 g, 96%). 1H-NMR (CDCl3, 400 MHz, ppm): δ 8.10 (s, 2H), 8.01 (d, J = 1.6 Hz, 2H) 7.77 (d, J = 8.4 Hz, 2H) 7.53 (dd, J = 8.4 Hz, 1.6 Hz, 2H), 4.87 (t, J = 6.8 Hz, 4H), 1.93–1.81 (m, 4H), 1.57–1.43 (m, 8H), 0.89–0.86 (m, 6H). 13C-NMR (CDCl3, 100 MHz, ppm): δ 142.8, 139.1, 138.4, 129.4, 125.7, 122.6, 121.0, 119.8, 115.8, 112.1, 75.0, 39.7, 31.9, 25.8, 22.8, 14.2. HRMS (ESI): m/z calcd. for C30H34BrNO2 [M + H]+ 520.1846, found 520.1686.
Synthesis of 2-bromo-5,11-dihexylindolo[3,2,1-jk]carbazole (6)
Compound 5 (7.0 g, 13.4 mmol) was dissolved in dichloromethane (250 mL), and then trifluoroacetic acid (6.1 g, 53.2 mmol) was added dropwise at 0 °C. Subsequently, triethylsilane (6.1 g, 53.2 mmol) was added dropwise at 0 °C, and the mixture was slowly warmed up to room temperature and stirred for 6 h. Then, the mixture was extracted with dichloromethane and dried over magnesium sulfate. The crude product was purified by silica gel column chromatography (dichloromethane/hexane, 1:8 v/v) and reprecipitated using hexane to afford compound 6 as a pure product (5.1 g, 78%). 1H-NMR (CDCl3, 400 MHz, ppm): δ 8.12 (s, 2H), 7.87 (d, J = 1.6 Hz, 2H) 7.76 (d, J = 8.4 Hz, 2H), 7.39 (dd, J = 8.0 Hz, 1.6 Hz, 2H), 2.81 (t, J = 7.6 Hz, 4H), 1.74–1.70 (m, 4H), 1.42–1.31 (m, 8H), 0.93–0.88 (m, 6H). 13C-NMR (CDCl3, 100 MHz, ppm): δ 142.6, 137.5, 136.8, 129.3, 128.0, 123.1, 122.2, 119.8, 115.5, 111.9, 36.2, 32.2, 32.0, 29.2, 22.8, 14.3. HRMS (ESI): m/z calcd. for C30H34BrN [M + H]+ 488.1947, found 488.1885.
Synthesis of 5,11-dihexyl-N,N-diphenylindolo[3,2,1-jk]carbazol-2-amine (7).
Compound 6 (4.0 g, 8.2 mmol), diphenyl amine (1.7 g, 9.8 mmol), tris(dibenzylideneacetone)dipalladium (0.3 g, 0.3 mmol), sodium tert-butoxide (2.4 g, 24.6 mmol), and tri-tert-butylphosphine solution (0.1 g, 0.6 mmol) were dissolved together in toluene under N2 atmosphere and refluxed overnight. After cooling to room temperature, the reaction mixture was extracted with ethyl acetate and dried over magnesium sulfate. The crude product was purified using silica gel column chromatography (dichloromethane/hexane, 1:8 v/v) and reprecipitated using diethyl ether to afford compound 7 as a yellow powder (3.7 g, 78%). 1H-NMR (CDCl3, 400 MHz, ppm): δ 7.88 (s, 2H), 7.82 (d, J = 1.6 Hz, 2H), 7.36 (dd, J = 8.4 Hz, 1.6 Hz, 2H), 7.25–7.22 (m, 4H), 7.14–7.12 (m, 4H), 7.00–6.95 (m, 2H), 2.78 (t, J = 7.6 Hz, 4H), 1.70–1.65 (m, 4H), 1.36–1.30 (m, 8H), 0.90–0.87 (m, 6H). 13C-NMR (CDCl3, 100 MHz, ppm): δ 149.4, 143.6, 142.2, 137.7, 136.5, 130.0, 129.2, 127.5, 123.2, 122.7, 121.6, 120.3, 119.3, 111.9, 36.2, 32.2, 32.0, 29.1, 22.8, 14.3. HRMS (ESI): m/z calcd. for C42H44N2 [M + H]+ 577.3577, found 577.3576.
Synthesis of N,N-bis(4-bromophenyl)-5,11-dihexylindolo[3,2,1-jk]carbazol-2-amine (8).
Compound 7 (3.5 g, 6.1 mmol) was dissolved in chloroform, and then N-bromosuccinimide (2.5 g, 13.9 mmol) was added dropwise at 0 °C. The mixture was stirred at 0 °C for 3 h and then extracted with dichloromethane and dried over magnesium sulfate. The crude product was purified using silica gel column chromatography (dichloromethane/hexane, 1:6 v/v) and reprecipitated using diethyl ether and dichloromethane to obtain compound 8 as a yellow powder (3.06 g, 69%). 1H-NMR (CDCl3, 400 MHz, ppm): δ 7.83 (d, J = 1.6 Hz, 2H), 7.80 (s, 2H), 7.78 (d, J = 8.4 Hz, 2H), 7.38 (dd, J = 8.0 Hz, 1.6 Hz, 2H), 7.33–7.31 (m, 4H), 7.00–6.97 (m, 4H), 2.79 (t, J = 7.6 Hz, 4H), 1.70–1.68 (m, 4H), 1.38–1.30 (m, 8H), 0.90–0.87 (m, 6H). 13C-NMR (CDCl3, 100 MHz, ppm): δ 148.0, 142.6, 142.3, 137.8, 136.7, 132.3, 129.8, 127.8, 124.2, 123.2, 119.9, 119.5, 114.3, 111.9, 36.2, 32.2, 32.0, 29.1, 22.8, 14.3. HRMS (ESI): m/z calcd. for C42H42Br2N2 [M + H]+ 733.1788, found 733.1756.
Synthesis of 5,11-dihexyl-N,N-bis(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) phenyl)indolo[3,2,1-jk]carbazol-2-amine (9).
Compound 8 (1.50 g, 2.04 mmol), [1,1′-bis(diphenylphosphino)ferrocene] dichloropalladium(II) (0.11 g, 0.14 mmol), potassium acetate (1.01 g, 24.48 mmol), and bis(pinacolato)diboron (3.32 g, 12.24 mmol) were dissolved in 1,4-dioxane under N2 atmosphere, and the mixture was stirred at 90 °C overnight. After cooling to room temperature, the mixture was extracted with dichloromethane and dried over magnesium sulfate. The crude product was purified using silica gel column chromatography (dichloromethane/hexane, 3:2 v/v) and reprecipitated using ether to afford compound 9 as a pure product (1.2 g, 61%). 1H-NMR (CDCl3, 400 MHz, ppm): δ 7.85 (s, 2H), 7.81 (d, J = 1.6 Hz, 2H), 7.78 (d, J = 8.0 Hz, 2H), 7.69 (d, J = 6.8 Hz, 4H), 7.36 (dd, J = 8.4 Hz, 1.6 Hz, 2H), 7.14 (d, J = 6.8 Hz, 4H), 2.78 (t, J = 8.0 Hz, 4H), 1.72–1.68 (m, 4H), 1.39–1.30 (m, 32H), 0.90–0.87 (m, 6H). 13C-NMR (CDCl3, 100 MHz, ppm): δ 151.5, 142.6, 142.4, 137.7, 136.6, 136.0, 129.9, 127.6, 123.2, 121.8, 120.5, 119.3, 111.9, 83.7, 36.2, 32.2, 31.9, 29.1, 25.0, 22.8, 14.3. HRMS (ESI): m/z calcd. for C54H66B2N2O4 [M + H]+ 829.5281, found 829.5295.
Polymerization of PICA.
The target polymers were synthesized via Suzuki polycondensation. To a mixture of N,N-bis(4-bromophenyl)-5,11-dihexylindolo[3,2,1-jk]carbazol-2-amine (0.24 g, 0.32 mmol), 5,11-dihexyl-N,N-bis(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)indolo[3,2,1-jk]carbazol-2-amine (0.27 g, 0.32 mmol), tetrakis(triphenylphosphine)palladium (0) (0.03 g, 0.02 mmol), anhydrous toluene (8 mL) and 2 M K2CO3 solution (8 mL) were added under Ar atmosphere. The solution was stirred at 110 °C for 36 h. To terminate the polymerization reaction, a small amount of bromobenzene was added. After 1 h, a small amount of phenylboronic acid was added, and the reaction mixture was stirred for an additional hour. The solution was then poured into a flask containing 200 mL of methanol. The crude polymer was purified by Soxhlet extraction using acetone, methanol, and hexane. The resultant polymer was collected, dissolved in chloroform, and reprecipitated in methanol to obtain the pure product (107.2 mg, 29%).
2.3. Measurements
1H and 13C NMR spectra were recorded on a Bruker Varian and Ascend 400 MHz spectrometer in CDCl3. UV–Vis absorption spectra were recorded on a Shimadzu UV-3600 spectrophotometer, and photoluminescence spectra were measured on a Hitachi F-7000 spectrophotometer. Cyclic voltammetry (CV) measurements were carried out with a CHI 600D system in acetonitrile solution containing 0.1 M tetrabutylammonium tetrafluoroborate (TBABF4) as the supporting electrolyte, Ag/AgNO3 as the reference electrode, a platinum wire as the counter electrode, and a platinum working electrode. The current density–voltage–luminance (I–V–L) characteristics were measured, and the electroluminescence (EL) spectra of the phosphorescent OLEDs were recorded using a Keithley 2400 source measurement unit and a CS-1000 spectrophotometer. A Keithley 2400 source measurement unit and a CS-1000 spectroradiometer were used to evaluate the device performance. The molecular weight was measured by gel permeation chromatography (GPC) with polystyrene standard and THF as the eluent using an Agilent 1260 Series instrument. The surface morphologies were measured by atomic force microscopy (AFM; Park Systems NX-20) in the non-contact scanning mode.
2.4. Fabrication of OLED Devices
We fabricated red and green OLED devices. The red devices had the following two structures:
Device (A): ITO/PEDOT:PSS (30 nm)/PICA:FPA (3 wt%)/TCTA:TPBi:Ir(mphmq)2tmd (50:50:3 wt%, 30 nm)/TPBi (33.5 nm)/LiF (1 nm)/Al (100 nm)
Device (B): ITO/PEDOT:PSS (30 nm) Poly-TPD:FPA (3 wt%)/TCTA:TPBi:Ir(mphmq)2tmd (50:50:3 wt%, 30 nm)/TPBi (28 nm)/Li (1 nm)/Al (100 nm).
The indium tin oxide (ITO) substrate was cleaned by sonication in acetone and isopropyl alcohol, followed by ultraviolet/ozone treatment for 20 min. A hole-injection layer (HIL) consisting of poly(3,4-ethylenedioxythiophene) doped with poly(styrene sulfonic acid) (PEDOT:PSS, AI4083) was spin-coated onto the ITO substrate at 2000 rpm for 40 s. An HTL of poly-TPD:FPA (3 wt%) or PICA:FPA (3 wt%) was deposited onto the PEDOT:PSS layer. The coated HTL was irradiated with UV light (254 nm, 80 mJ/cm2) for 5 s to form a crosslinked network. The EML of TCTA:TPBi:Ir(mphmq)2tmd (50:50:3 wt%) was spin-coated and then annealed at 110 °C for 30 min to remove the residual solvent. The electron transport layer (ETL) of TPBi was deposited onto the EML. Finally, a layer of lithium fluoride (LiF) as the electron-injecting layer (EIL) and a layer of aluminum (Al) as the cathode were deposited by thermal evaporation on top of the film through a mask under high vacuum (below 1.0 × 10−5 Torr, 2.7 mPa).
Green OLED devices with the following structure were fabricated through spin-coating and vacuum deposition: ITO/PEDOT:PSS (30 nm)/PICA:FPA (3 wt%) or poly-TPD:FPA (3 wt%)/CBP:PVK:Ir(mppy)3 (50:50:7 wt%, 35 nm)/TPBi (40 nm)/LiF (1 nm)/Al (100 nm). The fabrication procedure was identical to that used for the red OLEDs.
3. Results and Discussion
3.1. Synthesis and Characterization of PICA
Detailed synthetic procedures for the monomers and polymers were described above in the Experimental section. The hydroxyl groups on the side chains of compound 5 were successfully reduced using triethylsilane and trifluoroacetic acid to obtain compound 6. To obtain compound 7, diphenylamine was introduced at the 2-position of indolo[3,2,1-jk]carbazole using a Buchwald–Hartwig cross-coupling reaction, followed by bromination and borylation to obtain compounds 8 and 9, respectively.
PICA was successfully polymerized through the Suzuki polycondensation of compounds 8 and 9, and the resulting polymer was soluble in organic solvents such as chloroform, toluene, and chlorobenzene. The molecular weight of PICA was measured using GPC with THF as the eluent. The number-average molecular weight (Mn) and polydispersity index (PDI) of PICA were 8.16 kg/mol and 1.48, respectively. As a known HTL polymer, poly(4-butylphenyl-diphenylamine) (poly-TPD) with similar molecular weight was also prepared using the same Suzuki polycondensation as a reference material for comparing the hole-transporting properties. The Mn and PDI values of the synthesized poly-TPD were 6.92 kg/mol and 1.22, respectively. Thermal stability, optical properties, and HOMO-LUMO energy levels were measured to confirm whether PICA is suitable for application as a hole-transporting layer (HTL) of OLEDs.
The thermal properties of PICA were investigated using thermogravimetric analysis (TGA) under a nitrogen atmosphere. The 5% weight loss temperature (Td) of PICA was 360 °C, showing that the polymer has good thermal stability and is suitable for application in OLED devices. This temperature is comparable to that of poly-TPD (356 °C, Figure S25 in Electronic Supplementary Information (ESI†)).
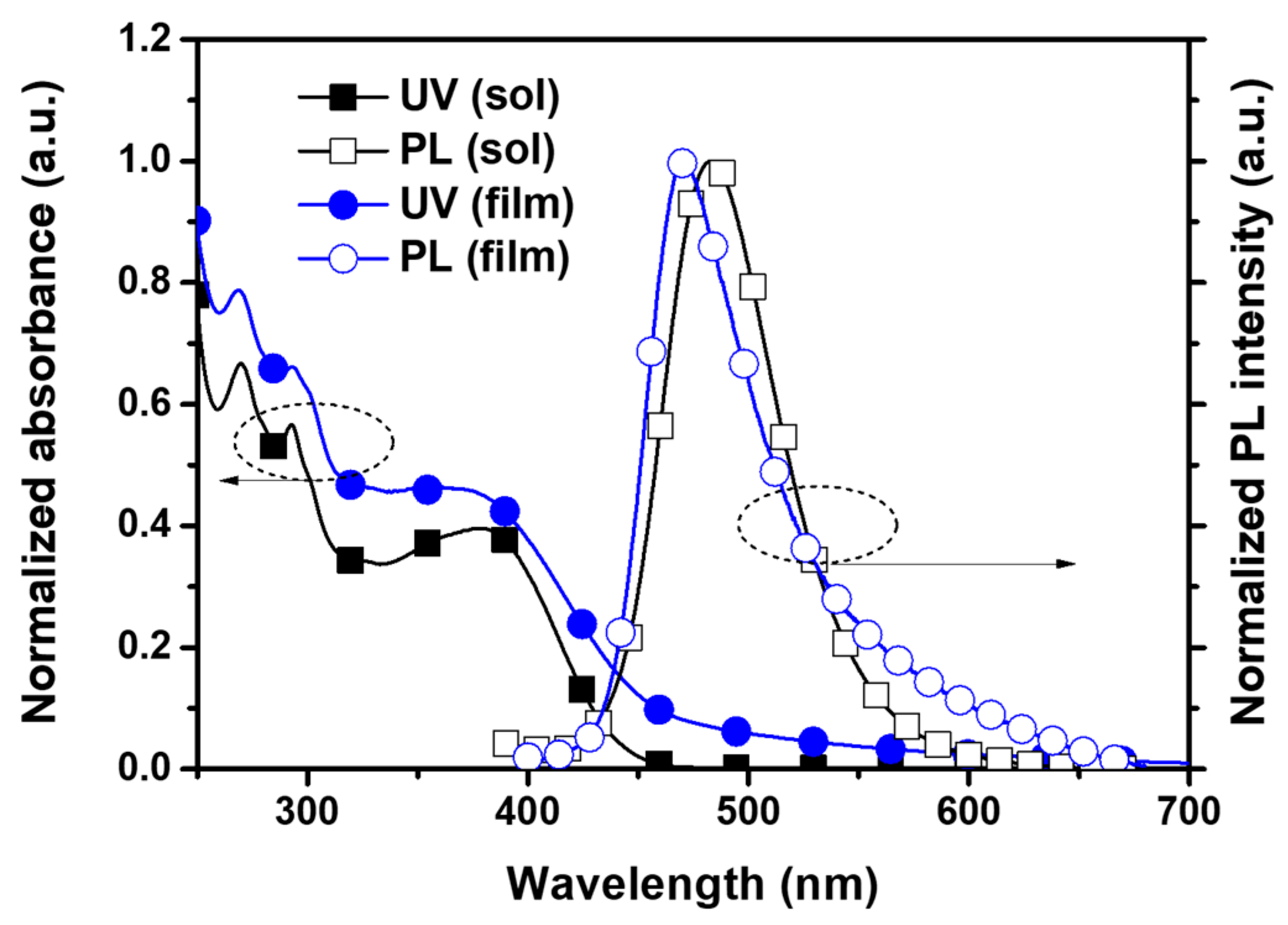
The UV–Vis absorption and photoluminescence (PL) spectra of PICA in solution (1 × 10−5 M in chloroform) and thin-film states were measured to investigate the optical properties. PICA showed an absorption maximum at 293 nm in both states (Figure 1, Table 1). The PL maximum of PICA in the solution and film states was at 483 and 471 nm, respectively. The triplet energy of PICA was measured to be −2.33 eV from the low-temperature PL maximum, and this value is slightly lower than that of poly-TPD (−2.31 eV, Figure S26, ESI†).
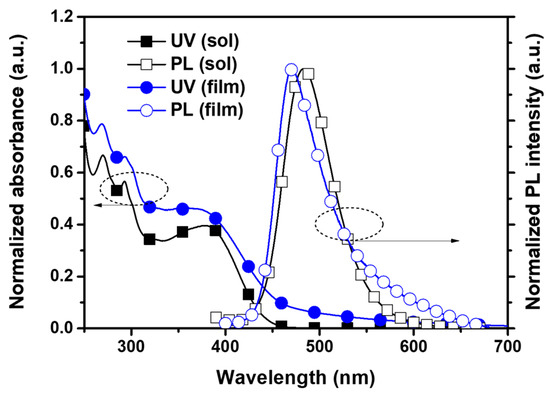
Figure 1.
UV–Vis absorption and PL spectra of PICA in solution and film states.

Table 1.
Optical and electrical properties of PICA.
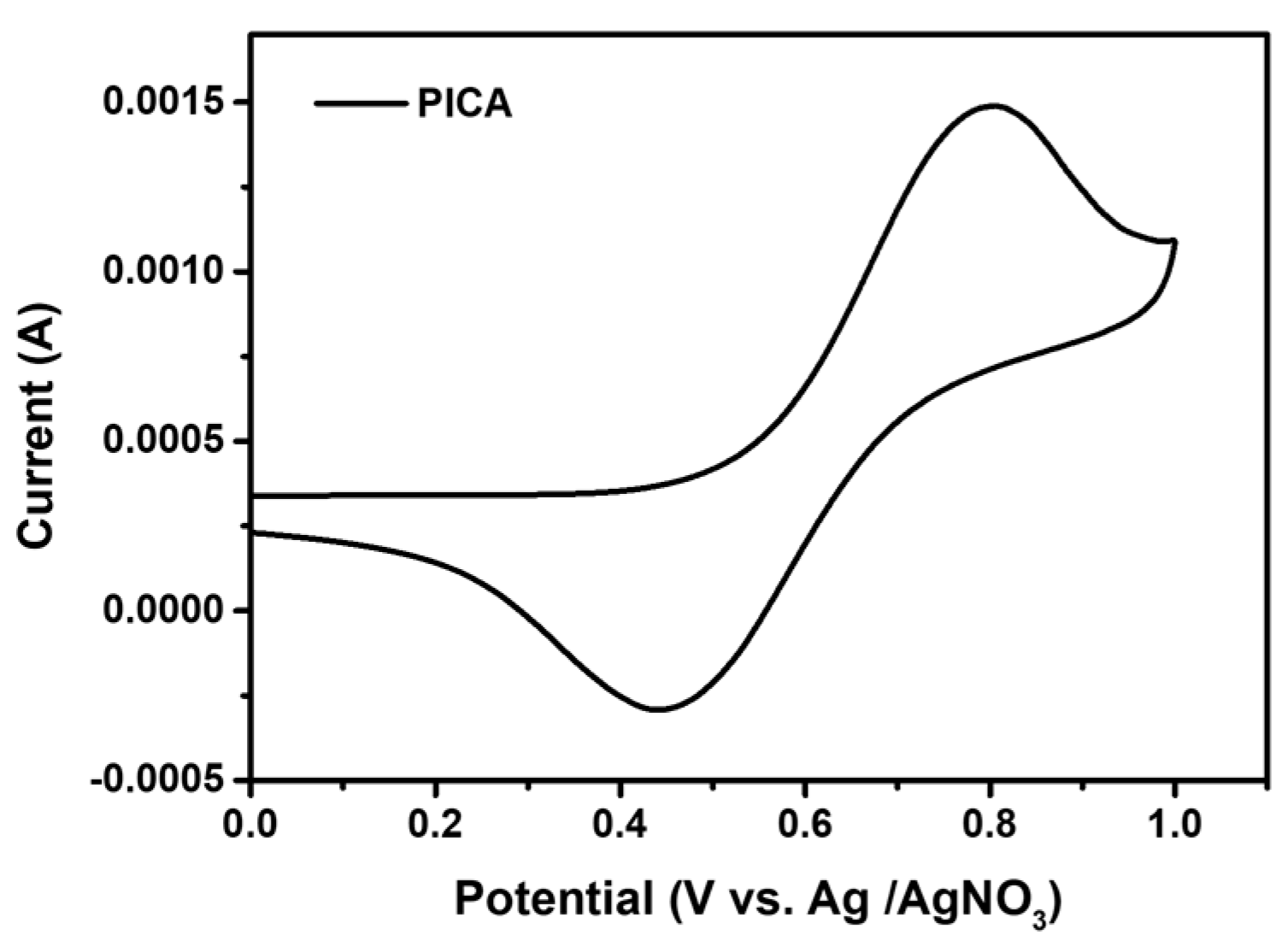
The electrochemical behavior of PICA was studied using CV in anhydrous acetonitrile (CH3CN) solution with 0.1 M tetrabutylammonium tetrafluoroborate as a supporting electrolyte (Figure 2). The HOMO energy level of PICA was determined by measuring the oxidation onset potential (Eox) of the PICA film coated on the Pt working electrode. This energy level was estimated to be −5.25 eV, slightly higher than that of poly-TPD (−5.30 eV). The lowest unoccupied molecular orbital (LUMO) energy level of PICA was estimated to be −2.46 eV using a combination of the abovementioned HOMO energy level and the energy bandgap (Egap) obtained from the absorption edge.
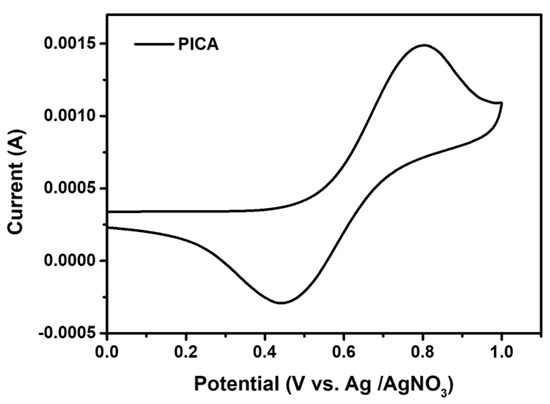
Figure 2.
Cyclic voltammogram of PICA.
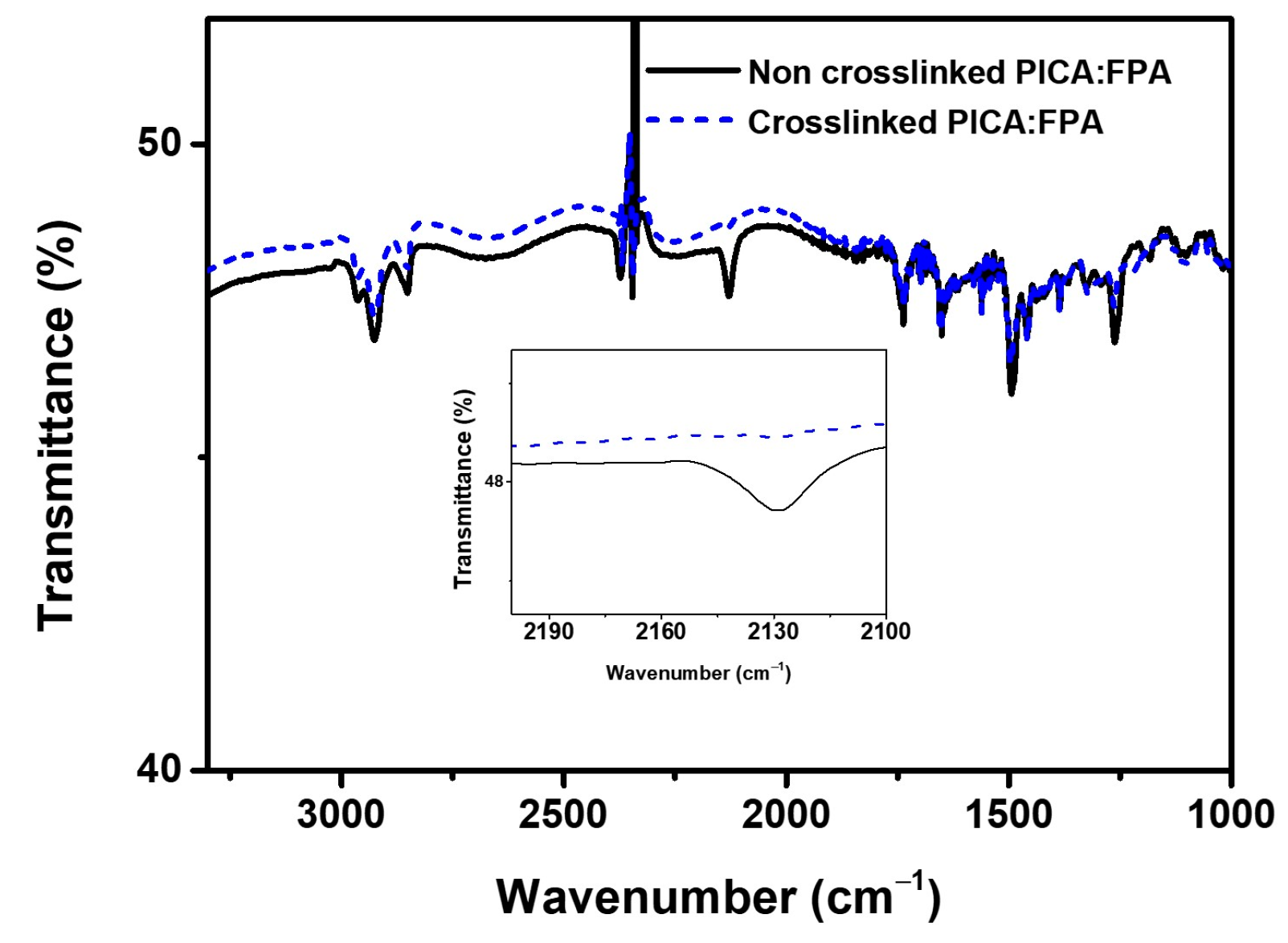
FT-IR spectroscopy was used to confirm photo-crosslinking in the blend of PICA and FPA after UV irradiation. The blended solution of PICA:FPA was coated onto a KBr pellet, and the pellet was irradiated by 254 nm UV light. The stretching peak intensity of the terminal azide (N=N=N) in FPA at 2129 cm−1 decreased after irradiation, which indirectly indicates that the azide was subsequently inserted into the benzylic positions in PICA to form a network (Figure 3). The successful photo-crosslinking of PICA is also supported by the solvent resistance test results in Section 3.2 below.
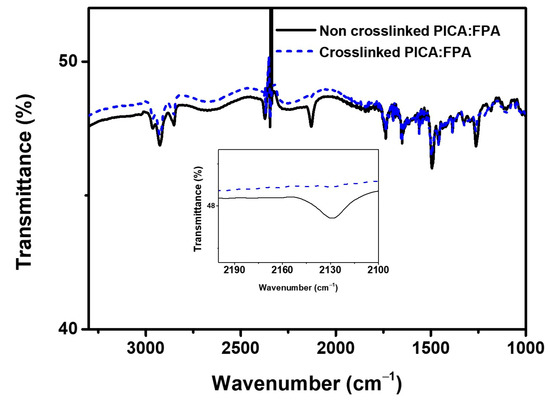
Figure 3.
FT-IR spectra of PICA:FPA (3 wt%) films before and after crosslinking, inset: enlarged spectra.
3.2. Crosslinking and Solvent Resistance Tests
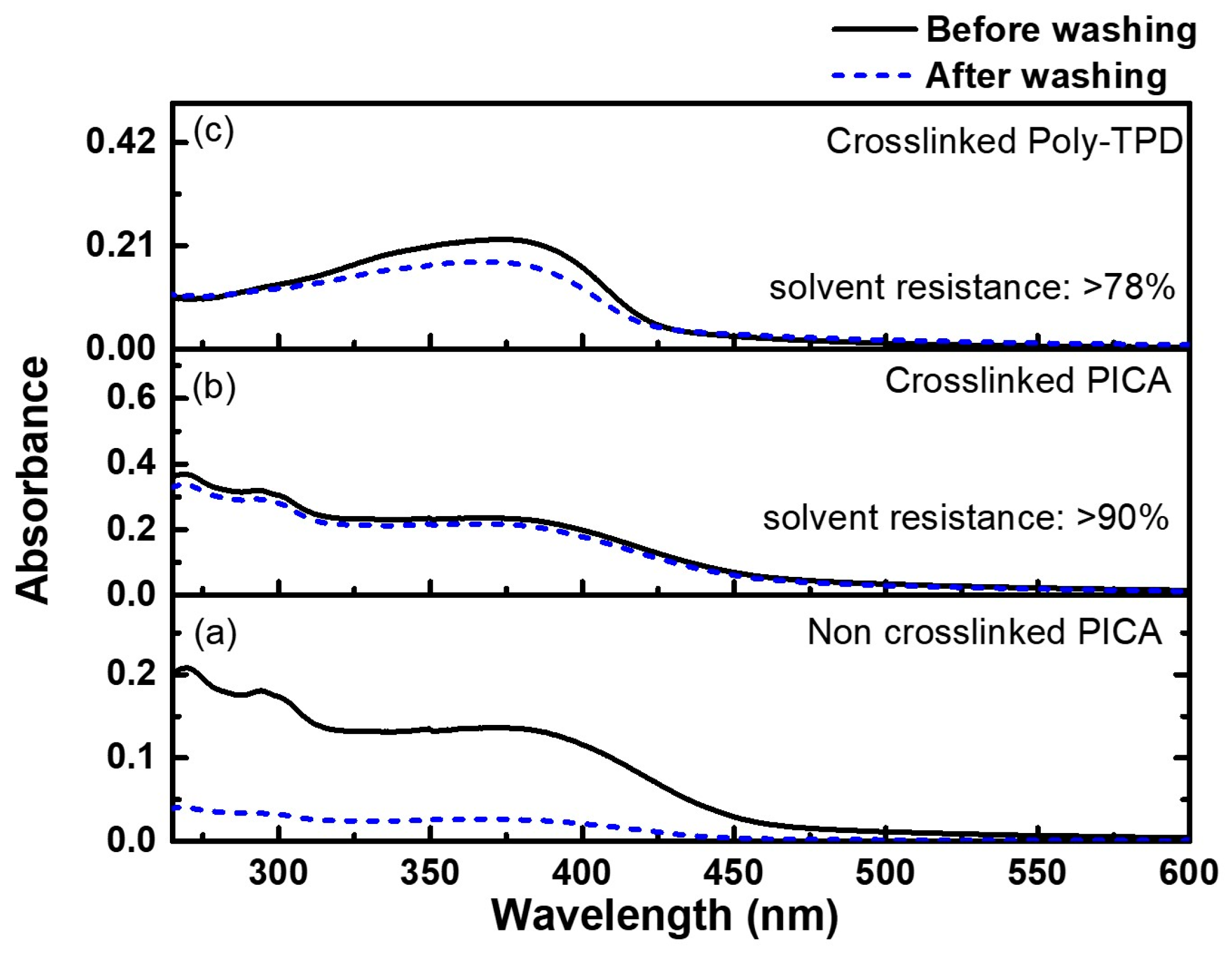
The photo-crosslinking conditions of the synthesized PICA were optimized by performing experiments at different FPA concentrations (1, 2, 3, and 5 wt%). A mixed solution of PICA and FPA in chlorobenzene was spin-coated onto the quartz substrate. The film was then photocured by UV irradiation at 254 nm (84 mJ/cm2) for 5 s. After rinsing the photo-crosslinked film in toluene for 1 min, the solvent resistance was evaluated by comparing the absorbance at maximum UV–Vis absorption (294 nm for PICA and 377 nm for poly-TPD) with that before rinsing. The non-crosslinked neat PICA film showed a solvent resistance of 19.7% at 293 nm after rinsing (Figure 4a). The photo-crosslinked PICA film with 3 wt% FPA exhibited a significantly better solvent resistance of 90% (Figure 4b), whereas the poly-TPD film with 3 wt% FPA showed 78% (Figure 4c). Moreover, the absorption and PL emission spectra of the PICA films were almost identical after crosslinking (Figure S27, ESI†). It indicated that the photo-crosslinked PICA film was rarely affected by the photophysical properties even after crosslinking.
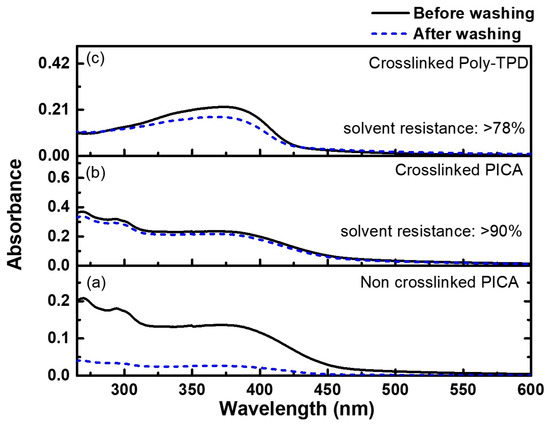
Figure 4.
UV–Vis absorption spectra of (a) non-crosslinked PICA, (b) photo-crosslinked PICA:FPA (3 wt%), and (c) photo-crosslinked poly-TPD:FPA (3 wt%) films before and after rinsing with toluene.
3.3. Hole-Only Devices Based on PICA
The hole-transporting abilities of the crosslinked PICA and poly-TPD films were evaluated by space-charge limited current (SCLC) measurements with a device structure of ITO/PEDOT:PSS (30 nm)/PICA (105 nm) or poly-TPD (95 nm)/MoO3(10 nm)/Al (100 nm). The carrier mobility (μh was calculated using the following equation of Mott-Gurney law:
where ε and ε0 are the relative dielectric constant and permittivity of free space, respectively, L is the thickness of the organic layer, and V is the applied voltage. The hole mobilities of the devices based on PICA and poly-TPD films were 2.9 × 10−5 and 1.3 × 10−5 cm2v–1s–1, respectively (Figure S29, ESI†). The fact that PICA has more than twice the hole mobility indicates that PICA with an arylamine structure, including an indolocarbazole unit, is more advantageous for hole transport than poly-TPD.
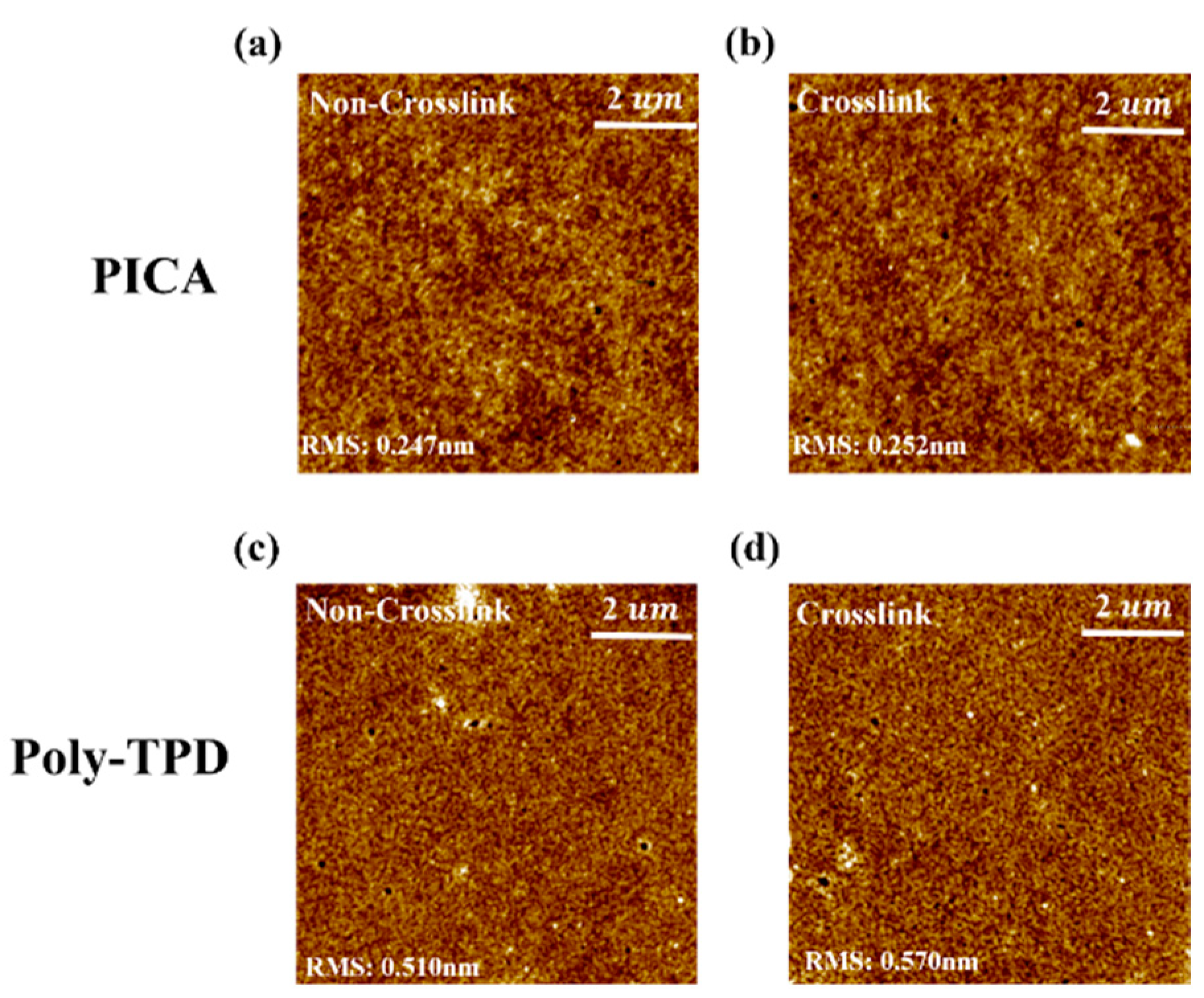
3.4. Surface Morphology of PICA and Poly-TPD
AFM images of the poly-TPD and PICA films were obtained to investigate changes in their surface morphology (Figure 5). The root-mean-square (RMS) roughness values of the non-crosslinked and crosslinked PICA films were 0.247 and 0.252 nm, and those of poly-TPD were 0.510 and 0.570 nm, respectively. Both the PICA and poly-TPD films showed a slight increase in RMS roughness after crosslinking, whereas PICA generated a more uniform and smoother film than poly-TPD before and after crosslinking.
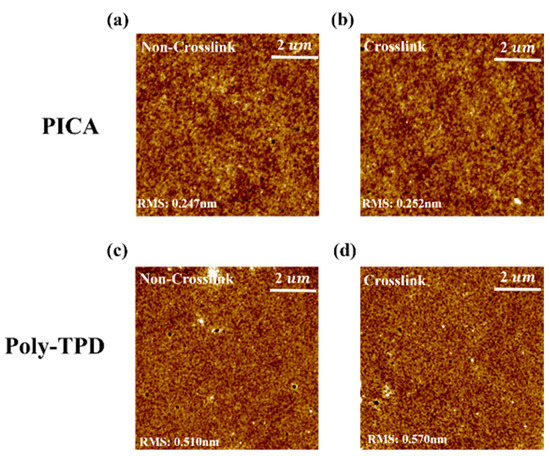
Figure 5.
AFM images of (a) non-crosslinked PICA, (c) non-crosslinked poly-TPD, (b) crosslinked PICA, and (d) crosslinked poly-TPD films.
3.5. Performances of OLEDs Based on PICA
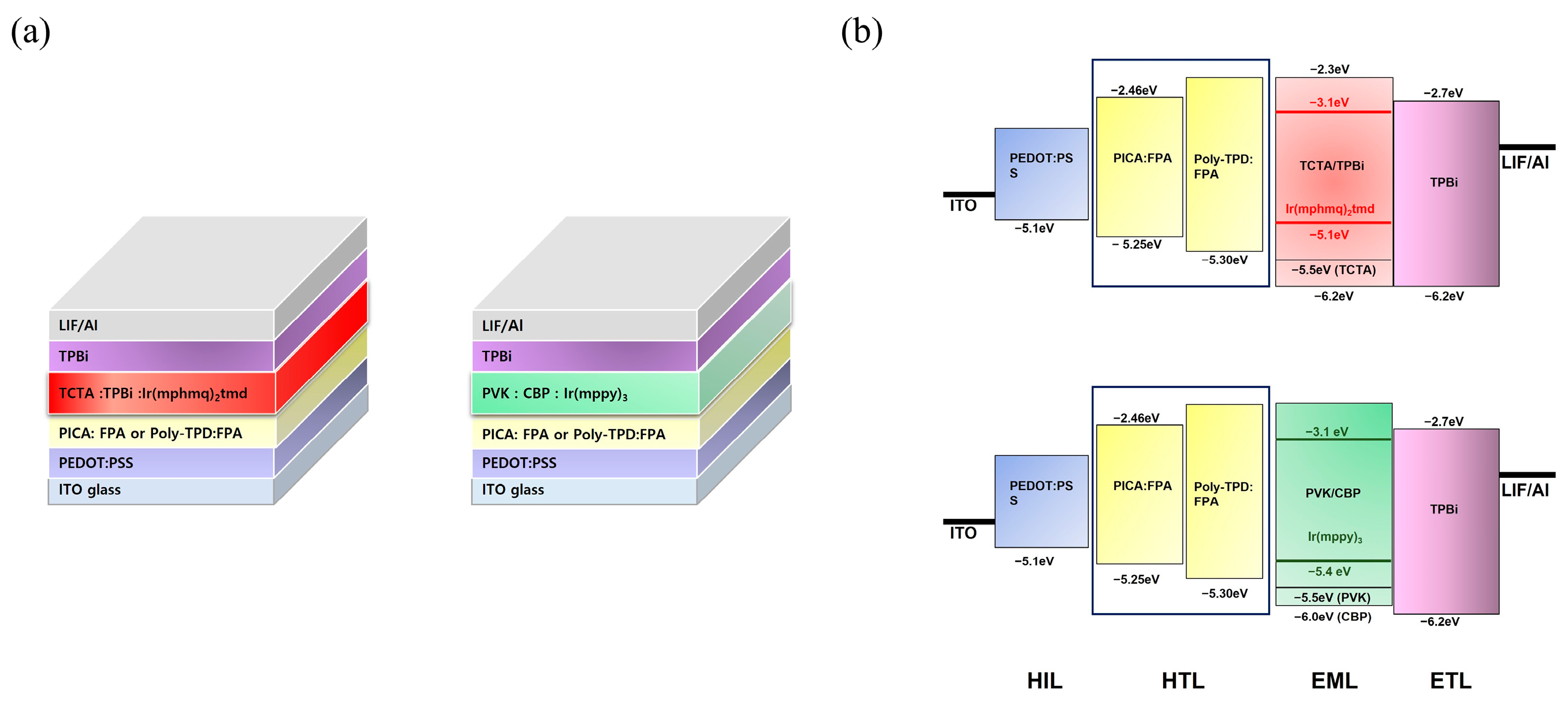
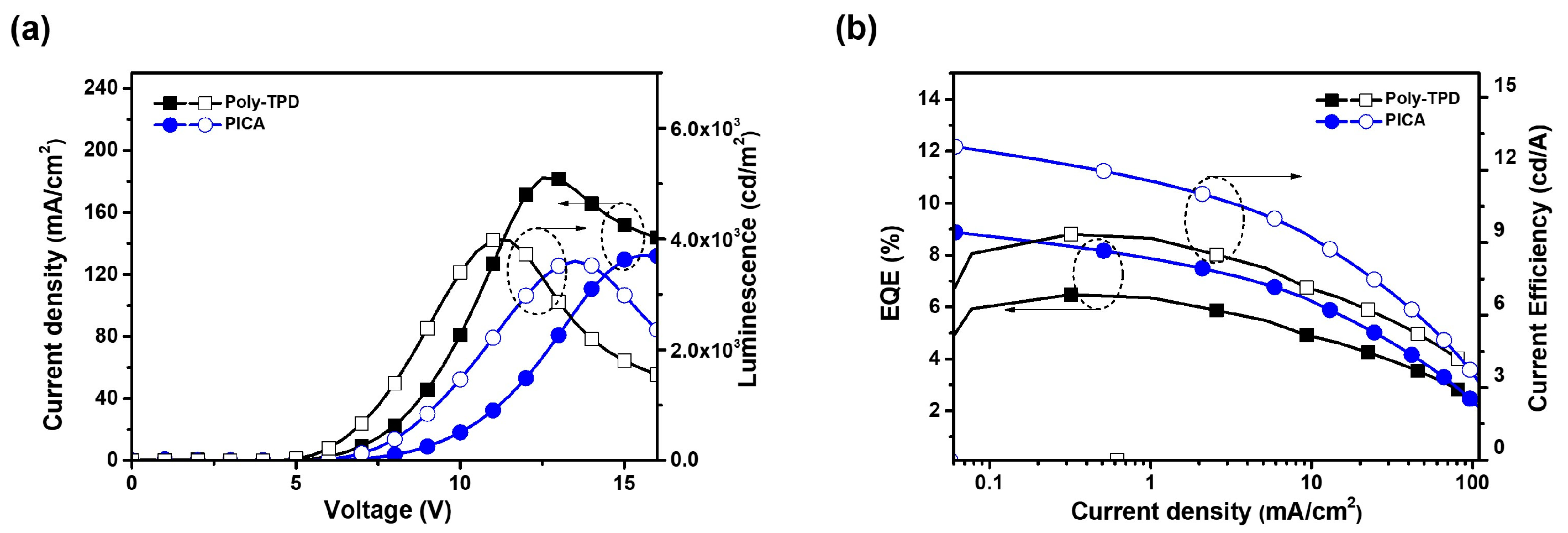
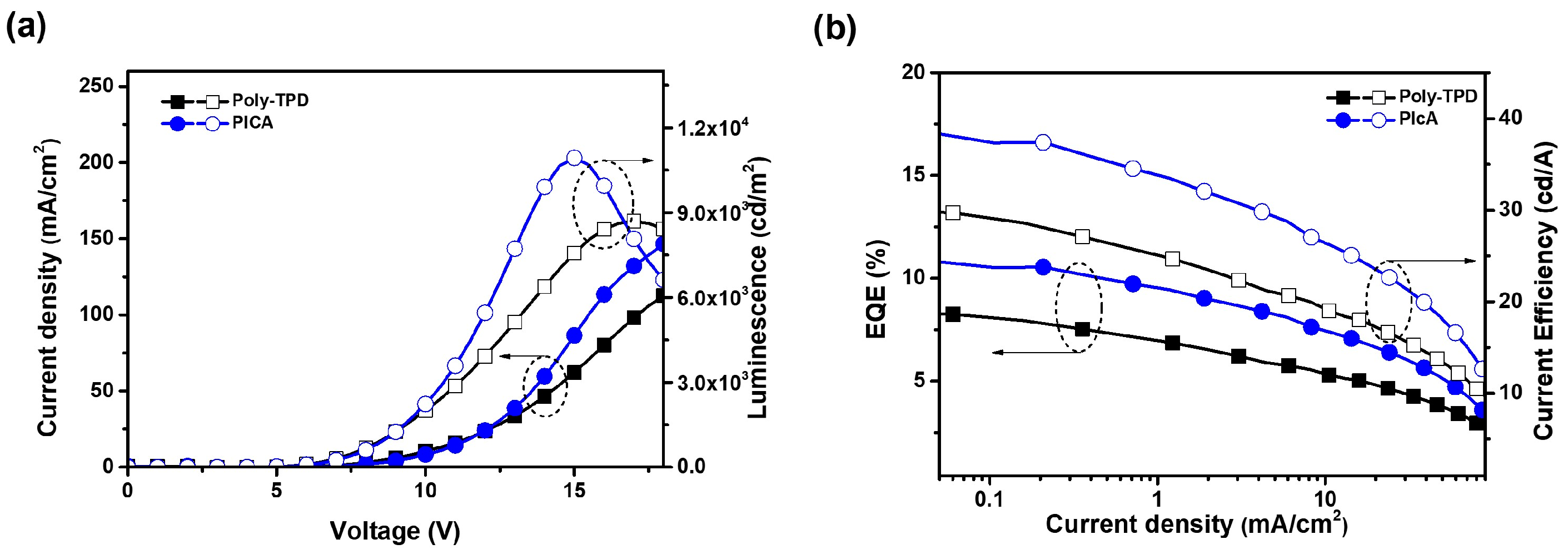
Red and green phosphorescent OLED devices were fabricated using crosslinked PICA to investigate the hole-transporting ability and device performance. The device structures and energy diagrams are shown in Figure 6. The device performance was compared with that of a reference device using poly-TPD. The device characteristics are listed in Table 2. Ir(mphmq)2(tmd) and Ir(mppy)3 were used as the red and green phosphorescent dopants, respectively. The red OLED device was fabricated with a structure of ITO/PEDOT:PSS (30 nm)/[PICA or poly-TPD]/TCTA:TPBi:Ir(mphmq)2tmd (50:50:3 wt%, 30 nm)/TPBi (33.5 nm for PICA and 28 nm for poly-TPD)/LiF (1 nm)/Al (100 nm). The turn-on voltage (Vturn-on) and maximum luminescence (Lmax) of the red OLED devices were 5.0 V and 3600 cd/m2 for PICA and 4.5 V and 4000 cd/m2 for poly-TPD, respectively. The red OLED device based on PICA was superior to that based on poly-TPD in terms of maximum external quantum efficiency (EQEmax, 8.88% vs. 6.47%), maximum luminous efficiency (LEmax, 12.97 vs. 9.35 cd/A), and maximum power efficiency (PEmax, 8.12 vs. 5.96 lm/W), as shown in Figure 7.
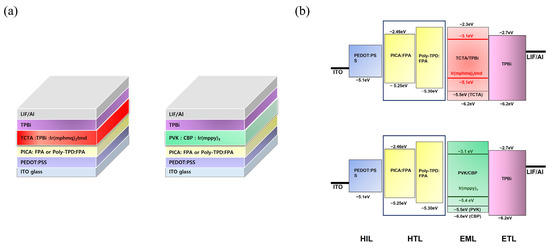
Figure 6.
(a) Red and green OLED structures. (b) Schematic energy diagrams of the device structures.

Table 2.
Device performances of red and green phosphorescent OLEDs.
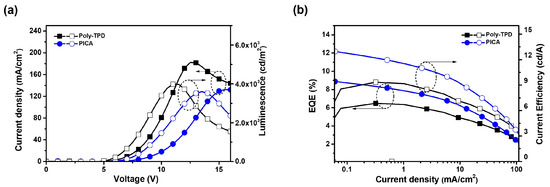
Figure 7.
(a) J–V–L and (b) EQE–J–CE (CE = current efficiency) curves of red OLED devices using crosslinked PICA or poly-TPD as the HTL.
Green OLED devices were fabricated using the same procedure as that for the red ones with the following device structure: ITO/PEDOT:PSS (30 nm)/[PICA or poly-TPD]/CBP:PVK:Ir(mppy)3 (50:50:7 wt%, 35 nm)/TPBi (40 nm)/LiF (1 nm)/Al (100 nm). The CIE coordinates of the green OLEDs with crosslinked PICA and poly-TPD were (0.29, 0.64) and (0.30, 0.64), respectively. The green OLED devices showed Vturn-on = 4.0 V and LEmax = 10,900 cd/m2 for PICA:FPA (3 wt%), and Vturn-on = 4.0 V and LEmax = 8700 cd/m2 for poly-TPD:FPA (3 wt%). In the green OLED devices, PICA performed better than poly-TPD in terms of EQEmax (10.84% vs. 8.40%), LEmax (38.47 vs. 30.19 cd/A), and PEmax (25.06 vs. 21.07 lm/W), as shown in Figure 8. We speculate that this improved device performance of PICA compared to poly-TPD as the HTL in solution-processed OLEDs is due to the higher hole mobility and more suitable energy matching of PICA. This resulted in better hole injection from PEDOT:PSS and better charge balance, hence the enhanced device performance.
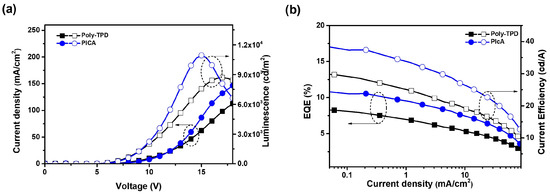
Figure 8.
(a) J–V–L and (b) EQE–J–CE (CE = current efficiency) curves of green OLED devices using crosslinked PICA or poly-TPD as the HTL.
4. Conclusions
We successfully synthesized a new cross-linkable material, PICA, for use as HTL in solution-processed OLEDs. Red and green OLED devices using photo-crosslinked PICA (EQE/LE/PE: 8.88%/12.97/8.12 in red devices, 10.84%/38.47 cd/A/25.06 lm/W in green devices) as the HTL exhibited better device performance than reference devices using poly-TPD. The higher solvent resistance of crosslinked PICA (90%) compared to that of poly-TPD (78%) might help prevent interlayer mixing and the dissolution of the HTL when forming an upper organic layer. Moreover, it is believed that PICA shows improved device performance compared to poly-TPD as an HTL for solution-processed OLEDs due to the higher hole mobility and better energy matching with the adjacent organic layers. These results show that PICA is a good candidate for photo-crosslinkable HTL material with highly efficient hole transport and good solvent resistance for solution-processed OLEDs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano13131934/s1: Figure S1–S24: 1H and 13C NMR spectrum of compound 1~12; Figure S25: TGA data of PICA and Poly-TPD; Figure S26: Photoluminescence spectra of Poly-TPD and PICA at 77K in MeTHF solutions; Figure S27: Photoluminescence spectra of PICA with crosslinking and non-crosslinking; Figure S28: Solvent resistance of PICA with the different ratios of FPA; Figure S29: Summary of SCLC data of hole-only devices of PICA and Poly-TPD.
Author Contributions
Conceptualization, J.Y.P. and D.-H.H.; methodology, J.Y.P.; formal analysis, J.Y.P. and S.L.K.; investigation, J.Y.P. and S.L.K.; data curation, S.L.K.; writing—original draft preparation, J.Y.P.; writing—review and editing, J.Y.P., S.L.K., H.J.P., and D.-H.H.; visualization, H.J.P.; supervision, D.-H.H.; project administration, D.-H.H.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) funded by the Korean government (MIST; grant number 2020R1A2C2008757) and the Technology Innovation Program (Industrial Strategic Technology Development Program No. K0001340) funded by the Ministry of Trade, Industry, and Energy (MOTIE, Korea).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Reineke, S.; Lindner, F.; Schwartz, G.; Seidler, N.; Walzer, K.; Lussem, B.; Leo, K. White organic light-emitting diodes with fluorescent tube efficiency. Nature 2009, 459, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Kim, G.H.; Lampande, R.; Ahn, D.H.; Im, J.B.; Moon, J.S.; Lee, J.K.; Lee, J.Y.; Lee, J.Y.; Kwon, J.H. A new rigid diindolocarbazole donor moiety for high quantum efficiency thermally activated delayed fluorescence emitter. J. Mater. Chem. C 2018, 6, 1343–1348. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Huang, Z.; Wang, H.; Zhao, J.; Yu, J.; Ma, D. High-efficiency red organic light-emitting diodes based on a double-emissive layer with an external quantum efficiency over 30%. J. Mater. Chem. C 2018, 6, 7042–7045. [Google Scholar] [CrossRef]
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Chiba, T.; Pu, Y.-J.; Miyazaki, R.; Nakayama, K.-I.; Sasabe, H.; Kido, J. Ultra-high efficiency by multiple emission from stacked organic light-emitting devices. Org. Electron. 2011, 12, 710–715. [Google Scholar] [CrossRef]
- Ahn, D.A.; Lee, S.; Chung, J.; Park, Y.; Suh, M.C. Impact of Interface Mixing on the Performance of Solution Processed Organic Light Emitting Diodes—Impedance and Ultraviolet Photoelectron Spectroscopy Study. ACS Appl. Mater. Interfaces 2017, 9, 22748–22756. [Google Scholar] [CrossRef]
- Liu, B.; Hu, S.; Zhang, L.; Xiao, P.; Huang, L.; Liu, C. Blue Molecular Emitter-Free and Doping-Free White Organic Light-Emitting Diodes With High Color Rendering. IEEE. Electron. Device Lett. 2021, 42, 387–390. [Google Scholar] [CrossRef]
- Baldo, M.A.; Thompson, M.E.; Forrest, S.R. High-efficiency fluorescent organic light-emitting devices using a phosphorescent sensitizer. Nature 2000, 403, 750–753. [Google Scholar] [CrossRef]
- Sasabe, H.; Kido, J. Multifunctional Materials in High-Performance OLEDs: Challenges for Solid-State Lighting. Chem. Mater. 2011, 23, 621–630. [Google Scholar] [CrossRef]
- Markham, J.P.J.; Lo, S.C.; Magennis, S.W.; Burn, P.L.; Samuel, I.D.W. High-efficiency green phosphorescence from spin-coated single-layer dendrimer light-emitting diodes. Appl. Phys. Lett. 2002, 80, 2645–2647. [Google Scholar] [CrossRef]
- Chang, S.-C.; Liu, J.; Bharathan, J.; Yang, Y.; Onohara, J.; Kido, J. Multicolor Organic Light-Emitting Diodes Processed by Hybrid Inkjet Printing Adv. Mater. 1999, 11, 734–737. [Google Scholar]
- Huang, F.; Cheng, Y.-J.; Zhang, Y.; Liu, M.S.; Jen, A.K.-Y. Crosslinkable hole-transporting materials for solution processed polymer light-emitting diodes. J. Mater. Chem. C 2008, 18, 4495–4509. [Google Scholar] [CrossRef]
- Jeong, C.H.; Godumala, M.; Yoon, J.; Choi, S.; Kim, Y.W.; Choi, D.H.; Cho, M.J.; Choi, D.H. Hole-Transporting Side-Chain Polymer Bearing a Thermally Crosslinkable Bicyclo[4.2.0]octa-1,3,5-trien-3-yl Group for High-Performing Thermally Activated Delayed Fluorescence OLED. ACS Appl. Mater. Interfaces 2019, 11, 17602–17609. [Google Scholar] [CrossRef]
- Ma, B.; Lauterwasser, F.; Deng, L.; Zonte, C.S.; Kim, B.J.; Fréchet, J.M.J. New Thermally Cross-Linkable Polymer and Its Application as a Hole-Transporting Layer for Solution Processed Multilayer Organic Light Emitting Diodes. Chem. Mater. 2007, 19, 4827–4832. [Google Scholar] [CrossRef]
- Park, J.; Lee, C.; Jung, J.; Kang, H.; Kim, K.-H.; Ma, B.; Kim, B.J. Facile Photo-Crosslinking of Azide-Containing Hole-Transporting Polymers for Highly Efficient, Solution-Processed, Multilayer Organic Light Emitting Devices. Adv. Funct. Mater. 2014, 24, 7588–7596. [Google Scholar] [CrossRef]
- Lee, T.-W.; Chung, Y.; Kwon, O.; Park, J.-J. Self-Organized Gradient Hole Injection to Improve the Performance of Polymer Electroluminescent Devices. Adv. Funct. Mater. 2007, 17, 390–396. [Google Scholar] [CrossRef]
- Ha, H.; Shim, Y.J.; Lee, D.H.; Park, E.Y.; Lee, I.-H.; Yoon, S.-K.; Suh, M.C. Highly Efficient Solution-Processed Organic Light-Emitting Diodes Containing a New Cross-linkable Hole Transport Material Blended with Commercial Hole Transport Materials. ACS Appl. Mater. Interfaces 2021, 13, 21954–21963. [Google Scholar] [CrossRef]
- Müller, C.D.; Falcou, A.; Reckefuss, N.; Rojahn, M.; Wiederhirn, V.; Rudati, P.; Frohne, H.; Nuyken, O.; Becker, H.; Meerholz, K. Multi-color organic light-emitting displays by solution processing. Nature 2003, 421, 829–833. [Google Scholar] [CrossRef]
- Jungermann, S.; Riegel, N.; Müller, D.; Meerholz, K.; Nuyken, O. Novel Photo-Cross-Linkable Hole-Transporting Polymers: Synthesis, Characterization, and Application in Organic Light Emitting Diodes. Macromolecules 2006, 39, 8911–8919. [Google Scholar] [CrossRef]
- Yan, H.; Scott, B.J.; Huang, Q.T.; Marks, J. Enhanced Polymer Light-Emitting Diode Performance Using a Crosslinked-Network Electron-Blocking Interlayer. Adv. Mater. 2004, 16, 1948–1953. [Google Scholar] [CrossRef]
- Lee, S.; Lyu, Y.Y.; Lee, S.H. The use of cross-linkable interlayers to improve device performances in blue polymer light-emitting diodes. Synth. Met. 2006, 156, 1004–1009. [Google Scholar] [CrossRef]
- Kwak, S.L.; Park, H.J.; Jang, J.-H.; Park, J.Y.; Park, J.M.; Lee, J.; Hwang, D.-H. Synthesis and characterization of thermally cross-linkable poly(iminoarylene)-based hole injection layer for solution-processed organic light-emitting diodes. Chem. Eng. J. 2023, 454, 139944. [Google Scholar] [CrossRef]
- Huang, C.W.; Chiang, F.-C.; Chu, Y.-L.; Lai, C.-C.; Lin, T.-E.; Zhu, C.-Y.; Kuo, S.-W. A solvent-resistant azide-based hole injection/transporting conjugated polymer for fluorescent and phosphorescent light-emitting diodes. J. Mater. Chem. C 2015, 3, 8142–8151. [Google Scholar] [CrossRef]
- Png, R.-Q.; Chia, P.J.; Tang, J.-C.; Liu, B.; Sivaramakrishnan, S.; Zhou, M.; Khong, S.-H.; Chan, H.S.O.; Burroughes, J.H.; Chua, L.L.; et al. High-performance polymer semiconducting heterostructure devices by nitrene-mediated photocrosslinking of alkyl side chains. Nat. Mater. 2010, 9, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hahm, D.; Kim, K.; Rhee, S.; Lee, M.; Kim, S.; Chang, J.H.; Park, H.W.; Lim, J.; Lee, M.; et al. High-resolution patterning of colloidal quantum dots via non-destructive, light-driven ligand crosslinking. Nat. Commun. 2020, 11, 2874. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Jang, J.-H.; Lee, J.-H.; Hwang, D.-H. Solution-processed organic light-emitting diodes using a photo-crosslinkable hole-trnasporting layer. J. Nanosci. Nanotechnol. 2020, 20, 4661–4665. [Google Scholar] [CrossRef] [PubMed]
- Wharton, S.-I.; Henry, J.B.; McNab, H.; Mount, A.R. The Production and Characterisation of Novel Conducting Redox-Active Oligomeric Thin Films From Electrooxidized Indolo[3,2,1-jk]carbazole. Chem. Eur. J. 2009, 15, 5482–5490. [Google Scholar] [CrossRef] [PubMed]
- Wakim, S.; Bouchard, J.; Simard, M.; Drolet, N.; Tao, Y.; Leclerc, M. Organic Microelectronics: Design, Synthesis, and Characterization of 6,12-Dimethylindolo[3,2-b]Carbazoles Chem. Mater. 2004, 16, 4386–4388. [Google Scholar]
- Li, Y.; Wu, Y.; Gardener, S.; Ong, B.S. Novel Peripherally Substituted Indolo[3,2-b]carbazoles for High-Mobility Organic Thin-Film Transistors. Adv. Mater. 2005, 17, 849–853. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Gar-Dener, S.; Ong, B.S. Indolo[3,2-b]carbazole-Based Thin-Film Transistors with High Mobility and Stability. J. Am. Chem. Soc. 2005, 127, 614–618. [Google Scholar] [CrossRef]
- Ma, X.-J.; Zhu, X.-D.; Wang, K.-L.; Igbari, F.; Yuan, Y.; Zhang, Y.; Gao, C.-H.; Jiang, Z.-Q.; Wang, Z.-K.; Liao, L.-S. Planar starburst hole-transporting materials for highly efficient perovskite solar cells. Nano Energy 2019, 63, 103865. [Google Scholar] [CrossRef]
- Cao, W.; Fang, M.; Chai, Z.; Xu, H.; Duan, T.; Li, Z.; Chen, X.; Qina, J.; Han, H. New D–p–A organic dyes containing a tert-butylcapped indolo[3,2,1-jk]carbazole donor with bithiophene unit as p-linker for dye-sensitized solar cells. RSC Adv. 2015, 5, 32967–32975. [Google Scholar] [CrossRef]
- Luo, C.; Bi, W.; Deng, S.; Zhang, J.; Chen, S.; Li, B.; Liu, Q.; Peng, H.; Chu, J. Indolo[3,2,1-jk]carbazole Derivatives-Sensitized Solar Cells: Effect of π-Bridges on the Performance of Cells. J. Phys. Chem. C 2014, 118, 14211–14217. [Google Scholar] [CrossRef]
- Henry, J.B.; Wharton, S.I.; Wood, E.R.; McNab, H.; Mount, A.R. Specific Indolo[3,2,1-jk]carbazole Conducting Thin-Film Materials Production by Selective Substitution. J. Phys. Chem. A 2011, 115, 5435–5442. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Cho, N.S.; Oh, H.-Y.; Yang, J.H.; Jeon, W.S.; Park, J.S.; Suh, M.C.; Kwon, J.H. Highly Efficient Red Phosphorescent Dopants in Organic Light-Emitting Devices. Adv. Mater. 2011, 23, 2721–2726. [Google Scholar] [CrossRef]
- Usluer, O.; Demic, S.; Egbe, D.A.M.; Birckner, E.; Tozlu, C.; Pivrikas, A.; Ramil, A.M.; Sariciftci, N.S. Fluorene-Carbazole Dendrimers: Synthesis, Thermal, Photophysical and Electroluminescent Device Properties. Adv. Funct. Mater. 2010, 20, 4152–4161. [Google Scholar] [CrossRef]
- Wu, C.-S.; Fang, S.-W.; Chen, Y. Solution-processable hole-transporting material containing fluorenyl core and triple-carbazolyl terminals: Synthesis and application to enhancement of electroluminescence. Phys. Chem. Chem. Phys. 2013, 15, 15121–15127. [Google Scholar] [CrossRef]
- Sundhoro, M.; Park, J.; Wu, B.; Yan, M. Synthesis of Polyphosphazenes by a Fast Perfluoroaryl Azide-Mediated Staudinger Reaction. Macromolecules 2018, 51, 4532–4540. [Google Scholar] [CrossRef]
- Konno, H.; Sasaki, Y. Selective One-pot Synthesis of Facial Tris-ortho-metalated Iridium(III) Complexes Using Microwave Irradiation. Chem. Lett. 2003, 32, 252–253. [Google Scholar] [CrossRef]
- Wang, P.-I.; Tsai, C.-Y.; Hsiao, Y.-J.; Jiang, J.-C.; Liaw, D.-J. High-Purity Semiconducting Single-Walled Carbon Nanotubes via Selective Dispersion in Solution Using Fully Conjugated Polytriarylamines. Macromolecules 2016, 49, 8520–8529. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).